Abstract
Background
The effectiveness of adjunctive perampanel has not been systematically assessed in seizure types other than its approved indications of focal seizures and primary generalised tonic–clonic seizures (PGTCS) in idiopathic generalised epilepsies (IGEs).
Objective
We aimed to identify and review available evidence on outcomes with perampanel in generalised seizures and epilepsies to examine its potential as a broad-spectrum anti-seizure medication.
Methods
Bibliographic databases of publications, clinical trials, and conference abstracts were searched up to August 2020 to identify studies reporting seizure or safety outcomes in patients of any age, with any type of epilepsy-associated generalised seizures treated with perampanel. Data extracted from selected records were tabulated by seizure type and syndrome, and analysed qualitatively (PROSPERO protocol CRD42020201564).
Results
Ninety-one reports met inclusion criteria and were selected: 15 reports of 1 randomised controlled trial (RCT), 8 reports of 4 non-randomised interventional studies, 37 reports of observational studies, 21 case reports and 10 systematic reviews and meta-analyses. Extracted data included 359 patients with PGTCS of any aetiology, 251 with myoclonic seizures, 112 with absence seizures, 50 with tonic seizures and 32 children with epileptic spasms. The most commonly reported epilepsy type was IGE (N = 378) and the most common syndromes were juvenile myoclonic epilepsy (N = 92), progressive myoclonic epilepsies (N = 59) and absence epilepsies (N = 43). The RCT provided Class I evidence of the efficacy and tolerability of adjunctive perampanel for PGTCS in patients aged ≥ 12 years with IGE. Data from other studies provides weaker (observational) evidence of its effectiveness in multiple generalised seizure types, including myoclonic, absence and tonic seizures. There were no patterns suggesting seizure worsening or aggravation in any seizure or epilepsy type.
Conclusions
The identified studies suggest the potential of perampanel as a broad-spectrum antiseizure medication. Much of the available data, however, come from non-randomised, non-controlled studies and are open to high risk of bias. Further studies are warranted to provide more robust evidence.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Digital Features for this article can be found at https://doi.org/10.6084/m9.figshare.14614611 |
Broad-spectrum anti-seizure medications are effective against focal and generalised seizures of any type and do not cause seizure aggravation. |
We systematically searched and evaluated data about efficacy and safety of perampanel in generalised seizures. |
Strong evidence supports the efficacy of perampanel in tonic–clonic seizures in idiopathic generalised epilepsy. |
Observational studies suggest (with high risk of bias) effectiveness in myoclonic, absence and tonic seizures, and generalised epilepsy syndromes. |
We found no evidence to suspect an association between perampanel and seizure worsening in generalised epilepsies. |
1 Introduction
Epilepsies are one of the most common chronic disorders of the brain, and pharmacotherapy with anti-seizure medications (ASMs) is central to management. Each ASM has a unique profile and needs to be tailored to individual patient factors, including the characteristics of seizures and specific epilepsy syndrome. Some ASMs have emerged as particularly effective in treating specific seizure types or specific syndromes, but may be less effective in other seizure types or even precipitate or worsen seizures in certain syndromes. ASMs are considered ‘broad-spectrum’ when they are effective against focal and generalised seizures of any type and do not usually cause seizure aggravation [1].
Perampanel is a selective non-competitive antagonist of the glutamate AMPA receptor ion channel that has been approved as adjunctive treatment of focal seizures in patients aged ≥ 4 years (and as monotherapy in the USA), and as adjunctive treatment of primary generalised tonic–clonic seizures (PGTCS) associated with idiopathic generalised epilepsy (IGE) in patients aged ≥ 12 years (and ≥ 7 years in the EU), on the basis of Class I evidence in patients aged ≥ 12 years [2, 3]. The effectiveness of perampanel in other generalised seizure types, and therefore whether it can be considered a broad-spectrum ASM, has not been systematically assessed.
The limited choice of broad-spectrum ASMs available, the serious nature of some seizure types, and the limited amount of high-quality data from randomised controlled trials (RCTs) led us to seek and evaluate all available data, include evidence from study types open to bias. Real-world evidence from observational studies can complement data from RCTs, and has value for understanding outcomes in routine clinical practice. This is particularly important for patient populations that are left out of RCTs, such as people with intellectual disability, in whom epilepsy is common. In these populations, real-world evidence may be the only available evidence. We feel the value of including a large quantity of data from observational, uncontrolled trials and case reports outweighs the limitations in interpreting such data and the weak certainty of conclusions.
To examine whether perampanel can be considered a broad-spectrum ASM, we systematically searched for studies of any type that reported clinical data on seizure outcomes or safety outcomes for patients of any age with epilepsy-associated generalised seizures treated with perampanel.
2 Methods
The report of this systematic review was made according to the recommendations of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement [4] and the Synthesis Without Meta-analysis in systematic reviews (SWiM) extension [5]. The study protocol was registered with the international prospective register of systematic reviews (PROSPERO; reference CRD42020201564), and this database was checked to ensure a similar review was not already underway.
We use the term ‘generalised seizure’ (occasionally ‘generalised-onset’ to avoid ambiguity) to indicate seizures with generalised onset, according to the ILAE 2017 classification of the epilepsies and seizure types [6, 7]. Terminology is therefore not always consistent with the source articles, which used variable terms and often used ‘generalised’ to refer to focal-to-bilateral tonic–clonic seizures.
2.1 Information Sources and Search Terms
We systematically searched electronic literature databases, conference databases, clinical trials databases between July and August 2020, and scanned reference lists of retrieved articles (December 2020). No language or date restrictions were applied, and English-language abstracts were used if authors were not proficient enough in the published language to screen for inclusions or extract relevant data. Abstracts at key epilepsy conferences were included (American Academy of Neurology [AAN], American Epilepsy Society [AES], European Academy of Neurology [EAN], European Congress on Epileptology [ECE] and International Epilepsy Congress [IEC]). Broad search terms were used (‘perampanel’, ‘fycompa’ and ‘E2007’) to identify studies of any type or design that reported outcomes in patients receiving perampanel (see electronic supplementary material ESM 1, Appendix A, for full details).
2.2 Eligibility Criteria
Studies of any type were eligible, including randomised, double-blind, controlled trials; randomised, open-label trials; non-randomised trials; observational studies (defined as data collected as part of routine care, in a defined population of patients receiving perampanel) and case series or case reports (defined as observational reports in a subset of patients or selected individual receiving perampanel).
