Abstract
Preservation of cognitive function is an important outcome in oncology. Optimal patient management requires an understanding of cognitive effects of the disease and its treatment and an efficacious approach to assessment and management of cognitive dysfunction, including selection of treatments to minimize the risk of cognitive impairment. Awareness is increasing of the potentially detrimental effects of cancer-related cognitive dysfunction on functional independence and quality of life. Prostate cancer occurs most often in older men, who are more likely to develop cognitive dysfunction than younger individuals; this population may be particularly vulnerable to treatment-related cognitive disorders. Prompt identification of treatment-induced cognitive dysfunction is a crucial aspect of effective cancer management. We review the potential etiologies of cognitive decline in patients with prostate cancer, including the potential role of androgen receptor pathway inhibitors; commonly used tools for assessing cognitive function validated in metastatic castration-resistant prostate cancer and adopted in non-metastatic castration-resistant prostate cancer trials; and strategies for management of cognitive symptoms. Many methods are currently used to assess cognitive function. The prevalence and severity of cognitive dysfunction vary according to the instruments and criteria applied. Consensus on the definition of cognitive dysfunction and on the most appropriate approaches to quantify its extent and progression in patients treated for prostate cancer is lacking. Evidence-based guidance on the appropriate tools and time to assess cognitive function in patients with prostate cancer is required.
Plain Language Summary
Men with prostate cancer are usually elderly and may have other health conditions. Old age, poor health, and some medications can all affect a person’s ability to think clearly, make sound judgments, remember things, and learn new information. These mental difficulties can make it hard for people to do day-to-day tasks such as shop** or making a meal, looking after themselves, or taking medications correctly. People may also have problems with friendships and relationships. Therefore, before a man starts treatment for prostate cancer, he and his doctor must consider the likelihood that his mental ability might be affected by the treatment in order to choose the most suitable treatment option. Initially, the doctor can use short screening tests, such as asking the patient to remember and repeat numbers or words, draw objects, name things in a picture, or complete a survey. If these tests show that the patient might be having mental difficulties, he may be invited for additional tests with a specialist, called a neuropsychologist. A patient with, or at risk of, mental difficulties will be offered a care plan, which might include one or more of the following: selecting or switching treatments to avoid side effects; using memory aids; treatment for pain, difficulty slee**, depression, or other problems that might affect mental function; sessions with a specialist to improve mental ability and develop techniques to adapt to worsening mental function; physical exercise; and getting help from others with household tasks, personal care, or taking medications.
Video abstract
Video Abstract (MP4 62137 kb)
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Digital Features for this article can be found at https://doi.org/10.6084/m9.figshare.19474502. |
In men with prostate cancer, older patients have an increased risk of develo** cognitive dysfunction compared with younger individuals, particularly as an adverse effect of anticancer treatment such as androgen receptor pathway inhibitor therapy for nonmetastatic castration-resistant prostate cancer. These cognitive effects can substantially limit patients’ functional independence and quality of life and their ability to participate in decisions about their healthcare. |
A wide range of cognitive tests (e.g., Trail Making Test, Hopkins Verbal Learning Test-Revised) and self-report measures (e.g., Functional Assessment of Cancer Therapy-Cognitive) are used to gauge cognitive function quantitatively and qualitatively. Validated objective neuropsychological evaluation is the gold standard. |
To limit the adverse impact of cognitive dysfunction in patients with prostate cancer, healthcare professionals need to understand how the disease and its treatment can affect cognitive function, so that they can select treatments that are associated with minimal impact on cognitive function, identify early signs of impairment, and arrange referral for further testing, counseling, and cognitive and pharmacological intervention. |
1 Introduction
There is growing awareness of the potentially detrimental effects of cancer-related cognitive dysfunction on patients’ functional capacity and quality of life (QoL). Cognitive decline is increasingly recognized as one of the most important treatment sequelae experienced by patients with cancer [1]. Various studies of chemotherapy and hormonal therapies have demonstrated associations between cognitive decline, malignancy, and anticancer therapy, although the biology underlying these relationships remains to be fully elucidated [2, 3]. Prostate cancer survivors may be particularly vulnerable to develo** cognitive dysfunction during treatment because the disease disproportionately affects older patients (median age at diagnosis is 66 years) [4]. As this patient population is also at risk for age-related cognitive dysfunction, minimizing the risk of treatment-related cognitive deficits emerges as an important consideration [2, 5, 6].
Treatment decisions for prostate cancer can be complex, potentially involving numerous clinical specialty teams, sequencing of systemic therapies, and local treatments with radiation or urological surgery. The International Society of Geriatric Oncology (SIOG) considers a cognitive evaluation to be mandatory to determine the ability of a patient to make informed decisions and to participate actively in his care [7, 8]. Although evidence is limited with respect to the cognitive effects of chemotherapy in men with prostate cancer, docetaxel (an antineoplastic, intracellular microtubule inhibitor) has been linked to cognitive impairment in patients with breast cancer [9]. Prolonged use of gonadotropin-releasing hormone agonists has been associated with cognitive decline in men with prostate cancer [10]. In addition, several second-generation androgen receptor (AR) inhibitors, including apalutamide and enzalutamide, have been associated with adverse central nervous system (CNS)-related events in men with prostate cancer, including fatigue and mental impairment disorders [11,12,13,14,15]. In contrast, limited data suggest fewer drug-related cognitive effects with the androgen synthesis inhibitor, abiraterone acetate, compared with enzalutamide [16,17,18]. In clinical trials, darolutamide, a structurally distinct AR inhibitor [19], in combination with androgen deprivation therapy (ADT) was not associated with a higher incidence of adverse cognitive effects compared to placebo and ADT [20].
Prompt identification of early signs and symptoms of an impending neurological disorder by the treating practitioner is an important component of a cancer management plan. We review the association between cognitive function and cancer, discuss some commonly used instruments for assessing cognitive dysfunction that have been validated in metastatic castration-resistant prostate cancer (mCRPC) and subsequently adopted in trials of nonmetastatic castration-resistant prostate cancer (nmCRPC), and provide guidance on how to judge appropriate adoption of these measures in a variety of clinical settings. Our aim is to inform the reader of effective and practical methods by which to evaluate cognition, and to discuss suggested approaches for managing cognitive dysfunction, with the principal goals of minimizing or mitigating its impact on patients’ functional capacity and QoL.
2 Literature Search
We performed a manual MEDLINE search for the following concepts, used separately or in combination: prostate cancer, cognitive function, cognitive dysfunction, cognitive impairment, cognitive decline, comorbidity, comorbidities, quality of life, cognitive assessment, cognitive management. We also searched for reports of phase III studies of second-generation AR-pathway inhibitors (abiraterone acetate, apalutamide, darolutamide, and enzalutamide). Only English-language articles were included, and the searches were limited to publications between January 1998 and September 2021. Relevant articles were selected for review on the basis of their abstracts. After elimination of duplications, a total of 94 papers were identified for inclusion. Additional supporting papers were identified through expert knowledge of the literature (19 in total) and through manual searches of source documents in included papers and ClinicalTrials.gov study details (13 in total), and relevant congress abstracts (five in total).
3 Cognitive Function in Patients with Cancer
The incidence of cognitive dysfunction has been estimated at up to 75% in patients with non-CNS cancer, although reported rates differ widely depending on the specific assessment instruments and the definitions of cognitive dysfunction used [2, 3]. A number of anticancer therapies are not highly specific, risking adverse effects on healthy organ systems, which include potential impacts on cognitive function through unintended CNS effects [5]. Cancer-related cognitive impairment can be enduring and incapacitating. It may interfere with normal roles and psychosocial functioning, instrumental activities of daily living, the ability to manage health, finances, and medications independently, and the ability to engage in meaningful family and social relationships [3].
The risk of cognitive impairment increases with age over 60 years [21], with data suggesting an incidence of 219 diagnoses of dementia per 100,000 individuals aged < 65 years vs 1964 per 100,000 in those aged ≥ 65 years (close to an eight-fold increase) [22]. Older patients with comorbidities and prodromal dementing processes may have an additional increased risk of cognitive decline and treatment-related cognitive adverse events (AEs) [2, 5, 6, 21]. Older patients with a history of cancer may be at greater risk of cognitive decline than older patients without cancer, as indicated by an analysis of data from adults aged 60 years and older who participated in the US National Health and Nutrition Examination Survey from 1999 to 2002 [23]. In that study, cognitive dysfunction was greater in cancer survivors (n = 408) than in non-cancer participants (n = 2639); for example, the average score on the Digit Symbol Substitution Test (which assesses response speed, sustained attention, visual spatial skills, and task shifting, with lower scores predicting possible future clinical/subclinical cognition and mobility disorders [24]) was 1.99 points lower for cancer survivors than for non-cancer participants (95% confidence interval [CI] −3.94, −0.05).
Poor cognitive performance on standardized neuropsychological tests is associated with increased mortality risk [25, 26]. Several studies also have linked cognitive dysfunction to an increased risk of mortality in older patients (age ≥ 65 years) with cancer. In a 2-year, single-center, longitudinal study of overall survival among patients with breast, prostate, or colorectal cancer, stratified by diagnosis and metastatic disease, Libert et al. found that cognitive dysfunction significantly affected survival in older patients with cancer (stratified hazard ratio for risk of death, 6.13; 95% CI 2.07, 18.09; p = 0.001) [27]. Katsoulis et al. reported an association between decreased Mini-Mental State Examination (MMSE) scores and increased overall cancer mortality in patients aged ≥ 65 years (hazard ratio 1.32; 95% CI 1.02, 1.70) [28], and Batty et al. observed an inverse association between cognition and cancer-related death in older patients (hazard ratio 1.21; 95% CI 1.10, 1.33) [26].
Cognitive dysfunction can profoundly affect QoL through its detrimental effects on function and autonomy [3, 29]. It may also impair the ability of patients with cancer to navigate their own treatment pathway: to understand and participate in complex decision-making processes, to weigh the often nuanced options of respective anticancer therapies, to understand the scope and the nature of associated risks, and to provide informed consent when needed [2, 4, 30]. Independent living can be compromised, and patients with cognitive dysfunction also may require close supervision by the oncology team and additional support to help them manage their therapeutic regimen [3, 30].
