Abstract
Background and Objective
Taspoglutide, a glucagon-like peptide-1 agonist, like native glucagon-like peptide-1, delays gastric emptying time and prolongs intestinal transit time, which may alter the pharmacokinetics of concomitantly administered oral drugs. The effect of taspoglutide on the pharmacokinetics of five oral drugs commonly used in patients with type 2 diabetes mellitus was assessed in healthy subjects.
Methods
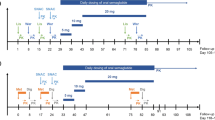
Five clinical pharmacology studies evaluated the potential drug–drug interaction between multiple subcutaneous taspoglutide doses and a single dose of lisinopril, warfarin, and simvastatin and multiple doses of digoxin and an oral contraceptive containing ethinylestradiol and levonorgestrel. The extent of interaction was quantified using geometric mean ratios and 90% confidence intervals for the maximum plasma concentration and area under the plasma concentration–time curve. In addition to pharmacokinetics, pharmacodynamic effects were assessed for warfarin and the oral contraceptive.
Results
Among the tested drugs, the effect of taspoglutide on the pharmacokinetics of simvastatin was most pronounced, on the day of taspoglutide administration, the average exposure to simvastatin was decreased by − 26% and − 58% for the area under the plasma concentration–time curve and maximum plasma concentration, respectively, accompanied by an increase in average exposure to its active metabolite, simvastatin β-hydroxy acid (+ 74% and + 23% for area under the plasma concentration–time curve and maximum plasma concentration, respectively). Although statistically significant changes in exposure were observed for other test drugs, the 90% confidence intervals for the geometric mean ratio for maximum plasma concentration and area under the plasma concentration–time curve were within the 0.7–1.3 interval. No clinically relevant changes on coagulation (for warfarin) and ovulation-suppressing activity (for the oral contraceptive) were apparent.
Conclusion
Overall, multiple doses of taspoglutide did not result in changes in the pharmacokinetics of digoxin, an oral contraceptive containing ethinylestradiol and levonorgestrel, lisinopril, warfarin, and simvastatin that would be considered of clinical relevance. Therefore, no dose adjustments are warranted upon co-administration.




Similar content being viewed by others
References
Meier JJ. GLP-1 receptor agonists for individualized treatment of type 2 diabetes mellitus. Nat Rev Endocrinol. 2012;8:728–42.
Htike ZZ, Zaccardi F, Papamargaritis D, Webb DR, Khunti K, Davies MJ. Efficacy and safety of glucagon-like peptide-1 receptor agonists in type 2 diabetes: a systematic review and mixed-treatment comparison analysis. Diabetes Obes Metab. 2017;19:524–36.
Raccah D. Safety and tolerability of glucagon-like peptide-1 receptor agonists: unresolved and emerging issues. Expert Opin Drug Saf. 2017;16(2):227–36.
Hjerpsted JB, Flint A, Brooks A, Axelsen MB, Kvist T, Blundell J. Semaglutide improves postprandial glucose and lipid metabolism, and delays first-hour gastric emptying in subjects with obesity. Diabetes Obes Metab. 2018;20(3):610–9.
Baggio LL, Drucker DJ. Biology of incretins: GLP-1 and GIP. Gastroenterology. 2007;132(6):2131–57.
Blase E, Taylor K, Gao HY, Wintle M, Fineman M. Pharmacokinetics of an oral drug (acetaminophen) administered at various times in relation to subcutaneous injection of exenatide (exendin-4) in healthy subjects. J Clin Pharmacol. 2005;45(5):570–7.
Tran KL, Park YI, Pandya S, Muliyil NJ, Jensen BD, Huynh K, Nguyen QT. Overview of glucagon-like peptide-1 receptor agonists for the treatment of patients with type 2 diabetes. Am Health Drug Benefits. 2017;10(4):178–88.
Nakatani Y, Maeda M, Matsumura M, Shimizu R, Banba N, Aso Y, Yasu T, Harasawa H. Effect of GLP-1 receptor agonist on gastrointestinal tract motility and residue rates as evaluated by capsule endoscopy. Diabetes Metab. 2017;43(5):430–7.
Loghin C, de la Pen˜a A, Cui X, Chien J. Gastric emptying effects of once weekly dulaglutide in patients with type 2 diabetes mellitus [poster]. In: 23rd Annual Scientific and Clinical Congress of the American Association of Clinical Endocrinologists; 19 May 2014. https://www.mdlinx.com/endocrinology/conference-abstract.cfm/12181/?nonus=0&searchstring=&coverage_day=0&page=1. Accessed 3 Mar 2019.
Drucker DJ, Buse JB, Taylor K, Kendall DM, Trautmann M, Zhuang D, Porter L, for the DURATION-1 Study Group. Exenatide once weekly versus twice daily for the treatment of type 2 diabetes: a randomised, open-label, non-inferiority study. Lancet. 2008;372:1240–50.
Bush M, Scott R, Watanalumlerd P, et al. Effects of multiple doses of albiglutide on the pharmacokinetics, pharmacodynamics, and safety of digoxin, warfarin, or a low-dose oral contraceptive. Postgrad Med. 2012;124(6):55–72.
