Abstract
Introduction
Elvitegravir/cobicistat/emtricitabine/tenofovir alafenamide (E/C/F/TAF) has been increasingly replaced by bictegravir/emtricitabine/tenofovir alafenamide (B/F/TAF) in the treatment of human immunodeficiency virus (HIV) owing to its more favorable pharmacokinetics and fewer drug–drug interactions. However, the effect of this switch on plasma lipids and lipidomic profiles remains poorly characterized.
Methods
HIV infected patients on an E/C/F/TAF regimen were recruited into the study and followed up every 12 weeks. Participants were divided into E/C/F/TAF and B/F/TAF groups depending on whether they were switched to B/F/TAF during follow-up. Clinical information and blood samples were collected at 0, 12, and 24 weeks, and lipidomic analysis was performed using liquid chromatography mass spectrometry.
Results
No significant differences were observed between the groups at baseline. At week 24, patients switched to B/F/TAF had lower triglyceride [mmol/L; 1.23 (0.62) versus 2.03 (0.75), P = 0.001] and very low-density lipoprotein cholesterol [mmol/L; 0.64 (0.26) versus 0.84 (0.32), P = 0.037) compared with patients who continued E/C/F/TAF therapy. Small decrease from baseline in Framingham general cardiovascular risk score (FRS) was observed in the B/F/TAF arm [week (W) 0: 2.59 (1.57) versus W24: 2.18 (1.01), P = 0.043]. Lipidomic analysis indicated that E/C/F/TAF treatment increased the levels of several diglycerides (DGs), triacylglycerols (TAGs), and lyso-phosphatidylcholines (LPCs), whereas switching to B/F/TAF led to increased sphingolipids and glycerophospholipids. After adjusting for demographic and clinical parameters, only DG (16:0/18:2), DG (18:2/22:6), DG (18:3/18:2), DG (20:5/18:2), TAG (18:3/18:2/21:5), TAG (20:5/18:2/22:6), and LPC (22:6) were found to be significantly associated with FRS (regression coefficient of 0.17–6.02, P < 0.05). Most of these FRS associate lipid species were significantly elevated in individuals treated with E/C/F/TAF instead of individuals treated with B/F/TAF.
Conclusion
E/C/F/TAF promotes the accumulation of lipid species closely associated with cardiovascular disease (CVD) risk among people living with HIV, whereas B/F/TAF has a decreased impact on CVD-related lipid profile and is associated with lower CVD risk.
A graphical abstract is available with this article.
Trial registration
ClinicalTrials.gov; identifier, NCT06019273.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
For HIV infected patients, the effect of switching from elvitegravir/cobicistat/emtricitabine/tenofovir alafenamide (E/C/F/TAF) to bictegravir/emtricitabine/tenofovir alafenamide (B/F/TAF) on lipidomic profiles and cardiovascular disease (CVD) risk remains poorly characterized. |
What was learned from the study? |
The mass spectrometry-based lipidomic approach allowed the identification of detailed lipid profiles, highlighting lipid perturbations upon antiretroviral therapy. |
E/C/F/TAF promotes the accumulation of lipid species closely associated with cardiovascular CVD risk instead of B/F/TAF. |
Novel metabolites, such as DG (16:0/18:2), DG (18:2/22:6), etc., were significantly associated with future CVD risk |
Digital Features
This article is published with digital features, including a graphical abstract, to facilitate understanding of the article. To view digital features for this article, go to https://doi.org/10.6084/m9.figshare.25225520.
Introduction
For people living with human immunodeficiency virus (PLWH), widespread application and earlier initiation of antiretroviral therapy (ART) has minimized the occurrence of opportunistic infections, and life expectancy has been dramatically prolonged, approaching that of the general population [1, 2]. However, aging with PLWH leads to a higher mortality related to conditions not related to acquired immune deficiency syndrome (AIDS), such as cardiovascular diseases (CVD), kidney disease, and liver disease [3, 4], which are closely related to lipid metabolism. The risk of subclinical atherosclerosis and CVD is higher for PLWH, even among those with suppressed human immunodeficiency virus (HIV) RNA levels, compared with that of the general population [5, 6]. In developed countries, CVD involving myocardial infarction, stroke, and sudden cardiac death have become leading causes of death among PLWH [2]. The underlying mechanism for this divergence in CVD risk between PLWH and HIV-negative people remains poorly understood, but it has been tentatively attributed to HIV infection-related factors such as persistent immune activation, ART toxicity, and HIV itself [7]. Dyslipidemia, which is a major risk factor for CVD, is common among PLWH, even in middle-income and low-income countries [8] and could be responsible for the association between the two disorders. Accordingly, monitoring and controlling lipid levels as a means to prevent and control the occurrence of CVD in the HIV-positive population is crucial.
Several studies have shown that old protease inhibitors, nucleotide reverse transcriptase inhibitors, and non-nucleotide reverse transcriptase inhibitors are closely associated with increases in triglyceride (TG), total cholesterol (TC), and low-density lipoprotein cholesterol (LDL-C) and decrease in high-density lipoprotein cholesterol (HDL-C). Currently, a once-daily single-tablet regimen (STR) containing a integrase strand transfer inhibitor (INSTI) is recommend as first-line ART regimen for PLWH owing to its demonstrated durable virologic efficacy, favorable tolerability, toxicity profiles, and potential adherence advantage [9]. However, the established elvitegravir, cobicistat, emtricitabine, and tenofovir alafenamide (E/C/F/TAF) regime has been associated with weight gain and dyslipidemia [10]. Because of its ease of administration and fewer drug–drug interactions, bictegravir, emtricitabine, tenofovir alafenamide (B/F/TAF) has gradually replaced E/C/F/TAF as the primary STR [11]. It has been reported that B/F/TAF-treated PLWH show superior lipid profiles in comparison to those treated with E/C/F/TAF, but relevant clinical data is lacking, so this needs further confirmation [12]. However, conventional lipid biomarkers in clinics may not adequately reflex perturbations in overall lipid profiles or sufficiently reflect CVD risk in PLWH [8].