Studies had to include patients with epilepsy-associated generalised seizure types, generalised epilepsies or generalised epilepsy syndromes who received oral perampanel as either adjunctive therapy or monotherapy. Participants of any age, sex, and ethnicity were eligible. Studies that included participants with exclusively non-epileptic seizure types (e.g. post-hypoxic seizures), exclusively non-generalised-onset seizure types, status epilepticus, or acute symptomatic seizures were excluded.
2.3 Outcome Measures
The core seizure outcome measures were change in seizure frequency relative to baseline, 50% responder rate (proportion of patients with ≥ 50% reduction in baseline seizure frequency) and seizure freedom. We also collected other relevant seizure data, including subjective and objective change in seizure severity relative to baseline, seizure worsening or aggravation and functional outcomes associated with seizures (e.g. activities of daily living). Retention rate (proportion of patients continuing on perampanel at endpoint, as a proportion of all those who started perampanel) was also recorded. We expected definitions of seizure outcomes to differ across studies, and we recorded data as reported in data extraction tables.
Safety outcomes included overall incidence of adverse events (AEs), the rate of individual AEs, AEs leading to discontinuation and AEs indicative of seizure worsening (e.g. status epilepticus, seizure clusters).
Reports that included patients with generalised seizures/epilepsy types but that did not report seizure or safety outcomes for generalised seizures separately from focal seizures were excluded. Only safety data (not seizure outcome data) were extracted from open-label extension (OLEx) studies of randomised controlled trials.
2.4 Study Selection, Data Extraction and Assessment of Risk of Bias
Two review authors (FR and BN) independently screened abstracts identified through searches of literature and conference databases to identify potentially relevant reports. Conference abstracts were further screened by KC and RW to remove duplicates (including unique but ‘encore’ abstracts that contained the same data as other abstracts), abstracts superseded by publications, and abstracts reporting interim data superseded by later reports.
KC screened results from clinical trials database searches, removed duplicates, and contacted study sponsors to request data (or interim data) for completed (and ongoing) studies.
Two authors (SL and TC) independently reviewed full texts of screened results and selected relevant reports for inclusion; RW and KC checked selections and any disagreement was resolved through discussion with a third review author (ET). FB, FR and BN manually searched reference lists of included studies for additional relevant reports.
RW extracted relevant data into Excel spreadsheets (Supplemental Table S1, see ESM 4) by study type (RCTs, non-randomised interventional trials, observational studies, case reports/series), and ordered by study size; KC checked accuracy of data extraction. The extracted variables included study authors, date of publication, number and top-line demographics of overall participants, dosing of perampanel, seizure outcomes and safety data in any generalised seizure populations or subpopulations (Supplemental Table S1, see ESM 4).
The risk of bias (RoB) of the included RCTs was assessed at the outcome level using the RoB 2 tool [8] by RS and RW; ET arbitrated any differences (Supplementary information: Appendix E, see ESM 2). RoB was not assessed individually for other study types (observational cohort studies and case reports). Instead, these study types were considered at high risk of bias and their data and conclusions were interpreted accordingly.
2.5 Data Synthesis
Data synthesis was qualitative, not quantitative, because of the anticipated heterogeneity of study designs and outcomes data collected. The aim of the synthesis strategy was to identify patterns that could suggest beneficial effect, lack of effect, or seizure worsening with perampanel in specific seizure types or syndromes. Therefore, data were tabulated for generalised epilepsy overall and then separately by seizure type and syndrome (where sufficient data were available), and grouped into summary tables giving the range of values. Because of heterogeneity in design of the included studies, data from different study types were listed separately, and data from case reports were not grouped or synthesised. Because of the preliminary and non-peer-reviewed nature of conference abstracts, these were reported separately from published, peer-reviewed literature.
3 Results
3.1 Study Selection
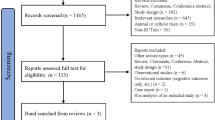
Database searches returned 2820 results (2419 from literature databases, 273 from conference proceedings, 128 from clinical trials databases); no additional records were identified in manual searches of reference lists. Relevant data was extracted from 91 items (Fig. 1).
PRISMA flow chart. Illustration of search results, screening and selection. Database searches returned 2820 results (2419 from literature databases, 273 from conference proceedings, 128 from clinical trials databases); no additional records were identified in manual searches of reference lists. Relevant data was extracted from 91 items. aOne full-text article was in a language not spoken by authors or contributors but had an abstract in English from which relevant data could be extracted [24]. bIncludes nine systematic reviews or meta-analyses. cIncludes one systematic review. PRISMA preferred reporting items for systematic reviews and meta-analyses, RCT randomised controlled trial, OLEx open-label extension
3.2 Study Characteristics
Characteristics of all selected studies are shown in Appendix B of the ESM 1.
One randomised, double-blind, placebo-controlled, parallel-group trial was identified. It recruited patients aged ≥ 12 years with independently confirmed refractory PGTCS associated with IGE (ClinicalTrials.gov identifier: NCT01393743). In addition to its primary report [3], four secondary publications and nine conference abstracts reported extractable data from subgroup analyses and long-term extension(s) (including NCT02427607, an OLEx in Japan), often pooled with other studies (Supplemental Table S2, see ESM 1).
Four non-randomised interventional studies were identified, reported in four full publications and four conference abstracts (Supplemental Table S3, see ESM 1).
Thirty-seven observational studies were identified; 23 from published literature (Supplemental Table S4a, see ESM 1), of which six were in predominantly paediatric populations, and 14 from conference abstracts (Supplemental Table S4b, see ESM 1), of which five were in predominantly paediatric populations.
Twenty-one reports of case studies were identified, 16 from published literature and five from conference abstracts (Supplemental Table S5, see ESM 1); most were focused on effectiveness in rare epilepsy types, and three focused on AEs.
3.3 Risk of Bias
The RCT [3] was judged to have a low risk of bias overall and for each domain assessed by the tool (bias arising from the randomisation process, due to deviations from the intended interventions, due to missing outcome data, in measurement of the outcome, in selection of the reported results) for the outcomes of retention rate, median percent change in PGTCS frequency per 28 days, 50% responder rate for PGTCS, and seizure-freedom rate for PGTCS (Appendix E, see ESM 2). The non-randomised interventional studies and observational studies were all assumed to have a high risk of bias and possible overestimation of efficacy, because of the lack of a control group and blinding/masking; in addition, the retrospective design of many of the observational studies increases the risk of bias.