Hausner H, Derving KJ, Holst AG, et al. Effect of semaglutide on the pharmacokinetics of metformin, warfarin, atorvastatin, and digoxin in healthy subjects. Clin Pharmacokinet. 2017;56(11):1391–401.
Malm-Erjefält M, Ekblom M, Vouis J, Zdravkovic M, Lennernäs H. Effect on the gastrointestinal absorption of drugs from different classes in the biopharmaceutics classification system, when treating with liraglutide. Mol Pharm. 2015;12(11):4166–73.
de la Peña A, Cui X, Geiser J, Loghin C. No dose adjustment is recommended for digoxin, warfarin, atorvastatin or a combination oral contraceptive when coadministered with dulaglutide. Clin Pharmacokinet. 2017;56(11):1415–27.
Byetta (exenatide), prescribing information. 2009. https://www.accessdata.fda.gov/drugsatfda_docs/label/2009/021773s9s11s18s22s25lbl.pdf. Accessed 13 Feb 2019.
Bydureon (exenatide extended-release), prescribing information. 2012. https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/022200s000lbl.pdf. Accessed 13 Feb 2019.
Lyxumia (lixisenatide), summary of product characteristics. 2013. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002445/WC500140401.pdf. Accessed 13 Feb 2019.
Dong JZ, Shen Y, Zhang J, Tsomaia N, Mierke DF, Taylor JE. Discovery and characterization of taspoglutide, a novel analogue of human glucagon-like peptide-1, engineered for sustained therapeutic activity in type 2 diabetes. Diabetes Obes Metab. 2011;13(1):19–25.
Sebokova E, Christ AD, Wang H, Sewing S, Dong JZ, Taylor J, Cawthorne MA, Culler MD. Taspoglutide, an analog of human glucagon-like peptide-1 with enhanced stability and in vivo potency. Endocrinology. 2010;151(6):2474–82.
Nauck M. Incretin therapies: highlighting common features and differences in the modes of action of glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors. Diabetes Obes Metab. 2016;18(3):203–16.
Kapitza C, Heise T, Birman P, Jallet K, Ramis J, Balena R. Pharmacokinetic and pharmacodynamic properties of taspoglutide, a once-weekly, human GLP-1 analogue, after single-dose administration in patients with type 2 diabetes. Diabet Med. 2009;26(11):1156–64.
Giraudon M, Sturm S, Lambert N, Niggli M, Brumm J, Mangold B, Schmitt C. Effect of varying degrees of renal impairment on the pharmacokinetics and tolerability of taspoglutide. Diabetes Obes Metab. 2017;19(4):537–44.
Sturm-Pellanda C, Abt M, Sanwald-Ducray P, Schmitt C. Subcutaneous bioavailability of taspoglutide at 3 different injection sites in healthy overweight/obese subjects. Clin Ther. 2015;37(11):2439–48.
Raz I, Fonseca V, Kipnes M, Durrwell L, Hoekstra J, Boldrin M, Balena R. Efficacy and safety of taspoglutide monotherapy in drug-naive type 2 diabetic patients after 24 weeks of treatment: results of a randomized, double-blind, placebo-controlled phase 3 study (T-Emerge 1). Diabetes Care. 2012;35(3):485–7.
Rosenstock J, Balas B, Charbonnel B, Bolli GB, Boldrin M, Ratner R, Balena R, T-Emerge 2 Study Group. The fate of taspoglutide, a weekly GLP-1 receptor agonist, versus twice-daily exenatide for type 2 diabetes: the T-Emerge 2 trial. Diabetes Care. 2013;36(3):498–504.
Henry RR, Mudaliar S, Kanitra L, Woloschak M, Balena R, T-Emerge 3 Study Group. Efficacy and safety of taspoglutide in patients with type 2 diabetes inadequately controlled with metformin plus pioglitazone over 24 weeks: T-Emerge 3 trial. J Clin Endocrinol Metab. 2012;97(7):2370–9.
Bergenstal RM, Forti A, Chiasson JL, Woloschak M, Boldrin M, Balena R. Efficacy and safety of taspoglutide versus sitagliptin for type 2 diabetes mellitus (T-Emerge 4 trial). Diabetes Ther. 2012;3(1):13.
Nauck M, Horton E, Andjelkovic M, Ampudia-Blasco FJ, Parusel CT, Boldrin M, Balena R, T-Emerge 5 Study Group. Taspoglutide, a once-weekly glucagon-like peptide 1 analogue, vs. insulin glargine titrated to target in patients with type 2 diabetes: an open-label randomized trial. Diabet Med. 2013;30(1):109–13.
Pratley RE, Urosevic D, Boldrin M, Balena R, T-Emerge 6 Study Group. Efficacy and tolerability of taspoglutide versus pioglitazone in subjects with type 2 diabetes uncontrolled with sulphonylurea or sulphonylurea-metformin therapy: a randomized, double-blind study (T-Emerge 6). Diabetes Obes Metab. 2013;15(3):234–40.