Lipid metabolism is thought to be closely associated with the onset and progression of several conditions such as CVD, diabetes, and non-alcoholic fatty liver disease (NAFLD) [13]. Clinical lipidomics is an emerging field that aims to identify multiple different plasma lipid species and measure their levels, providing a more comprehensive and sensitive means to detect potential changes in lipidomic profile [14]. The identification of these signatures could be applied to enumerate novel biomarkers and future intervention targets for disease treatment. A few published studies have explored the impact of HIV infection and ART on the aspects of lipid profiles that could be related to CVD [15, 16]. Wong et al. reported altered levels of 74 lipid species and 8 lipid classes including ceramide (Cer), diglyceride (DGs), phosphatidylinositol (PI) and triacylglycerol (TAG) et al. were significantly associated with future cardiovascular events in individuals with HIV [17]. However, prospective lipidomic data for PLWH are still scarce and further studies are required to explore the long-term effects of different ART regimens on lipid metabolism and underlying mechanisms associated with CVD risk. Accordingly, in the present study, we explored the effects of E/C/F/TAF and B/F/TAF on lipidomic profiles and the lipid subspecies associated with CVD.
Methods
Study Design and Participants
This is a prospective, single-center, observational clinical study conducted at National Clinical Research Center for Infectious Diseases Zhejiang, China. PLWH ≥ 18 years old receiving stable E/C/F/TAF therapy with HIV RNA < 50 copies/mL for ≥ 6 months and without other comorbidities or concomitant medications were recruited into the study. Participants were divided into E/C/F/TAF and B/F/TAF groups depending on whether they were switched to B/F/TAF during follow-up. The exclusion criteria included a history of severe medical conditions, the use of medications that may interfere with lipid metabolism (such as statins/fibrates, antidiabetic), and pregnancy during the study. Subjects who were lost to follow-up were also excluded. For full inclusion and exclusion criteria see supplemental Text 1. Written informed consent was obtained from all participants, and the protocol of this trial were approved by the Institutional Ethics Committee at the First Affiliated Hospital, Zhejiang University School of Medicine (approval number: ITT20220125B-R1). This study was registered with ClinicalTrials.gov (NCT06019273).
Procedures
This is a single-center cohort study of prospectively collected data. Before enrollment, demographic data [age, sex, body mass index (BMI), smoking habits], past medical history (particularly including history of hypertension, diabetes, and medication), and HIV-related factors (time since diagnosis, time on ART, time on E/C/F/TAF, prior treatments, comorbidities) were recorded. Clinical data collection and laboratory biochemical examination were also carried out before enrollment. Subjects who met the inclusion criteria but not the exclusion criteria were enrolled. Enrolled participants were systematically scheduled for a principal visit every 12 weeks, which included a comprehensive laboratory evaluation and the procurement of biological specimens. The initial 24 weeks post-enrollment were designated as the observation period. Individuals who switched to other than B/F/TAF ART regimen were excluded from the final analysis. Subjects maintaining their E/C/F/TAF regimen throughout the observational phase were allocated to the E/C/F/TAF cohort, with their enrollment date established as their baseline. Subjects opting for a B/F/TAF regimen during the observation period were assigned to the B/F/TAF cohort. Prior to regimen conversion, they underwent a visit for comprehensive evaluation and biological specimen collection, followed by scheduled visits every 12 weeks. The consultation preceding the regimen change was designated as the baseline for the B/F/TAF cohort. The visit point post 24 weeks of treatment with either E/C/F/TAF or B/F/TAF was marked as the end timepoint. The laboratory biochemical examination mainly included full blood count, liver/kidney function tests, and conventional metabolic assessments, i.e., TC, TG, HDL-C, LDL-C, fasting blood glucose (FBG), as well as CD4+ and CD8+ T-lymphocyte cell and HIV RNA counts. Plasma samples were collected in standardized conditions at the time of interview following a minimum of 8 h fasting and stored at −80 °C until analysis. Lipidomic samples preparation was shown in Supplementary Material Text 1. Lipidomic profiles were obtain at the baseline timepoint, 12 weeks posttreatment and end timepoint for both E/C/F/TAF and B/F/TAF groups.
Cardiovascular Disease Risk and Non-alcoholic Fatty Liver Disease Assessment
Based on age, sex, lipid levels, blood pressure, smoking, and presence of diabetes, the Framingham general cardiovascular risk score (FRS) was calculated to estimate 10-year CVD risk, with higher scores indicating higher risk of cardiovascular events [18]. According to previous studies, the hepatic steatosis index (HSI) was used as a surrogate marker for NAFLD. HSI = 8 × [alanine aminotransferase (ALT)/aspartate aminotransferase (AST)] + BMI + (2, if diabetes mellitus) + (2, if female), with values < 30 ruling out and values > 36 ruling in steatosis [19]. The cardiovascular disease risk and NAFLD assessment of the participants was performed at the baseline time point and end time point.