4 Drug Treatment and Other Factors Associated with Cognitive Dysfunction
The clinical status of older patients with cancer is often complicated by chronic comorbidities that interfere with a straightforward disease management plan [4]. The observed changes are not simply cognitive in nature, but also include fatigue, mood changes, pain, and sleep disorders [2]. Disease stage may also adversely influence cognitive function [2, 5, 31]. Declining executive function has been observed in patients with more advanced malignancies compounded by comorbidities [32], and affective disorders have been associated with cognitive complaints [33]. Genetic mutations associated with Alzheimer’s disease have been identified in patients with prostate cancer [34, 35], and variants of several genes, including the apolipoprotein E gene and the rs1047776 single-nucleotide polymorphism in GNB3, may increase the risk of cognitive dysfunction [6, 10, 36]. However, Buskbjerg et al. found that patients with prostate cancer who were carriers of the catechol-O-methyltransferase Met allele experienced a larger decline in visuospatial memory compared with Val allele carriers after ADT. No association between apolipoprotein E, brain-derived neurotrophic factor genotype, and treatment-related cognitive decline was observed. Patients with prostate cancer treated with ADT did not demonstrate alterations in brain connectome metrics over time compared to healthy controls [37]. Crawford et al. also review the case for a potential correlation between higher serum levels of follicle-stimulating hormone, ADT, and poorer cognitive performance, suggesting a biological rationale for cognitive change in patients with prostate cancer receiving ADT [38].
Cognitive dysfunction has been associated with drug toxicity, which may prompt modifications to anticancer therapy. The connection between chemotherapy and cognitive dysfunction is well established, although most research has been conducted in patients with breast cancer [5, 39]. In the ELCAPA01 study of elderly patients with cancer (n = 375) [40], a univariate analysis revealed that cognitive dysfunction was associated with changes to pharmacotherapy, defined as: intensification via the addition of one or more modalities, i.e., surgery, chemotherapy, hormonal therapy, radiotherapy, and supportive care; decrease via modality removal/treatment replacement with supportive care; or postponement of treatment (the study does not detail which changes were associated with cognitive dysfunction). A greater proportion of patients in the group that changed therapy exhibited cognitive decline compared with those who maintained their original regimen (38.5% vs 24.9%, respectively; p = 0.023). Of those who changed treatment regimens, 63 (80.8%) reduced their treatment intensity, with the majority (85.7%) transitioning from an active modality to supportive care [8, 30, 40]. Thus, cognitive decline in this study may reflect treatment toxicity, but also may represent the adverse CNS effects of progressive disease.
Although generally well tolerated, ADT-induced hypogonadism and AR-pathway inhibitors have been linked to the development of cognitive dysfunction in men with prostate cancer, although argument for causation has been inconsistent [41,42,43]. Separate evidence suggests that testosterone may exert neuroprotective effects [44], as its depletion has been associated with learning and memory deficits [10, 41, 42, 44, 45]. These findings correlate with data that associate low serum testosterone levels with dementia risk in aging men without prostate cancer [44]. Plata-Bello et al. noted that accelerated testosterone decreases in patients with prostate cancer treated with ADT may negatively affect normal brain aging, and that the increase in white matter lesions and loss of gray matter volume found in their study may make these patients more susceptible to the development of cognitive impairment, particularly if they present with a low cognitive reserve [46]. A study by Cherrier et al. found reduced, task-related, functional magnetic resonance imaging activation in patients treated with ADT compared with controls (p = 0.0032 for recognition, p = 0.0031 for mental rotation matching) [47]. Similarly, Chao et al. observed diminished regional brain activations during stop-signal tasks in patients with nonmetastatic prostate cancer treated with ADT for 6 months compared with baseline, whereas patients in a matched control group with nonmetastatic prostate cancer who did not receive ADT had no change in brain activation [48]. However, in that study, there were no differences between the ADT-treated and untreated cohorts in the performance of cognitive tasks or in health-related QoL (HRQoL) at 6 months [48].
The fact that some studies investigating the effect of ADT on cognitive function suggest a clear association [10, 41, 45, 49,50,51,52,53,54], while others fail to identify one [55,56,57,58,59,60,61,62], could be attributed to the diverse definitions of cognitive dysfunction, and to differences in the assessment instruments selected and to how they were applied [63]. Furthermore, differences in patient populations, such as age, education level, comorbidities, pre-existence of genetic mutations, or choice of ADT may affect the propensity for cognitive deterioration in patients treated with ADT [34, 35, 52, 57, 61]. As a result, some patients may experience severe cognitive decline while others experience no adverse effect of ADT [64]. Importantly, an analysis of MedWatch (the Food and Drug Administration Safety Information and Adverse Event Reporting Program Studies) failed to show an association between ADT and Alzheimer’s disease or cognitive dysfunction when reported AEs were assessed using calculated proportional reporting ratios [65,66,67]. However, other claims-based investigations demonstrate a possible association between ADT and dementia, emphasizing a lack of data consistency in this area of research [68]. The SIOG recommends careful assessment of the risk–benefit ratio of ADT for localized prostate cancer, including a brief cognitive screening assessment, with referral of patients who meet screening criteria for potential cognitive risk to undergo full neuropsychological evaluation by specialists [4]. Given that ADT is increasingly being used in combination with second-generation AR-pathway inhibitors, any indicators of cognitive function during fixed-duration use of ADT in the localized prostate cancer setting should be taken into consideration when assessing the potential risk of cognitive dysfunction during combination therapy for advanced disease.
In recent years, several AR-pathway inhibitors with different mechanisms of action have been incorporated into the treatment pathway for patients with prostate cancer, including the three second-generation AR inhibitors, apalutamide, darolutamide, and enzalutamide, as well as the androgen biosynthesis inhibitor abiraterone acetate. Clinical studies of these agents that include outcomes related to cognitive dysfunction are summarized in Table 1 [11,12,13, 15,16,17, 20, 65, 69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102]. Rigorous cognitive function testing was not conducted in the phase III studies, although all included validated measures of general or prostate cancer-specific HRQoL such as the EuroQol 5-dimension questionnaire (EQ-5D) and the Functional Assessment of Cancer Treatment-Prostate questionnaire (FACT-P; both described in further detail below), which address some aspects of mental health and ability to undertake daily activities [13, 69,70,71,72, 76,77,78,79,80, 82, 84,85,86, 88,89,90,91,92,93,94,95]. In these analyses of phase III studies, which included relatively fit patients without overt cognitive impairment, HRQoL was generally maintained with AR-pathway inhibitor therapy, often with minimal differences between active therapy and placebo arms, and the time to deterioration in HRQoL was typically significantly longer in AR-pathway inhibitor arms than in placebo arms (Table 1) [13, 70, 71, 74, 77, 79, 82, 85, 86, 88, 90, 92, 94, 95]. Of note, in the phase II COSMIC study, changes in FACT-P scores over time favored abiraterone acetate plus prednisone over enzalutamide in patients aged ≥ 75 years, but not in younger patients [99].
Most studies of AR-pathway inhibitor therapy that focus specifically on measures of cognitive function involve patients treated with abiraterone acetate and enzalutamide (Table 1), with some evidence suggesting that the incidence of cognitive dysfunction may be higher in patients treated with enzalutamide. Cognitive dysfunction may be more prevalent in real-world populations than in the carefully selected populations included in phase III studies. For example, in the observational AQUARiUS study, clinically meaningful worsening in perceived cognitive impairment was reported by 49% of patients treated with abiraterone acetate and 76% of those who received enzalutamide (p = 0.05) [96]. By contrast, in REAAcT, COSMIC, GUTG-001, and TOPCOP1, patients treated with abiraterone acetate or enzalutamide had almost no change from baseline in cognitive function over time (Table 1) [16, 97, 99, 102].
In the phase III studies, the impact of treatment on cognitive function can also be gleaned from CNS-related terms reported as AEs. In the pivotal studies of abiraterone acetate in patients with mCRPC, fatigue was the most widely reported CNS-associated AE, occurring in 14% of abiraterone-treated patients with metastatic hormone-sensitive prostate cancer in LATITUDE and 40–47% of patients with mCRPC in the COU-AA-301 and COU-AA-302 studies; the incidence was 15% and 35–44% in the respective placebo arms [76, 79, 84]. Other potentially CNS-related AEs reported were asthenia in 5% of patients treated with abiraterone acetate vs 5% of placebo recipients in LATITUDE, asthenia in 15% vs 14% in COU-AA-301, and insomnia in 15% vs 12% in COU-AA-302 [76, 79, 84]. Clinical trials of enzalutamide in patients with mCRPC have reported CNS-associated AEs, including fatigue [14, 15].
In the final analysis of the phase III PROSPER trial in patients with nmCRPC, CNS-associated AEs occurred more frequently in patients randomized to enzalutamide plus ADT than to ADT alone: fatigue (37% vs 16%), dizziness (12% vs 6%), headache (11% vs 5%), asthenia (10% vs 7%), and cognitive and memory impairment (8% vs 2%) [11]. In the final analysis of the phase III SPARTAN trial in patients with nmCRPC, the CNS-associated AE of fatigue was reported more frequently in the apalutamide plus ADT group than in the placebo plus ADT group (33% vs 21%) [12]. In addition, at the primary analysis, dizziness (9.3% vs 6.3%) and mental impairment disorders (5.1% vs 3.0%) were reported more frequently with apalutamide than placebo [13]; the incidences of these events were not presented at the final analysis. Results from the final analysis of the phase III ARAMIS trial in men with nmCRPC showed that the only CNS-associated AE with an incidence > 10% that occurred more frequently with darolutamide plus ADT than with placebo plus ADT was fatigue (13.2% vs 8.3%) [20]. Incidences of other CNS-associated AEs were low and comparable between the darolutamide and placebo arms [20]. Specifically, the incidence of asthenic conditions was 4.0% vs 3.1% and the incidence of mental impairment disorders was 2.0% vs 1.8% for darolutamide vs placebo [20]. Additionally, although final analysis data have not been presented for dizziness, its incidence was comparable between the darolutamide arm (4.5%) and the placebo arm (4.0%) at the primary analysis [71]. These results suggest that darolutamide has a favorable safety profile with minimal additional risk of CNS-related AEs commonly associated with other AR inhibitors [20].
