Abstract
Leptomeningeal metastases represent an aggressive stage of cancer with few durable treatment options. Improved understanding of cancer biology, neoplastic reliance on oncogenic driver mutations, and complex immune system interactions have resulted in an explosion in cancer-directed therapy in the last two decades to include small molecule inhibitors and immune checkpoint inhibitors. Most of these therapeutics are underexplored in patients with leptomeningeal metastases, limiting extrapolation of extracranial and even intracranial efficacy outcomes to the unique leptomeningeal space. Further confounding our interpretation of drug activity in the leptomeninges is an incomplete understanding of drug penetration through the blood–cerebrospinal fluid barrier of the choroid plexus. Nevertheless, a number of retrospective studies and promising prospective trials provide evidence of leptomeningeal activity of several small molecule and immune checkpoint inhibitors and underscore potential areas of further therapeutic development for patients harboring leptomeningeal disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Several small molecule inhibitors, such as osimertinib and lorlatinib, demonstrate high penetrance into the cerebrospinal fluid, potent leptomeningeal activity, and superior survival outcomes relative to historical controls. |
Immune checkpoint inhibitors can induce leptomeningeal responses in select patients with immunotherapy-responsive cancers, though the bioactivity of these agents may be hampered by a dysfunctional leptomeningeal immune microenvironment and relatively low cerebrospinal fluid drug penetrance with intravenous administration. |
Clinical trials designed specifically for patients with leptomeningeal metastases, with inclusion of cerebrospinal fluid pharmacokinetic analyses, are needed to define the leptomeningeal bioactivity of novel agents in this patient population. |
1 Introduction
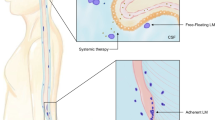
Leptomeningeal metastases (LM) represent an aggressive stage of advanced cancer, defined by the entry of metastatic cancer cells into the cerebrospinal fluid (CSF) [1, 2]. Upon influx into this unique nutrient-sparse microenvironment, LM disseminate along the entire neuraxis as free-floating cells in the CSF and adherent plaques to the brain and spinal cord [3,4,5,6]. The incidence of LM varies by cancer type, ranging from 5-20% based on population and autopsy studies, and is likely increasing as patients live longer with cancer [7,8,9,10]. Historical survival following the diagnosis of LM is 2–5 months [11, 12]. This grim prognosis is, to some extent, improving in the modern era of targeted small molecule inhibitors and immunotherapy. Prior to the advent of modern cancer therapeutics in the last two decades, the treatment of LM from solid tumor malignancies has traditionally involved intrathecal chemotherapy [13,14,15,16,17,18], palliative involved-field radiation therapy to bulky or symptomatic disease [19, 20], and CSF-penetrating systemic chemotherapies [21]. None of these approaches have demonstrated a survival benefit for patients with LM in prospective studies. Very recently, proton craniospinal irradiation has emerged as an efficacious and life-prolonging strategy compared with conventional involved-field radiation [22, 23].
Small molecule inhibitors and immune checkpoint inhibitors have revolutionized the treatment of both localized and advanced cancer, demonstrating a significant improvement in cancer control and patient survival in large, controlled clinical trials. Patients with central nervous system (CNS) metastases, most particularly LM, have been excluded from the majority of these studies for several reasons. First, patients with CNS metastases possess a worse prognosis compared with those with extracranial-only disease, making them challenging to study in a larger patient population for efficacy endpoints. Second, assessing LM response to cancer therapeutics is inherently challenging given the dynamic disease state, and standardized grading systems for LM response have not been fully validated. Third, genomic divergence between the primary tumor and intracranial metastases underscores a potential opportunity for discordant intracranial and extracranial responses [24]. Last and very importantly, the existence of distinct blood–brain (BBB) and blood–CSF (BCSFB) barrier systems leads to uncertainty regarding drug access into the CNS, with inconsistent permeability potential between the brain parenchyma and intrathecal space [25]. For example, the well-established P-glycoprotein drug efflux transporter appears to have opposing functions at the BBB and BCSFB, transporting drugs out of the brain parenchyma but into the CSF [26, 27]. Comparative brain tissue and CSF pharmacokinetic sampling is essential to better understand differential drug transport into these two compartments of the CNS, and one should not assume that lack of BBB penetration has equivalent consequences at the BCSFB (or vice versa).
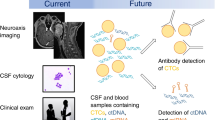
As a result of these limitations, the efficacy of targeted therapies and immune checkpoint blockade in the leptomeningeal space is largely unexplored, with potential therapeutic impact based on case-reported responses, retrospective institutional studies, post-hoc analyses, and small prospective studies designed specifically for patients with CNS metastases. A wide range of CSF drug penetrance has been established for numerous agents (Table 1), though variability in patient characteristics, pharmacokinetic assays, and reporting standards add complexity when making intra-class drug comparisons. Nevertheless, these encouraging studies provide proof-of-concept evidence of leptomeningeal activity and highlight several areas worthy of further investigation.
2 Small Molecule Inhibitors
The oncologic community has seen an enormous shift in cancer-directed therapy from cytotoxic chemotherapy to small molecule inhibitors [62]. Lazertinib is currently under investigation in combination with amivantamab, an EGFR-MET bispecific antibody with immune cell-directing activity [63], in patients with brain and leptomeningeal metastases in a two-arm phase II study (NCT04965090).
2.2 MET Inhibitors
Acquired resistance to EGFR inhibitors in NSCLC is common after 6–12 months of treatment by two non-mutually exclusive mechanisms: secondary resistance mutations (such as exon 20 T790M in 50–60% of patients) and ‘oncogene kinase switch’ pathways (including MET gene amplification in 5–22% of patients) [64].
MET (or c-MET) gene amplification results in upregulation of the MET RTK on the cellular membrane, resulting in signal transduction through the PI3K/Akt and MAPK pathways. Numerous genetic alterations (including point mutations, deletions, insertions, and indels) have also been shown to induce MET exon 14 skip**, which results in impairment in CBL-mediated receptor degradation, subsequent MET receptor accumulation, and aberrant MET oncogene signaling [65]. MET exon 14 mutations occur in approximately 3–4% of patients with NSCLC, seen more commonly in older patients, those with a smoking history, and pleomorphic carcinoma or adenosquamous cell carcinoma subtypes [66].
Two highly selective and brain-penetrant MET inhibitors, capmatinib and tepotinib, are FDA-approved for the treatment of advanced MET exon 14-skip** mutant NSCLC [67, 68]. There are no prospective studies investigating the activity of either MET inhibitor in patients with LM. However, a case report of capmatinib in a patient with MET exon 14 mutant NSCLC demonstrated symptom resolution and LM radiographic improvement after 2 months of treatment [69]. Duration of disease control was not reported. Two case reports also demonstrate leptomeningeal response to tepotinib lasting for at least 5 months [70, 71]. A CSF penetration rate between the two patients was calculated to be 1.2–1.8%, which is greater than the known half-maximal inhibitory concentration (IC50) of tepotinib and therefore suggestive of pharmacokinetic potential in the leptomeninges.
2.3 ALK Inhibitors
Genetic rearrangements affecting the ALK gene resulting in RTK overexpression are also often implicated in tumor biology, present in approximately 5% of patients with NSCLC [72]. ALK-fusion proteins retain a C-terminus tyrosine kinase, joined to a unique protein at the N-terminus, resulting in kinase overactivation and signal transduction of cell survival and proliferation pathways. In the case of NSCLC, the most common ALK rearrangement is the echinoderm microtubule-associated protein-like 4 (EML4)-ALK fusion protein, which is enriched in the young, non-smoker population [73, 74]. Patients with EML4-ALK rearrangements have a higher rate of CNS metastases at both initial presentation and relapse, and approximately 5% of patients will develop LM at an advanced stage of disease [75].
Crizotinib, a first generation ALK and c-ros oncogene 1 (ROS1) inhibitor, was associated with reasonable extracranial disease control but displayed a less robust intracranial response, with the CNS as a new site of disease in 20% of patients at the time of progression [76]. Case reports of leptomeningeal response to crizotinib tend to be disappointing, likely owing to the low CSF-to-plasma drug ratios (range 0.0006–0.026) [77, 78]. Second-generation (ceritinib [79], alectinib [80,81,82,102, 103]. The intracranial activity of sotorasib was demonstrated in a post-hoc analysis of patients with KRAS G12C mutant NSCLC and stable brain metastases in the phase I/II CodeBreaK100 study [104]. Forty patients with asymptomatic, treated brain metastases achieved a median PFS of 5.3 months and OS of 8.3 months following initiation of sotorasib, compared with 6.7 months and 13.6 months, respectively, in 132 patients without baseline brain metastases. Adagrasib, a second covalent inhibitor of KRAS-G12C, offers a longer half-life than sotorasib and demonstrated a comparable intracranial PFS of 5.4 months in patients with stable brain metastases [105]. The intracranial activity of sotorasib and adagrasib against untreated, active brain metastases is also suggested in case reports and preliminary findings from the KRYSTAL-1 study (NCT03785249) [106, 107].
To date, there are no published case reports or prospective studies highlighting the leptomeningeal activity of sotorasib or adagrasib. CSF penetration is confirmed, however, with 2 patients treated with adagrasib in the KRYSTAL-1 study achieving CSF concentrations of 24.2 nM and 34.6 nM at steady state and with correlative regression of their untreated brain metastases [108].
2.5 HER2 Inhibitors
The overexpression of HER2 is present in approximately 30% of patients with breast cancer, propagating cancer cell progression via RTK-mediated PI3K/Akt and MAPK pathway activation [109]. HER2 is also implicated in a number of other primary malignancies, including lung, esophageal, colon, ovarian, and endometrial cancer [110]. Patients with HER2+ breast cancer have high rates of intracranial metastases, likely owing to both intrinsic neurotropism of HER2+ malignancies and the poor BBB penetrance of trastuzumab and pertuzumab, two first-line HER2-targeting monoclonal antibodies [111].The rate of LM in patients with HER2+ breast cancer is estimated between 6-7% [112].
Several small molecule HER2 inhibitors have been developed in attempts to improve outcomes and CNS control, primarily in HER2+ breast cancer. Lapatinib and neratinib represent second-generation HER2-targeting agents that are FDA-approved for the treatment of recurrent HER2+ breast cancer. Lapatinib reversibly targets both HER2 and EGFR, whereas neratinib is an irreversible pan-HER inhibitor [113]. Despite their small molecular weight (suggestive of BBB penetration), the intracranial benefit afforded these agents has been poor as monotherapies, with heterogenous uptake in brain metastasis pharmacokinetic studies [114,115,116,117,118,119]. Lapatinib and neratinib both demonstrate improved intracranial control in prospective studies when in combination with capecitabine, with CNS ORR of 20–66% in trials [114,115,116,117]. The CSF penetration rates of lapatinib (1250 mg single dose, 0.9–1.3%) and neratinib (250 mg for 7–21 days, undetectable < 1.50 ng/mL in CSF) are low in a few pharmacokinetic studies [120, 121]. Case reports of these agents suggest leptomeningeal control lasting for 1–7 months for combination neratinib/capecitabine [117] and 6–12 months for lapatinib/capecitabine [118, 122]; however, attribution is challenging as capecitabine monotherapy is also associated with case-reportable leptomeningeal activity [123,124,125,126].
Tucatinib, a third-generation reversible and highly selective HER2 inhibitor, gained FDA approval in April 2020 after its use was associated with improvements in both CNS-PFS (9.9 vs 4.2 months) and OS (18.1 vs 12.0 months), when used in combination with trastuzumab and capecitabine in those with stable or active brain metastases [35, 127]. While this study excluded patients with LM, a subsequent case report suggested leptomeningeal disease control lasting 10 months with whole brain radiotherapy (WBRT) followed by combination tucatinib and capecitabine in a patient with HER2-activating variant breast cancer [128]. To appropriately investigate this question, a phase II study is currently underway investigating combination tucatinib, trastuzumab, and capecitabine in patients with HER2+ breast cancer and LM (NCT03501979), with encouraging preliminary results supportive of durable leptomeningeal activity [129]. Paired CSF and plasma samples in 15 patients demonstrate detectable tucatinib (range 0.57–25 ng/mL, IC50 3.3 ng/mL) and its metabolite, ONT-993 (range 0.28–4.7 ng/mL), in the CSF as early as 2 hours following tucatinib administration [130], with steady state CSF levels of tucatinib approaching that of unbound plasma levels.
HER2-targeted treatments in breast cancer continue to evolve. Two HER2 antibody-drug conjugates, trastuzumab emtansine (T-DM1) and trastuzumab deruxtecan (T-DXd), have gained FDA approval in recent years and have shown potent intracranial activity [131,132,133,134,135,136,137]. As bulky molecules with large molecular weights (> 100 kDa), the extent to which either agent penetrates the blood–CSF barrier remains to be determined. While these agents are not small molecule inhibitors and therefore beyond the scope of this review, they are worth mentioning as their leptomeningeal activity is an emerging topic of future study (NCT04420598). Additional non-small molecule inhibitor HER2-targeting agents, such as intrathecal trastuzumab [138, 139] and investigational intrathecal HER2 chimeric antigen receptor (CAR) T cells (NCT03696030), have been used in the HER2+ LM arena.