Lipidomic Data Acquisition and Analysis
A Dionex Ultimate 3000 RS ultrahigh performance liquid chromatography fitted with Q-Exactive quadrupole-Orbitrap mass spectrometer equipped with heated electrospray ionization (ESI) source (Thermo Fisher Scientific, Waltham, MA, USA) was used to analyze the metabolic profiling in both ESI positive and ESI negative ion modes. An ACQUITY UPLC BEH C18 column (1.7 μm, 2.1 mm × 100 mm) were employed in both positive and negative modes. The binary gradient elution system consisted of (A) acetonitrile: water (60:40, v:v, containing 10 mmol/L ammonium formate) and (B) acetonitrile: isopropanol (10:90, v:v, containing 10 mmol/L ammonium formate) and separation was achieved using the following gradient: 0 min, 5% B; 0.5 min, 5% B; 2 min, 43% B; 32.1 min, 52% B; 8.5 min, 53% B; 8.6 min,75% B;114 min, 90% B, 14.5in, 100% B, 15.5 min, 100% B, 15.7 min, 5% B and 18 min, 5% B. The flow rate was 0.4 mL/min and column temperature was 60 °C. All the samples were kept at 4 °C during the analysis. The injection volume was 5 μL. Positive samples: heater temperature was 350 °C, sheath gas flow rate was 50 arb, auxiliary gas flow rate 15 arb, sweep gas flow rate was 1 arb, spray voltage was 3.8 kV, capillary temperature was 320 °C, and S-lens RF level was 75%. MS1 scan ranged from 135 to 2000. Negative samples: heater temperature was 350 °C, sheath gas flow rate was 50 arb, auxiliary gas flow rate was 15 arb, sweep gas flow rate was 1 arb, spray voltage was 3.0 kV, capillary temperature was 320 °C, and S-lens RF level was 75%. MS1 scan ranged from 135 to 2000. The quality controls (QCs) were injected at regular intervals (every eight samples) throughout the analytical run to provide a set of data from which repeatability can be assessed. The original Q Exactive liquid chromatography mass spectrometry (LC–MS) data in raw format were processed by software Lipid Search for MSn and the exact mass-to-charge ratio (m/z) of parent ions was established. The molecular structure of lipids and the additive mode of its positive and negative ions were identified according to the parent ions and multi-stage mass spectrometry data of each individual sample. The results were aligned according to a certain retention time range and combined into a single report to sort out the original data matrix.
In each sample, all peak signals were normalized (that is, the signal intensity of each peak is converted to the relative intensity in the spectrum, and then multiplied by 10,000). The extracted data were then further processed by removing any peaks with a missing value (ion intensity of 0) in more than 50% in groups and by replacing the zero value by half of the minimum value. A data matrix was combined from the positive and negative ion data.
Statistical Analysis
Categorical variables were described as frequency (%) and continuous data were summarized with means [standard deviation (SD)] or median [interquartile range (IQR)]. For comparisons within-group and between groups, continuous and categorical variables were tested for statistical significance using t tests, Wilcoxon rank-sum tests, or chi-squared tests, as appropriate. For plasma lipidome data, principal component analysis (PCA) was used to observe the overall distribution among the samples and the stability of the whole analysis process. Orthogonal partial least-squares-discriminant analysis (OPLS-DA) and Student’s t test were utilized to identify the metabolites that differed between groups. Significantly altered metabolites were defined as those with variable importance in the projection (VIP) values > 1 in OPLS-DA analysis and two-tailed P values < 0.05 in univariate analysis. For comparisons between groups, those with |fold change| > 2 and P < 0.05 were considered differentially expressed lipid species. Short time-series expression miner (STEM) analysis [20] was used to investigate the expression tendency of lipid species. Profiles with P < 0.05 based on the number of metabolites clustered were considered significantly enriched clusters. Pearson correlation was used to measure the strength and direction of linear relationships between the significantly altered lipid species and clinical parameters. Multiple comparisons were performed by multivariable-adjusted analysis using a linear regression model. Statistical analyses were performed in SPSS 25.0 (IBM SPSS, Chicago, IL, USA) and R software (version 3.5.3). Multivariate modeling using OPLS-DA was performed using MetaboAnalyst 5.0 (www.metaboanalyst.ca). The graphical representations were based on the graphical environment of R, GraphPad Prism software (version 9.0), and TB tools (version 1.12) [21].
Results
Baseline Characteristics
Patient recruitment for this study commenced in November 2021 and concluded in January 2022. Fifty patients receiving stable E/C/F/TAF therapy were included in the study and were followed up every 12 weeks. Two participants switched to the dolutegravir (DTG)-based ART regimen (DTG + lamivudine AND DTG + rilpivirine) due to adverse effects (dyslipidemia and weight gain). Of the remaining 48 subjects screened and enrolled, 22 chose to switch to B/F/TAF regimen and 26 chose to continue on E/C/F/TAF treatment during the 1 year follow-up period. Three patients in the B/F/TAF arm and one in the E/C/F/TAF arm were lost to follow-up, leaving 44 participants for final analysis (Fig. 1).
Of the 44 subjects included in the study, 95.50% (n = 42) were male, the median age was 32.00 (IQR 27.00–37.00), and the mean FRS at baseline was low (3.44 SD 4.39). The median time since HIV diagnosis and cumulative E/C/F/TAF exposure was 4.00 (2.00–7.00) years and 15.00 (7.00–22.00) months, respectively. Participant demographics and baseline characteristics were not different statistically and comparable across the two study groups (Table 1).
Effect of Switching from E/C/F/TAF to B/F/TAF: Decreased TG and CVD Risk
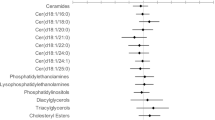
At baseline, no differences of serum lipid profile and other conventional biochemical parameters were observed between groups. After 6 months of follow-up, switching to B/F/TAF was found to be associated with decreases in TG and VLDL-C levels, whereas increases in TG and VLDL-C levels were observed for continued use of E/C/F/TAF (Fig. 2, Table S1). At week 24, compared with the E/C/F/TAF group, TG and VLDL-C were significantly lower in the B/F/TAF group (mean difference (md) 0.80 mmol/L, 95% confidence interval (CI) 0.37–1.23, P = 0.001 for TG; md 0.20 mmol/L, 95% CI 0.13–0.38, P = 0.037 for VLDL-C). Switching to B/F/TAF was associated with a TC reduction of 0.42 mmol/L (95% CI 0.08–0.77, P = 0.019) after 24 weeks, compared with 0.22 mmol/L (95% CI 0.02–0.43, P = 0.048) with the continuous use of E/C/F/TAF (between-group difference 0.011, 95% CI 0.54–0.57, P = 0.968). However, a statistically significant reduction in LDL-C was observed in the E/C/F/TAF group at week 24 (md 0.23 mmol/L, 95% CI 0.04–0.42, P = 0.023), while no statistically significant decrease in LDL-C was found in the switched group (md 0.25 mmol/L, 95% CI −0.04 to 0.52, P = 0.083). The HDL-C and TC/HDL-C ratio exhibited a non-significant reduction from baseline in both groups to a comparable degree. Additionally, after 24 weeks, uric acid and total bilirubin levels were increased significantly in the E/C/F/TAF and B/F/TAF groups, respectively (Fig. S1, Table S1).