The CNS-related AEs reported with apalutamide and enzalutamide may be due to their penetration of the blood–brain barrier, whereas darolutamide has shown limited blood–brain barrier penetration in preclinical and clinical studies [101, 103]. A head-to-head, quantitative, whole-body autoradiography study in rats reported comparable, early post-dose, homogenous distribution of darolutamide and enzalutamide in organs and tissues with the exception of brain and adipose tissue. Considerably limited brain penetration was observed for darolutamide compared with enzalutamide [103]. A single-dose pharmacokinetic study of apalutamide and an in vivo organ distribution study in rats compared [14C]apalutamide with previously reported [14C]enzalutamide and [14C]darolutamide data through quantitative whole-body autoradiography. At 8 h post-dose, quantitative whole-body autoradiography demonstrated persistent brain concentrations of 1.82 μg.eq/g for apalutamide and 3.25 μg.eq/g for enzalutamide and similar brain-to-blood ratios of 0.847 and 0.807, respectively; the qualitative brain distribution of darolutamide at 8 h was 0.069 μg.eq/g with a brain-to-blood ratio of 0.079 [104]. These results are supported by functional neuroimaging data from healthy human volunteers. In a phase I study, changes in cerebral blood flow were assessed in response to enzalutamide and darolutamide treatment. Arterial spin-labeled magnetic resonance imaging served as a proxy for brain penetration of these AR inhibitors. Whole-brain gray matter analysis demonstrated a 3.5% and 3.3% reduction in cerebral blood flow for enzalutamide compared with placebo and darolutamide, respectively. In addition, localized reductions in cerebral blood flow were detected in the hippocampus and frontal cortex following enzalutamide treatment compared with placebo or darolutamide [101].
5 Assessment of Patient Cognitive Function
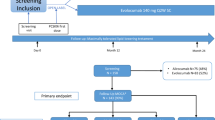
To determine whether cognitive function is affected by treatment, and the nature and extent of this impact, it is important to understand the baseline presence and pattern of cognitive deficits before treatment initiation [5]. Patients may present with self-reported difficulties in concentration, memory, attention, and executive functioning, and a sense of declining linguistic and arithmetic competence, the severity of which may vary. Baseline cognitive function is not routinely measured in clinical studies, a notable exception being the study by Gonzalez et al., who included an estimated full-scale intelligence quotient assessment at baseline as a measure of cognitive reserve [10]. Routine use of such measures would provide a quantifiable benchmark for declining cognitive function with treatment. Screening for cognitive dysfunction is considered an integral part of standard management before initiation of a prostate cancer treatment plan in elderly patients [4], and multidisciplinary team interaction in decision making is recommended [8]. Brief screening with simple tools such as the Montreal Cognitive Assessment (MoCA), MMSE, or Mini-Cog™ may be useful initially to determine whether the patient presents with gross signs of cognitive dysfunction, although subtle expressions of a mild, yet disruptive, cognitive disorder may go undetected because of inadequate test sensitivity. Screening tests may be supplemented with symptom assessment tools that measure patient-reported outcomes to eliminate other factors that may impact cognitive function, such as depression or fatigue. Referral for specialized neuropsychological assessment, typically more comprehensive and definitive in nature, may follow if cognitive dysfunction is either identified on a screening measure or clinically suspected. In addition, as cognitive function can be affected by various comorbidities and complaints commonly observed in patients with prostate cancer (e.g., fatigue and pain), comprehensive assessment usually includes evaluation of these symptoms (see Fig. 1 for a suggested cognitive assessment process).
Decision process: cognitive function assessment in patients with prostate cancer (PC). FACT-Cog Functional Assessment of Cancer Treatment-Cognitive Function, FACT-P Functional Assessment of Cancer Treatment-Prostate, G8 geriatric screening tool, IQ intelligence quotient, MMSE Mini-Mental State Examination, MoCA Montreal Cognitive Assessment, OTC over the counter
Currently, a large variety of methods are adopted to evaluate cognitive function, with variable outcomes depending on the psychometric properties of the assessment tool (e.g., sensitivity and validity) [42]. As with other clinical populations studied to date, a lack of consensus prevails on which patient factors, characteristics, and symptoms place a patient at risk for cognitive dysfunction, and on the appropriate assessments to identify cognitive dysfunction in patients receiving treatment for prostate cancer [41, 42].
A wide range of cognitive testing instruments (e.g., Wechsler Adult Intelligence Scale, Controlled Word Association, Trail Making Test, Hopkins Verbal Learning Test-Revised) and self-report measures (e.g., Functional Assessment of Cancer Therapy [FACT]-Cognitive, Patients Assessment of Own Functioning Inventory) have been used to identify cognitive dysfunction [41, 42]. Unfortunately, the lack of a consistent core set of tools or measures has limited the reliability of cross-study comparisons [42]. Common definitions of cognitive dysfunction and standardized assessments are needed to make more uniform comparisons of effects on cognitive function across clinical studies, as different definitions of dysfunction based on cognitive test performance or self-reported criteria may either understate or exaggerate the prevalence of neuropsychological deficits [41, 42]. Furthermore, many studies exclude patients who are unable to attend clinics or understand the study instructions given the known impact of the impact of sociodemographic, language, or learning barriers on these assessment tools; additional studies in these vulnerable populations are required.
There is an unmet need for practical guidance to provide the urologist or oncologist with an algorithm for straightforward and rapid identification of cognitive dysfunction, and for a reproducible method of prospectively assessing cognitive function. However, assessment and management of cognitive dysfunction transcend the expertise of the community urologist or oncologist, and referral to a neuropsychologist may be preferable if cognitive dysfunction is apparent or suspected. Although test administration is relatively straightforward, interpretation of scores relies on psychodiagnostic skills, knowledge of functional neuroanatomy, test construction and psychometrics, appreciation of the contribution of social and cultural factors, and the interviewing skills of the clinician [31].
6 Definition of Cognitive Dysfunction
Clinicians should initiate cognitive assessment with a thorough patient interview, including medical and surgical histories to determine the pre-cancer level of functioning, if possible, and to gain information on the educational level and occupation of the patient, all with the primary aim of identifying a reliable pre-cancer cognitive baseline. The initial interview also should aim to uncover any developmental disability and glean information on chronic comorbidities and comedications. Knowledge of the presence or absence of chronic nonmalignant comorbidities such as cardiovascular disease, and of the type of anticancer therapy administered may clarify the underlying etiology of the observed cognitive dysfunction. The timeline of onset and course of cognitive symptoms should be charted and the clinician should consider how these relate to the cancer diagnosis and treatment [5].
Cognitive decline after treatment with systemic chemotherapy tends to follow a pattern suggestive of frontal-subcortical dysfunction. It may include impairments in executive functioning, processing speed, and rapid motor coordination, as well as in learning and memory. These deficits tend to manifest in complaints of short-term memory loss, decreased concentration and attention span, organization, and multi-tasking [5, 31]. It bears mentioning that the cognitive dysfunction observed in patients receiving AR-directed therapy for prostate cancer may not routinely rise to the level of severity required to meet any clinically defined criteria for dementia. Therefore, neuropsychological tests, as opposed to exclusively clinical criteria, may more accurately capture the alterations in cognitive function in patients with cancer, and quantify their individual level of pre-morbid functioning [5].
A thorough neuropsychological evaluation also should include assessment of pain, fatigue, and affect; these factors may accompany cognitive dysfunction, may adversely impact cognitive performance, and frequently are associated with cognitive complaints. Neuropsychologists are uniquely qualified to objectively characterize the nuances and extent of cognitive impairment, applying a range of objective instruments designed specifically for this purpose. Ideally, neuropsychological evaluations should be performed prior to treatment initiation, thereby establishing a pre-therapeutic baseline. However, in clinical practice, this often does not occur. A neuropsychologist can assess the level of cognitive dysfunction, determine any relative contributions of mood and other factors to cognitive dysfunction, and develop a management plan to mitigate cognitive AEs. An understanding of the different types, degrees, and stages of cognitive dysfunction can aid the treating clinician to determine when next-level referral should occur for further assessment and symptom management [63].
Neuropsychological assessment requires the selection of instruments with satisfactory psychometric properties (e.g., reliability, validity) that are appropriate for the specific patient and their clinical situation, considering the educational and cultural background of the patient, medical history, including medications and dietary supplements, and diagnostic and treatment considerations. In 2011, the International Cognition and Cancer Task Force released guidance on recommendations for neuropsychological tests in research settings, common criteria for defining cognitive dysfunction, and approaches to improve homogeneity of study methodology [63]. An aspirational goal for neuropsychological evaluation is that the results provide a measure of functional loss in daily life. It is well recognized that standardized neuropsychological tests can directly measure the organ system being assessed (i.e., the brain), but that many intervening variables such as life demands, compensatory strategies, and external supports affect the relationship between cognitive test results and functional independence [105]. With this in mind, it is not surprising and perhaps not a reasonable goal for cognitive test results alone to correlate robustly with functional independence in daily life, i.e., ‛ecological’ validity. This emphasizes the need for testing to occur in the context of comprehensive evaluations conducted by clinical neuropsychologists.
7 Available Assessment Tools
7.1 Screening Tools
Screening tools are a logical first step for the clinician, but it is important to acknowledge the limitations of these and understand their respective error types. No effective brief screening tool for cancer-related cognitive dysfunction has yet been identified, although numerous measures that screen cognitive function are available (Table 2) [4, 30, 106,107,108,109,110,111].
The MMSE was developed as a screening tool for dementia. While often useful in providing a snapshot of global cognition, it lacks adequate sensitivity to identify declines in cognitive performance over time that are commonly observed in non-CNS oncology settings. In addition, the MMSE often fails to identify cognitive impairment in certain patient subgroups (e.g., highly educated elderly individuals) [106,107,108, 112].