2.6 PARP Inhibitors
The poly(ADP-ribose) polymerase (PARP) family of proteins are involved in the base-excision repair system of DNA single-stranded breaks (SSBs) [140]. The accumulation of SSBs leads to the development of double-stranded DNA breaks, which necessitate different DNA repair pathways such as homologous recombination and nonhomologous end joining. Certain malignancies, namely breast cancer susceptibility proteins 1 (BRCA1) or BRCA2-mutated breast and ovarian cancer, have defective homologous recombination repair enzymes and therefore are more dependent on an intact SSB repair pathway to propagate [141]. The use of PARP inhibitors in this scenario invokes the concept of synthetic lethality, whereby combinatorial disruption of both DNA pathways results in cell death [142].
There are currently four FDA-approved PARP inhibitors (olaparib, rucaparib, niraparib, and talazoparib), with others in development (veliparib), for the treatment of homologous recombination-deficient (HRD) cancers. Breast cancer brain metastases have been shown to contain a higher HRD mutational burden relative to matched primary tumor [143], raising the question of enhanced sensitivity of brain metastases to PARP inhibitors and with activity suggested in small studies and case reports [144,145,146,147]. The use of PARP inhibition is of particular value to patients with triple negative breast cancer, who are enriched in the BRCA1 subtype and oftentimes lack additional targetable therapies [148, 149]. Data regarding the CSF penetration of these agents is lacking, with preclinical suggestion of superior brain and/or CSF penetration of veliparib and niraparib compared with other PARP inhibitors [150, 151]. Veliparib’s CSF penetration rate is calculated to be 57% that of plasma in non-human primates [152]. Case reports have hinted at durable LM responses with olaparib lasting 12–14 months for BRCA-mutated ovarian cancer [153, 154] and 19 months in BRCA-mutated breast cancer patients [155]. Further investigation of both CSF penetration and leptomeningeal activity of PARP inhibitors is warranted.
2.7 Combinatorial Endocrine Therapy and CDK4/6 Inhibition
The addition of cyclin-dependent kinase (CDK)4 and CDK6 inhibitors, such as ribociclib, abemaciclib, and palbociclib, to endocrine therapy (ET) has significantly improved outcomes in patients with hormone receptor (HR)-positive breast cancer. As LM most commonly represents a late stage of advanced cancer, the use of such agents in a heavily pretreated patient population is challenging due to prior exposure to CDK4/6 inhibitors and acquired endocrine resistance.
ET is the therapeutic backbone of HR+ breast cancer patients, with data to suggest BBB penetrability of tamoxifen, letrozole, and anastrozole [156, 157]. Tamoxifen can also be detected in trace amounts in the CSF, with a CSF-to-serum ratio < 0.2 [158]. A few case reports of durable leptomeningeal control with various ET monotherapies have been published (neurologic PFS of 16 months with letrozole, 12 months with exemestane, 10 months for tamoxifen), consistent with CSF penetration [159,160,161,162,163]. The largest study of ET in patients with HR+ brain and leptomeningeal metastases found a retrospective survival benefit among LM patients that received ET (7 vs 3 months), which on multivariate analysis was independent of the use of aromatase inhibitors, tamoxifen, or fulvestrant [164]. Significantly fewer patients with LM received ET compared with those with only parenchymal metastases (26.7% vs 47.6%), likely a consequence of acquired endocrine resistance at later stages of disease. As a result, hormonal therapy is likely insufficient in isolation for most patients with LM and should be used as combinatorial treatment with other agents when possible.
Justification for combinatorial ET and CDK4/6 inhibition strategies arises from the observation that cell dysregulation is a common cause of endocrine resistance [165]. The efficacy of combination ET and CDK4/6 inhibition in patients with HR+ breast, brain and leptomeningeal metastases has been studied in one prospective phase II study, in which 10 patients with HR+ breast cancer LM (HR+ HER2− n = 7; HR+ HER2+ n = 3) received either abemaciclib with or without ET or trastuzumab [166]. CSF was collected at steady state, with roughly equivalent CSF-to-plasma concentrations which exceeded the IC50 for both CDK4 and CDK6 inhibition. The best intracranial response in the HER2− LM cohort was stable disease in 2 of 7 patients, with a combined PFS of 5.9 months (95% CI 0.7–8.6) and a median OS of 8.4 months (95% CI 3.3–23.5). In the HER2+ LM cohort, the best intracranial response was stable disease in 1 patient lasting < 6 months. Of note, cytologic data was only available in 20% of the LM patients on this study at study entry, limiting interpretation of the leptomeningeal burden of disease. Currently, there are no published case reports of the efficacy of ribociclib or palbociclib in LM, despite reports of intracranial activity for parenchymal metastases [167,168,169]. However, the CSF concentration of ribociclib in a phase 0 glioblastoma study at the time of surgery was equal to 0.374 μmol/L, with a CSF-to-plasma unbound ratio of 1.8 (0.6–4.4) [170]. Drug concentration in the CSF was less than that in enhancing and non-enhancing tumor, but still greater than the in vitro CDK4/6 IC50.
2.8 PI3K/Akt/mTOR Inhibitors
In addition to cell cycle dysregulation, resistance to endocrine therapies in breast cancer may also occur through upregulation of the PI3K/Akt/mTOR pathway [171]. In fact, aberrant activation of this pathway occurs in almost every human malignancy. Downstream signaling from the PI3K/Akt/mTOR pathway influences cellular proliferation, motility, metabolism, and angiogenesis [171]. Despite the ubiquitous nature of this master regulator, data supporting the use of any PI3K/Akt/mTOR inhibitor in LM from solid tumor malignancies has been largely disappointing.
Alpelisib, a selective PI3Kα inhibitor, has demonstrated activity in combination with ET for breast cancer brain metastases, with greater clinical activity among those harboring PI3Kα mutations [172,173,174]. Despite FDA approval in 2019 for advanced PIK3CA-mutated HR+HER2− breast cancer, to date there is no published literature on the CSF penetration or leptomeningeal activity of this agent. In clinical practice, the use of PI3K inhibitors is limited in patients with brain metastases due to toxicities of mood disturbances and hyperglycemia, which are both challenging (and often dose-limiting) side effects for patients requiring corticosteroids for cerebral edema [175]. Psychiatric side effects were of particular concern for buparlisib, a CNS-penetrant oral pan-PI3K inhibitor, ultimately leading to discontinuation of study in the breast cancer population [176]. Preliminary results of a dose-finding phase I study of paxalisib, a CNS-penetrant dual PI3K/mTOR inhibitor, in combination with WBRT for patients with brain or leptomeningeal metastases from PI3K-mutated malignancies showed this drug to be well tolerated [177]. The dose expansion arm of this study is currently recruiting (NCT04192981).
The combination of everolimus, a CNS-penetrant mTOR inhibitor, with exemestane is a long-accepted regimen for patients with metastatic HR+HER2− breast cancer following progression on aromatase inhibitors [178]. Despite known CNS activity of everolimus, the brain and leptomeningeal activity of this combination is not well characterized in the literature. The role of everolimus for breast cancer brain metastases has been studied in various chemotherapy-containing combinations [179, 180], with 3-month clinical benefit rate ranging approximately 65–90%, but again with exclusion of patients with LM.
While PI3K and mTOR inhibitors have been previously established as treatment strategies for HR+ breast cancer and with some CNS activity, Akt inhibitors are only recent additions to the investigational landscape. Two small molecule Akt inhibitors, capivasertib and ipatasertib, are under investigation for patients with metastatic HR+ breast cancer [181,182,183]. Further studies are required to determine the brain and leptomeningeal activity of these agents. Of note, preclinical studies suggest that ipatasertib may have CNS-active properties in breast cancer brain metastasis animal models [184] and is also currently under investigation for glioblastoma (NCT03673787).
2.9 BRAF/MEK Inhibitors
Approximately 40–60% of patients with melanoma harbor an activating BRAF mutation, which results in constitutively active cytoplasmic serine–threonine kinase and MAPK pathway [185]. Patients with BRAF-mutated melanoma tend to be younger and with a more aggressive phenotype, with a high frequency of brain metastases [186, 187]. The BRAF/MEK inhibitor combinations, dabrafenib/trametinib and encorafenib/binimetinib, in patients with BRAF-mutant melanoma brain metastases carry an intracranial clinical benefit rate of approximately 60–90% for a duration of 4–8 months [188, 189].
Despite ample data supporting BRAF/MEK inhibitor activity in parenchymal metastases, there are no prospective studies investigating these agents for patients with LM. CSF penetration of vemurafenib and dabrafenib are both predicted to be low based on available data. In an untimed analysis of 6 patients, mean vemurafenib CSF concentrations were 0.47 ± 0.37 mg/L with a CSF-to-plasma ratio of 0.98 ± 0.84% [190]. In non-human primates, CSF penetration of dabrafenib has been calculated as 0.57 ± 0.18% [191]. Nevertheless, case reports suggest leptomeningeal activity of these agents in small patient numbers. The largest retrospective review of this nature reported an OS of 7.2 months in 3 patients treated with BRAF/MEK inhibition alone, and 6.2 and 12.5 months in 2 patients treated with BRAF/MEK inhibition plus immune checkpoint blockade [192]. Vemurafenib monotherapy has been associated with LM-symptom, radiographic and cytologic improvement lasting 4–16 months, with longer duration of control in combination with WBRT [193,194,195]. A similar rapid improvement in LM symptoms and imaging abnormalities was associated with dabrafenib monotherapy, lasting at least 3–4 months [196]. Dabrafenib and trametinib have induced LM disease control for at least 5–9 months in 2 patients [197, 198]. Extrapolation of such data to true LM control rates of BRAF/MEK inhibition is challenging to determine given the high variability in patient characteristics, use of radiation, and prior immunotherapy or BRAF/MEK exposure among these case reports.
Prospective studies of BRAF/MEK inhibition in patients with LM are currently underway, including a phase I investigation of PF-07284890 in combination with binimetinib in patients with intracranial BRAF V600 mutant solid tumors (NCT04543188), and a phase II study of encorafenib, binimetinib, and nivolumab versus ipilimumab and nivolumab in BRAF V600 mutant melanoma and CNS metastases (NCT04511013).
2.10 VEGF Inhibitors
Vascular Endothelial Growth Factor (VEGF) is a master regulator of angiogenesis in carcinogenesis, but also plays a key role in tumor environment immune modulation and cellular proliferation via the MAPK and PI3K/Akt pathways [199]. CSF levels of VEGF are elevated in the presence of LM, which has been associated with inferior response to therapy and poor patient outcomes [200,201,202].
Currently approved VEGF receptor small molecule inhibitors include sorafenib, sunitinib, and pazopanib. Subgroup analysis of the phase III TARGET study of sorafenib versus placebo in patients with renal cell carcinoma revealed a lower rate of brain metastasis development in those who received sorafenib versus placebo (3% vs 12%), suggesting CNS activity [203]. Sorafenib is also a radiosensitizer, and in patients with breast cancer brain metastases was shown to be well tolerated in combination with WBRT with a CNS-PFS of 12.8 months (95% CI 6.7–NR) [204]. There are no available data regarding the CSF penetration of this agent, however a case report of a patient with renal cell carcinoma revealed a sorafenib-induced radiographic LM response lasting at least 10 weeks [205]. Considering the impact of anti-VEGF therapy on gadolinium uptake on MRI, further investigations of VEGF small molecule inhibitors should incorporate both radiographic and cytologic changes to measure response.
2.11 NTRK Inhibitors
Neurotrophic tropomyosin receptor kinase (NTRK) gene fusions are rare oncogenic driver mutations present in a wide variety of cancers [206]. The NTRK1, NTRK2, and NTRK3 genes encode tropomyosin receptor kinase (TRK) receptors TRKA, TRKB, and TRKC, respectively. Under normal conditions, transmembrane TRK receptors are expressed primarily in neuronal tissues. Upon binding of neutrophin growth factors to the extracellular binding domain, TRK receptors dimerize to support neuronal function including neural development, cell growth, and synaptic plasticity. However, in cancer biology, activating fusion mutations of the NTRK gene with a 5′ fusion partner encoding a dimerization domain results in constitutive activation the TRK receptor, with downstream signaling through the MAPK, PI3K, and phospholipase C-ɣ1 pathways. In adults, NTRK gene fusions are enriched primarily in secretory carcinomas of the breast, secretory salivary gland carcinoma, and thyroid cancer, but can also be detected in a minority of patients with more common cancers such as melanoma, colon cancer, lung adenocarcinoma, and various sarcomas [207]. Larotrectinib, a highly selective TRKA/B/C inhibitor, and entrectinib, a multikinase inhibitor with activity against TRKA/B/C, are two CNS-active small molecule inhibitors with demonstrated clinical activity amongst cancers harboring NTRK fusion mutations, including durable responses in primary and metastatic brain tumors [208,209,210].