Changes in plasma lipid concentrations after 24 weeks of treatment. Data are presented as mean ± SEM. *P < 0.05; **P < 0.01; ***P < 0.001. TG triglyceride, TC total cholesterol, HDL-C high-density lipoprotein cholesterol, LDL-C low-density lipoprotein cholesterol, VLDL-C very-low-density lipoprotein cholesterol, W week
Despite the low baseline mean FRS score levels in the study population, patients who switched to B/F/TAF regimen experienced a pronounced reduction after 24 weeks of follow-up (md 0.41, 95% CI 0.02–0.81, P = 0.043) and a mild increase of FRS was observed in the E/C/F/TAF group over the same time, although the difference was not statistically significant.
No apparent changes in HSI were detected in either group of participants during the follow-up period. However, the incidence of NAFLD, identified by HSI, was mildly elevated in the E/C/F/TAF group (8.0% versus 20.0%), whereas no significant changes were seen in the B/F/TAF group throughout the follow-up period (Table S1). At week 24, the prevalence of NAFLD was not significantly different between the two groups (20.0% versus 26.3%, P = 0.620).
Lipidomic Profile Alteration is Associated with CVD Risk in the E/C/F/TAF Group but not the B/F/TAF Group
A total of 1093 lipid species including ceramide (Cer), diglyceride (DG), lyso-phosphatidylcholine (LPC), phosphatidylcholine (PC), phosphatidylethanolamine (PE), phosphatidylinositol (PI), phosphatidylserine (PS), sphingomyelin (SM), triacylglycerol (TAG), and others were identified and subjected to statistical analysis (Fig. S2). We first applied a time-series analysis to understand the impact of using E/C/F/TAF or B/F/TAF on plasma lipid metabolism. It identified seven and four significant lipidomic profiles with different expression behaviors in the two groups, respectively, during follow-up (Fig. S3). Combined with univariate analysis (Tables S2–5), it showed different end-point effects on lipid metabolism according to different ARTs (Fig. 3A–C). Levels of four DGs [DG (16:0/18:2), DG (16:1/18:3), DG (18:3/18:2), DG (20:5/18:2)] and three LPCs [LPC (20:4), LPC (22:1), LPC (22:6)] were significantly elevated in the E/C/F/TAF group. Nevertheless, the B/F/TAF group revealed a substantially greater change in plasma lipid species with the significant increase in one fatty acid (FA) [FA (19:3)], one O-acyl-(gamma-hydroxy) fatty acid [OAHFA (38:2)], one digalactosyl diacylglycerol [DGDG (16:0/18:2)], six LPCs, one lyso-phosphatidyl ethanolamine (LPE) [LPE (16:0p)], five PCs, one phosphatidylglycerol [PG (40:2)], one PI [PI (24:3/16:0)], five Cers, one phytosphingosine [phSM(d42:2)], four SMs, six sphingoshines (So), and a constant decreases in Cer (d18:1/24:1) and PI (18:0/20:3) (Fig. 3C).
Plasma lipidomic alterations associated with different antiretroviral therapies. Venn diagram displaying the number of significantly differentially expressed lipid species during the 24 weeks of treatment with E/C/F/TAF (A) and B/F/TAF (B). Set_1 is the significantly differentially expressed metabolites identified by time series analysis during the 24-week follow-up (FDR < 0.05). Set_2 is the significantly changed metabolites between week 0 and week 12 (P < 0.05, VIP > 1), and Set_3 is the significantly changed metabolites between week 0 and week 24 (P < 0.05, VIP > 1). The 7 and 35 overlapped metabolites were indicated in the two groups, respectively. C Heatmap of the significantly changed metabolites in the plasma of the participants. The abundance of each metabolite was normalized by Z score normalization. The lipid species class is shown on the left, and the group information is annotated above the heatmap. D–G. Linear regression plots showing correlations of FRS with different lipid species levels. r Pearson correlation coefficient, black dotted lines: linear regressions curves. FRS Framingham general cardiovascular risk score, Cer ceramide, DG diglyceride, DGDG digalactosyl diacylglycerol, FA fatty acid, LPC lyso-phosphatidylcholine, LPE lyso-phosphatidyl ethanolamine, OAHFA O-acyl-(gamma-hydroxy) fatty acid, PC phosphatidylcholine, PG phosphatidylglycerol, phSM phytosphingosine, PI phosphatidylinositol, SM sphingomyelin, SO sphingoshine, TAG triacylglycerol
Among all the participants, these 42 significantly altered lipid species were highly associated with conventional biochemical parameter levels (Fig. S4). Levels of DGs, elevated in the E/C/F/TAF group, were observed to be positively correlated with FBG, TG, and VLDL-C; and negatively correlated with HDL-C. However, Cers, LPCs, SMs, and Sos, which were increased significantly in the B/F/TAF group, showed completely opposite correlations with conventional biochemical parameters. Interestingly, the levels of DGs, Cers, LPCs, and Sos were significantly associated with FRS and HSI (Fig. S4). When adjusted for age, sex and BMI, TC, and HDL-C, the levels of DG (16:0/18:2), DG (18:3/18:2), DG (20:5/18:2), and LPC (22:6) were independently positively correlated with FRS (r = 0.348–0.523, P < 0.05) (Fig. 3D–G, Table S6).