The MoCA, though used infrequently to assess mild cognitive impairment is a slightly more rigorous and sensitive alternative to the MMSE as it includes a somewhat greater diversity of tasks. However, it too is clearly limited when compared with a more comprehensive battery. The MoCA subtest scores exhibit poor accuracy in predicting cognitive impairment within specific domains of memory, attention, processing speed, language, visuospatial ability, and executive functioning. Selective interpretation of cognitive performance based on a particular test item with high specificity or sensitivity in a given cognitive domain does not assure accurate identification of cognitive impairment [113].
The Mini-Cog tool is recommended by SIOG as first-line screening for cognitive health status in elderly patients with prostate cancer [4], if the score is abnormal, a referral for neuropsychological assessment is recommended [4, 30]. The G8, a geriatric functional assessment questionnaire, provides an overview of the general health status of elderly patients. One of its components addresses neuropsychological problems, scoring the severity of dementia or depression on a descending scale of 0–2, with 0 suggesting severe impairment [4]. To detect frailty and cognitive dysfunction in elderly patients, SIOG recommends as a first-line analysis administering the validated G8 tool concurrently with the Mini-Cog [4]. This approach can then serve as the basis for determining the necessity of a comprehensive geriatric assessment and help to guide appropriate selection of therapy [4].
7.2 Self-Reported Symptom Assessment Tools
Comprehensive neuropsychological evaluation frequently assesses common symptoms experienced by patients with cancer. The presence of clinically significant symptoms benefits from management and interventions to improve patient function, well-being, and QoL [5, 63, 112, 114, 115]. Patient self-reported cognitive dysfunction should elicit objective evaluation of cognitive function as well as comprehensive evaluation of the aforementioned symptoms, followed by patient education [39, 112, 115, 116].
We include here several widely used clinical screening tools that may be used by the clinician to measure patient-reported outcomes in patients with cancer (Table 3) [117,118,119,120,121,122,123,124,125,126,127,128]. Patient-reported outcome measures should be selected carefully, based on their purpose, context, and the issue/symptom to be investigated. The choice of instrument may vary depending on whether the clinician intends to use it for screening symptoms or clinical research, and with which identifiable symptoms the patient presents (e.g., the FACT-Cognitive Function asks about very different domains compared with the FACT-P) [117, 118].
7.3 Neuropsychological Assessment Tools
Objective neuropsychological evaluation with cognitive tests that are psychometrically sound (e.g., evidence of adequate validity and test-retest reliability) represents the gold standard for assessing cognitive function [63]. Instruments should be sensitive to even subtle changes in those functions that are most susceptible to chemotherapy — learning and memory, processing speed, executive function, and fine motor control. Androgen deprivation therapy for prostate cancer has also been shown to affect visuospatial abilities [45], such that assessment of this domain may be of importance.
Table 4 lists examples of tests that have been adopted clinically by neuropsychologists to evaluate cognitive function in patients with cancer [33, 63, 129,130,141].
The Cambridge Neuropsychological Test Automated Battery (CANTAB) is a modular system for assessing cognitive dysfunction. It includes tests of attention and psychomotor speed, executive function, memory, emotion, and social cognition [142, 143]. Selected modules from CANTAB have been incorporated into the randomized phase II ARACOG (Androgen Receptor Directed Therapy on Cognitive Function in Patients Treated With Darolutamide or Enzalutamide; AFT-47; ClinicalTrials.gov identifier NCT04335682) trial of AR-pathway inhibitor therapy to measure cognitive performance in patients with prostate cancer. This study will compare cognitive outcomes between patients with prostate cancer treated with darolutamide or enzalutamide, and aims to test the suitability of this cognitive assessment tool in the prostate cancer setting [144].
A further area of research interest will be the integration of cognitive testing with the growing uptake of telehealth services, prompted in large part by the COVID-19 pandemic. Currently, it is too early to confirm the long-term place of telehealth modalities, and more research is needed on how best to use telehealth to assess and care for patients.
8 Management of Cognitive Dysfunction
Limited guidance exists for the assessment and management of cancer-related cognitive dysfunction given the lack of complete understanding of its etiology and underlying mechanisms [3, 5, 29]. Compensatory strategies, which mitigate the impact of cognitive decline on daily function and environmental modifications, are often used to maximize the daily functional independence of patients with cancer treatment-related cognitive decline [2, 5].
Incorporation of neuropsychological evaluation into multidisciplinary patient care can assist in evaluating and modifying therapeutic regimens if significant treatment-related neurotoxicities are detected. Consideration may be given to adjusting therapy type, dose, and treatment schedule, to balance maintenance of disease control with preservation of HRQoL and function [2]. This assessment and feedback can be helpful in itself, enabling the treating clinician to address questions and manage anxieties, as it is important to the patient that their concerns are acknowledged and supported by the healthcare provider [2, 29]. A targeted management plan that identifies where support is most needed can also assist patients to regain control over their personal situation [2]. Older patients, especially those with multiple comorbidities, may require additional support and environmental adjustments [5]. The management of related neuropsychiatric comorbidities, such as depression, disordered sleep cycles, fatigue, or pain should be optimized [145, 146]. Psychostimulant medications have been prescribed to attenuate the fatigue experienced by some patients with cancer. Donepezil also has been prescribed to manage cognitive symptoms, but most studies targeting cognition or fatigue have been small, limited in duration, and of variable quality [5].
Patient/family education and counseling is suggested as a way of fostering self-management and co** strategies [145], and expanded social support may be required to assist the patient in managing the daily demands of self-care and instrumental activities of daily living, such as finances or medication administration [30]. Lifestyle management can include co**/organizational strategies, for example, memory aids, goal-focused compensatory interventions, and behavioral strategies [145].
Cognitive rehabilitation interventions, including occupational and speech therapy, psychotherapy, and group training programs to reinforce memory skills have been shown to improve self-reported cognitive function and fatigue [147, 148]. Physical exercise is also recommended; it has been associated with improved cognitive functioning in patients with Alzheimer’s disease, with self-reported QoL improvement [5, 30], and with positive effects on self-reported cognitive function [112, 149]. Beneficial effects of exercise on social and cognitive functioning were also found in a meta-analysis of randomized controlled trials involving patients with prostate cancer [150].
9 Discussion
Cognitive dysfunction is increasingly recognized as a major clinical challenge in cancer management, which is likely to gain in importance as the aging population grows [2, 4]. In addition to the potential impact on QoL via its effects on patient functioning and autonomy [3, 29], cognitive dysfunction may impede the ability of the patient with prostate cancer to navigate his own therapeutic course [2, 30].
It is essential for the maintenance of health-related QoL to identify symptoms of cognitive dysfunction and to manage them effectively, and where possible to avoid or mitigate their development [3, 29, 41]. Knowledge of the precise etiology of the cognitive sequelae of non-CNS malignancies and their medical management is limited [3]. In addition, as a lack of consensus remains on the definition of cognitive dysfunction and on the most appropriate tools by which to identify it, further study is needed [39].
Accumulating evidence shows that anticancer therapy can have detrimental effects on patients’ cognitive function; the risk may vary depending on the patient’s age and comorbidities [4, 5, 26] and the therapeutic agent being considered (Table 1). It is imperative that treatment decisions take into account the potential risk for each patient and that effective treatments are selected that are associated with minimal risk of cognitive dysfunction. The majority of studies that have evaluated the impact of prostate cancer and combined ADT and AR-pathway inhibitor therapies on cognition have focused on effects within the first year during and after treatment, while the true impact of long-term androgen ablation on cognitive status has yet to be determined [63]. Results from ARACOG are expected to add to the literature on the cognitive effects of treatment for prostate cancer. This and other prostate cancer trials using an objective assessment of cognitive function are expected to be more sensitive than the clinician-reported and coordinator-reported AEs that have been used as a measure of cognitive dysfunction historically.
The published literature has not identified the optimal timing for behavioral intervention; therefore, it is premature to definitively state whether cognitive interventions during or even before initiation of anticancer therapy could elicit a preventive effect [39]. With the overriding objectives of generating more accurate estimates of the incidence and course of post-treatment cognitive decline and creating a framework to support data sharing across investigators, the International Cognition and Cancer Task Force proposed minimum criteria for neuropsychological studies of cognitive dysfunction [63]. These criteria included a core set of neuropsychological tests; a common criterion for defining cognitive impairment; and common methodologies for combining data across studies [63]. Some or all of these recommendations could be applied to the creation of a large data source that would serve to accelerate our progress in future studies of AR-pathway inhibitors for their association with cognitive dysfunction in patients with nmCRPC.
Objective measures of cognitive function that can be broadly distributed and used to assess patients with prostate cancer before and during treatment in routine clinical practice are urgently needed. While neuropsychologists are well equipped with the requisite expertise and standardized, validated assessment instruments to reliably identify and quantify the severity of cancer-associated and treatment-associated cognitive impairment in nmCRPC, community oncologists and urologists do not have the skill set to conduct these evaluations in the clinical setting and may not have easy access to neuropsychological expertise in their area [3]. This has fostered interest in a brief screening tool that can be easily incorporated into standard clinical workflows, the administration of which would not require clinical expertise. However, further research is required to formulate validated novel paradigms that readily translate to clinical assessment and that are platform-independent, reliable, and user friendly in a practice environment [109]. Currently, the G8 and the Mini-Cog represent two assessment instruments that are recommended by SIOG for adoption for screening in clinical practice [4]. In combination, these tools provide an assessment of functional status and cognition that could prompt referral for further evaluation and support services. Cognition is assessed by one item of the G8, while physiological status is assessed by an item of the Mini-Cog. The MoCA also can be used as a cognitive screening tool in clinical practice, given that it encompasses the eight cognitive domains of short-term memory recall, visuospatial abilities, executive function, attention, concentration, working memory, language, and orientation to time and place [30, 110].
As patient-reported outcome measures have not been validated to assess cognitive function in clinical practice, further evaluation is needed to guide clinical application of these reporting tools. Moreover, no single instrument to date sufficiently reflects the patient experience of nmCRPC and its therapeutic impact. Thus, examining new and effective methods to measure the effects of treatment for nmCRPC, such as conceptual modeling of the patient experience with both disease processes and the physical and psychosocial signs, symptoms, and impacts of its treatment [111, 119], may serve to stimulate discussion about which concepts should be reflected in study endpoints and practice-based QoL assessments.