A few clinical cases and preclinical experiments highlight potential CSF activity of NTRK inhibitors, particularly entrectinib. Rat toxicology modeling suggests superior CSF-to-unbound plasma concentrations of entrectinib (0.22) compared with larotrectinib (0.03) following intravenous administration [211]. One case report outlines a patient with undifferentiated uterine sarcoma who developed widespread LM after treatment with larotrectinib for 3 years [212]. No drug resistance mutations were identified on meningeal biopsy. She subsequently received WBRT and transitioned to entrectinib, but unfortunately experienced neurologic deterioration after 1 month of treatment and transitioned to hospice. CNS progression on larotrectinib therapy was also demonstrated in a patient with NSCLC and an acquired TPM3-NTRK1 fusion mutation, though the authors do not comment on whether the resistance mutation was present in the leptomeninges [213]. The clinical activity of entrectinib was, however, demonstrated in two pediatric patients with CSF-disseminated ROS1/NTRK-fusion high-grade gliomas [214]. A partial radiographic response was observed in 1 patient after initiation of entrectinib monotherapy, and combination of entrectinib with other treatments (including radiation and intrathecal therapy) appeared to be well tolerated with controlled leptomeningeal tumors for at least 5–8 months. Entrectinib concentration in the CSF steadily increased over time, approaching approximately 25 nM after 40 days of treatment in 1 patient. In summary, CSF penetration and activity of entrectinib is suggested in gliomas in small patient numbers, but the performance of either agent in patients with LM from extracranial malignancies remains to be determined.
3 Immune Checkpoint Inhibitors
The immune checkpoint pathways, cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4) and programmed death 1 (PD-1), are two signaling pathways integral to T-cell maturation and the downregulation of T cells reactive to self-antigens. These two molecules compete with costimulatory signals upon T-cell receptor (TCR) binding to the antigen-presenting major histocompatibility complex (MHC). By blocking the costimulatory signal from activating the maturing T-lymphocyte, the CTLA-4 and PD-1 pathways induce T-cell anergy either in the priming phase of T-lymphocyte development within the lymph nodes (for CTLA-4) or in the effector stage in the peripheral tissues (for PD-1). Cancer cells evade this system by downregulating MHC expression and by upregulating the CTLA-4 and PD ligand-1 (PD-L1) molecules. The use of monoclonal antibodies directed against CTLA-4 (ipilimumab), PD-1 (nivolumab, pembrolizumab, cemiplimab), and PD-L1 (atezolizumab, durvalumab, avelumab) inhibitors remove the cancer-expressing “brakes” on the immune system and result in increased T-lymphocyte-mediated destruction. Immune checkpoint inhibitors have confirmed intracranial activity in patients with brain metastases from a wide variety of malignancies, including melanoma [215, 216], NSCLC [217,218,219], renal cell carcinoma [220,221,222], and breast cancer [223, 224]. The combination of intracranial radiation with immunotherapy may augment this response, in part through the radiation-mediated release of cancer antigens [225,226,227].
Despite demonstrable activity of immunotherapy for parenchymal brain metastases, the efficacy of these agents in LM has been slower to crystalize. A few retrospective reviews and prospective studies do provide a suggestion of potential benefit in select patients.
Three phase II studies treated patients with LM with various immune checkpoint blockade regimens. One study for patients with melanoma brain metastases investigated combination ipilimumab/nivolumab versus nivolumab monotherapy for intracranial control, and included a cohort C designed for those with poor prognosis (brain metastasis recurrent after local therapy, neurologic symptoms, and/or presence of LM) to receive nivolumab monotherapy [228]. Four of the 16 patients in this cohort had LM; none of them responded intracranially to nivolumab monotherapy, and the best intracranial response was progressive disease in 81% of the entire cohort. A second phase II study studied pembrolizumab monotherapy in patients with solid tumor LM, which included predominantly breast cancer patients [229]. The median OS was 3.6 months (90% CI 2.2–5.2), with a trend for higher pre-treatment CSF lymphocyte percentage among those surviving longer than 3 months. No difference in survival was observed on the basis of HR or HER2 status. The best intracranial response to pembrolizumab was stable disease in 11 of 16 evaluable patients. A third study investigated combination ipilimumab and nivolumab in 18 patients with LM from solid tumor malignancies, with breast cancer representing 44% of the study population [230]. The median OS was 2.9 months (90% CI 1.6–5.0) with a median intracranial PFS of 1.93 months (90% CI 1.28–2.66 months).
Given the lack of lung cancer representation in the available prospective studies, a retrospective review of 19 patients with NSCLC LM treated with immune checkpoint inhibitors was performed across seven European institutions [231]. Lung cancer patients obtained a median PFS of 2.0 months (range 1.8–2.2) and a median OS of 3.7 months (range 0.9–6.6) with immune checkpoint blockade. Patients classified as ‘good risk’ by the National Comprehensive Cancer Center Network LM prognostic classification had a longer 6-month PFS compared with those considered ‘poor risk’ (40% vs 0%), however without a significant difference in 6- and 12-month survival rates between the two groups. Neurologic symptoms only improved in 1 patient, with all others with stable or worsening condition while on treatment. Combining immunotherapy with other agents might yield a more robust response in this patient population. A case report of the IMpower150 (IMP150) regimen (atezolizumab, bevacizumab, paclitaxel, carboplatin) in a patient with ‘good risk’ PD-L1-positive NSCLC suggests improvement in clinical, radiographic, and cytologic abnormalities lasting for at least 6 cycles with ongoing response [232]. The leptomeningeal activity of IMP150 is further supported by a multi-institutional retrospective review including 21 patients with NSCLC LM: ORR was 43%, DCR was 81%, PFS was 4.3 months (95% CI 3.5–9.9), and median OS was 7.1 months (95% CI 4.6–14.0) [233]. The authors refrain from commenting on the rate of CSF conversion, owing to the logistical challenge of measuring leptomeningeal responses in an uncontrolled retrospective review; however, the durable OS when employing combination therapy is encouraging and suggests superiority over regimens using immunotherapy alone.
Certain trends have emerged in a systematic review of 61 patients with solid tumor LM across 14 published studies treated with immune checkpoint inhibitors, alone or in combination with other treatments [234]. Median PFS and OS were 5.1 and 6.3 months, respectively, but with significantly lower survival among patients treated with steroids. There were no statistically significant differences in survival outcomes between tumor types. Immunotherapy adverse events were found in 68.7% of patients, however the majority of these toxicities were mild and self-limited.
Intravenous administration of immune checkpoint inhibitors results in some penetration of drug into the CSF. Paired CSF and serum levels of steady-state pembrolizumab suggests a mean CSF-to-plasma ratio of only 0.009 (95% CI 0.004–0.014) in patients with glioblastoma [235]. However, this small concentration of drug remained capable of reducing PD-1-expressing T-lymphocyte percentage in the leptomeninges from 39.3% to 3.8%, suggestive of biochemical activity in the CSF. In patients with solid tumor CNS metastases receiving nivolumab, the CSF nivolumab concentration ranged from 14.5 ng/mL to 304 ng/mL, with again low corresponding CSF-to-plasma ratios [236]. Given the lack of more robust responses in prospective studies, intrathecal delivery of immune checkpoint inhibitors to increase CSF drug concentration is one potential strategy to improve patient outcomes. The concept of direct CSF T-lymphocyte activation is also not a unique concept; previous investigations of intrathecal IL-2 in patients with melanoma LM resulted in a median survival of 7.8 months (range 0.4–90.8) but with transient intracranial pressure complications [237]. Intrathecal nivolumab is currently being investigated in patients with solid tumor LM (NCT05112549) and in combination with intravenous nivolumab for melanoma LM (NCT03025256). Preliminary data suggests intrathecal nivolumab to be well tolerated with potential clinical benefit by current survival analysis [238]. The compassionate use of intrathecal pembrolizumab in a patient with triple negative breast cancer LM demonstrated tolerability without acute infusion reactions after two cycles of intrathecal drug administration, however the patient died 3 weeks later due to progressive neurologic symptoms [239].
In addition to the drug penetration and durability issues faced by all leptomeningeal-directed therapies, the activity of immune checkpoint blockade in LM also requires a functional immune system within the CSF, a topic of much debate. The presence of cancer cells in the leptomeninges certainly invokes an inflammatory response of both lymphoid and myeloid lineage [240], hence the historical term ‘neoplastic meningitis’ to describe this clinical syndrome. However, single cell sequencing of melanoma-containing skin, brain, and CSF samples reveals three immunologically distinct microenvironments, with the leptomeninges generally harboring a more immunosuppressive phenotype enriched with exhausted or inactivated CD4 and CD8 cells [241]. Immune checkpoint inhibition is associated with an abundance of CSF CD8+ T-lymphocytes with a proliferating phenotype compared with pre-treatment baseline [242]. These CD8+ T-lymphocytes demonstrate heightened gene expression related to antitumoral IFN-γ signaling and effector function, correlating with IFN-γ response within the tumor cells at equivalent time points. Further investigation into anatomic site-specific leptomeningeal immune responses to immune checkpoint blockade is warranted, with particular attention on to what degree this therapy can ‘revitalize’ exhausted T-lymphocytes in the intrathecal space and achieve leptomeningeal responses.
In conclusion, a wide spectrum of clinical outcomes is evident when comparing the relatively shorter survival in the prospective studies with superior outcomes in larger retrospective reviews, leading to several hypotheses. Survival estimates of 2–4 months in prospective studies with intravenous immune checkpoint blockade mirrors the historical survival benchmarks in the pre-immunotherapy era and underperforms relative to targeted therapy with small molecule inhibitors. While this suggests leptomeningeal bioactivity, it also raises questions regarding the potency of this strategy in isolation. Patient selection is also critically important. The retrospective reviews tended to be enriched in patients with known immunotherapy-responsive tumors and saw higher rates of immune checkpoint combinations with chemotherapy, anti-angiogenic agents, or immediately following radiation therapy. The relatively low CSF penetrability of immune checkpoint inhibitors into the CSF, compounded by dysfunctional and paucicellular immune repertoire in the leptomeninges, underscores the need for innovative strategies to amplify immunotherapeutic responses in the spinal fluid. In addition to intrathecal immune checkpoint inhibitor approaches (NCT05112549, NCT03025256), several other immunotherapy combinatorial strategies are under investigation for patients with LM, such as combination with WBRT (NCT03719768), the multi-kinase VEGFR inhibitor lenvatinib (NCT04729348), encorafenib and binimetinib (NCT04511013), and EGFR inhibition (NCT04833205).
4 Conclusion
Modern cancer therapeutics have evolved tremendously in the last two decades since the FDA approval of several small molecule inhibitors and immune checkpoint inhibitors for several malignancies, primarily lung, breast, and melanoma. A number of these agents have demonstrable activity in the leptomeningeal space based on retrospective series and select clinical trials designed specifically for patients with LM, providing a glimmer of hope for patients with historically poor outcomes and high unmet need. This benefit is most impressively demonstrated in patients with solid tumors harboring targetable driver mutations, such as EGFR-mutant and ALK/ROS1-positive NSCLC, with robust improvements in clinical performance status and unprecedented survival benefits for select patients with LM. Ongoing studies will hopefully soon illustrate whether this benefit may also be seen using modern HER2-targeting strategies in patients with HER2+ breast cancer. Considering the high penetrance of TKIs into the leptomeningeal space as demonstrated in a few pharmacokinetic studies, the incorporation of such treatment strategies in patients with LM should be prioritized whenever possible. The extrapolation of immunotherapeutic approaches in patients with LM have been slower to crystalize, likely due to the relatively lower penetrance of immune checkpoint inhibitors into the CSF, the dysfunctional immune microenvironment in the leptomeninges, the need for optimal patient selection with immunotherapy-responsive tumors, and consideration of combinatorial strategies for this patient population.
As the neuro-oncologic community continues to develop clinical trials dedicated to patients with CNS metastases, further prospective studies of both existing and emerging oncogene- and immune-targeted therapies will soon illuminate the efficacy of these modern therapeutics in the leptomeningeal space. Pharmacokinetic sampling of matched CSF, plasma, and, when appropriate, brain metastasis tissue should always be considered in clinical trial design in order to define drug permeability through the unique blood–brain and blood–CSF barriers. Investigation of these agents in combination with our currently available therapies for LM, such as intrathecal chemotherapy and radiation, is also essential to devise the optimal sequential treatment strategy in this subset of patients and determine when potentially neurotoxic standard therapies might even be delayed or deferred in favor of modern CNS-active therapies.
References
Le Rhun E, Weller M, Brandsma D, Van den Bent M, de Azambuja E, Henriksson R, et al. EANO-ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up of patients with leptomeningeal metastasis from solid tumours. Ann Oncol. 2017;28(suppl_4):iv84–99.
Chamberlain M, Junck L, Brandsma D, Soffietti R, Rudà R, Raizer J, et al. Leptomeningeal metastases: a RANO proposal for response criteria. Neuro Oncol. 2016;19(4):484–92.