Switching to B/F/TAF Shows Less Effect on TAGs and DGs
To further explore the lipid metabolism in response to different ARTs, the fold changes (FCs) and P values were calculated for comparison between groups. The FCs for E/C/F/TAF and B/F/TAF at the 24-week time point (after normalization to baseline levels) are displayed in Fig. 4A and Table S7. DG (18:2/22:6), DG (20:5/18:2), and TAGs, especially TAG (18:1/22:0/22:6), TAG (18:3/18:2/21:5), and TAG (20:5/18:2/22:6) were significantly altered in the E/C/F/TAF group compared to the levels in the B/F/TAF group (FC 2.57–252.53, P < 0.05). In addition, at 24 weeks, switching from E/C/F/TAF to B/F/TAF was associated with a significant increases in some PEs [PE (38:4e), PE (42:2e), and PE (20:0p/22:6)].
Distinct impacts of E/C/F/TAF and B/F/TAF on lipid metabolism. A Volcano plot showing the significantly differentially expressed metabolites in the two groups (|fold change| > 2 and P < 0.05 by Student’s t test). B Heat map correlations between differential lipid species inter-groups and conventional biochemical parameters. FC fold change, NS non-significance, DG diglyceride, PE phosphatidylethanolamine, PG phosphatidylglycerol, TAG triacylglycerol, ALP alkaline phosphatase, ALT alanine aminotransferase, AST aspartate aminotransferase, FBG fasting blood glucose, FRS Framingham risk score, GFR glomerular filtration rate, γ-GT gamma-glutamyl transferase, HDL-C high-density lipoprotein cholesterol, HSI hepatic steatosis index, LDL-C low-density lipoprotein cholesterol, Scr serum creatinine, TC total cholesterol, TG triglyceride, UA uric acid, VLDL-C very low-density lipoprotein cholesterol. *P < 0.05; **P < 0.01; ***P < 0.001
Correlation of differentially expressed metabolites inter-group and clinical indicators are shown in Fig. 4B. DGs and TAGs, significantly elevated in E/C/F/TAF group, showed slight positive associations with TG, VLDL-C, FRS, and HSI, but not with LDL-C (Fig. 4B). After adjusting for age, sex and BMI, TC, HDL-C, the levels of DG (18:2/22:6), DG (20:5/18:2), TAG (18:3/18:2/21:5), and TAG (20:5/18:2/22:6) remained significantly correlated with FRS (Table S7). Furthermore, no correlation was found between FRS and PE (38:4e), PE (42:2e), and PE (20:0p/22:6).
Discussion
As a second-generation STR, B/F/TAF is recommended as a preferable ART regimen for PLWH [22]. B/F/TAF, unlike E/C/F/TAF, seems to have little effect on blood lipids [23]. However, longitudinal studies comparing the effects of B/F/TAF and E/C/F/TAF on blood lipids are scarce. In this prospective study, we investigate changes in the conventional blood lipid and lipidomic profiles of PLWH treated with E/C/F/TAF and B/F/TAF. For conventional blood lipids analysis, the levels of TG and VLDL at week 24 were better in the B/F/TAF group, while the levels of TC, LDL-C, and HDL-C, as well as the TC/HDL-C ratio at week 24 were not significantly different between the two groups. Additionally, FRS decreased after switching to the B/F/TAF regimen.
Our non-targeted lipidomic approach revealed that E/C/F/TAF exerted a particular effect on glycerolipids and glycerophospholipids, particularly the upregulation of DGs, TAGs, and LPCs, which are associated with FRS. In the B/F/TAF group, significant increases were observed for sphingolipids and glycerophospholipids, which were found to be inversely related to CVD risk. Finally, our fully adjusted analyses identified seven lipid species, i.e., DG (16:0/18:2), DG (18:2/22:6), DG (18:3/18:2), DG (20:5/18:2), TAG (18:3/18:2/21:5), TAG (20:5/18:2/22:6), and LPC (22:6), that were significantly associated with future CVD risk, independent of clinical factors.
Previous studies have demonstrated that E/C/F/TAF is associated with increased TG, TC, LDL-C, and HDL-C [24] and that switching from E/C/F/TAF to B/F/TAF is strongly related to blood lipid improvement [12]. However, this blood lipid improvement was only observed in a cohort of women living with HIV. It was well known that blood lipid levels vary significantly between genders and ethnicities. To the best of our knowledge, this study is the first prospective cohort to observe changes in blood lipid levels and lipidomic profiles after switching from E/C/F/TAF to B/F/TAF in an Asian population.
In this study, it was observed that switching to B/F/TAF is associated with lower TG and VLDL-C levels, which is in line with previous reports [12]. It is important to note, however, that LDL-C, which is considered a conventional cardiovascular risk factor, was decreased in the E/C/F/TAF group in our study, which is different from the results of a previous study [10]. This could be explained by the short follow-up duration (24 weeks), low median age (32.00, IQR 27.00–37.00), and small sample size (44 patients). Furthermore, it has been demonstrated that conventional blood lipid analysis including that of LDL-C cannot encompass perturbations in overall lipid profiles and may not adequately assess the heterogeneity in interindividual atherogenic vulnerability [25]. In some cases, lower LDL-C may not be associated with improved cardiovascular outcomes [26]. Consistent with previous work, it was observed in our study that despite the reduction of LDL-C levels in the E/C/F/TAF group, individual lipid species associated with CVD risk are persistently elevated during the follow-up.