10 Conclusions
Cognitive decline related to symptomatic disease progression and/or pharmacotherapy can profoundly affect independence in conducting activities of daily living, and diminish HRQoL in patients with prostate cancer [2]. A better understanding of the relationship between cognitive function, prostate cancer, and available treatments will help to ensure that the most appropriate therapeutic management strategies are adopted. Awareness of potential sources of cognitive dysfunction, including certain anticancer therapies, and prompt identification of early treatment-related symptoms are vital components to improving the standard of care in prostate cancer management. The effects of ADT and AR-directed therapies on cognitive function and the CNS should be considered carefully to ensure that any effect on functional capacity and QoL is minimized.
Several challenges must be overcome to facilitate the effective assessment of cognitive function in particular in men with nmCRPC receiving AR-directed treatment. We have highlighted some assessment methods that can be adopted by the clinician to measure and manage cognitive function in these patients. As cognitive dysfunction continues to increase in recognition as a significant AE of cancer and anticancer therapy, it is hoped that further contributions will be made to the corpus of literature on cognitive function in patients with prostate cancer, providing reliable and consistent clinical evidence and metrics on the patterns and etiologies of cognitive dysfunction.
Change history
28 November 2022
A peer-reviewed Video Abstract was retrospectively added to this publication
References
Kayl AE, Collins R, Wefel JS. Neuropsychological assessment of adults with cancer. In: Meyers CA, editor. Cognition and cancer. Cambridge: Cambridge University Press; 2008. p. 44–55.
Noll KR, Bradshaw ME, Rexer J, Wefel JS. Neuropsychological practice in the oncology setting. Arch Clin Neuropsychol. 2018;33(3):344–53.
Jean-Pierre P, McDonald BC. Neuroepidemiology of cancer and treatment-related neurocognitive dysfunction in adult-onset cancer patients and survivors. Handb Clin Neurol. 2016;138:297–309.
Droz JP, Albrand G, Gillessen S, Hughes S, Mottet N, Oudard S, et al. Management of prostate cancer in elderly patients: recommendations of a task force of the International Society of Geriatric Oncology. Eur Urol. 2017;72(4):521–31.
Bradshaw ME, Wefel JS. Neuropsychological assessment of older adults with a history of cancer. In: Ravdin L, Katzen H, editors. Handbook on the neuropsychology of aging and dementia. New York: Springer; 2013. p. 427–42.
Qian J, Wolters FJ, Beiser A, Haan M, Ikram MA, Karlawish J, et al. APOE-related risk of mild cognitive impairment and dementia for prevention trials: an analysis of four cohorts. PLoS Med. 2017;14(3):e1002254.
Boyle HJ, Alibhai S, Decoster L, Efstathiou E, Fizazi K, Mottet N, et al. Updated recommendations of the International Society of Geriatric Oncology on prostate cancer management in older patients. Eur J Cancer. 2019;116:116–36.
Wildiers H, Heeren P, Puts M, Topinkova E, Janssen-Heijnen ML, Extermann M, et al. International Society of Geriatric Oncology consensus on geriatric assessment in older patients with cancer. J Clin Oncol. 2014;32(24):2595–603.
Lange M, Heutte N, Rigal O, Noal S, Kurtz JE, Lévy C, et al. Decline in cognitive function in older adults with early-stage breast cancer after adjuvant treatment. Oncologist. 2016;21(11):1337–48.
Gonzalez BD, Jim HS, Booth-Jones M, Small BJ, Sutton SK, Lin HY, et al. Course and predictors of cognitive function in patients with prostate cancer receiving androgen-deprivation therapy: a controlled comparison. J Clin Oncol. 2015;33(18):2021–7.
Sternberg CN, Fizazi K, Saad F, Shore ND, De Giorgi U, Penson DF, et al. Enzalutamide and survival in nonmetastatic, castration-resistant prostate cancer. N Engl J Med. 2020;382(23):2197–206.
Smith MR, Saad F, Chowdhury S, Oudard S, Hadaschik BA, Graff JN, et al. Apalutamide and overall survival in prostate cancer. Eur Urol. 2021;79(1):150–8.
Smith MR, Saad F, Chowdhury S, Oudard S, Hadaschik BA, Graff JN, et al. Apalutamide treatment and metastasis-free survival in prostate cancer. N Engl J Med. 2018;378(15):1408–18.
Higano CS, Beer TM, Taplin ME, Efstathiou E, Hirmand M, Forer D, et al. Long-term safety and antitumor activity in the phase 1–2 study of enzalutamide in pre- and post-docetaxel castration-resistant prostate cancer. Eur Urol. 2015;68(5):795–801.
Pilon D, Behl AS, Ellis LA, Robitaille MN, Lefebvre P, Dawson NA. Assessment of real-world central nervous system events in patients with advanced prostate cancer using abiraterone acetate, bicalutamide, enzalutamide, or chemotherapy. Am Health Drug Benefits. 2017;10(3):143–53.
Shore ND, Saltzstein D, Sieber P, Mehlhaff B, Gervasi L, Phillips J, et al. Results of a real-world study of enzalutamide and abiraterone acetate with prednisone tolerability (REAAcT). Clin Genitourin Cancer. 2019;17(6):457-63.e6.
Thiery-Vuillemin A, Hvid Poulsen M, Lagneau E, Ploussard G, Birtle A, Dourthe LM, et al. Impact of abiraterone acetate plus prednisone or enzalutamide on fatigue and cognition in patients with metastatic castration-resistant prostate cancer: initial results from the observational AQUARiUS study. ESMO Open. 2018;3(5):e000397.
Batra A, Marchioni M, Hashmi AZ, Lonergan PE, Morgans AK, Nead KT, et al. Cognition and depression effects of androgen receptor axis-targeted drugs in men with prostate cancer: a systematic review. J Geriatr Oncol. 2021;12(5):687–95.
Moilanen AM, Riikonen R, Oksala R, Ravanti L, Aho E, Wohlfahrt G, et al. Discovery of ODM-201, a new-generation androgen receptor inhibitor targeting resistance mechanisms to androgen signaling-directed prostate cancer therapies. Sci Rep. 2015;5:12007.
Fizazi K, Shore N, Tammela TL, Ulys A, Vjaters E, Polyakov S, et al. Nonmetastatic, castration-resistant prostate cancer and survival with darolutamide. N Engl J Med. 2020;383(11):1040–9.
Salthouse TA. Neuroanatomical substrates of age-related cognitive decline. Psychol Bull. 2011;137(5):753–84.
Noone AM, Howlader N, Krapcho M, Miller D, Brest A, Yu M, et al. SEER Cancer Statistics Review, 1975-2015, National Cancer Institute. Bethesda, MD. https://seer.cancer.gov/archive/csr/1975_2015/. Accessed 29 Mar 2022.
Williams AM, Janelsins MC, van Wijngaarden E. Cognitive function in cancer survivors: analysis of the 1999–2002 National Health and Nutrition Examination Survey. Support Care Cancer. 2016;24(5):2155–62.
Jaeger J. Digit symbol substitution test: the case for sensitivity over specificity in neuropsychological testing. J Clin Psychopharmacol. 2018;38(5):513–9.
Hayat SA, Luben R, Dalzell N, Moore S, Hogervorst E, Matthews FE, et al. Understanding the relationship between cognition and death: a within cohort examination of cognitive measures and mortality. Eur J Epidemiol. 2018;33(11):1049–62.
Batty GD, Deary IJ, Zaninotto P. Association of cognitive function with cause-specific mortality in middle and older age: follow-up of participants in the English Longitudinal Study of Ageing. Am J Epidemiol. 2016;183(3):183–90.
Libert Y, Dubruille S, Borghgraef C, Etienne AM, Merckaert I, Paesmans M, et al. Vulnerabilities in older patients when cancer treatment is initiated: does a cognitive impairment impact the two-year survival? PLoS ONE. 2016;11(8):e0159734.
Katsoulis M, Kyrozis A, Trichopoulou A, Bamia C, Trichopoulos D, Lagiou P. Cognitive impairment and cancer mortality: a biological or health care explanation? Cancer Causes Control. 2014;25(11):1565–70.
Boykoff N, Moieni M, Subramanian SK. Confronting chemobrain: an in-depth look at survivors’ reports of impact on work, social networks, and health care response. J Cancer Surviv. 2009;3(4):223–32.
Magnuson A, Mohile S, Janelsins M. Cognition and cognitive impairment in older adults with cancer. Curr Geriatr Rep. 2016;5(3):213–9.
Wefel JS, Kayl AE, Meyers CA. Neuropsychological dysfunction associated with cancer and cancer therapies: a conceptual review of an emerging target. Br J Cancer. 2004;90(9):1691–6.
Mandelblatt JS, Stern RA, Luta G, McGuckin M, Clapp JD, Hurria A, et al. Cognitive impairment in older patients with breast cancer before systemic therapy: is there an interaction between cancer and comorbidity? J Clin Oncol. 2014;32(18):1909–18.
Pendergrass JC, Targum SD, Harrison JE. Cognitive impairment associated with cancer: a brief review. Innov Clin Neurosci. 2018;15(1–2):36–44.
Lehrer S, Rheinstein PH. Alzheimer gene BIN1 may simultaneously influence dementia risk and androgen deprivation therapy dosage in prostate cancer. Am J Clin Oncol. 2020;43(10):685–9.
Lehrer S, Rheinstein PH. Co-occurrent alterations of Alzheimer’s genes and prostate cancer genes in prostate cancer. Cancer Genomics Proteomics. 2020;17(3):271–5.
Nicoll JA, Savva GM, Stewart J, Matthews FE, Brayne C, Ince P. Association between APOE genotype, neuropathology and dementia in the older population of England and Wales. Neuropathol Appl Neurobiol. 2011;37(3):285–94.
Buskbjerg CR, Amidi A, Buus S, Gravholt CH, Hadi Hosseini SM, Zachariae R. Androgen deprivation therapy and cognitive decline: associations with brain connectomes, endocrine status, and risk genotypes. Prostate Cancer Prostatic Dis. 2021. https://doi.org/10.1038/s41391-021-00398-1 (Epub ahead of print).