Boire A, Zou Y, Shieh J, Macalinao DG, Pentsova E, Massagué J. Complement component 3 adapts the cerebrospinal fluid for leptomeningeal metastasis. Cell. 2017;168(6):1101-1113.e13.
Kokkoris CP. Leptomeningeal carcinomatosis. How does cancer reach the pia-arachnoid? Cancer. 1983;51(1):154–60.
Yao H, Price TT, Cantelli G, Ngo B, Warner MJ, Olivere L, et al. Leukaemia hijacks a neural mechanism to invade the central nervous system. Nature. 2018;560(7716):55–60.
Remsik J, Chi Y, Tong X, Sener U, Derderian C, Park A, et al. Leptomeningeal metastatic cells adopt two phenotypic states. Cancer Rep. 2020;5: e1236.
Patchell RA, Posner JB. Neurologic complications of systemic cancer. Neurol Clin. 1985;3(4):729–50.
Jeyapalan SA, Batchelor TT. Diagnostic evaluation of neurologic metastases. Cancer Invest. 2000;18(4):381–94.
Glass JP, Melamed M, Chernik NL, Posner JB. Malignant cells in cerebrospinal fluid (CSF): the meaning of a positive CSF cytology. Neurology. 2011;77(20):1793.
Kesari S, Batchelor TT. Leptomeningeal metastases. Neurol Clin. 2003;21(1):25–66.
Le Rhun E, Devos P, Weller J, Seystahl K, Mo F, Compter A, et al. Prognostic validation and clinical implications of the EANO ESMO classification of leptomeningeal metastasis from solid tumors. Neuro Oncol. 2021;23(7):1100–12.
Clarke JL, Perez HR, Jacks LM, Panageas KS, Deangelis LM. Leptomeningeal metastases in the MRI era. Neurology. 2010;74(18):1449–54.
Grossman SA, Finkelstein DM, Ruckdeschel JC, Trump DL, Moynihan T, Ettinger DS. Randomized prospective comparison of intraventricular methotrexate and thiotepa in patients with previously untreated neoplastic meningitis. Eastern Cooperative Oncology Group. J Clin Oncol. 1993;11(3):561–9.
Glantz MJ, Jaeckle KA, Chamberlain MC, Phuphanich S, Recht L, Swinnen LJ, et al. A randomized controlled trial comparing intrathecal sustained-release cytarabine (DepoCyt) to intrathecal methotrexate in patients with neoplastic meningitis from solid tumors. Clin Cancer Res. 1999;5(11):3394–402.
Hitchins RN, Bell DR, Woods RL, Levi JA. A prospective randomized trial of single-agent versus combination chemotherapy in meningeal carcinomatosis. J Clin Oncol. 1987;5(10):1655–62.
Gutin PH, Levi JA, Wiernik PH, Walker MD. Treatment of malignant meningeal disease with intrathecal thioTEPA: a phase II study. Cancer Treat Rep. 1977;61(5):885–7.
Groves MD, Glantz MJ, Chamberlain MC, Baumgartner KE, Conrad CA, Hsu S, et al. A multicenter phase II trial of intrathecal topotecan in patients with meningeal malignancies. Neuro Oncol. 2008;10(2):208–15.
Le Rhun E, Wallet J, Mailliez A, Le Deley MC, Rodrigues I, Boulanger T, et al. Intrathecal liposomal cytarabine plus systemic therapy versus systemic chemotherapy alone for newly diagnosed leptomeningeal metastasis from breast cancer. Neuro Oncol. 2020;22(4):524–38.
El Shafie RA, Böhm K, Weber D, Lang K, Schlaich F, Adeberg S, et al. Palliative radiotherapy for leptomeningeal carcinomatosis–analysis of outcome, prognostic factors, and symptom response. Front Oncol. 2019;8:641.
Buszek SM, Chung C. Radiotherapy in leptomeningeal disease: a systematic review of randomized and non-randomized trials. Front Oncol. 2019;9:1224.
Kapke JT, Schneidewend RJ, Jawa ZA, Huang CC, Connelly JM, Chitambar CR. High-dose intravenous methotrexate in the management of breast cancer with leptomeningeal disease: case series and review of the literature. Hematol Oncol Stem Cell Ther. 2019;12(4):189–93.
Yang TJ, Wijetunga NA, Yamada J, Wolden S, Mehallow M, Goldman DA, et al. Clinical trial of proton craniospinal irradiation for leptomeningeal metastases. Neuro Oncol. 2021;23(1):134–43.
Yang JT, Wijetunga NA, Pentsova E, Wolden S, Young RJ, Correa D, et al. Randomized phase II trial of proton craniospinal irradiation versus photon involved-field radiotherapy for patients with solid tumor leptomeningeal metastasis. J Clin Oncol. 2022. https://doi.org/10.1200/JCO.22.01148.
Brastianos PK, Carter SL, Santagata S, Cahill DP, Taylor-Weiner A, Jones RT, et al. Genomic characterization of brain metastases reveals branched evolution and potential therapeutic targets. Cancer Discov. 2015;5(11):1164–77.
Jacus MO, Daryani VM, Harstead KE, Patel YT, Throm SL, Stewart CF. Pharmacokinetic properties of anticancer agents for the treatment of central nervous system tumors: update of the literature. Clin Pharmacokinet. 2016;55(3):297–311.
Rao VV, Dahlheimer JL, Bardgett ME, Snyder AZ, Finch RA, Sartorelli AC, et al. Choroid plexus epithelial expression of MDR1 P glycoprotein and multidrug resistance-associated protein contribute to the blood-cerebrospinal-fluid drug-permeability barrier. Proc Natl Acad Sci USA. 1999;96(7):3900–5.
Cordon-Cardo C, O’Brien JP, Casals D, Rittman-Grauer L, Biedler JL, Melamed MR, et al. Multidrug-resistance gene (P-glycoprotein) is expressed by endothelial cells at blood-brain barrier sites. Proc Natl Acad Sci USA. 1989;86(2):695–8.
Zhong L, Li Y, **ong L, Wang W, Wu M, Yuan T, et al. Small molecules in targeted cancer therapy: advances, challenges, and future perspectives. Signal Transduct Target Ther. 2021;6(1):201.
Rubin BP, Duensing A. Mechanisms of resistance to small molecule kinase inhibition in the treatment of solid tumors. Lab Invest. 2006;86(10):981–6.
Roskoski R Jr. Properties of FDA-approved small molecule protein kinase inhibitors: a 2022 update. Pharmacol Res. 2022;175: 106037.
Paluch-Shimon S, Cardoso F. PARP inhibitors coming of age. Nat Rev Clin Oncol. 2021;18(2):69–70.
Peng Y, Wang Y, Zhou C, Mei W, Zeng C. PI3K/Akt/mTOR pathway and its role in cancer therapeutics: are we making headway? Front Oncol. 2022;12: 819128.
Ramalingam SS, Vansteenkiste J, Planchard D, Cho BC, Gray JE, Ohe Y, et al. Overall survival with osimertinib in untreated, EGFR-mutated advanced NSCLC. N Engl J Med. 2019;382(1):41–50.
Shaw AT, Bauer TM, de Marinis F, Felip E, Goto Y, Liu G, et al. First-line lorlatinib or crizotinib in advanced ALK-positive lung cancer. N Engl J Med. 2020;383(21):2018–29.
Murthy RK, Loi S, Okines A, Paplomata E, Hamilton E, Hurvitz SA, et al. Tucatinib, trastuzumab, and capecitabine for HER2-positive metastatic breast cancer. N Engl J Med. 2019;382(7):597–609.
Robert C, Karaszewska B, Schachter J, Rutkowski P, Mackiewicz A, Stroiakovski D, et al. Improved overall survival in melanoma with combined dabrafenib and trametinib. N Engl J Med. 2014;372(1):30–9.
Seshacharyulu P, Ponnusamy MP, Haridas D, Jain M, Ganti AK, Batra SK. Targeting the EGFR signaling pathway in cancer therapy. Expert Opin Ther Targets. 2012;16(1):15–31.
Sharma SV, Bell DW, Settleman J, Haber DA. Epidermal growth factor receptor mutations in lung cancer. Nat Rev Cancer. 2007;7(3):169–81.
Hatanpaa KJ, Burma S, Zhao D, Habib AA. Epidermal growth factor receptor in glioma: signal transduction, neuropathology, imaging, and radioresistance. Neoplasia. 2010;12(9):675–84.
Kumari N, Singh S, Haloi D, Mishra SK, Krishnani N, Nath A, et al. Epidermal growth factor receptor mutation frequency in squamous cell carcinoma and its diagnostic performance in cytological samples: a molecular and immunohistochemical study. World J Oncol. 2019;10(3):142–50.
Melosky B, Kambartel K, Häntschel M, Bennetts M, Nickens DJ, Brinkmann J, et al. Worldwide prevalence of epidermal growth factor receptor mutations in non-small cell lung cancer: a meta-analysis. Mol Diagn Ther. 2022;26(1):7–18.
Ge M, Zhuang Y, Zhou X, Huang R, Liang X, Zhan Q. High probability and frequency of EGFR mutations in non-small cell lung cancer with brain metastases. J Neurooncol. 2017;135(2):413–8.
Li YS, Jiang BY, Yang JJ, Tu HY, Zhou Q, Guo WB, et al. Leptomeningeal metastases in patients with NSCLC with EGFR mutations. J Thorac Oncol. 2016;11(11):1962–9.
Togashi Y, Masago K, Masuda S, Mizuno T, Fukudo M, Ikemi Y, et al. Cerebrospinal fluid concentration of gefitinib and erlotinib in patients with non-small cell lung cancer. Cancer Chemother Pharmacol. 2012;70(3):399–405.
Katayama T, Shimizu J, Suda K, Onozato R, Fukui T, Ito S, et al. Efficacy of erlotinib for brain and leptomeningeal metastases in patients with lung adenocarcinoma who showed initial good response to gefitinib. J Thorac Oncol. 2009;4(11):1415–9.
Lee E, Keam B, Kim DW, Kim TM, Lee SH, Chung DH, et al. Erlotinib versus gefitinib for control of leptomeningeal carcinomatosis in non-small-cell lung cancer. J Thorac Oncol. 2013;8(8):1069–74.
Jackman DM, Holmes AJ, Lindeman N, Wen PY, Kesari S, Borras AM, et al. Response and resistance in a non-small-cell lung cancer patient with an epidermal growth factor receptor mutation and leptomeningeal metastases treated with high-dose gefitinib. J Clin Oncol. 2006;24(27):4517–20.
Jackman DM, Cioffredi LA, Jacobs L, Sharmeen F, Morse LK, Lucca J, et al. A phase I trial of high dose gefitinib for patients with leptomeningeal metastases from non-small cell lung cancer. Oncotarget. 2015;6(6):4527–36.
Tamiya A, Tamiya M, Nishihara T, Shiroyama T, Nakao K, Tsuji T, et al. Cerebrospinal fluid penetration rate and efficacy of afatinib in patients with EGFR mutation-positive non-small cell lung cancer with leptomeningeal carcinomatosis: a multicenter prospective study. Anticancer Res. 2017;37(8):4177–82.
Jänne PA, Yang JC-H, Kim D-W, Planchard D, Ohe Y, Ramalingam SS, et al. AZD9291 in EGFR inhibitor-resistant non-small-cell lung cancer. N Engl J Med. 2015;372(18):1689–99.
Soria J-C, Ohe Y, Vansteenkiste J, Reungwetwattana T, Chewaskulyong B, Lee KH, et al. Osimertinib in untreated EGFR-mutated advanced non-small-cell lung cancer. N Engl J Med. 2017;378(2):113–25.
Kim D, Yang J, Cross D, Ballard P, Yang P, Yates J, et al. Preclinical evidence and clinical cases of Azd9291 activity in Egfr-mutant non-small cell lung cancer (Nsclc) brain metastases (Bm). Ann Oncol. 2014;25:iv152.
Ballard P, Yates JWT, Yang Z, Kim D-W, Yang JC-H, Cantarini M, et al. Preclinical comparison of osimertinib with other EGFR-TKIs in EGFR-mutant NSCLC brain metastases models, and early evidence of clinical brain metastases activity. Clin Cancer Res. 2016;22(20):5130–40.
Varrone A, Varnäs K, Jucaite A, Cselényi Z, Johnström P, Schou M, et al. A PET study in healthy subjects of brain exposure of (11)C-labelled osimertinib—a drug intended for treatment of brain metastases in non-small cell lung cancer. J Cereb Blood Flow Metab. 2020;40(4):799–807.
Wu YL, Ahn MJ, Garassino MC, Han JY, Katakami N, Kim HR, et al. CNS efficacy of osimertinib in patients with T790M-positive advanced non-small-cell lung cancer: data from a randomized phase III trial (AURA3). J Clin Oncol. 2018;36(26):2702–9.
Goss G, Tsai CM, Shepherd FA, Ahn MJ, Bazhenova L, Crinò L, et al. CNS response to osimertinib in patients with T790M-positive advanced NSCLC: pooled data from two phase II trials. Ann Oncol. 2018;29(3):687–93.