Prospective data from previous studies show that the mortality of PLWH from liver disease was second only to AIDS-related mortality, and NAFLD could soon emerge as the most common liver disease in PLWH [27, 28]. Liver biopsy is the gold standard for diagnosis of NAFLD, but it is difficult to perform in clinical practice. Hence, in the present study, the HSI scoring system, based on BMI, ALT/AST ratio, gender, and history of diabetes, was used to evaluate and identify NAFLD in the participants [19]. In our study, incidence of NAFLD was elevated in the E/C/F/TAF group, although this difference was not statistically significant. Previous studies have demonstrated that the development of NAFLD is a long-term and multiple-step process [29]; hence, it is necessary to be alert to the adverse effects of E/C/F/TAF on the development of NAFLD. Notably, although effective for predicting hepatic steatosis in the general populations, HSI has not been extensively validated in other populations, such as PLWH. Therefore, our findings are exploratory, and further studies with larger sample sizes and more sensitive and accurate NAFLD diagnosis methods are required. In addition, in this study, FRS was applied to predict 10-year cardiovascular risk. Switching to B/F/TAF was found to be associated with decreased FRS, whereas FRS was found to increase mildly in the E/C/F/TAF group, indicating that B/F/TAF instead of E/C/F/TAF could be related to cardiovascular risk reduction. In a prospective study involving 2283 PLWH, FRS was found to accurately reflect risk of CVD events [30]. Thus, we have reason to believe that switching to B/F/TAF reduces cardiovascular risk in PLWH.
To investigate the long-term effects of different ART regimen on lipid metabolism, detailed lipidomic analysis was used to identify the specific lipid species that are significantly altered over 24 weeks treatment. In the longitudinal analysis, treatment with E/C/F/TAF was accompanied by a persistent elevation of several DGs and LPCs, while switching to B/F/TAF appeared to induce a substantially greater change in plasma lipid species, with increased levels of Cers, LPCs, PCs, PGs SMs, and Sos. We then made a horizontal comparison at week 24 to assess the lipid metabolism characteristics of the different ART regimens. It was observed that in comparison with E/C/F/TAF, B/F/TAF seems to have less effect on DGs (FC 0.004–0.389) and TAGs (FC 0.070–0483). Additionally, we identified significant associations of multiple TAGs and DGs with future CVD risk in this prospective cohort, which is in accordance with previous studies [31,32,33]. Notably, however, several DGs and TAGs such as DG (16:0/18:2), DG (18:2/22:6), DG (18:3/18:2), DG (20:5/18:2), TAG (18:3/18:2/21:5), and TAG (20:5/18:2/22:6) were newly identified to be associated with CVD risk after fully adjusted analyses. LPCs have been previously proposed as possible proinflammatory and proatherosclerotic lipids [34]. Further lipidomic research has revealed that the fatty acid composition of LPC particles plays a crucial role in their function. LPCs containing saturated fatty acids (SaFAs) are proinflammatory, while LPCs containing polyunsaturated fatty acids (PUFAs) are antiinflammatory [34]. Elevated circulating SaFAs are associated with a greater risk of CVD, whereas increased PUFA-containing LPCs appear to be associated with a lower risk of CVD and mortality in PLWH [35, 36]. In addition, Chai et al. found that lipid species associated with the risk of carotid artery plaque in LPCs have lower carbon numbers and double-bond contents, which is in line with our results [37]. In this study, LPC (34:2), LPC (34:3), and LPC (38:5), increased in the B/F/TAF group, were negatively associated with FRS. However, LPC (22:6), a PUFA-containing LPC with six double bonds, was found to be positively correlated with FRS, and the association remains robust when adjusting for age, sex, BMI, TC, and HDL-C. Further work is required to assess the relationship between LPC levels and CVD risk.
Sphingolipids and especially Cers are important bioactive lipids involved in atherosclerosis, and their predictive value for cardiovascular risk is superior to that of LDL-C [38, 39]. In the present study, Cer (d18:1/24:1) was found to increase with B/F/TAF treatment and to be positively associated with FRS, which is consistent with previous results in PLWH [40]. Distinct from previous related studies, Cer (d30:0), Cer (d32:0), Cer (d33:0), Cer (d36:0), and Cer (d38:0) were inversely correlated with FRS. This difference may be explained by the distinct fatty acid compositions of Cer particles and requires further study. Sphingoshines, another sphingolipid increased in the B/F/TAF group, was observed to be upregulated in PLWH despite receiving ART treatment [41]. Sphingoshines act as first and second messengers, playing a crucial role in regulating pathobiological processes, such as cancer, inflammation, and infectious diseases [42]. However, the relationship between sphingoshines and clinical cardiovascular disease remains poorly defined and warrants further investigation.
Together, our data show significant differences in lipid metabolism between the two groups, but the reason for this discrepancy remains obscure. Although TAF-containing regimens were previously thought to be related to a worsening of lipid profile, TAF exposure is comparable for the E/C/F/TAF and B/F/TAF groups due to the inhibitory effect of cobicistat on p-glycoprotein (P-gp) and breast cancer resistance protein (BCRP) transporters [43]. Cobicistat, a pharmacological booster, shows a lower potential for worsening lipid metabolism compared with ritonavir, but further investigation into the effects of cobicistat on lipid dysregulation is warranted [44].
To our knowledge, this is the first prospective lipidomic study that has identified lipid species that may be related to a better metabolism profile and lower CVD risk with the use of B/F/TAF rather than E/C/F/TAF. However, our study does have some limitations. First, the study was limited by a small sample size and residual confounding biases. Nevertheless, we selected stable PLWH with no other comorbidities or medications that might have an impact on the metabolic profile and to eliminate heterogeneity within the sample. Additionally, there were no significant demographic, clinical, or laboratory differences at baseline between the two groups.
Second, the study was limited by a short duration of follow-up and a lack of incidence of CVD events. Previous studies have indicated that significant alterations of lipidomic can happen in a short time frame [45], and in this study, STEM analysis was used to identified continuously changing metabolites. Although the FRS, a well-recognized CVD risk-assessment tool, was used to estimate the future risk of CVD for PLWH, we have to admit that direct measurement of CVD events is the gold standard for assessing cardiovascular risk. Therefore, longer-term studies to evaluate how alterations in lipidomic profiles may influence the incidence of actual CVD events are required.