3.4 Results of Individual Studies
3.4.1 Randomised, Double-Blind, Placebo-Controlled, Parallel Group Clinical Trial
The primary report from the identified RCT (NCT01393743; study 332) showed significantly greater reduction in seizure frequency in patients treated with adjunctive perampanel versus placebo-treated patients for PGTCS and for all seizures, significantly greater responder rate for PGTCS, and numerically greater seizure-free rate for PGTCS and for all seizures (no statistical comparison) [3]. Incidence of seizure worsening was not reported as an efficacy outcome, but AEs suggestive of seizure worsening were infrequent (Supplemental Table S1, see ESM 4). Overall rates of treatment-emergent AEs (TEAEs) were higher in the perampanel group than placebo, and the most common TEAEs were dizziness, fatigue, headache, somnolence and irritability. TEAEs suggestive of seizure worsening occurred in one patient in each group (severe status epilepticus with perampanel 6 mg/day, which resolved on discontinuation; moderate status epilepticus with placebo).
One post-hoc analysis of this phase III study explored outcomes by seizure type (Sect. 3.5.1.2–3, Supplemental Tables S6 and S7, see ESM 1). The other analyses added little information relevant to our objective; except for an apparently lower efficacy in the few patients taking concomitant enzyme-inducing ASMs, seizure and safety outcomes were broadly consistent regardless of age, age at diagnosis, concomitant ASMs, and ethnicity in patients with PGTCS associated with IGE (see Supplemental Table S1 and Appendix C in the ESM 1 for full details and references).
3.4.2 Non-Randomised Interventional Studies
In paediatric patients, two prospective, open-label, multicentre studies had extractable outcome data in generalised seizures: Study 311 (NCT02849626) [9] and Study 232 (NCT01527006) [10]. In Study 311, from a population of 180 patients aged 4 to < 12 years with focal seizure or PGTCS, 31 patients had generalised seizures, and among the 22 with PGTCS at baseline, seizure frequency was reduced by a median of 69% (95% CI 18–100; IQR 82) [9]. The responder rate for PGTCS was 64.0% (14/22) and the seizure-free rate for PGTCS was 55% at 23 weeks (12/22). Outcomes were not reported for other generalised seizure types. TEAEs were reported in 84% (26/31), serious TEAEs in 13% (4/31) and the most common TEAEs were somnolence (5/31), dizziness (5/31) and irritability (5/31). Three patients recorded serious TEAEs possibly suggestive of seizure worsening (one had ‘petit mal epilepsy’; one ‘epilepsy’; one ‘seizure’ and ‘seizure cluster’). Extension data and analyses by subgroups (age, number of concomitant ASMs, and IGE versus non-IGE) were broadly consistent with results for the overall generalised seizure population, but numbers were small (Supplemental Tables S8 and S9, see ESM 1).
In study 232, of 50 patients aged ≥ 2 to < 12 years, 22 had generalised seizures, with a median 35.8% reduction in overall seizure frequency and a responder rate of 59.1% in the 11-week core study treatment phase. Seizure data were presented by age subgroups for these 50 patients, but with small numbers and highly variable data (Supplemental Tables S8 and S9, see ESM 1) [10]. Safety outcomes were not reported for the generalised seizure subgroup.
Two prospective studies in non-paediatric populations were identified. In 49 people (mean age 36.6 years) with progressive myoclonus epilepsy (PME) of various aetiologies, 46.7% of patients (21/45) were classed as having improved myoclonic seizures (those with ≥ 1-point improvement in minimal myoclonus scale [MMS]), and 100% of patients (17/17) were classed as responders for PGTCS (≥ 50% reduction from baseline in PGTCS frequency, in patients with ≥ 2 PGTCS/month at baseline) [11]. In 10 people aged 15–41 years with Lafora disease, 3/7 (42.9%) reported reduction in myoclonic seizure frequency and 4/6 (66.7%) in PGTCS frequency; worsening of PGTCS frequency occurred in 2/6 (33.3%) (Supplemental Tables S8 and S9, see ESM 1) [12].
3.4.3 Observational Studies
We identified reports in 257 patients of predominantly paediatric ages who received perampanel (182 in published literature and 75 in conference abstracts). The largest observational study with perampanel in paediatric patients (Hwang et al.) reported data for 118 patients in generalised seizures, but no breakdown by seizure type or syndrome [13].
Other reports included data in smaller paediatric populations, with sample sizes ranging from 5 to 25 patients in generalised epilepsy, Dravet syndrome, lissencephaly, Lennox Gastaut syndrome (LGS), severe epileptic encephalopathies, and juvenile myoclonic epilepsy (JME) (Supplemental Table S1 and Appendix C, see ESM 1).
We identified reports in 692 adult patients and mixed-age populations treated with perampanel (548 in published literature and 144 patients in conference abstracts). The largest study (Villanueva et al.) provided outcomes by seizure type and syndrome in 149 patients aged ≥12 years with IGE, including JME, childhood absence epilepsy (CAE), juvenile absence epilepsy (JAE), and GTCS alone [14]. Other publications reported data in smaller adult/mixed populations, with sample sizes ranging from 8 to 114 patients, including those with refractory myoclonic epilepsies and PMEs (Supplemental Table S1 and Appendix C, see ESM 1).
3.4.4 Case Studies/Series
Case studies and case series in 24 patients were identified and are listed in Supplemental Table S5, with full details in Supplemental Table S1 (see ESM 4).
3.4.5 Meta-Analyses
We identified nine published systematic reviews and meta-analyses and one conference abstract reporting a systematic review, which did not provide any additional data as the studies they identified had already been captured by our searches (Appendix C, see ESM 1).
3.5 Synthesis of Results
3.5.1 Outcomes by Seizure Type
3.5.1.1 PGTCS
Outcomes for PGTCS are reported for 81 people treated with perampanel in one RCT, 54 people in three non-randomised interventional studies, 215 people in 14 observational studies, and nine people in case studies (total N = 359) (Table 1).