Crawford ED, Schally AV, Pinthus JH, Block NL, Rick FG, Garnick MB, et al. The potential role of follicle-stimulating hormone in the cardiovascular, metabolic, skeletal, and cognitive effects associated with androgen deprivation therapy. Urol Oncol. 2017;35(5):183–91.
Wefel JS, Kesler SR, Noll KR, Schagen SB. Clinical characteristics, pathophysiology, and management of noncentral nervous system cancer-related cognitive impairment in adults. CA Cancer J Clin. 2015;65(2):123–38.
Caillet P, Canoui-Poitrine F, Vouriot J, Berle M, Reinald N, Krypciak S, et al. Comprehensive geriatric assessment in the decision-making process in elderly patients with cancer: ELCAPA study. J Clin Oncol. 2011;29(27):3636–42.
Nelson CJ, Lee JS, Gamboa MC, Roth AJ. Cognitive effects of hormone therapy in men with prostate cancer: a review. Cancer. 2008;113(5):1097–106.
Treanor CJ, Li J, Donnelly M. Cognitive impairment among prostate cancer patients: an overview of reviews. Eur J Cancer Care (Engl). 2017;26(6):e12642.
Marandino L, Vignani F, Buttigliero C, Gamba T, Necchi A, Tucci M, et al. Evaluation of cognitive function in trials testing new-generation hormonal therapy in patients with prostate cancer: a systematic review. Cancers (Basel). 2020;12(9):2568.
Holland J, Bandelow S, Hogervorst E. Testosterone levels and cognition in elderly men: a review. Maturitas. 2011;69(4):322–37.
McGinty HL, Phillips KM, Jim HS, Cessna JM, Asvat Y, Cases MG, et al. Cognitive functioning in men receiving androgen deprivation therapy for prostate cancer: a systematic review and meta-analysis. Support Care Cancer. 2014;22(8):2271–80.
Plata-Bello J, Plata-Bello A, Pérez-Martín Y, Fajardo V, Concepción-Massip T. Androgen deprivation therapy increases brain ageing. Aging (Albany NY). 2019;11(15):5613–27.
Cherrier MM, Borghesani PR, Shelton AL, Higano CS. Changes in neuronal activation patterns in response to androgen deprivation therapy: a pilot study. BMC Cancer. 2010;10:1.
Chao HH, Uchio E, Zhang S, Hu S, Bednarski SR, Luo X, et al. Effects of androgen deprivation on brain function in prostate cancer patients: a prospective observational cohort analysis. BMC Cancer. 2012;12:371.
Jim HS, Small BJ, Patterson S, Salup R, Jacobsen PB. Cognitive impairment in men treated with luteinizing hormone-releasing hormone agonists for prostate cancer: a controlled comparison. Support Care Cancer. 2010;18(1):21–7.
Sari Motlagh R, Quhal F, Mori K, Miura N, Aydh A, Laukhtina E, et al. The risk of new onset dementia and/or Alzheimer disease among patients with prostate cancer treated with androgen deprivation therapy: a systematic review and meta-analysis. J Urol. 2021;205(1):60–7.
Holtfrerich SKC, Knipper S, Purwins J, Castens J, Beyer B, Schlomm T, et al. The impact of long-term androgen deprivation therapy on cognitive function and socioeconomic decision making in prostate cancer patients. Psychooncology. 2020;29(8):1338–46.
Hong JH, Huang CY, Chang CH, Muo CH, Jaw FS, Lu YC, et al. Different androgen deprivation therapies might have a differential impact on cognition: an analysis from a population-based study using time-dependent exposure model. Cancer Epidemiol. 2020;64:101657.
Tae BS, Jeon BJ, Shin SH, Choi H, Bae JH, Park JY. Correlation of androgen deprivation therapy with cognitive dysfunction in patients with prostate cancer: a nationwide population-based study using the National Health Insurance Service Database. Cancer Res Treat. 2019;51(2):593–602.
Ceylan Y, Gunlusoy B, Koskderelioglu A, Gedizlioglu M, Degirmenci T. The depressive effects of androgen deprivation therapy in locally advanced or metastatic prostate cancer: a comparative study. Aging Male. 2020;23(5):733–9.
Joly F, Alibhai SM, Galica J, Park A, Yi QL, Wagner L, et al. Impact of androgen deprivation therapy on physical and cognitive function, as well as quality of life of patients with nonmetastatic prostate cancer. J Urol. 2006;176(6 Pt 1):2443–7.
Alibhai SM, Breunis H, Timilshina N, Marzouk S, Stewart D, Tannock I, et al. Impact of androgen-deprivation therapy on cognitive function in men with nonmetastatic prostate cancer. J Clin Oncol. 2010;28(34):5030–7.
Sánchez-Martínez V, Buigues C, Navarro-Martínez R, García-Villodre L, Jeghalef N, Serrano-Carrascosa M, et al. Analysis of brain functions in men with prostate cancer under androgen deprivation therapy: a one-year longitudinal study. Life (Basel). 2021;11(3):227.
Cinar O, Turunc T, Kazaz IO, Yildirim O, Deliktas H, Cihan A, et al. Effects of androgen deprivation therapy on cognitive functions in patients with metastatic prostate cancer: a multicentric, prospective study of the Society of Urological Surgery Andrology group. Int J Clin Pract. 2021;75(6):e14095.
Alonso Quiñones HJ, Stish BJ, Hagen C, Petersen RC, Mielke MM. Prostate cancer, use of androgen deprivation therapy, and cognitive impairment: a population-based study. Alzheimer Dis Assoc Disord. 2020;34(2):118–21.
Alonso-Quiñones H, Stish BJ, Aakre JA, Hagen CE, Petersen RC, Mielke MM. Androgen deprivation therapy use and risk of mild cognitive impairment in prostate cancer patients. Alzheimer Dis Assoc Disord. 2021;35(1):44–7.
Alibhai SM, Timilshina N, Duff-Canning S, Breunis H, Tannock IF, Naglie G, et al. Effects of long-term androgen deprivation therapy on cognitive function over 36 months in men with prostate cancer. Cancer. 2017;123(2):237–44.
Morote J, Tabernero ÁJ, Álvarez-Ossorio JL, Ciria JP, Domínguez-Escrig JL, Vázquez F, et al. Cognitive function in patients on androgen suppression: a prospective, multicentric study. Actas Urol Esp (Engl Ed). 2018;42(2):114–20.
Wefel JS, Vardy J, Ahles T, Schagen SB. International Cognition and Cancer Task Force recommendations to harmonise studies of cognitive function in patients with cancer. Lancet Oncol. 2011;12(7):703–8.
Wilding S, Downing A, Wright P, Selby P, Watson E, Wagland R, et al. Cancer-related symptoms, mental well-being, and psychological distress in men diagnosed with prostate cancer treated with androgen deprivation therapy. Qual Life Res. 2019;28(10):2741–51.
Lehrer S, Rheinstein PH, Rosenzweig KE. No relationship of anti-androgens to Alzheimer’s disease or cognitive disorder in the MedWatch Database. J Alzheimers Dis Rep. 2018;2(1):123–7.
Nead KT, Gaskin G, Chester C, Swisher-McClure S, Dudley JT, Leeper NJ, et al. Androgen deprivation therapy and future Alzheimer’s disease risk. J Clin Oncol. 2016;34(6):566–71.
Kim JH, Lee B, Han DH, Chung KJ, Jeong IG, Chung BI. Discrepancies on the association between androgen deprivation therapy for prostate cancer and subsequent dementia: meta-analysis and meta-regression. Oncotarget. 2017;8(42):73087–97.
Nead KT, Gaskin G, Chester C, Swisher-McClure S, Leeper NJ, Shah NH. Association between androgen deprivation therapy and risk of dementia. JAMA Oncol. 2017;3(1):49–55.
Scher HI, Fizazi K, Saad F, Taplin ME, Sternberg CN, Miller K, et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med. 2012;367(13):1187–97.
Cella D, Ivanescu C, Holmstrom S, Bui CN, Spalding J, Fizazi K. Impact of enzalutamide on quality of life in men with metastatic castration-resistant prostate cancer after chemotherapy: additional analyses from the AFFIRM randomized clinical trial. Ann Oncol. 2015;26(1):179–85.
Fizazi K, Shore N, Tammela TL, Ulys A, Vjaters E, Polyakov S, et al. Darolutamide in nonmetastatic, castration-resistant prostate cancer. N Engl J Med. 2019;380(13):1235–46.
Smith MR, Shore N, Tammela TL, Ulys A, Vjaters E, Polyakov S, et al. Darolutamide and health-related quality of life in patients with non-metastatic castration-resistant prostate cancer: an analysis of the phase III ARAMIS trial. Eur J Cancer. 2021;154:138–46.
Armstrong AJ, Szmulewitz RZ, Petrylak DP, Holzbeierlein J, Villers A, Azad A, et al. ARCHES: A randomized, phase III study of androgen deprivation therapy with enzalutamide or placebo in men with metastatic hormone-sensitive prostate cancer. J Clin Oncol. 2019;37(32):2974–86.
Stenzl A, Dunshee C, De Giorgi U, Alekseev B, Iguchi T, Szmulewitz RZ, et al. Effect of enzalutamide plus androgen deprivation therapy on health-related quality of life in patients with metastatic hormone-sensitive prostate cancer: an analysis of the ARCHES randomised, placebo-controlled, phase 3 study. Eur Urol. 2020;78(4):603–14.
de Bono JS, Logothetis CJ, Molina A, Fizazi K, North S, Chu L, et al. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med. 2011;364(21):1995–2005.
Fizazi K, Scher HI, Molina A, Logothetis CJ, Chi KN, Jones RJ, et al. Abiraterone acetate for treatment of metastatic castration-resistant prostate cancer: final overall survival analysis of the COU-AA-301 randomised, double-blind, placebo-controlled phase 3 study. Lancet Oncol. 2012;13(10):983–92.
Harland S, Staffurth J, Molina A, Hao Y, Gagnon DD, Sternberg CN, et al. Effect of abiraterone acetate treatment on the quality of life of patients with metastatic castration-resistant prostate cancer after failure of docetaxel chemotherapy. Eur J Cancer. 2013;49(17):3648–57.