Third, the study population was mainly middle-aged Chinese men, preventing extrapolation of our findings to other populations. We acknowledge that this gender distribution may introduce a bias in interpreting the impact of ART on lipidomic profile changes and its subsequent cardiovascular risk implications. Men and women exhibit significant physiological and metabolic differences that can influence the impact of ART on lipid profiles. For instance, differences in sex hormone levels can affect lipid metabolism, with women typically having higher levels of HDL-C and a lower risk of cardiovascular diseases [46]. In addition, gender may influence individuals’ responses to ART, including drug metabolism rates, the incidence of side effects, and treatment efficacy [47]. In future research endeavors, it is necessary to increase the sample sizes and ensure a more balanced representation of gender ratios within study populations so as to enhance the applicability and generalizability of our findings.
Finally, even though over a thousand individual lipids were identified in this study, the non-targeted lipidomic approach was restricted to lipid compounds known in the database, possibly overlooking some interesting but uncharacterized compounds. Furthermore, the levels of lipid species were semi-quantified without absolute values, and thus a targeted lipidomic on specific interesting lipid species is required to better understand the underlying biological mechanisms.
Conclusions
In summary, this lipidomic study identified a significant distinction in lipid metabolism between PLWH on E/C/F/TAF and those on B/F/TAF regimens. The different alteration lipid profiles in participants between the groups suggests that E/C/F/TAF may promote DG, TAG, and LPC accumulation, which is closely associated with future CVD risk, whereas B/F/TAF has a beneficial effect on lipid profile and is associated with CVD risk reduction. In addition, we newly identified a range of individual lipid species associated with risk of future CVD, independent of clinical factors.
Overall, our findings provide potential opportunities for new intervention strategies (e.g., switching ART regimen, lifestyle, drugs) to prevent or mitigate CVD progression by modifying lipid metabolism. Further exploration with larger cohorts is required to validate our preliminary findings and determine the exact role of the lipid species in CVD.
Data Availability
The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.
References
Rodger AJ, Lodwick R, Schechter M, et al. Mortality in well controlled HIV in the continuous antiretroviral therapy arms of the SMART and ESPRIT trials compared with the general population. AIDS (London, England). 2013;27(6):973–9. https://doi.org/10.1097/QAD.0b013e32835cae9c.
Croxford S, Kitching A, Desai S, et al. Mortality and causes of death in people diagnosed with HIV in the era of highly active antiretroviral therapy compared with the general population: an analysis of a national observational cohort. Lancet Public Health. 2017;2(1):e35–46. https://doi.org/10.1016/s2468-2667(16)30020-2.
Deeks SG, Lewin SR, Havlir DV. The end of AIDS: HIV infection as a chronic disease. Lancet (London, England). 2013;382(9903):1525–33. https://doi.org/10.1016/s0140-6736(13)61809-7.
Guaraldi G, Milic J, Mussini C. Aging with HIV. Curr HIV/AIDS Rep. 2019;16(6):475–81. https://doi.org/10.1007/s11904-019-00464-3.
Hanna DB, Post WS, Deal JA, et al. HIV infection is associated with progression of subclinical carotid atherosclerosis. Clin Infect Dis Off Publ Infect Dis Soc Am. 2015;61(4):640–50. https://doi.org/10.1093/cid/civ325.
Freiberg MS, Chang CC, Kuller LH, et al. HIV infection and the risk of acute myocardial infarction. JAMA Intern Med. 2013;173(8):614–22. https://doi.org/10.1001/jamainternmed.2013.3728.
So-Armah K, Benjamin LA, Bloomfield GS, et al. HIV and cardiovascular disease. Lancet HIV. 2020;7(4):e279–93. https://doi.org/10.1016/s2352-3018(20)30036-9.
Friis-Møller N, Ryom L, Smith C, et al. An updated prediction model of the global risk of cardiovascular disease in HIV-positive persons: the Data-collection on Adverse Effects of Anti-HIV Drugs (D:A:D) study. Eur J Prev Cardiol. 2016;23(2):214–23. https://doi.org/10.1177/2047487315579291.
[Chinese guidelines for diagnosis and treatment of HIV/AIDS (2021 edition)]. Zhonghua nei ke za zhi. 2021;60(12):1106–28. https://doi.org/10.3760/cma.j.cn112138-20211006-00676.
Kuo PH, Sun HY, Chuang YC, Wu PY, Liu WC, Hung CC. Weight gain and dyslipidemia among virally suppressed HIV-positive patients switching to co-formulated elvitegravir/cobicistat/emtricitabine/tenofovir alafenamide. Int J Infect Dis IJID Off Publ Int Soc Infect Dis. 2020;92:71–7. https://doi.org/10.1016/j.ijid.2019.12.029.
Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the Use of Antiretroviral Agents in Adults and Adolescents with HIV. Department of Health and Human Services. https://clinicalinfo.hiv.gov/en/guidelines/adult-and-adolescent-arv.
Kityo C, Hagins D, Koenig E, et al. Switching to fixed-dose bictegravir, emtricitabine, and tenofovir alafenamide (B/F/TAF) in virologically suppressed HIV-1 infected women: a randomized, open-label, multicenter, active-controlled, phase 3, noninferiority trial. J Acquir Immune Defic Syndr (1999). 2019;82(3):321–8. https://doi.org/10.1097/qai.0000000000002137.
Meikle TG, Huynh K, Giles C, Meikle PJ. Clinical lipidomics: realizing the potential of lipid profiling. J Lipid Res. 2021;62: 100127. https://doi.org/10.1016/j.jlr.2021.100127.
Lam SM, Shui G. Lipidomics as a principal tool for advancing biomedical research. J Genet Genom Yi chuan xue bao. 2013;40(8):375–90. https://doi.org/10.1016/j.jgg.2013.06.007.