Nanjo S, Hata A, Okuda C, Kaji R, Okada H, Tamura D, et al. Standard-dose osimertinib for refractory leptomeningeal metastases in T790M-positive EGFR-mutant non-small cell lung cancer. Br J Cancer. 2018;118(1):32–7.
Ahn MJ, Chiu CH, Cheng Y, Han JY, Goldberg SB, Greystoke A, et al. Osimertinib for patients with leptomeningeal metastases associated with EGFR T790M-positive advanced NSCLC: the AURA leptomeningeal metastases analysis. J Thorac Oncol. 2020;15(4):637–48.
Yang JCH, Kim SW, Kim DW, Lee JS, Cho BC, Ahn JS, et al. Osimertinib in patients with epidermal growth factor receptor mutation-positive non-small-cell lung cancer and leptomeningeal metastases: the BLOOM study. J Clin Oncol. 2020;38(6):538–47.
Park S, Lee MH, Seong M, Kim ST, Kang JH, Cho BC, et al. A phase II, multicenter, two cohort study of 160 mg osimertinib in EGFR T790M-positive non-small-cell lung cancer patients with brain metastases or leptomeningeal disease who progressed on prior EGFR TKI therapy. Ann Oncol. 2020;31(10):1397–404.
**ng L, Pan Y, Shi Y, Shu Y, Feng J, Li W, et al. Biomarkers of osimertinib response in patients with refractory, EGFR-T790M–positive non-small cell lung cancer and central nervous system metastases: the APOLLO study. Clin Cancer Res. 2020;26(23):6168–75.
Nagasaka M, Zhu VW, Lim SM, Greco M, Wu F, Ou S-HI. Beyond osimertinib: the development of third-generation EGFR tyrosine kinase inhibitors for advanced EGFR+ NSCLC. J Thorac Oncol. 2021;16(5):740–63.
Park K, Haura EB, Leighl NB, Mitchell P, Shu CA, Girard N, et al. Amivantamab in EGFR Exon 20 insertion-mutated non-small-cell lung cancer progressing on platinum chemotherapy: initial results from the CHRYSALIS phase I study. J Clin Oncol. 2021;39(30):3391–402.
Zhang Z, Yang S, Wang Q. Impact of MET alterations on targeted therapy with EGFR-tyrosine kinase inhibitors for EGFR-mutant lung cancer. Biomark Res. 2019;7(1):27.
Awad MM, Lee JK, Madison R, Classon A, Kmak J, Frampton GM, et al. Characterization of 1,387 NSCLCs with MET exon 14 (METex14) skip** alterations (SA) and potential acquired resistance (AR) mechanisms. J Clin Oncol. 2020;38(15_suppl):9511–9511.
Awad MM, Oxnard GR, Jackman DM, Savukoski DO, Hall D, Shivdasani P, et al. MET Exon 14 mutations in non-small-cell lung cancer are associated with advanced age and stage-dependent MET genomic amplification and c-Met overexpression. J Clin Oncol. 2016;34(7):721–30.
Wolf J, Seto T, Han J-Y, Reguart N, Garon EB, Groen HJM, et al. Capmatinib in MET Exon 14–mutated or MET-amplified non-small-cell lung cancer. N Engl J Med. 2020;383(10):944–57.
Paik PK, Felip E, Veillon R, Sakai H, Cortot AB, Garassino MC, et al. Tepotinib in non-small-cell lung cancer with MET Exon 14 skip** mutations. N Engl J Med. 2020;383(10):931–43.
Cravero P, Vaz N, Ricciuti B, Clifford SE, DiUbaldi G, Drevers D, et al. Leptomeningeal response to capmatinib after progression on crizotinib in a patient with MET Exon 14-mutant NSCLC. JTO Clin Res Rep. 2020;1(4):100072–100072.
Ninomaru T, Okada H, Fujishima M, Irie K, Fukushima S, Hata A. Lazarus response to tepotinib for leptomeningeal metastases in a patient with MET Exon 14 skip** mutation-positive lung adenocarcinoma: case report. JTO Clin Res Rep. 2021;2(3): 100145.
Tanaka H, Taima K, Makiguchi T, Nakagawa J, Niioka T, Tasaka S. Activity and bioavailability of tepotinib for leptomeningeal metastasis of NSCLC with MET exon 14 skip** mutation. Cancer Commun. 2021;41(1):83–7.
Chia PL, Mitchell P, Dobrovic A, John T. Prevalence and natural history of ALK positive non-small-cell lung cancer and the clinical impact of targeted therapy with ALK inhibitors. Clin Epidemiol. 2014;6:423–32.
Soda M, Choi YL, Enomoto M, Takada S, Yamashita Y, Ishikawa S, et al. Identification of the transforming EML4–ALK fusion gene in non-small-cell lung cancer. Nature. 2007;448(7153):561–6.
Shaw AT, Yeap BY, Mino-Kenudson M, Digumarthy SR, Costa DB, Heist RS, et al. Clinical features and outcome of patients with non-small-cell lung cancer who harbor EML4-ALK. J Clin Oncol. 2009;27(26):4247–53.
Gainor JF, Ou S-HI, Logan J, Borges LF, Shaw AT. The central nervous system as a sanctuary site in ALK-positive non–small-cell lung cancer. J Thorac Oncol. 2013;8(12):1570–3.
Costa DB, Shaw AT, Ou SH, Solomon BJ, Riely GJ, Ahn MJ, et al. Clinical experience with crizotinib in patients with advanced ALK-rearranged non-small-cell lung cancer and brain metastases. J Clin Oncol. 2015;33(17):1881–8.
Okimoto T, Tsubata Y, Hotta T, Hamaguchi M, Nakao M, Hamaguchi SI, et al. A low crizotinib concentration in the cerebrospinal fluid causes ineffective treatment of anaplastic lymphoma kinase-positive non-small cell lung cancer with carcinomatous meningitis. Intern Med. 2019;58(5):703–5.
Costa DB, Kobayashi S, Pandya SS, Yeo W-L, Shen Z, Tan W, et al. CSF concentration of the anaplastic lymphoma kinase inhibitor crizotinib. J Clin Oncol. 2011;29(15):e443–5.
Crinò L, Ahn MJ, De Marinis F, Groen HJ, Wakelee H, Hida T, et al. Multicenter phase II study of whole-body and intracranial activity with ceritinib in patients with ALK-rearranged non-small-cell lung cancer previously treated with chemotherapy and crizotinib: results from ASCEND-2. J Clin Oncol. 2016;34(24):2866–73.
Gadgeel SM, Shaw AT, Govindan R, Gandhi L, Socinski MA, Camidge DR, et al. Pooled analysis of CNS response to alectinib in two studies of pretreated patients with ALK-positive non-small-cell lung cancer. J Clin Oncol. 2016;34(34):4079–85.
Gadgeel SM, Gandhi L, Riely GJ, Chiappori AA, West HL, Azada MC, et al. Safety and activity of alectinib against systemic disease and brain metastases in patients with crizotinib-resistant ALK-rearranged non-small-cell lung cancer (AF-002JG): results from the dose-finding portion of a phase 1/2 study. Lancet Oncol. 2014;15(10):1119–28.
Gainor JF, Sherman CA, Willoughby K, Logan J, Kennedy E, Brastianos PK, et al. Alectinib salvages CNS relapses in ALK-positive lung cancer patients previously treated with crizotinib and ceritinib. J Thorac Oncol. 2015;10(2):232–6.
Zou Z, **ng P, Hao X, Wang Y, Song X, Shan L, et al. Intracranial efficacy of alectinib in ALK-positive NSCLC patients with CNS metastases-a multicenter retrospective study. BMC Med. 2022;20(1):12.
Camidge DR, Kim DW, Tiseo M, Langer CJ, Ahn MJ, Shaw AT, et al. Exploratory analysis of brigatinib activity in patients with anaplastic lymphoma kinase-positive non-small-cell lung cancer and brain metastases in two clinical trials. J Clin Oncol. 2018;36(26):2693–701.
Geraud A, Mezquita L, Bigot F, Caramella C, Planchard D, Le Pechoux C, et al. Prolonged leptomeningeal responses with brigatinib in two heavily pretreated <em>ALK</em>-rearranged non-small cell lung cancer patients. J Thorac Oncol. 2018;13(11):e215–7.
Gaye E, Geier M, Bore P, Guilloïque M, Lucia F, Quéré G, et al. Intra-cranial efficacy of brigatinib in an ALK-positive non-small cell lung cancer patient presenting leptomeningeal carcinomatosis. Lung Cancer. 2019;133:1–3.
Shaw AT, Felip E, Bauer TM, Besse B, Navarro A, Postel-Vinay S, et al. Lorlatinib in non-small-cell lung cancer with ALK or ROS1 rearrangement: an international, multicentre, open-label, single-arm first-in-man phase 1 trial. Lancet Oncol. 2017;18(12):1590–9.
Bauer TM, Shaw AT, Johnson ML, Navarro A, Gainor JF, Thurm H, et al. Brain penetration of lorlatinib: cumulative incidences of CNS and non-CNS progression with lorlatinib in patients with previously treated ALK-positive non-small-cell lung cancer. Target Oncol. 2020;15(1):55–65.
Solomon BJ, Besse B, Bauer TM, Felip E, Soo RA, Camidge DR, et al. Lorlatinib in patients with ALK-positive non-small-cell lung cancer: results from a global phase 2 study. Lancet Oncol. 2018;19(12):1654–67.
Felip E, Shaw AT, Bearz A, Camidge DR, Solomon BJ, Bauman JR, et al. Intracranial and extracranial efficacy of lorlatinib in patients with <em>ALK</em>-positive non-small-cell lung cancer previously treated with second-generation ALK TKIs. Ann Oncol. 2021;32(5):620–30.
Collier TL, Normandin MD, Stephenson NA, Livni E, Liang SH, Wooten DW, et al. Synthesis and preliminary PET imaging of 11C and 18F isotopologues of the ROS1/ALK inhibitor lorlatinib. Nat Commun. 2017;8(1):15761.
Bauer T, Shaw A, Johnson M, Navarro A, Gainor J, Thurm H, et al. MA08.05 brain penetration of lorlatinib and cumulative incidence rates for CNS and non CNS progression from a phase 1/2 study. J Thorac Oncol. 2018;13(10):S382–3.
Sun S, Pithavala YK, Martini J-F, Chen J. Evaluation of lorlatinib cerebrospinal fluid concentrations in relation to target concentrations for anaplastic lymphoma kinase (ALK) inhibition. J Clin Pharmacol. 2022;62(9):1170–6.
Li Z, Li P, Yan B, Gao Q, Jiang X, Zhan Z, et al. Sequential ALK inhibitor treatment benefits patient with leptomeningeal metastasis harboring non-EML4-ALK rearrangements detected from cerebrospinal fluid: a case report. Thorac Cancer. 2020;11(1):176–80.
Sun MG, Kim IY, Kim YJ, Jung TY, Moon KS, Jung S, et al. Lorlatinib therapy for rapid and dramatic control of brain and spinal leptomeningeal metastases from ALK-positive lung adenocarcinoma. Brain Tumor Res Treat. 2021;9(2):100–5.
Gafer H, de Waard Q, Compter A, and van den Heuvel M. Rapid regression of neurological symptoms in patients with metastasised ALK+ lung cancer who are treated with lorlatinib: a report of two cases. BMJ Case Rep. 2019:12(7):e227299.
Zhu VW, Lin Y-T, Kim D-W, Loong HH, Nagasaka M, To H, et al. An international real-world analysis of the efficacy and safety of lorlatinib through early or expanded access programs in patients with tyrosine kinase inhibitor-refractory ALK-positive or ROS1-positive NSCLC. J Thorac Oncol. 2020;15(9):1484–96.
Frost N, Christopoulos P, Kauffmann-Guerrero D, Stratmann J, Riedel R, Schaefer M, et al. Lorlatinib in pretreated ALK- or ROS1-positive lung cancer and impact of TP53 co-mutations: results from the German early access program. Ther Adv Med Oncol. 2021;13:1758835920980558.
Bauer TM, Felip E, Solomon BJ, Thurm H, Peltz G, Chioda MD, et al. Clinical management of adverse events associated with lorlatinib. Oncologist. 2019;24(8):1103–10.
Liu P, Wang Y, Li X. Targeting the untargetable KRAS in cancer therapy. Acta Pharm Sin B. 2019;9(5):871–9.
Prior IA, Lewis PD, Mattos C. A comprehensive survey of Ras mutations in cancer. Cancer Res. 2012;72(10):2457–67.
Hong DS, Fakih MG, Strickler JH, Desai J, Durm GA, Shapiro GI, et al. KRASG12C inhibition with sotorasib in advanced solid tumors. N Engl J Med. 2020;383(13):1207–17.
Skoulidis F, Li BT, Dy GK, Price TJ, Falchook GS, Wolf J, et al. Sotorasib for lung cancers with KRAS p.G12C mutation. N Engl J Med. 2021;384(25):2371–81.