Sternberg CN, Molina A, North S, Mainwaring P, Fizazi K, Hao Y, et al. Effect of abiraterone acetate on fatigue in patients with metastatic castration-resistant prostate cancer after docetaxel chemotherapy. Ann Oncol. 2013;24(4):1017–25.
Rathkopf DE, Smith MR, de Bono JS, Logothetis CJ, Shore ND, de Souza P, et al. Updated interim efficacy analysis and long-term safety of abiraterone acetate in metastatic castration-resistant prostate cancer patients without prior chemotherapy (COU-AA-302). Eur Urol. 2014;66(5):815–25.
Ryan CJ, Smith MR, de Bono JS, Molina A, Logothetis CJ, de Souza P, et al. Abiraterone in metastatic prostate cancer without previous chemotherapy. N Engl J Med. 2013;368(2):138–48.
Davis ID, Martin AJ, Stockler MR, Begbie S, Chi KN, Chowdhury S, et al. Enzalutamide with standard first-line therapy in metastatic prostate cancer. N Engl J Med. 2019;381(2):121–31.
Stockler MR, Martin AJ, Davis ID, Dhillon HM, Begbie SD, Chi KN, et al. Health-related quality of life in metastatic, hormone-sensitive prostate cancer: ENZAMET (ANZUP 1304), an international, randomized phase III trial led by ANZUP. J Clin Oncol. 2022;10(40):837-46.
Fizazi K, Tran N, Fein L, Matsubara N, Rodriguez-Antolin A, Alekseev BY, et al. Abiraterone plus prednisone in metastatic, castration-sensitive prostate cancer. N Engl J Med. 2017;377(4):352–60.
Fizazi K, Tran N, Fein L, Matsubara N, Rodriguez-Antolin A, Alekseev BY, et al. Abiraterone acetate plus prednisone in patients with newly diagnosed high-risk metastatic castration-sensitive prostate cancer (LATITUDE): final overall survival analysis of a randomised, double-blind, phase 3 trial. Lancet Oncol. 2019;20(5):686–700.
Chi KN, Protheroe A, Rodríguez-Antolín A, Facchini G, Suttman H, Matsubara N, et al. Patient-reported outcomes following abiraterone acetate plus prednisone added to androgen deprivation therapy in patients with newly diagnosed metastatic castration-naive prostate cancer (LATITUDE): an international, randomised phase 3 trial. Lancet Oncol. 2018;19(2):194–206.
Beer TM, Armstrong AJ, Rathkopf DE, Loriot Y, Sternberg CN, Higano CS, et al. Enzalutamide in metastatic prostate cancer before chemotherapy. N Engl J Med. 2014;371(5):424–33.
Armstrong AJ, Lin P, Tombal B, Saad F, Higano CS, Joshua AM, et al. Five-year survival prediction and safety outcomes with enzalutamide in men with chemotherapy-naïve metastatic castration-resistant prostate cancer from the PREVAIL trial. Eur Urol. 2020;78(3):347–57.
Loriot Y, Miller K, Sternberg CN, Fizazi K, De Bono JS, Chowdhury S, et al. Effect of enzalutamide on health-related quality of life, pain, and skeletal-related events in asymptomatic and minimally symptomatic, chemotherapy-naive patients with metastatic castration-resistant prostate cancer (PREVAIL): results from a randomised, phase 3 trial. Lancet Oncol. 2015;16(5):509–21.
Hussain M, Fizazi K, Saad F, Rathenborg P, Shore N, Ferreira U, et al. Enzalutamide in men with nonmetastatic, castration-resistant prostate cancer. N Engl J Med. 2018;378(26):2465–74.
Tombal B, Saad F, Penson D, Hussain M, Sternberg CN, Morlock R, et al. Patient-reported outcomes following enzalutamide or placebo in men with non-metastatic, castration-resistant prostate cancer (PROSPER): a multicentre, randomised, double-blind, phase 3 trial. Lancet Oncol. 2019;20(4):556–69.
Saad F, Cella D, Basch E, Hadaschik BA, Mainwaring PN, Oudard S, et al. Effect of apalutamide on health-related quality of life in patients with non-metastatic castration-resistant prostate cancer: an analysis of the SPARTAN randomised, placebo-controlled, phase 3 trial. Lancet Oncol. 2018;19(10):1404–16.
Oudard S, Hadaschik B, Saad F, Cella D, Basch E, Graff JN, et al. Health-related quality of life at the SPARTAN final analysis of apalutamide for nonmetastatic castration-resistant prostate cancer patients receiving androgen deprivation therapy. Eur Urol Focus. 2021. https://doi.org/10.1016/j.euf.2021.08.005 (Epub ahead of print).
Chi KN, Agarwal N, Bjartell A, Chung BH, Pereira de Santana Gomes AJ, Given R, et al. Apalutamide for metastatic, castration-sensitive prostate cancer. N Engl J Med. 2019;381(1):13–24.
Chi KN, Chowdhury S, Bjartell A, Chung BH, Pereira de Santana Gomes AJ, Given R, et al. Apalutamide in patients with metastatic castration-sensitive prostate cancer: final survival analysis of the randomized, double-blind, phase III TITAN study. J Clin Oncol. 2021;39(20):2294–303.
Agarwal N, McQuarrie K, Bjartell A, Chowdhury S, Pereira de Santana Gomes AJ, Chung BH, et al. Health-related quality of life after apalutamide treatment in patients with metastatic castration-sensitive prostate cancer (TITAN): a randomised, placebo-controlled, phase 3 study. Lancet Oncol. 2019;20(11):1518–30.
Thiery-Vuillemin A, Poulsen MH, Lagneau E, Ploussard G, Birtle A, Dourthe LM, et al. Impact of abiraterone acetate plus prednisone or enzalutamide on patient-reported outcomes in patients with metastatic castration-resistant prostate cancer: final 12-mo analysis from the observational AQUARiUS study. Eur Urol. 2020;77(3):380–7.
Gotto G, Drachenberg DE, Chin J, Casey R, Fradet V, Sabbagh R, et al. Real-world evidence in patient-reported outcomes (PROs) of metastatic castrate-resistant prostate cancer (mCRPC) patients treated with abiraterone acetate + prednisone (AA+P) across Canada: final results of COSMiC. Can Urol Assoc J. 2020;14(12):E616–20.
Khalaf DJ, Annala M, Taavitsainen S, Finch DL, Oja C, Vergidis J, et al. Optimal sequencing of enzalutamide and abiraterone acetate plus prednisone in metastatic castration-resistant prostate cancer: a multicentre, randomised, open-label, phase 2, crossover trial. Lancet Oncol. 2019;20(12):1730–9.
Khalaf DJ, Sunderland K, Eigl BJ, Kollmannsberger CK, Ivanov N, Finch DL, et al. Health-related quality of life for abiraterone plus prednisone versus enzalutamide in patients with metastatic castration-resistant prostate cancer: results from a phase II randomized trial. Eur Urol. 2019;75(6):940–7.
Khalaf DJ, Sunderland K, Eigl BJ, Finch DL, Oja CD, Vergidis J, et al. Quality of life (QOL) for the treatment sequence of abiraterone acetate plus prednisone (AAP) followed by enzalutamide (ENZ) versus the opposite sequence for metastatic castration-resistant prostate cancer (mCRPC): results from a phase II randomized clinical trial. J Clin Oncol. 2020;38(15 Suppl):abstract 5578.
Williams S, Mazibuko N, O’Daly O, Zurth C, Patrick F, Wooldridge C, et al. Significant localized reduction in cerebral blood flow (CBF) in regions relevant to cognitive function with enzalutamide (ENZA) compared to darolutamide (DARO) and placebo (PBO) in healthy volunteers. J Clin Oncol. 2020;38(Suppl.):abstract 326.
Alibhai SMH, Breunis H, Feng G, Timilshina N, Hansen A, Warde P, et al. Association of chemotherapy, enzalutamide, abiraterone, and radium 223 with cognitive function in older men with metastatic castration-resistant prostate cancer. JAMA Netw Open. 2021;4(7):e2114694.
Zurth C, Sandmann S, Trummel D, Seidel D, Gieschen H. Blood-brain barrier penetration of [14C] darolutamide compared with [14C] enzalutamide in rats using whole body autoradiography. J Clin Oncol. 2018;36(Suppl.):abstract 345.
Zurth C, Sandman S, Trummel D, Seidel D, Nubbemeyer R, Gieschen H. Higher blood–brain barrier penetration of [14C] apalutamide and [14C] enzalutamide compared to [14C] darolutamide in rats using whole-body autoradiography. J Clin Oncol. 2019;37(Suppl.):abstract156.
World Health Organization. International Classification of Functioning, Disability and Health (ICF). https://www.who.int/classifications/international-classification-of-functioning-disability-and-health. Accessed 29 Mar 2022.
Brown PD, Buckner JC, O’Fallon JR, Iturria NL, Brown CA, O’Neill BP, et al. Effects of radiotherapy on cognitive function in patients with low-grade glioma measured by the Folstein mini-mental state examination. J Clin Oncol. 2003;21(13):2519–24.
Iconomou G, Mega V, Koutras A, Iconomou AV, Kalofonos HP. Prospective assessment of emotional distress, cognitive function, and quality of life in patients with cancer treated with chemotherapy. Cancer. 2004;101(2):404–11.
Meyers CA, Wefel JS. The use of the mini-mental state examination to assess cognitive functioning in cancer trials: no ifs, ands, buts, or sensitivity. J Clin Oncol. 2003;21(19):3557–8.
Nasreddine ZS, Phillips NA, Bédirian V, Charbonneau S, Whitehead V, Collin I, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53(4):695–9.
Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–98.
Borson S, Scanlan JM, Chen P, Ganguli M. The Mini-Cog as a screen for dementia: validation in a population-based sample. J Am Geriatr Soc. 2003;51(10):1451–4.
Evans JD, Jethwa KR, Ost P, Williams S, Kwon ED, Lowe VJ, et al. Prostate cancer-specific PET radiotracers: a review on the clinical utility in recurrent disease. Pract Radiat Oncol. 2018;8(1):28–39.