Studies included populations with different baseline characteristics and wide levels of refractoriness, and the responder rates ranged from 14% in highly refractory paediatric populations [15] to 100% in a small population of JME patients [16]. In the largest studies of routine perampanel use, responder rates were generally > 50%, and freedom from PGTCS was reported in 63% of 115 patients with IGE in the study with the largest sample size [14].
3.5.1.2 Myoclonic Seizures
Outcomes for myoclonic seizures are reported for 24 people with myoclonic seizures associated with IGE in a post-hoc analysis of the RCT [17], in 59 patients treated with perampanel in two non-randomised interventional studies [11, 12] and 151 patients in 14 observational studies (Table 2). These included 66 patients with IGE (mostly JME) [14, 16, 18], 76 patients with PMEs [11, 12, 18,19,20] and three with Dravet syndrome [21].
The 17 case studies included 13 cases of PMEs [22,23,24,25,26,27,28,29,30,31,32], one of JME [33], one of graft versus host disease [34] and one patient with Angelman syndrome [29] (total N = 251) (Table 2).
In a post-hoc analysis of the RCT, seizure outcomes in 24 patients with myoclonic seizures were explored [17]. Results were inconclusive, as the study was designed to assess changes in PGTCS frequency and did not have sufficient statistical power to identify statistically significant differences in the frequency of myoclonic seizures between the treatment arms. With small group sizes (perampanel N = 24, placebo N = 23), imbalance in demographic and prognostic factors is possible. Further, seizure frequency may be an appropriate outcome measure for PGTCS but cannot always be counted accurately for myoclonic seizures. The median frequency of myoclonic seizures at baseline was 13.8 in the perampanel group (range 0.5–719.9 per 28 days), and 3.5 in the placebo group (range 0.5–250.5 per 28 days). Freedom from myoclonic seizures was reported in 16.7% of patients (4/24) in the perampanel group and 13.0% (3/23) in the placebo group, and increased myoclonic seizure frequency was reported in 29.2% (7/24; perampanel) and 30.4% (7/23; placebo) (Supplemental Tables S6 and S7, see ESM 1) [17].
In the largest observational cohort of patients with myoclonic seizures (N = 48 with IGE), there was a mean 65% reduction in days with myoclonic seizures 1 year after addition of perampanel, and 65% of patients were free of myoclonic seizures [14]. Data on myoclonic seizure worsening were reported in six studies; four reported no worsening, one reported worsening in one out of three patients, and one reported physician-assessed worsening of intensity/duration in 1/46 patients at 12 months and increase in days with myoclonic seizures in 3/48 patients (6.3%) at 12 months [14]. Some of these studies also recorded functional improvements (e.g. in speech, eating, movement) and improvements in activities of daily living (Supplemental Table S1, see ESM 4) [18, 19, 35].
Case studies reported improvement (usually dramatic) in myoclonic seizure frequency or severity in 14 of 17 patients following perampanel addition [22,23,24, 26,27,28, 30,31,32,33,34, 36], lack of clinical success in one case [25], and onset of atypical absence seizures and non-convulsive status epilepticus in two patients [29].
3.5.1.3 Absence Seizures
Outcomes for absence seizures are reported for 27 people with absence seizures associated with IGE in a post-hoc analysis of the RCT, in 83 people treated with perampanel in 9 observational studies, and two in case studies (N = 112; Table 3).
In a post-hoc analysis of the RCT, seizure outcomes in patients with absence seizures were explored [17]. Results were inconclusive, as the study was designed to study changes in PGTCS frequency and did not have sufficient statistical power to identify statistically significant differences in the frequency of absence seizures between the treatment arms. With small group sizes (perampanel N = 27, placebo N = 33), imbalance in demographic and prognostic factors is possible. Further, seizure frequency may be an appropriate outcome measure for PGTCS but cannot always be counted accurately for absence seizures. The median frequency of absence seizures at baseline was 13.0 in the perampanel group (range 0.4–1403.0 per 28 days), and 8.2 in the placebo group (range 0.4–572.0 per 28 days). Freedom from absence seizures was reported in 22.2% of patients (6/27) in the perampanel group and 12.1% (4/33) in the placebo group, and increased absence seizure frequency was observed in 29.6% (8/27; perampanel) and 45.5% (15/33; placebo) patients (Supplemental Tables S6 and S7, see ESM 1) [17].
3.5.1.4 Tonic Seizures
Outcomes for tonic seizures are reported for 48 people treated with perampanel in observational studies, and two in case studies (Appendix D, Supplemental Table S10, see ESM 1). The population was predominantly young children (from 6 months of age), up to young adults, with responder rates ranging from 20 to 80% over 6 months of treatment [15]. No evidence of seizure worsening emerged; one case of seizure clusters and status epilepticus possibly caused by pharmacokinetic interactions and reduced serum levels of concomitant ASMs was reported [37].
3.5.1.5 Epileptic Spasms
Outcomes for epileptic spasms are reported for 32 children treated with perampanel in four observational studies, and one case study (Appendix D, Supplemental Table S11, see ESM 1). No patients became free of epileptic spasms, and a 50% reduction in baseline seizure frequency was found in 31–67% of patients at 3–12 months after addition of perampanel [15, 38,39,40].
3.5.2 Outcomes by Syndrome
3.5.2.1 IGE Overall
Seizure and efficacy outcomes are reported for 81 patients with IGE treated with adjunctive perampanel in one RCT [3], 24 patients in one non-randomised interventional study [41], 271 patients in ten observational studies, and two case studies (total N = 378) (Appendix D, Supplemental Table S1, see ESM 4). The RCT demonstrated statistically significant greater reductions in seizure frequency and higher responder rates with perampanel compared with placebo; due to the low risk of bias of this trial (see Sect. 3.3), the results from other study types characterised by higher sources of bias have not been synthesised here for IGE overall. Full outcomes for all study types are provided in Supplemental Table S1 (see ESM 4).
3.5.2.2 Juvenile Myoclonic Epilepsy
Outcomes in JME are reported in 91 people from four observational studies and one case study (total N = 92, Table 4). The largest of these included 60 patients with JME, of whom 61.7% were free of all seizures at 12 months and for the previous 6 months (37/60; Table 4). The seizure-free rate was 61.9% for PGTCS (26/42), 68.2% for myoclonic seizures (30/44), and 56.3% for absence seizures (30/44). AEs were reported by 46.7% (28/60), and led to discontinuation in 10.0% (6/60) (Supplemental Table S1, see ESM 4). Results from smaller studies were broadly consistent, with high rates of seizure freedom (Table 4).