Ramalingam S, Skoulidis F, Govindan R, Velcheti V, Li B, Besse B, et al. P52. 03 efficacy of sotorasib in KRAS P. G12C-mutated NSCLC with stable brain metastases: a post-hoc analysis of CodeBreaK 100. J Thorac Oncol. 2021;16(10):S1123.
Jänne PA, Riely GJ, Gadgeel SM, Heist RS, Ou S-HI, Pacheco JM, et al. Adagrasib in non–small-cell lung cancer harboring a KRASG12C mutation. N Engl J Med. 2022;387(2):120–31.
Sabari JK, Spira AI, Heist RS, Janne PA, Pacheco JM, Weiss J, et al. Activity of adagrasib (MRTX849) in patients with KRASG12C-mutated NSCLC and active, untreated CNS metastases in the KRYSTAL-1 trial. J Clin Oncol. 2022;40(17_suppl):LBA9009.
Koster KL, Appenzeller C, Lauber A, Früh M, and Schmid S. Sotorasib Shows Intracranial Activity in Patients with <b><i>KRAS G12C-</i></b>Mutated Adenocarcinoma of the Lung and Untreated Active Brain Metastases. Case Rep Oncol. 2022;720–5.
Sabari JK, Velcheti V, Shimizu K, Strickland MR, Heist RS, Singh M, et al. Activity of adagrasib (MRTX849) in brain metastases: preclinical models and clinical data from patients with KRASG12C-mutant non-small cell lung cancer. Clin Cancer Res. 2022;28(15):3318–28.
Slamon DJ, Godolphin W, Jones LA, Holt JA, Wong SG, Keith DE, et al. Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science. 1989;244(4905):707–12.
Iqbal N, Iqbal N. Human epidermal growth factor receptor 2 (HER2) in cancers: overexpression and therapeutic implications. Mol Biol Int. 2014;2014: 852748.
Lower EE, Drosick DR, Blau R, Brennan L, Danneman W, Hawley DK. Increased rate of brain metastasis with trastuzumab therapy not associated with impaired survival. Clin Breast Cancer. 2003;4(2):114–9.
Bendell JC, Domchek SM, Burstein HJ, Harris L, Younger J, Kuter I, et al. Central nervous system metastases in women who receive trastuzumab-based therapy for metastatic breast carcinoma. Cancer. 2003;97(12):2972–7.
Conlon NT, Kooijman JJ, van Gerwen SJC, Mulder WR, Zaman GJR, Diala I, et al. Comparative analysis of drug response and gene profiling of HER2-targeted tyrosine kinase inhibitors. Br J Cancer. 2021;124(7):1249–59.
Lin NU, Diéras V, Paul D, Lossignol D, Christodoulou C, Stemmler HJ, et al. Multicenter phase II study of lapatinib in patients with brain metastases from HER2-positive breast cancer. Clin Cancer Res. 2009;15(4):1452–9.
Bachelot T, Romieu G, Campone M, Diéras V, Cropet C, Dalenc F, et al. Lapatinib plus capecitabine in patients with previously untreated brain metastases from HER2-positive metastatic breast cancer (LANDSCAPE): a single-group phase 2 study. Lancet Oncol. 2013;14(1):64–71.
Shawky H, Tawfik H. All-oral combination of lapatinib and capecitabine in patients with brain metastases from HER2-positive breast cancer–a phase II study. J Egypt Natl Canc Inst. 2014;26(4):187–94.
Freedman RA, Gelman RS, Anders CK, Melisko ME, Parsons HA, Cropp AM, et al. TBCRC 022: a phase II trial of neratinib and capecitabine for patients with human epidermal growth factor receptor 2-positive breast cancer and brain metastases. J Clin Oncol. 2019;37(13):1081–9.
Morikawa A, de Stanchina E, Pentsova E, Kemeny MM, Li BT, Tang K, et al. Phase I study of intermittent high-dose lapatinib alternating with capecitabine for HER2-positive breast cancer patients with central nervous system metastases. Clin Cancer Res. 2019;25(13):3784–92.
Cortés J, Dieras V, Ro J, Barriere J, Bachelot T, Hurvitz S, et al. Afatinib alone or afatinib plus vinorelbine versus investigator’s choice of treatment for HER2-positive breast cancer with progressive brain metastases after trastuzumab, lapatinib, or both (LUX-Breast 3): a randomised, open-label, multicentre, phase 2 trial. Lancet Oncol. 2015;16(16):1700–10.
Gori S, Lunardi G, Inno A, Foglietta J, Cardinali B, Del Mastro L, et al. Lapatinib concentration in cerebrospinal fluid in two patients with HER2-positive metastatic breast cancer and brain metastases. Ann Oncol. 2014;25(4):912–3.
Freedman RA, Gelman RS, Agar NYR, Santagata S, Randall EC, Gimenez-Cassina Lopez B, et al. Pre- and postoperative neratinib for HER2-positive breast cancer brain metastases: translational breast cancer research consortium 022. Clin Breast Cancer. 2020;20(2):145-151.e2.
Nakao T, Okuda T, Fujita M, Kato A. A case of leptomeningeal metastases of human epidermal growth factor receptor 2-positive breast cancer that responded well to lapatinib plus capecitabine. Surg Neurol Int. 2019;10:131.
Ekenel M, Hormigo AM, Peak S, Deangelis LM, Abrey LE. Capecitabine therapy of central nervous system metastases from breast cancer. J Neurooncol. 2007;85(2):223–7.
Maur M, Omarini C, Piacentini F, Fontana A, Pettorelli E, Cascinu S. Metronomic capecitabine effectively blocks leptomeningeal carcinomatosis from breast cancer: a case report and literature review. Am J Case Rep. 2017;18:208–11.
Rogers LR, Remer SE, Tejwani S. Durable response of breast cancer leptomeningeal metastasis to capecitabine monotherapy. Neuro Oncol. 2004;6(1):63–4.
Tham YL, Hinckley L, Teh BS, Elledge R. Long-term clinical response in leptomeningeal metastases from breast cancer treated with capecitabine monotherapy: a case report. Clin Breast Cancer. 2006;7(2):164–6.
Lin NU, Borges V, Anders C, Murthy RK, Paplomata E, Hamilton E, et al. Intracranial efficacy and survival with tucatinib plus trastuzumab and capecitabine for previously treated HER2-positive breast cancer with brain metastases in the HER2CLIMB trial. J Clin Oncol. 2020;38(23):2610–9.
Yan F, Rinn KJ, Kullnat JA, Wu AY, Ennett MD, Scott EL, et al. Response of leptomeningeal metastasis of breast cancer with a HER2/neu activating variant to tucatinib: a case report. J Natl Compr Canc Netw. 2022;20(7):745–52.
Murthy RK, O’Brien B, Berry DA, Singareeka-Raghavendra A, Monroe MG, Johnson J, et al. Abstract PD4-02: Safety and efficacy of a tucatinib-trastuzumab-capecitabine regimen for treatment of leptomeningeal metastasis (LM) in HER2-positive breast cancer: Results from TBCRC049, a phase 2 non-randomized study. Can Res. 2022;82(4_Supplement):PD4-02.
Stringer-Reasor EM, O’Brien BJ, Topletz-Erickson A, White JB, Lobbous M, Riley K, et al. Pharmacokinetic (PK) analyses in CSF and plasma from TBCRC049, an ongoing trial to assess the safety and efficacy of the combination of tucatinib, trastuzumab and capecitabine for the treatment of leptomeningeal metastasis (LM) in HER2 positive breast cancer. J Clin Oncol. 2021;39(15_suppl):1044–1044.
Montemurro F, Delaloge S, Barrios CH, Wuerstlein R, Anton A, Brain E, et al. Trastuzumab emtansine (T-DM1) in patients with HER2-positive metastatic breast cancer and brain metastases: exploratory final analysis of cohort 1 from KAMILLA, a single-arm phase IIIb clinical trial. Ann Oncol. 2020;31(10):1350–8.
Krop IE, Lin NU, Blackwell K, Guardino E, Huober J, Lu M, et al. Trastuzumab emtansine (T-DM1) versus lapatinib plus capecitabine in patients with HER2-positive metastatic breast cancer and central nervous system metastases: a retrospective, exploratory analysis in EMILIA. Ann Oncol. 2015;26(1):113–9.
Ricciardi GRR, Russo A, Franchina T, Schifano S, Mastroeni G, Santacaterina A, et al. Efficacy of T-DM1 for leptomeningeal and brain metastases in a HER2 positive metastatic breast cancer patient: new directions for systemic therapy—a case report and literature review. BMC Cancer. 2018;18(1):97.
Ferraro E, Drago JZ, Modi S. Implementing antibody-drug conjugates (ADCs) in HER2-positive breast cancer: state of the art and future directions. Breast Cancer Res. 2021;23(1):84.
Modi S, Saura C, Yamashita T, Park YH, Kim S-B, Tamura K, et al. Trastuzumab deruxtecan in previously treated HER2-positive breast cancer. N Engl J Med. 2019;382(7):610–21.
Modi S, Jacot W, Yamashita T, Sohn J, Vidal M, Tokunaga E, et al. Trastuzumab deruxtecan in previously treated HER2-low advanced breast cancer. N Engl J Med. 2022;387(1):9–20.
Bartsch R, Berghoff AS, Furtner J, Marhold M, Bergen ES, Roider-Schur S, et al. Trastuzumab deruxtecan in HER2-positive breast cancer with brain metastases: a single-arm, phase 2 trial. Nat Med. 2022;28(9):1840-1847.
Bonneau C, Paintaud G, Trédan O, Dubot C, Desvignes C, Dieras V, et al. Phase I feasibility study for intrathecal administration of trastuzumab in patients with HER2 positive breast carcinomatous meningitis. Eur J Cancer. 2018;95:75–84.
Malani R, Fleisher M, Kumthekar P, Lin X, Omuro A, Groves MD, et al. Cerebrospinal fluid circulating tumor cells as a quantifiable measurement of leptomeningeal metastases in patients with HER2 positive cancer. J Neurooncol. 2020;148(3):599–606.
Morales J, Li L, Fattah FJ, Dong Y, Bey EA, Patel M, et al. Review of poly (ADP-ribose) polymerase (PARP) mechanisms of action and rationale for targeting in cancer and other diseases. Crit Rev Eukaryot Gene Expr. 2014;24(1):15–28.
Jm L, Ledermann JA, Kohn EC. PARP Inhibitors for BRCA1/2 mutation-associated and BRCA-like malignancies. Ann Oncol. 2014;25(1):32–40.
Lord CJ, Ashworth A. PARP inhibitors: synthetic lethality in the clinic. Science. 2017;355(6330):1152–8.
Diossy M, Reiniger L, Sztupinszki Z, Krzystanek M, Timms KM, Neff C, et al. Breast cancer brain metastases show increased levels of genomic aberration-based homologous recombination deficiency scores relative to their corresponding primary tumors. Ann Oncol. 2018;29(9):1948–54.
Litton JK, Ettl J, Hurvitz SA, Martin M, Roche H, Lee K-H, et al. Clinical outcomes in patients (pts) with a history of central nervous system (CNS) metastases receiving talazoparib (TALA) or physician’s choice of chemotherapy (PCT) in the phase 3 EMBRACA trial. J Clin Oncol. 2021;39(15_suppl):1090–1090.
Pascual T, Gonzalez-Farre B, Teixidó C, Oleaga L, Oses G, Ganau S, et al. Significant clinical activity of olaparib in a somatic BRCA1-mutated triple-negative breast cancer with brain metastasis. JCO Precis Oncol. 2019;3:1–6.
Wang Q, Zhang F, Gao H, Xu Y. Successful treatment of a patient with brain metastases from endometrial cancer using Niraparib: a case report. Ann Palliative Med. 2021;10(1):818–27.
Gray S, Khor XY, Yiannakis D. Niraparib as maintenance therapy in a patient with ovarian cancer and brain metastases. BMJ Case Rep. 2019;12(8): e230738.
Atchley DP, Albarracin CT, Lopez A, Valero V, Amos CI, Gonzalez-Angulo AM, et al. Clinical and pathologic characteristics of patients with BRCA-positive and BRCA-negative breast cancer. J Clin Oncol. 2008;26(26):4282–8.
Chen H, Wu J, Zhang Z, Tang Y, Li X, Liu S, et al. Association between BRCA status and triple-negative breast cancer: a meta-analysis. Front Pharmacol. 2018;9:909–909.
Gada K, Sharma G, Kmett C, Minthorn E, Lugo D, Gehman A, et al. Tissue distribution and brain penetration of niraparib in tumor bearing mouse models and its clinical relevance. J Clin Oncol. 2021;39(15_suppl):e15066.
Nguyen M, Robillard L, Harding TC, **ao JJ, Simmons AD, Kristeleit H, et al. Abstract 3888: Intracranial evaluation of the in vivo pharmacokinetics, brain distribution, and efficacy of rucaparib in BRCA-mutant, triple-negative breast cancer. Cancer Res. 2019;79(13_Supplement):3888.