Dautzenberg G, Lijmer J, Beekman A. Diagnostic accuracy of the Montreal Cognitive Assessment (MoCA) for cognitive screening in old age psychiatry: determining cutoff scores in clinical practice. Avoiding spectrum bias caused by healthy controls. Int J Geriatr Psychiatry. 2020;35(3):261–9.
Hermelink K, Küchenhoff H, Untch M, Bauerfeind I, Lux MP, Bühner M, et al. Two different sides of “chemobrain”: determinants and nondeterminants of self-perceived cognitive dysfunction in a prospective, randomized, multicenter study. Psychooncology. 2010;19(12):1321–8.
Wu LM, Tanenbaum ML, Dijkers MP, Amidi A, Hall SJ, Penedo FJ, et al. Cognitive and neurobehavioral symptoms in patients with non-metastatic prostate cancer treated with androgen deprivation therapy or observation: a mixed methods study. Soc Sci Med. 2016;156:80–9.
Hutchinson AD, Hosking JR, Kichenadasse G, Mattiske JK, Wilson C. Objective and subjective cognitive impairment following chemotherapy for cancer: a systematic review. Cancer Treat Rev. 2012;38(7):926–34.
Marzouk S, Naglie G, Tomlinson G, Duff Canning S, Breunis H, Timilshina N, et al. Impact of androgen deprivation therapy on self-reported cognitive function in men with prostate cancer. J Urol. 2018;200(2):327–34.
Esper P, Mo F, Chodak G, Sinner M, Cella D, Pienta KJ. Measuring quality of life in men with prostate cancer using the Functional Assessment of Cancer Therapy-Prostate instrument. Urology. 1997;50(6):920–8.
Von Ah D, Jansen CE, Allen DH. Evidence-based interventions for cancer- and treatment-related cognitive impairment. Clin J Oncol Nurs. 2014;18:17–25.
Yellen SB, Cella DF, Webster K, Blendowski C, Kaplan E. Measuring fatigue and other anemia-related symptoms with the Functional Assessment of Cancer Therapy (FACT) measurement system. J Pain Symptom Manage. 1997;13(2):63–74.
Okuyama T, Akechi T, Kugaya A, Okamura H, Shima Y, Maruguchi M, et al. Development and validation of the cancer fatigue scale: a brief, three-dimensional, self-rating scale for assessment of fatigue in cancer patients. J Pain Symptom Manage. 2000;19(1):5–14.
Hinz A, Mehnert A, Kocalevent RD, Brähler E, Forkmann T, Singer S, et al. Assessment of depression severity with the PHQ-9 in cancer patients and in the general population. BMC Psychiatry. 2016;16:22.
Teng E, Becker BW, Woo E, Knopman DS, Cummings JL, Lu PH. Utility of the functional activities questionnaire for distinguishing mild cognitive impairment from very mild Alzheimer disease. Alzheimer Dis Assoc Disord. 2010;24(4):348–53.
Marshall GA, Zoller AS, Lorius N, Amariglio RE, Locascio JJ, Johnson KA, et al. Functional activities questionnaire items that best discriminate and predict progression from clinically normal to mild cognitive impairment. Curr Alzheimer Res. 2015;12(5):493–502.
Morris C, Gibbons E, Fitzpatrick R. A structured review of patient-reported outcome measures for men with prostate cancer, 2010. https://www.ndph.ox.ac.uk/files/research/prostate-cancer-final-2010.pdf. Accessed 29 Mar 2022.
Ware JE Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30(6):473–83.
Pickard AS, Ray S, Ganguli A, Cella D. Comparison of FACT- and EQ-5D-based utility scores in cancer. Value Health. 2012;15(2):305–11.
Brazier J, Jones N, Kind P. Testing the validity of the EuroQOL and comparing it with the SF-36 health survey questionnaire. Qual Life Res. 1993;2(3):169–80.
Kurita GP, Sandvad M, Lundorff L, De Mattos-Pimenta CA, Højsted J, Sjøgren P. Assessment of cognitive function in patients with metastatic cancer: are we using the right tools? Palliat Support Care. 2018;16(1):80–9.
Reitan RM. Validity of the trail making test as an indicator of organic brain damage. Percept Mot Skills. 1958;8:271–6.
Xu X, Rahardjo TB, **ao S, Hogervorst E. The Hopkins Verbal Learning Test and detection of MCI and mild dementia: a literature review. J Alzheimers Dis Parkinsonism. 2014;4(6):166.
Brandt J. The Hopkins Verbal Learning Test: development of a new memory test with six equivalent forms. Clin Neuropsychol. 1991;5(2):125–42.
Malek-Ahmadi M, Small BJ, Raj A. The diagnostic value of controlled oral word association test-FAS and category fluency in single-domain amnestic mild cognitive impairment. Dement Geriatr Cogn Disord. 2011;32(4):235–40.
Loring DW, Bauer RM. Testing the limits: cautions and concerns regarding the new Wechsler IQ and Memory scales. Neurology. 2010;74(8):685–90.
Banken JA. Clinical utility of considering Digits Forward and Digits Backward as separate components of the Wechsler Adult Intelligence Scale-Revised. J Clin Psychol. 1985;41(5):686–91.
Choi HJ, Lee DY, Seo EH, Jo MK, Sohn BK, Choe YM, et al. A normative study of the digit span in an educationally diverse elderly population. Psychiatry Investig. 2014;11(1):39–43.
Joy S, Kaplan E, Fein D. Speed and memory in the WAIS-III Digit Symbol-Coding subtest across the adult lifespan. Arch Clin Neuropsychol. 2004;19(6):759–67.
Stephens R. Age-related decline in Digit-Symbol performance: eye-movement and video analysis. Arch Clin Neuropsychol. 2006;21(1):101–7.
Merten T. Factor structure of the Hooper Visual Organization Test: a cross-cultural replication and extension. Arch Clin Neuropsychol. 2005;20(1):123–8.
Johnstone B, Wilhelm KL. The construct validity of the Hooper Visual Organization Test. Assessment. 1997;4(3):243–8.
Gualtieri CT, Johnson LG. Reliability and validity of a computerized neurocognitive test battery, CNS Vital Signs. Arch Clin Neuropsychol. 2006;21(7):623–43.
Cambridge Cognition. CANTAB cognitive research software. https://www.cambridgecognition.com/cantab/. Accessed 29 Mar 2022.
Robbins TW, James M, Owen AM, Sahakian BJ, McInnes L, Rabbitt P. Cambridge Neuropsychological Test Automated Battery (CANTAB): a factor analytic study of a large sample of normal elderly volunteers. Dementia. 1994;5(5):266–81.
Ryan C. Management of fatigue, cognition/dementia. Advanced Prostate Cancer Consensus Conference: APCCC 2019. https://www.apccc.org/fileadmin/files/2019/apccc2019/slides/Session_7/Session_7_Talk_4_Ryan.pdf. Accessed 29 Mar 2022.
Andersen BL, DeRubeis RJ, Berman BS, Gruman J, Champion VL, Massie MJ, et al. Screening, assessment, and care of anxiety and depressive symptoms in adults with cancer: an American Society of Clinical Oncology guideline adaptation. J Clin Oncol. 2014;32(15):1605–19.
Howell D, Keller-Olaman S, Oliver TK, Hack TF, Broadfield L, Biggs K, et al. A pan-Canadian practice guideline and algorithm: screening, assessment, and supportive care of adults with cancer-related fatigue. Curr Oncol. 2013;20(3):e233–46.
Gehring K, Sitskoorn MM, Gundy CM, Sikkes SA, Klein M, Postma TJ, et al. Cognitive rehabilitation in patients with gliomas: a randomized, controlled trial. J Clin Oncol. 2009;27(22):3712–22.
Cherrier MM, Anderson K, David D, Higano CS, Gray H, Church A, et al. A randomized trial of cognitive rehabilitation in cancer survivors. Life Sci. 2013;93(17):617–22.
Livingston PM, Craike MJ, Salmon J, Courneya KS, Gaskin CJ, Fraser SF, et al. Effects of a clinician referral and exercise program for men who have completed active treatment for prostate cancer: a multicenter cluster randomized controlled trial (ENGAGE). Cancer. 2015;121(15):2646–54.
Fang YY, Lee YH, Chan JC, Chiou PY, Chou XY, Chiu WT, et al. Effects of exercise interventions on social and cognitive functioning of men with prostate cancer: a meta-analysis. Support Care Cancer. 2020;28(5):2043–57.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
Bayer HealthCare Pharmaceuticals, Inc. (Whippany, NJ, USA) provided funding, in line with GPP3 guidelines, for medical writing support from Rebecca Hopkins, BSc, and editorial assistance from Annabel Ola, MSc, of Scion (London, UK) in the development of the initial draft and for medical writing and editorial support from Sara Black, ISMPP CMPP™, of OPEN Health Communications (London, UK) for formatting for journal submission and addressing reviewer comments. The authors retain ultimate responsibility for opinions, conclusions, and data interpretation.
Conflicts of interest/Competing interests
JSW has acted as a consultant for Angiochem, Bayer, Juno, Novocure, Vanquish Oncology, and Magnolia Tejas, and has been an advisory board member for AbbVie, Bayer, Blueprint Medicines, and Magnolia Neurosciences. CJR has received honoraria from Janssen, Bayer, and Sanofi Aventis. JV and JJ declare no conflicts of interest. AKM has received honoraria from AstraZeneca, Astellas, Bayer, Clovis, Dedreon, Myovant, Pfizer, Novartis, Blue Earth, Janssen, Sanofi, Genentech, and Seattle Genetics, and has received research support from Bayer, Astellas, and Seattle Genetics.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and material
Not applicable.
Code availability
Not applicable.
Authors’ contributions
All authors made substantial contributions to the conception and planning of this review, to the analysis and interpretation of the literature search results, and to the drafting and revision of this manuscript. All authors approved the final submitted version of the manuscript.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Wefel, J.S., Ryan, C.J., Van, J. et al. Assessment and Management of Cognitive Function in Patients with Prostate Cancer Treated with Second-Generation Androgen Receptor Pathway Inhibitors. CNS Drugs 36, 419–449 (2022). https://doi.org/10.1007/s40263-022-00913-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40263-022-00913-5