3.5.2.3 Absence Epilepsies
Outcomes with perampanel are reported in 43 people with absence epilepsy syndromes in two observational studies [14, 42] (Supplemental Table S12, see ESM 1). The largest cohort was 37 patients aged ≥ 12 years (N = 21with JAE, N = 10 with CAE, and N = 6 with adult-onset absence epilepsy) [14]. In these 37 patients, PGTCS frequency was reduced by 71.4% from baseline at 1 year after addition of perampanel, and 51.4% of patients (19/37) were free of all seizures at 12 months and since a 6-month visit (67.9% [19/28] free of PGTCS, 33.3% [1/3] free of myoclonic, and 48.4% [15/31] free of absence seizures).
3.5.2.4 Progressive Myoclonic Epilepsies
Outcomes with perampanel are reported in 59 patients with progressive myoclonic epilepsies in two non-randomised interventional studies [11, 12], 45 patients in five observational studies, and 14 patients in case studies (total N = 118) (Table 5). Reductions in myoclonic seizures frequency and severity were seen across study types and across different aetiologies, including Lafora disease [12, 43] and Unverricht–Lundborg disease [19]; reductions in PGTCS were also often reported. Considering the uncontrolled nature of these studies and different populations and settings, results are reasonably consistent with meaningful response in 50–80% of patients (Table 5).
3.5.2.5 Dravet Syndrome
Outcomes with perampanel are reported in 13 people with Dravet syndrome in three observational studies (Supplemental Table S13, see ESM 1). Approximately 50% of patients experienced a ≥ 50% reduction in overall seizure frequency, and reductions were also reported in individual seizure types (PGTCS, clonic, myoclonic and atypical absence) [15, 21, 40]. Seizure worsening appears rare, with ≥50% increase in seizure frequency seen in 0/10 patients [21] and 1/2 patients [40].
3.5.2.6 Lennox-Gastaut Syndrome
Outcomes with perampanel are reported in 21 patients with LGS in observational studies (Supplemental Table S14, see ESM 1). In these small studies, seizure responder rates ranged from 0/1 [15] to 6/6 [44].
3.5.2.7 West Syndrome
Outcomes with perampanel are reported in five patients with West syndrome across two observational studies (Supplemental Table S15, see ESM 1). None of the participants were considered responders, and seizure frequency increased by ≥ 50% in one of the three patients included in one study [40].
4 Discussion
4.1 Summary of Evidence
The studies identified in this comprehensive review of the literature confirmed the effectiveness of add-on perampanel in the control of PGTCS in patients aged ≥ 12 years with IGE and suggested its potential role in the treatment of different generalised seizure types and syndromes.
Pronounced reduction or abolition of PGTCS was reported in epilepsy syndromes other than IGE, including PMEs, and the highest rates of seizure response and seizure freedom were observed among patients with JME. Adjunctive perampanel was particularly effective in the control of myoclonic seizures in the context of JME; clinically meaningful reductions in myoclonic seizures frequency and severity were also seen in patients with PMEs across different aetiologies, including Lafora disease and Unverricht-Lundborg disease. Adjunctive perampanel was also useful in the treatment of absence seizures in the context of IGE; the size of treatment effect was generally smaller than that observed for myoclonic seizures, but estimates were based on fewer patients.
Data for tonic seizures as well as for epileptic encephalopathies like Dravet syndrome and LGS relied upon very small cohorts of patients and no firm conclusions can be drawn. However, we found no reasons to recommend against a therapeutic trial of adjunctive perampanel in these patients, particularly considering the severity of these syndromes and the limited therapeutic options available. Results in West syndrome and epileptic spasms were mixed, and both the limited sample sizes and unclear measurements of clinical outcomes, including the lack of information on EEG pattern and resolution of hypsarrhythmia, need to be acknowledged.
Importantly, no evidence emerged to suspect any associations between perampanel and seizure aggravation in generalised epilepsies. Some individuals did experience increased frequency or severity of seizures after addition of perampanel, but in the context of larger numbers of patients reporting improvement. Causality regarding seizure worsening cannot be proved in observational studies due to the natural variability in seizure course, possible changes in concomitant medications, and concurrent illness; these and other factors able to exacerbate seizure frequency and severity were not addressed in individual reports.
The tolerability profile was consistent with that reported in RCTs of adjunctive perampanel in patients with focal seizures and with PGTCS—somnolence, dizziness/unsteadiness, irritability, and altered behaviour were the most commonly reported AEs. Although rates of discontinuation due to AEs were not often reported for generalised seizure populations, retention rates were typically > 70% across the studies and suggested that adjunctive perampanel was overall well tolerated.
There is an emerging neurophysiological rationale to support a broad-spectrum effect of perampanel in epilepsy. A recent study explored the effect of perampanel on excitability in cortico-subcortical networks, by measuring high-frequency oscillations of somatosensory evoked potentials in 15 people with epilepsy [53]. A reduction was observed in the area of total high-frequency oscillations, mainly on the early burst related to thalamo-cortical pathways. This suggests a mechanism via which antagonism of AMPA receptors by perampanel could be broadly effective across various seizure types in epilepsy.
4.2 Strengths and Limitations
The strengths of this systematic review include the comprehensive search strategy aimed to include studies of all types, ‘grey literature’ represented by non-peer-reviewed conference abstracts, and ongoing studies identified through clinical trials registries. Results from individual studies were often reported multiple times, and our careful checks for ‘encore’ conference abstracts and exclusion of interim data that was superseded by later reports ensured that the majority of evidence we gathered came from unique patients. Inclusion of data from conference abstracts and unpublished studies posted in clinical trials registries minimised the risk of (positive) publication bias.