Muscal JA, Thompson PA, Giranda VL, Dayton BD, Bauch J, Horton T, et al. Plasma and cerebrospinal fluid pharmacokinetics of ABT-888 after oral administration in non-human primates. Cancer Chemother Pharmacol. 2010;65(3):419–25.
Favier L, Truc G, Boidot R, Bengrine-Lefevre L. Long-term response to Olaparib in carcinomatous meningitis of a BRCA2 mutated ovarian cancer: a case report. Molecular and clinical oncology. 2020;13(1):73–5.
Bangham M, Goldstein R, Walton H, Ledermann JA. Olaparib treatment for BRCA-mutant ovarian cancer with leptomeningeal disease. Gynecol Oncol Rep. 2016;18:22–4.
Exman P, Mallery RM, Lin NU, Parsons HA. Response to olaparib in a patient with germline BRCA2 mutation and breast cancer leptomeningeal carcinomatosis. npj Breast Cancer. 2019;5(1):46.
Lien EA, Wester K, Lønning PE, Solheim E, Ueland PM. Distribution of tamoxifen and metabolites into brain tissue and brain metastases in breast cancer patients. Br J Cancer. 1991;63(4):641–5.
Miyajima M, Kusuhara H, Takahashi K, Takashima T, Hosoya T, Watanabe Y, et al. Investigation of the effect of active efflux at the blood-brain barrier on the distribution of nonsteroidal aromatase inhibitors in the central nervous system. J Pharm Sci. 2013;102(9):3309–19.
Lien EA, Solheim E, Lea OA, Lundgren S, Kvinnsland S, Ueland PM. Distribution of 4-hydroxy-N-desmethyltamoxifen and other tamoxifen metabolites in human biological fluids during tamoxifen treatment. Cancer Res. 1989;49(8):2175–83.
Ozdogan M, Samur M, Bozcuk HS, Sagtas E, Yildiz M, Artac M, et al. Durable remission of leptomeningeal metastasis of breast cancer with letrozole: a case report and implications of biomarkers on treatment selection. Jpn J Clin Oncol. 2003;33(5):229–31.
Navarro Martín LM, Fernández AO, Rodríguez Sánchez CA, Martín IR, Cruz Hernández JJ. Durable clinical benefit with exemestane in leptomeningeal metastasis of breast cancer. Clin Transl Oncol. 2005;7(8):358–60.
Boogerd W, Dorresteijn LD, van Der Sande JJ, de Gast GC, Bruning PF. Response of leptomeningeal metastases from breast cancer to hormonal therapy. Neurology. 2000;55(1):117–9.
Zoghi B, Elledge R. Endocrine therapy for leptomeningeal metastases from ER-positive breast cancer: case report and a review of the literature. Breast J. 2016;22(2):218–23.
Fernandes L, Matos LVd, Cardoso D, Saraiva M, Medeiros-Mirra R, Coelho A, et al. Endocrine therapy for the treatment of leptomeningeal carcinomatosis in luminal breast cancer: a comprehensive review. CNS Oncol. 2020;9(4):CNS65.
Bergen ES, Berghoff AS, Medjedovic M, Rudas M, Fitzal F, Bago-Horvath Z, et al. Continued endocrine therapy is associated with improved survival in patients with breast cancer brain metastases. Clin Cancer Res. 2019;25(9):2737–44.
Presti D, Quaquarini E. The PI3K/AKT/mTOR and CDK4/6 pathways in endocrine resistant HR+/HER2- metastatic breast cancer: biological mechanisms and new treatments. Cancers (Basel). 2019;11(9):1242.
Tolaney SM, Sahebjam S, Le Rhun E, Bachelot T, Kabos P, Awada A, et al. A phase II study of abemaciclib in patients with brain metastases secondary to hormone receptor-positive breast cancer. Clin Cancer Res. 2020;26(20):5310.
Cottu PH, De Laurentiis M, Marchetti P, Coltelli L, Califaretti N, Debled M, et al. Ribociclib (RIB) + letrozole (LET) in patients (pts) with hormone receptor-positive (HR+), human epidermal growth factor receptor-2–negative (HER2–) advanced breast cancer (ABC) and central nervous system (CNS) metastases: subgroup analysis from the phase IIIb CompLEEment-1 trial. Ann Oncol. 2019;30: v118.
Cottu P, Ring A, Abdel-Razeq H, Marchetti P, Cardoso F, Salvador Bofill J, et al. Ribociclib plus letrozole in subgroups of special clinical interest with hormone receptor–positive, human epidermal growth factor receptor 2–negative advanced breast cancer: subgroup analysis of the phase IIIb CompLEEment-1 trial. Breast. 2022;62:75–83.
Brastianos PK, Kim AE, Wang N, Lee EQ, Ligibel J, Cohen JV, et al. Palbociclib demonstrates intracranial activity in progressive brain metastases harboring cyclin-dependent kinase pathway alterations. Nat Cancer. 2021;2(5):498–502.
Tien A-C, Li J, Bao X, Derogatis A, Kim S, Mehta S, et al. A phase 0 trial of ribociclib in recurrent glioblastoma patients incorporating a tumor pharmacodynamic- and pharmacokinetic-guided expansion cohort. Clin Cancer Res. 2019;25(19):5777–86.
Yang J, Nie J, Ma X, Wei Y, Peng Y, Wei X. Targeting PI3K in cancer: mechanisms and advances in clinical trials. Mol Cancer. 2019;18(1):26.
Mayer IA, Abramson VG, Formisano L, Balko JM, Estrada MV, Sanders ME, et al. A phase Ib study of alpelisib (BYL719), a PI3Kα-specific inhibitor, with letrozole in ER+/HER2- metastatic breast cancer. Clin Cancer Res. 2017;23(1):26–34.
André F, Ciruelos E, Rubovszky G, Campone M, Loibl S, Rugo HS, et al. Alpelisib for PIK3CA-mutated, hormone receptor-positive advanced breast cancer. N Engl J Med. 2019;380(20):1929–40.
Batalini F, Moulder SL, Winer EP, Rugo HS, Lin NU, Wulf GM. Response of Brain Metastases From PIK3CA-Mutant Breast Cancer to Alpelisib. JCO Precis Oncol. 2020;4.
Nunnery SE, Mayer IA. Management of toxicity to isoform α-specific PI3K inhibitors. Ann Oncol. 2019;30(Suppl_10):x21–6.
Di Leo A, Johnston S, Lee KS, Ciruelos E, Lønning PE, Janni W, et al. Buparlisib plus fulvestrant in postmenopausal women with hormone-receptor-positive, HER2-negative, advanced breast cancer progressing on or after mTOR inhibition (BELLE-3): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2018;19(1):87–100.
Yang J, Mann J, Pike L, Zinovoy M, Young R, Offin M, et al. MMAP-05 phase I study of concurrent paxalisib and radiation therapy in patients with solid tumor brain metastases or leptomeningeal metastases harboring pi3k pathway mutations: results from the dose-escalation cohort. Neuro-Oncol Adv. 2022;4(Supplement_1):i15–6.
Baselga J, Campone M, Piccart M, Burris HA, Rugo HS, Sahmoud T, et al. Everolimus in postmenopausal hormone-receptor–positive advanced breast cancer. N Engl J Med. 2011;366(6):520–9.
Van Swearingen AED, Siegel MB, Deal AM, Sambade MJ, Hoyle A, Hayes DN, et al. LCCC 1025: a phase II study of everolimus, trastuzumab, and vinorelbine to treat progressive HER2-positive breast cancer brain metastases. Breast Cancer Res Treat. 2018;171(3):637–48.
Hurvitz S, Singh R, Adams B, Taguchi JA, Chan D, Dichmann RA, et al. Phase Ib/II single-arm trial evaluating the combination of everolimus, lapatinib and capecitabine for the treatment of HER2-positive breast cancer with brain metastases (TRIO-US B-09). Ther Adv Med Oncol. 2018;10:1758835918807339.
Smyth LM, Tamura K, Oliveira M, Ciruelos EM, Mayer IA, Sablin M-P, et al. Capivasertib, an AKT kinase inhibitor, as monotherapy or in combination with fulvestrant in patients with AKT1E17K-mutant, ER-positive metastatic breast cancer. Clin Cancer Res. 2020;26(15):3947–57.
Turner N, Dent RA, O’Shaughnessy J, Kim S-B, Isakoff SJ, Barrios C, et al. Ipatasertib plus paclitaxel for PIK3CA/AKT1/PTEN-altered hormone receptor-positive HER2-negative advanced breast cancer: primary results from cohort B of the IPATunity130 randomized phase 3 trial. Breast Cancer Res Treat. 2022;191(3):565–76.
Howell SJ, Casbard A, Carucci M, Ingarfield K, Butler R, Morgan S, et al. Fulvestrant plus capivasertib versus placebo after relapse or progression on an aromatase inhibitor in metastatic, oestrogen receptor-positive, HER2-negative breast cancer (FAKTION): overall survival, updated progression-free survival, and expanded biomarker analysis from a randomised, phase 2 trial. Lancet Oncol. 2022;23(7):851–64.
Ippen FM, Grosch JK, Subramanian M, Kuter BM, Liederer BM, Plise EG, et al. Targeting the PI3K/Akt/mTOR pathway with the pan-Akt inhibitor GDC-0068 in PIK3CA-mutant breast cancer brain metastases. Neuro Oncol. 2019;21(11):1401–11.
Colombino M, Capone M, Lissia A, Cossu A, Rubino C, De Giorgi V, et al. BRAF/NRAS mutation frequencies among primary tumors and metastases in patients with melanoma. J Clin Oncol. 2012;30(20):2522–9.
Rabbie R, Ferguson P, Wong K, Couturier D-L, Moran U, Turner C, et al. The mutational landscape of melanoma brain metastases presenting as the first visceral site of recurrence. Br J Cancer. 2021;124(1):156–60.
El-Osta H, Falchook G, Tsimberidou A, Hong D, Naing A, Kim K, et al. BRAF mutations in advanced cancers: clinical characteristics and outcomes. PLoS ONE. 2011;6(10): e25806.
Davies MA, Saiag P, Robert C, Grob JJ, Flaherty KT, Arance A, et al. Dabrafenib plus trametinib in patients with BRAF(V600)-mutant melanoma brain metastases (COMBI-MB): a multicentre, multicohort, open-label, phase 2 trial. Lancet Oncol. 2017;18(7):863–73.
Holbrook K, Lutzky J, Davies MA, Davis JM, Glitza IC, Amaria RN, et al. Intracranial antitumor activity with encorafenib plus binimetinib in patients with melanoma brain metastases: a case series. Cancer. 2020;126(3):523–30.
Sakji-Dupré L, Le Rhun E, Templier C, Desmedt E, Blanchet B, Mortier L. Cerebrospinal fluid concentrations of vemurafenib in patients treated for brain metastatic BRAF-V600 mutated melanoma. Melanoma Res. 2015;25(4):302–5.
Rodgers L, C MLM, Gross A, Peer C, Cruz R, Figg WD, et al. TRTH-33 comparative plasma and cerebrospinal fluid pharmacokinetics of BRAF and MEK inhibitors in a nonhuman primate model. Neuro Oncol. 2017;19(1522-8517 (Print))
Arasaratnam M, Hong A, Shivalingam B, Wheeler H, Guminksi AD, Long GV, et al. Leptomeningeal melanoma—a case series in the era of modern systemic therapy. Pigment Cell Melanoma Res. 2018;31(1):120–4.
Floudas CS, Chandra AB, Xu Y. Vemurafenib in leptomeningeal carcinomatosis from melanoma: a case report of near-complete response and prolonged survival. Melanoma Res. 2016;26(3):312–5.
Lee JM, Mehta UN, Dsouza LH, Guadagnolo BA, Sanders DL, Kim KB. Long-term stabilization of leptomeningeal disease with whole-brain radiation therapy in a patient with metastatic melanoma treated with vemurafenib: a case report. Melanoma Res. 2013;23(2):175–8.
Schäfer N, Scheffler B, Stuplich M, Schaub C, Kebir S, Rehkämper C, et al. Vemurafenib for leptomeningeal melanomatosis. J Clin Oncol. 2013;31(11):e173–4.
Wilgenhof S, Neyns B. Complete cytologic remission of V600E BRAF-mutant melanoma-associated leptomeningeal carcinomatosis upon treatment with dabrafenib. J Clin Oncol. 2015;33(28):e109–11.
Glitza IC, Ferguson SD, Guha-Thakurta N. Rapid resolution of leptomeningeal disease with targeted therapy in a metastatic melanoma patient. J Neurooncol. 2017;133(3):663–5.
Kim DW, Barcena E, Mehta UN, Rohlfs ML, Kumar AJ, Penas-Prado M, et al. Prolonged survival of a patient with metastatic leptomeningeal melanoma treated with BRAF inhibition-based therapy: a case report. BMC Cancer. 2015;15(1):400.
Ntellas P, Mavroeidis L, Gkoura S, Gazouli I, Amylidi A-L, Papadaki A, et al. Old player-new tricks: non angiogenic effects of the VEGF/VEGFR pathway in cancer. Cancers. 2020;12(11):3145.