Hollander P, Lasko B, Barnett AH, Bengus M, Kanitra L, Pi-Sunyer FX, Balena R. Effects of taspoglutide on glycemic control and body weight in obese patients with type 2 diabetes (T-Emerge 7 study). Obesity. 2013;21(2):238–47 (Silver Spring).
Ratner R, Nauck M, Kapitza C, Asnaghi V, Boldrin M, Balena R. Safety and tolerability of high doses of taspoglutide, a once-weekly human GLP-1 analogue, in diabetic patients treated with metformin: a randomized double-blind placebo-controlled study. Diabet Med. 2010;27(5):556–62.
Heinig K, Wirz T. Determination of taspoglutide in human and animal plasma using liquid chromatography-tandem mass spectrometry with orthogonal column-switching. Anal Chem. 2009;81(10):3705–13.
Leary AC, Dowling M, Wilson A, McKenna B, Rothwell J. A single-dose, randomised, crossover study to compare the rate and extent of absorption of lisinopril solution versus tablets in healthy volunteers. Paediatr Perinatal Drug Ther. 2006;7(4):178–82.
Kothare PA, Soon DK, Linnebjerg H, Park S, Chan C, Yeo A, Lim M, Mace KF, Wise SD. Effect of exenatide on the steady-state pharmacokinetics of digoxin. J Clin Pharmacol. 2005;45:1032–7.
Vickers S, Duncan CA, Chen IW, Rosegay A, Duggan DE. Metabolic disposition studies on simvastatin, a cholesterol-lowering prodrug. Drug Metab Dispos. 1990;18(2):138–45.
Vree TB, Dammers E, Ulc I, Horkovics-Kovats S, Ryska M, Merkx IJ. Variable plasma/liver and tissue esterase hydrolysis of simvastatin in healthy volunteers after a single oral dose. Clin Drug Invest. 2001;21:643–52.
Prueksaritanont T, Ma B, Yu N. The human hepatic metabolism of simvastatin hydroxy acid is mediated primarily by CYP3A, and not CYP2D6. Br J Clin Pharmacol. 2003;56(1):120–4.
Tsamandouras N, Dickinson G, Guo Y, Hall S, Rostami-Hodjegan A, Galetin A, Aarons L. Development and application of a mechanistic pharmacokinetic model for simvastatin and its active metabolite simvastatin acid using an integrated population PBPK approach. Pharm Res. 2015;32(6):1864–83.
Tang BK, Kalow W. Variable activation of lovastatin by hydrolytic enzymes in human plasma and liver. Eur J Clin Pharmacol. 1995;47:449–51.
Kothare PA, Linnebjerg H, Skrivanek Z, Reddy S, Mace K, Pena A, Han J, Fineman M, Mitchell M. Exendatide effects on statin pharmacokinetics and lipid response. Int J Clin Pharmacol Ther. 2007;45:114–20.
Nguyen KA, Li L, Deshun L, Yazdanparast A, Wang L, Kreutz RP, Whipple EC, Schleyer TK. A comprehensive review and meta-analysis of risk factors for statin-induced myopathy. Eur J Clin Pharmacol. 2018;74:1099–109.
Araki M, Maeda M, Motojima K. Hydrophobic statins induce autophagy and cell death in human rhabdomyosarcoma cells by depleting geranylgeranyl diphosphate. Eur J Pharmacol. 2012;674:95–103.
Lee AJ, Maddix DS. Rhabdomyolysis secondary to a drug interaction between simvastatin and clarithromycin. Ann Pharmacother. 2001;3(1):26–31.
Jacobson TA. Comparative pharmacokinetic interaction profiles of pravastatin, simvastatin, and atorvastatin when coadministered with cytochrome P450 inhibitors. Am J Cardiol. 2004;94(9):1140–6.
Zocor (simvastatin) prescribing information. 2012. https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/019766s085lbl.pdf. Accessed 13 Feb 2019.
Acknowledgements
The authors thank Elke Zwanziger, Katja Heinig, Jon Talbot, Christelle Vistuer, and Nathalie Lambert for providing scientific review, operational, and technical support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was funded by F. Hoffmann-La Roche Ltd.
Conflict of interest
Katrijn Bogman, Jochen Brumm, Carsten Hofmann, Mylène Giraudon, Markus Niggli, Carolina Sturm-Pellanda, Annette Sauter, Stefan Sturm, and Christophe Schmitt are employees of F. Hoffmann-La Roche Ltd. Katrijn Bogman, Carsten Hofmann, Carolina Sturm-Pellanda, Annette Sauter, Stefan Sturm, Christophe Schmitt, and Bernhard Mangold are shareholders of F. Hoffmann-La Roche Ltd. Bernhard Mangold was an employee of F. Hoffmann-La Roche Ltd. at the time the studies were conducted and reported.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Bogman, K., Brumm, J., Hofmann, C. et al. Assessment of Drug–Drug Interactions between Taspoglutide, a Glucagon-Like Peptide-1 Agonist, and Drugs Commonly Used in Type 2 Diabetes Mellitus: Results of Five Phase I Trials. Clin Pharmacokinet 58, 1205–1214 (2019). https://doi.org/10.1007/s40262-019-00757-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40262-019-00757-1