A major limitation is that the certainty of the evidence provided by observational, non-controlled, and non-randomised studies is low due to many sources of bias, including the placebo effect, natural fluctuations in seizure frequency, lack of a control group and blinding/masking, overestimation of efficacy and regression to the mean. Definitive conclusions on comparative safety and efficacy can only be obtained through well-designed RCTs. However, real-world evidence does have a place in healthcare decision making. For example, data from observational studies and case reports can help to extrapolate findings from clinical trials to more representative patient populations, including those who are often excluded from clinical trials. Real-world evidence can also give a ‘signal’ of effectiveness in seizures and epilepsy types that are often excluded from clinical trial populations, such as rare and serious epilepsies like PMEs, or patients with intellectual disability. Ideally, RCTs will follow this hypothesis generation to formally assess efficacy and safety. So our summary of evidence should be used not as an indication of what seizure outcomes could be expected in other patients with similar seizure types, but to indicate general populations where a therapeutic trial of perampanel may be warranted.
Another limitation was the heterogeneity in populations, outcome measures and definitions used in the identified studies, which did not allow a quantitative data synthesis and also hampered the qualitative analysis. In particular, seizure freedom was sometimes reported in the completer population and sometimes in the full intent-to-treat population, with unclear definitions of ‘freedom’. The reporting of findings from observational studies was often of poor quality; outcome data were provided for subgroups that had no corresponding demographic data and sometimes no N-numbers, settings of the studies were often not given, denominators/populations were often undefined (e.g. completer population or intent-to-treat population) and ‘response’ was sometimes not defined. These issues highlight the need for better and consistent outcome reporting in real-world evidence in epilepsy, highlight a lack of familiarity with applicable reporting standards (e.g. STROBE), and reflect the lack of a specific reporting standard that matches these single-cohort, non-comparative, observational study designs.
5 Conclusions
Management of epilepsy can be particularly challenging when the presenting seizure type is unclear or more seizure types coexist. In such situations, broad-spectrum ASMs are a rational choice. Furthermore, many patients have generalised seizure types that are not well represented in RCTs, and treatment decisions often have to be made in the absence of Class I evidence.
By gathering all currently available evidence, including observational real-world data, we can begin to look for patterns and identify seizure types where adjunctive use of perampanel might be beneficial and justifies further research. The data we have collected suggest, although with weak certainty due to the observational nature and high risk of bias of most of the included studies, that perampanel can be effective and tolerated in multiple generalised seizure types in absence of any suspicion of seizure worsening or aggravation (see summary video in ESM 3). In addition to the high-quality evidence already supporting the effectiveness of perampanel in PGTCS and in focal seizures, these data play in favour of its potential as a broad-spectrum ASM. Therapeutic trials of adjunctive perampanel may be particularly warranted in patients with JME, myoclonic seizures associated with PMEs and absence seizures. We encourage well-designed interventional studies with perampanel in these epilepsies to provide additional and more robust findings and to better delineate the antiseizure activity and profile of perampanel.
References
Potschka H, Trinka E. Perampanel: Does it have broad-spectrum potential? Epilepsia. 2019;60(suppl 1):22–36.
Steinhoff BJ, Ben-Menachem E, Ryvlin P, Shorvon S, Kramer L, Satlin A, et al. Efficacy and safety of adjunctive perampanel for the treatment of refractory partial seizures: a pooled analysis of three phase III studies. Epilepsia. 2013;54:1481–9.
French JA, Krauss GL, Wechsler RT, Wang X-F, DiVentura B, Brandt C, et al. Perampanel for tonic-clonic seizures in idiopathic generalized epilepsy A randomized trial. Neurology. 2015;85:950–7.
Moher D, Liberati A, Tetzlaff J, Altman D, The PRISMA Group. preferred reporting items for systematic reviews and meta-analyses: the PRISMA Statement. PLoS Med. 2009;6:e1000097.
Campbell M, McKenzie JE, Sowden A, Katikireddi SV, Brennan SE, Ellis S, et al. Synthesis without meta-analysis (SWiM) in systematic reviews: reporting guideline. BMJ. 2020;368:l6890.
Scheffer IE, Berkovic S, Capovilla G, Connolly MB, French J, Guilhoto L, et al. ILAE classification of the epilepsies: Position paper of the ILAE Commission for Classification and Terminology. Epilepsia. 2017;58:512–21.
Fisher RS, Cross JH, French JA, Higurashi N, Hirsch E, Jansen FE, et al. Operational classification of seizure types by the International League Against Epilepsy: Position Paper of the ILAE Commission for Classification and Terminology. Epilepsia. 2017;58:522–30.
Sterne JAC, Savovic J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898.
Fogarasi A, Flamini R, Milh M, Phillips S, Yoshitomi S, Patten A, et al. Open-label study to investigate the safety and efficacy of adjunctive perampanel in pediatric patients (4 to <12 years) with inadequately controlled focal seizures or generalized tonic-clonic seizures. Epilepsia. 2020;61:125–37.
Renfroe JB, Mintz M, Davis R, Ferreira J, Dispoto S, Ferry J, et al. Adjunctive perampanel oral suspension in pediatric patients from ≥2 to <12 years of age with epilepsy: Pharmacokinetics, safety, tolerability, and efficacy. J Child Neurol. 2019;34:284–94.
Canafoglia L, Sebastiano DR, Visani E, Franceschetti S, Barbella G, Ferlazzo E, et al. An Italian multicentre study of perampanel in progressive myoclonus epilepsies. Epilepsy Res. 2019;156:106191.
Goldsmith D, Minassian BA. Efficacy and tolerability of perampanel in ten patients with Lafora disease. Epilepsy Behav. 2016;62:132–5.
Hwang SK, Lee YJ, Kwon S, Nam SO, Kim WS, Kim JS, et al. Real-life effectiveness and tolerability of perampanel in pediatric patients aged 4 years or older with epilepsy: A Korean national multicenter study. J Clin Neurol (Korea). 2020;16:53–9.
Villanueva V, Garces M, Gomez A, Montoya J, Castillo A, Mauri-Llerda JA, et al. Perampanel in routine clinical use in idiopathic generalized epilepsy: The 12-month GENERAL study. Epilepsia. 2018;59:1740–52.
Swiderska N, Tan HJ, Martland T, Rajai A, Silwal A, Desurkar A. Effectiveness and tolerability of Perampanel in children, adolescents and young adults with refractory epilepsy: A UK national multicentre study. Seizure. 2017;52:63–70.
Wang N. Perampanel in refractory juvenile myoclonic epilepsy: a case series. Abstract 2.184. In: AES Annual Meeting Abstract Database AESnet.org. 2017. https://www.aesnet.org/. Accessed 28 Jan 2021.