Groves MD, Hess KR, Puduvalli VK, Colman H, Conrad CA, Gilbert MR, et al. Biomarkers of disease: cerebrospinal fluid vascular endothelial growth factor (VEGF) and stromal cell derived factor (SDF)-1 levels in patients with neoplastic meningitis (NM) due to breast cancer, lung cancer and melanoma. J Neurooncol. 2009;94(2):229–34.
Herrlinger U, Wiendl H, Renninger M, Förschler H, Dichgans J, Weller M. Vascular endothelial growth factor (VEGF) in leptomeningeal metastasis: diagnostic and prognostic value. Br J Cancer. 2004;91(2):219–24.
van de Langerijt B, Gijtenbeek JM, de Reus HPM, Sweep FCGJ, Geurts-Moespot A, Hendriks JCM, et al. CSF levels of growth factors and plasminogen activators in leptomeningeal metastases. Neurology. 2006;67(1):114.
Massard C, Zonierek J, Gross-Goupil M, Fizazi K, Szczylik C, Escudier B. Incidence of brain metastases in renal cell carcinoma treated with sorafenib. Ann Oncol. 2010;21(5):1027–31.
Morikawa A, Grkovski M, Patil S, Jhaveri KL, Tang K, Humm JL, et al. A phase I trial of sorafenib with whole brain radiotherapy (WBRT) in breast cancer patients with brain metastases and a correlative study of FLT-PET brain imaging. Breast Cancer Res Treat. 2021;188(2):415–25.
Ranze O, Hofmann E, Distelrath A, Hoeffkes HG. Renal cell cancer presented with leptomeningeal carcinomatosis effectively treated with sorafenib. Onkologie. 2007;30(8–9):450–1.
Kummar S, Lassen UN. TRK inhibition: a new tumor-agnostic treatment strategy. Target Oncol. 2018;13(5):545–56.
Rosen EY, Goldman DA, Hechtman JF, Benayed R, Schram AM, Cocco E, et al. TRK fusions are enriched in cancers with uncommon histologies and the absence of canonical driver mutations. Clin Cancer Res. 2020;26(7):1624–32.
Drilon A, Laetsch TW, Kummar S, DuBois SG, Lassen UN, Demetri GD, et al. Efficacy of larotrectinib in TRK fusion-positive cancers in adults and children. N Engl J Med. 2018;378(8):731–9.
Doebele RC, Drilon A, Paz-Ares L, Siena S, Shaw AT, Farago AF, et al. Entrectinib in patients with advanced or metastatic NTRK fusion-positive solid tumours: integrated analysis of three phase 1–2 trials. Lancet Oncol. 2020;21(2):271–82.
Doz F, van Tilburg CM, Geoerger B, Højgaard M, Øra I, Boni V, et al. Efficacy and safety of larotrectinib in TRK fusion-positive primary central nervous system tumors. Neuro Oncol. 2022;24(6):997–1007.
Fischer H, Ullah M, de la Cruz CC, Hunsaker T, Senn C, Wirz T, et al. Entrectinib, a TRK/ROS1 inhibitor with anti-CNS tumor activity: differentiation from other inhibitors in its class due to weak interaction with P-glycoprotein. Neuro Oncol. 2020;22(6):819–29.
Lanman T, Hayden Gephart M, Bui N, Toland A, Nagpal S. Isolated leptomeningeal progression in a patient with NTRK fusion+ uterine sarcoma: a case report. Case Rep Oncol. 2021;14(3):1841–6.
Choi H, Kim J, Lee YH, Kim L, Kim NH, Chae YK. Abstract 4120: Acquired TPM3-NTRK1 fusion resistant to larotrectinib in a non-small cell lung cancer with EML4-ALK fusion progressed on lorlatinib. Can Res. 2022;82(12_Supplement):4120.
Mayr L, Guntner AS, Madlener S, Schmook MT, Peyrl A, Azizi AA, et al. Cerebrospinal fluid penetration and combination therapy of entrectinib for disseminated ROS1/NTRK-fusion positive pediatric high-grade glioma. J Pers Med. 2020;10(4):290.
Tawbi HA, Forsyth PA, Algazi A, Hamid O, Hodi FS, Moschos SJ, et al. Combined nivolumab and ipilimumab in melanoma metastatic to the brain. N Engl J Med. 2018;379(8):722–30.
Tawbi HA, Forsyth PA, Hodi FS, Algazi AP, Hamid O, Lao CD, et al. Long-term outcomes of patients with active melanoma brain metastases treated with combination nivolumab plus ipilimumab (CheckMate 204): final results of an open-label, multicentre, phase 2 study. Lancet Oncol. 2021;22(12):1692–704.
Gauvain C, Vauléon E, Chouaid C, Le Rhun E, Jabot L, Scherpereel A, et al. Intracerebral efficacy and tolerance of nivolumab in non–small-cell lung cancer patients with brain metastases. Lung Cancer. 2018;116:62–6.
Li S, Zhang H, Liu T, Chen J, Dang J. The effect of asymptomatic and/or treated brain metastases on efficacy of immune checkpoint inhibitors in metastatic non–small cell lung cancer: a meta-analysis. Front Oncol. 2021;11.
Nadal E, Massuti B, Huidobro G, Castro RL, Estival A, Mosquera J, et al. OA09.02 Atezo-brain: single arm phase II study of atezolizumab plus chemotherapy in stage IV NSCLC with untreated brain metastases. J Thorac Oncol. 2021;16(10):S863.
Uezono H, Nam D, Kluger HM, Sznol M, Hurwitz M, Yu JB, et al. Outcomes of stereotactic radiosurgery and immunotherapy in renal cell carcinoma patients with brain metastases. Am J Clin Oncol. 2021;44(9).
Flippot R, Dalban C, Laguerre B, Borchiellini D, Gravis G, Négrier S, et al. Safety and efficacy of nivolumab in brain metastases from renal cell carcinoma: results of the GETUG-AFU 26 NIVOREN multicenter phase II study. J Clin Oncol. 2019;37(23):2008–16.
Emamekhoo H, Olsen MR, Carthon BC, Drakaki A, Percent IJ, Molina AM, et al. Safety and efficacy outcomes with nivolumab plus ipilimumab in patients with advanced renal cell carcinoma and brain metastases: results from the CheckMate 920 trial. J Clin Oncol. 2021;39(15_suppl):4515.
Emens LA, Adams S, Barrios CH, Diéras V, Iwata H, Loi S, et al. First-line atezolizumab plus nab-paclitaxel for unresectable, locally advanced, or metastatic triple-negative breast cancer: IMpassion130 final overall survival analysis. Ann Oncol. 2021;32(8):983–93.
Bailleux C, Eberst L, Bachelot T. Treatment strategies for breast cancer brain metastases. Br J Cancer. 2021;124(1):142–55.
Lanier CM, Hughes R, Ahmed T, LeCompte M, Masters AH, Petty WJ, et al. Immunotherapy is associated with improved survival and decreased neurologic death after SRS for brain metastases from lung and melanoma primaries. Neurooncol Pract. 2019;6(5):402–9.
Hadi I, Roengvoraphoj O, Bodensohn R, Hofmaier J, Niyazi M, Belka C, et al. Stereotactic radiosurgery combined with targeted/ immunotherapy in patients with melanoma brain metastasis. Radiat Oncol. 2020;15(1):37.
Minniti G, Anzellini D, Reverberi C, Cappellini GCA, Marchetti L, Bianciardi F, et al. Stereotactic radiosurgery combined with nivolumab or Ipilimumab for patients with melanoma brain metastases: evaluation of brain control and toxicity. J Immunother Cancer. 2019;7(1):102.
Long GV, Atkinson V, Lo S, Sandhu S, Guminski AD, Brown MP, et al. Combination nivolumab and ipilimumab or nivolumab alone in melanoma brain metastases: a multicentre randomised phase 2 study. Lancet Oncol. 2018;19(5):672–81.
Brastianos PK, Lee EQ, Cohen JV, Tolaney SM, Lin NU, Wang N, et al. Single-arm, open-label phase 2 trial of pembrolizumab in patients with leptomeningeal carcinomatosis. Nat Med. 2020;26(8):1280–4.
Brastianos PK, Strickland MR, Lee EQ, Wang N, Cohen JV, Chukwueke U, et al. Phase II study of ipilimumab and nivolumab in leptomeningeal carcinomatosis. Nat Commun. 2021;12(1):5954.
Hendriks LEL, Bootsma G, Mourlanette J, Henon C, Mezquita L, Ferrara R, et al. Survival of patients with non-small cell lung cancer having leptomeningeal metastases treated with immune checkpoint inhibitors. Eur J Cancer. 2019;116:182–9.
Remon J, Castro-Henriques M, Esteller L, and Vives J. Combination of atezolizumab, bevacizumab, and chemotherapy (IMpower 150) in a patient with NSCLC having leptomeningeal metastases. JTO Clin Res Rep. 2021;2(1).
Itchins M, Ainsworth H, Alexander M, Dean S, Dharmaraj D, Pavlakis N et al. A multi-center real-world experience of IMpower150 in oncogene driven tumors and CNS metastases. Clin Lung Cancer. 2022;23(8):702-708.
Palmisciano P, Haider AS, Nwagwu CD, Wahood W, Yu K, Ene CI, et al. The role of immune checkpoint inhibitors in leptomeningeal disease: a systematic review. Anticancer Res. 2021;41(11):5333.
Portnow J, Wang D, Blanchard MS, Tran V, Alizadeh D, Starr R, et al. Systemic anti-PD-1 immunotherapy results in PD-1 blockade on T cells in the cerebrospinal fluid. JAMA Oncol. 2020;6(12):1947–51.
Pluim D, Ros W, van Bussel MTJ, Brandsma D, Beijnen JH, Schellens JHM. Enzyme linked immunosorbent assay for the quantification of nivolumab and pembrolizumab in human serum and cerebrospinal fluid. J Pharm Biomed Anal. 2019;164:128–34.
Glitza IC, Rohlfs M, Guha-Thakurta N, Bassett RL Jr, Bernatchez C, Diab A, et al. Retrospective review of metastatic melanoma patients with leptomeningeal disease treated with intrathecal interleukin-2. ESMO Open. 2018;3(1): e000283.
John I, Foster AP, Haymaker CL, Bassett RL, Lee JJ, Rohlfs ML, et al. Intrathecal (IT) and intravenous (IV) nivolumab (N) for metastatic melanoma (MM) patients (pts) with leptomeningeal disease (LMD). J Clin Oncol. 2021;39(15_suppl):9519.
Ensign SPF, Yancey E, Anderson KS, Mrugala MM. Safety and feasibility of intrathecal pembrolizumab infusion in refractory triple negative breast cancer with leptomeningeal disease: a case report. Curr Probl Cancer Case Rep. 2021;4: 100103.
Chi Y, Remsik J, Kiseliovas V, Derderian C, Sener U, Alghader M, et al. Cancer cells deploy lipocalin-2 to collect limiting iron in leptomeningeal metastasis. Science. 2020;369(6501):276–82.
Smalley I, Chen Z, Phadke M, Li J, Yu X, Wyatt C, et al. Single-cell characterization of the immune microenvironment of melanoma brain and leptomeningeal metastases. Clin Cancer Res. 2021;27(14):4109–25.
Prakadan SM, Alvarez-Breckenridge CA, Markson SC, Kim AE, Klein RH, Nayyar N, et al. Genomic and transcriptomic correlates of immunotherapy response within the tumor microenvironment of leptomeningeal metastases. Nat Commun. 2021;12(1):5955.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The study and its open access publication was funded by National Institutes of Health (US) (Grant nos. P30 CA008748, 5R01CA245499).
Conflicts of interest/competing interests
JAW has received research funding from the American Society of Clinical Oncology. AAB has received research funding from The Pew Charitable Trusts, Pershing Square Sohn Cancer Research Alliances, Joe W. and Dorothy Dorsett Brown Foundation, W.M. Keck Foundation, American Brain Tumor Association, Alan and Sandra Gerry Metastasis and Tumor Ecosystems Center, AACR, National Institutes of Health, STARR Cancer Consortium, MSKCC Center for Experimental Therapeutics, FM Kirby Foundation. AAB holds an unpaid position on the Scientific Advisory Board for Evren Scientific. AAB is an inventor on the following patents: 62/258,044, 10/413,522, and 63/052,139.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and material
No datasets were generated or analyzed during the current study.
Code availability
Not applicable.
Author contributions
JAW and AAB both contributed to manuscript conceptualization. JAW performed the literature search, data analysis, and manuscript preparation. AAB critically revised the manuscript for intellectual content. All authors contributed substantially to the final manuscript. All authors approve the final manuscript for submission.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Wilcox, J.A., Boire, A.A. Leveraging Molecular and Immune-Based Therapies in Leptomeningeal Metastases. CNS Drugs 37, 45–67 (2023). https://doi.org/10.1007/s40263-022-00975-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40263-022-00975-5