Brandt C, Wechsler RT, O’Brien TJ, Patten A, Malhotra M, Ngo LY, et al. Adjunctive perampanel and myoclonic and absence seizures: post hoc analysis of data from study 332 in patients with idiopathic generalized epilepsy. Seizure. 2020;80:115–23.
Gil-Lopez FJ, Donaire A, Carreno M, Montoya J, Falip M, Aparicio J, et al. Retrospective study of perampanel efficacy and tolerability in myoclonic seizures. Acta Neurol Scand. 2018;138:122–9.
Crespel A, Gelisse P, Tang NPL, Genton P. Perampanel in 12 patients with Unverricht-Lundborg disease. Epilepsia. 2017;58:543–7.
Kim HJ, Yum MS, Yeh HR, Lee BH, Ko TS. Efficacy of perampanel on progressive myoclonic epilepsy in patients with Gaucher’s disease. Epilepsia. 2018;59(suppl 3):194 (Abstract).
Yoshitomi S, Takahashi Y, Yamaguchi T, Imai K, Inoue Y, Ishii A, et al. Efficacy and tolerability of perampanel in pediatric patients with Dravet syndrome. Epilepsy Res. 2019;154:34–8.
Dirani M, Nasreddine W, Beydoun A, Abdulla F. Seizure control and improvement of neurological dysfunction in Lafora disease with perampanel. Epilepsy Behav Case Rep. 2014;2:164–6.
Schorlemmer K, Bauer S, Belke M, Hermsen A, Klein KM, Reif PS, et al. Sustained seizure remission on perampanel in progressive myoclonic epilepsy (Lafora disease). Epilepsy Behav Case Rep. 2013;1:118–21.
Oi Y, Kobayashi K, Hitomi T, Matsumoto R, Takahashi R, Ikeda A. Low-dose perampanel improved cortical myoclonus and basophobia in a patient with Unverricht-Lundborg disease: A case report. Clinical Neurology. 2018;58:622–5.
Mohamadpour M, Gabriel G, Grant AC. A native Haitian woman with Unverricht-Lundborg disease. Case Rep Neurol. 2017;9:284–8.
Iijima M, Kobayashi M, Kitagawa K, Oguni H. Perampanel improved intractable myoclonus in two patients with myoclonus epilepsy. eNeurologicalSci. 2019;17:100215.
So ECT, Mak CM, Ng GSF, Tsui KW, Ma KH, Yeung WL. Reduction in myoclonus and ataxia following the use of perampanel in patient with sialidosis type 1. Pediatr Neurol. 2020;109:91–3.
Hu SC, Hung KL, Chen HJ, Lee WT. Seizure remission and improvement of neurological function in sialidosis with perampanel therapy. Epilepsy Behav Case Rep. 2018;10:32–4.
Rodriguez AD, Extremera VC, Penas JJG, Rojas MLR-F, Fernandez MG. Induccion de crisis de ausencia atipica durante el tratamiento con perampanel Induced atypical absence seizures during treatment with perampanel. Ann Pediatr. 2019;91:346–8.
Wong LC, Hsu CJ, Lee WT. Perampanel attenuates myoclonus in a patient with neuronal ceroid lipofuscinoses type 2 disease. Brain Dev. 2019;41:817–9.
Shiraishi H, Egawa K, Ito T, Kawano O, Asahina N, Kohsaka S. Efficacy of perampanel for controlling seizures and improving neurological dysfunction in a patient with dentatorubral-pallidoluysian atrophy (DRPLA). Epilepsy Behav Case Rep. 2017;8:44–6.
Shiloh-Malawsky Y, Zook Lewis C. Improved myoclonus and seizure control and dramatic improvement of neurological function in patients with dentatorubral pallidoluysian atrophy (DRPLA) treated with perampanel. Abstract 2.261. In: AES Annual Meeting Abstract Database AESnet.org. 2019. https://www.aesnet.org/. Accessed 28 Jan 2021.
Lanzone J, Ricci L, Tombini M, Assennza G. Dramatic effect of perampanel in a patient with JME and persistent myoclonic jerks. Epilepsia. 2018;59(suppl 3):371 (Abstract).
Robuccio A, Ssentongo P, Gilliam FG, Sather MD, Claxton DF. Intractable myoclonic seizures in an allogeneic stem cell transplant recipient: a rare case of myoclonic epilepsy. Epilepsy Behav Case Rep. 2015;4:48–51.
Oi K, Neshige S, Hitomi T, Kobayashi K, Tojima M, Fujii D, et al. Low-dose perampanel improves refractory cortical myoclonus by the dispersed and suppressed paroxysmal depolarization shifts in the sensorimotor cortex. Clin Neurophysiol. 2019;130:1804–12.
Khalid K. Unverricht-Lundborg disease. J Neurol Sci. 2019;405(suppl):0386.
Novy J, Rossetti AO, Rothuizen LE, Buclin T. Perampanel: a significant liver enzyme inducer in some patients? Eur Neurol. 2014;72:213–6 (Abstract).
Ishikawa N, Tateishi Y, Tani H, Kobayashi Y, Kobayashi M. Clinical profiles associated with serum perampanel concentrations in children with refractory epilepsy. Epilepsy Behav. 2019;94:82–6.
Ikemoto S, Hamano SI, Hirata Y, Matsuura R, Koichihara R. Efficacy and serum concentrations of perampanel for treatment of drug-resistant epilepsy in children, adolescents, and young adults: comparison of patients younger and older than 12 years. Seizure. 2019;73:75–8.
Biro A, Muller A, Selch C, Staudt M, Kluger G, Stephani U, et al. Effectiveness and tolerability of perampanel in children and adolescents with refractory epilepsies: first experiences. Neuropediatrics. 2015;46:110–6.
Nordli Jr DR, Arzimanoglou A, Patten A, Ngo LY. Long-term efficacy and safety of adjunctive perampanel in pediatric patients with primary generalized tonic-clonic seizures of idiopathic generalized epilepsy: post hoc analysis of study 311. Abstract 3.306. In: AES Annual Meeting Abstract Database AESnet.org. 2019. https://www.aesnet.org/. Accessed 28 Jan 2021.
Salas-Puig J, Cambrodí R, Abraira L, Quintana M, Santamarina E, Jose Jurado M, et al. Perampanel in refractory idiopathic generalized epilepsies. Abstract 2.141. In: AES Annual Meeting Abstract Database AESnet.org. 2018. https://www.aesnet.org/. Accessed 28 Jan 2021.
Genton P, Jovic NJ, Lesca G, Kecmanovic M. Is adjunctive perampanel an option for intractable seizures in Lafora disease? Epilepsia. 2015;56(suppl 1):0203 (Abstract).
Vajjala VS, Treiman DM. Fycompa (perampanel) experience in a tertiary epilepsy center. Abstract 2.237. In: AES Annual Meeting Abstract Database AESnet.org. 2018. https://www.aesnet.org/. Accessed 28 Jan 2021.
Gutierrez FJM, Roman MD, Albert DC. Electro-clinical analysis of epilepsy patients with generalized seizures on adjunctive perampanel treatment. Epilepsy Res. 2020;165:78.
Morano A, Fattouch J, Albini M, Casciato S, Fanella M, Basili LM, et al. Perampanel as adjunctive therapy in highly refractory epilepsies: real-world data from an Italian tertiary care epilepsy centre. J Neurol Sci. 2018;390:67–74.
Suchita IA, Rashid S, Morgan LC, Leary LD, Szabo CA. Therapeutic response and adverse effects of perampanel (Fycompa) as adjunctive antiepileptic therapy. Abstract 2.291. In: AES Annual Meeting Abstract Database AESnet.org. 2018. https://www.aesnet.org/. Accessed 28 Jan 2021.
Dolton E, Choudry A. Perampanel and challenging behaviour in intellectual disability and epilepsy: a management dilemma. Case Rep Psychiatry. 2014;p.: 409209.
Shimabukuro K, Gibbon F, Kerstetter J, Tinsley C, Ashwal S. DRESS associated with perampanel administration in a child with drug-resistant epilepsy. Neurology. 2014;83:2188.
Volkov I, Volkova O. Beneficial effect of add-on perampanel on GTCS in patients with Lafora disease. Nevrologiya Neiropsikhiatriya Psikhosomatika. 2018;10:98 (Abstract).
Ikemoto S, Hamano SI, Hirata Y, Matsuura R, Koichihara R. Perampanel in lissencephaly-associated epilepsy. Epilepsy Behav Case Rep. 2019;11:67–9.
Sakurai M, Azuma J, Yamamoto T, Sakai N, Hamada Y. Early juvenile Tay-Sachs disease with atypical symptoms. Pediatr Int. 2019;61:611–3.
Lanzone J, Boscarino M, Ricci L, Insola A, Tombini M, Di Lazzaro V, et al. Effects of the noncompetitive AMPA receptor antagonist perampanel on thalamo-cortical excitability: a study of high-frequency oscillations in somatosensory evoked potentials. Clin Neurophysiol. 2021;132:1049–56.
Acknowledgements
We thank Roseanne Wilkinson for medical writing support, funded by Eisai Inc. Support included formatting and uploading the study protocol, screening and checking search results, coordinating searches, managing databases, coordinating the risk of bias assessment, extracting data from selected full-text articles, creating tables, and reviewing/editing the introduction, methods and results. We thank Katy Oak and Catriona Organ (Royal Cornwall Hospitals Library, Truro, UK) for conducting literature searches (funded by Eisai Inc.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
Open access funding was provided by Paracelsus Medical University. Eisai Inc. provided funding for medical writing support, which was provided by Kate Carpenter and Roseanne Wilkinson, and for librarian support in conducting literature searches.
Conflicts of interest/Competing interests
BN, FB, FR, TC have nothing to disclose. ET has nothing to disclose related to the submitted work. Outside the submitted work, ET has received consultancy fees from Arvelle, Argenix, Clexio, Celegene, UCB, Eisai, Epilog, Bial, Medtronic, Everpharma, Biogen, Takeda, Liva-Nova, Newbridge, Sunovion, GW Pharmaceuticals and Marinus; speaker fees from Arvelle, Bial, Biogen, Böhringer Ingelheim, Eisai, Everpharma, GSK, GW Pharmaceuticals, Hikma, Liva-Nova, Newbridge, Novartis, Sanofi, Sandoz and UCB; research funding (directly, or to his institution) from GSK, Biogen, Eisai, Novartis, Red Bull, Bayer and UCB. ET receives grants from Austrian Science Fund (FWF), Österreichische Nationalbank, and the European Union. ET is the CEO of Neuroconsult Ges.m.b.H. SL has nothing to disclose related to the submitted work. Outside the submitted work, SL has received speaker or consultancy fees from Eisai, UCB Pharma and GW Pharmaceuticals and has served on advisory boards for GW Pharmaceuticals and Arvelle Therapeutics. KC received financial support from Eisai Inc. for writing work on this manuscript, and has received fees from Eisai for other writing and consultancy work unrelated to this manuscript. RS has nothing to disclose related to the submitted work. Outside the submitted work, RS reports institutional research grants and fees from LivaNova, Eisai, Bial, UCB, Desitin, Averelle and GW pharma, and is a stakeholder in epilepsy checklists and monitoring systems.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and material
We have made all data that were extracted for this manuscript available in the electronic supplementary material (Supplemental Table S1)
Code availability
Not applicable.
Authors’ contributions
ET initiated the project. ET, SL FB, RS and KC developed the protocol. FB, BN, RW and FR manually searched conference abstract databases. FB, BN and FR screened retrieved abstracts. FB, BN and FR manually reviewed reference lists of retrieved article for additional reports. KC manually searched clinical trial databases and screened for relevance. SL and TC reviewed full texts for selection/exclusion. KC and RW checked screening and selection. ET made decisions on inclusion where reviewers disagreed. RS conducted risk of bias assessment. RW extracted data from selected reports. KC checked data extraction. All authors reviewed the outline. KC and RW prepared tables. KC drafted the Methods and Results sections. ET and SL drafted the Introduction and Discussion. All authors reviewed and provided intellectual contributions to the draft manuscript. All authors approved the final draft.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file3 (MP4 20203 kb)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Trinka, E., Lattanzi, S., Carpenter, K. et al. Exploring the Evidence for Broad-Spectrum Effectiveness of Perampanel: A Systematic Review of Clinical Data in Generalised Seizures. CNS Drugs 35, 821–837 (2021). https://doi.org/10.1007/s40263-021-00831-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40263-021-00831-y