Jao J, Balmert LC, Sun S, et al. Distinct lipidomic signatures in people living with HIV: combined analysis of ACTG 5260s and MACS/WIHS. J Clin Endocrinol Metab. 2022;107(1):119–35. https://doi.org/10.1210/clinem/dgab663.
Mena A, Clavero E, Díaz-Díaz JL, Castro A. Similar plasma lipidomic profile in people living with HIV treated with a darunavir-based or an integrase inhibitor-based antiretroviral therapy. Sci Rep. 2019;9(1):17184. https://doi.org/10.1038/s41598-019-53761-7.
Wong G, Trevillyan JM, Fatou B, et al. Plasma lipidomic profiling of treated HIV-positive individuals and the implications for cardiovascular risk prediction. PLoS One. 2014;9(4): e94810. https://doi.org/10.1371/journal.pone.0094810.
D’Agostino RB Sr, Vasan RS, Pencina MJ, et al. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation. 2008;117(6):743–53. https://doi.org/10.1161/circulationaha.107.699579.
Lee JH, Kim D, Kim HJ, et al. Hepatic steatosis index: a simple screening tool reflecting nonalcoholic fatty liver disease. Digest Liver Dis Off J Ital Soc Gastroenterol Ital Assoc Study Liver. 2010;42(7):503–8. https://doi.org/10.1016/j.dld.2009.08.002.
Ernst J, Bar-Joseph Z. STEM: a tool for the analysis of short time series gene expression data. BMC Bioinform. 2006;7:191. https://doi.org/10.1186/1471-2105-7-191.
Chen C, Chen H, Zhang Y, et al. TBtools: an integrative toolkit developed for interactive analyses of big biological data. Mol Plant. 2020;13(8):1194–202. https://doi.org/10.1016/j.molp.2020.06.009.
European AIDS Clinical Society. EACS guidelines (Version 11.1). (2022–10). https://www.eacsociety.org/guidelines/eacs-guidelines/.
Molina JM, Ward D, Brar I, et al. Switching to fixed-dose bictegravir, emtricitabine, and tenofovir alafenamide from dolutegravir plus abacavir and lamivudine in virologically suppressed adults with HIV-1: 48 week results of a randomised, double-blind, multicentre, active-controlled, phase 3, non-inferiority trial. Lancet HIV. 2018;5(7):e357–65. https://doi.org/10.1016/s2352-3018(18)30092-4.
Sax PE, Wohl D, Yin MT, et al. Tenofovir alafenamide versus tenofovir disoproxil fumarate, coformulated with elvitegravir, cobicistat, and emtricitabine, for initial treatment of HIV-1 infection: two randomised, double-blind, phase 3, non-inferiority trials. Lancet (London, England). 2015;385(9987):2606–15. https://doi.org/10.1016/s0140-6736(15)60616-x.
Nurmohamed NS, Kraaijenhof JM, Mayr M, et al. Proteomics and lipidomics in atherosclerotic cardiovascular disease risk prediction. Eur Heart J. 2023;44(18):1594–607. https://doi.org/10.1093/eurheartj/ehad161.
Crowson CS, Rollefstad S, Ikdahl E, et al. Impact of risk factors associated with cardiovascular outcomes in patients with rheumatoid arthritis. Ann Rheum Dis. 2018;77(1):48–54. https://doi.org/10.1136/annrheumdis-2017-211735.
Joshi D, O’Grady J, Dieterich D, Gazzard B, Agarwal K. Increasing burden of liver disease in patients with HIV infection. Lancet (London, England). 2011;377(9772):1198–209. https://doi.org/10.1016/s0140-6736(10)62001-6.
Verna EC. Non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in patients with HIV. Lancet Gastroenterol Hepatol. 2017;2(3):211–23. https://doi.org/10.1016/s2468-1253(16)30120-0.
** J, Valanejad L, Nguyen TP, et al. Activation of CDK4 triggers development of non-alcoholic fatty liver disease. Cell Rep. 2016;16(3):744–56. https://doi.org/10.1016/j.celrep.2016.06.019.
Thompson-Paul AM, Lichtenstein KA, Armon C, et al. Cardiovascular disease risk prediction in the HIV outpatient study. Clin Infect Dis Off Publ Infect Dis Soc Am. 2016;63(11):1508–16. https://doi.org/10.1093/cid/ciw615.
Wang Z, Peters BA, Usyk M, et al. Gut microbiota, plasma metabolomic profiles, and carotid artery atherosclerosis in HIV infection. Arterioscler Thromb Vasc Biol. 2022;42(8):1081–93. https://doi.org/10.1161/atvbaha.121.317276.
Stegemann C, Drozdov I, Shalhoub J, et al. Comparative lipidomics profiling of human atherosclerotic plaques. Circ Cardiovasc Genet. 2011;4(3):232–42. https://doi.org/10.1161/circgenetics.110.959098.
Stegemann C, Pechlaner R, Willeit P, et al. Lipidomics profiling and risk of cardiovascular disease in the prospective population-based Bruneck study. Circulation. 2014;129(18):1821–31. https://doi.org/10.1161/circulationaha.113.002500.
Akerele OA, Cheema SK. Fatty acyl composition of lysophosphatidylcholine is important in atherosclerosis. Med Hypotheses. 2015;85(6):754–60. https://doi.org/10.1016/j.mehy.2015.10.013.
Sacks FM, Lichtenstein AH, Wu JHY, et al. Dietary fats and cardiovascular disease: a presidential advisory from the American Heart Association. Circulation. 2017;136(3):e1–23. https://doi.org/10.1161/cir.0000000000000510.
Bowman ER, Kulkarni M, Gabriel J, et al. Altered lipidome composition is related to markers of monocyte and immune activation in antiretroviral therapy treated human immunodeficiency virus (HIV) infection and in uninfected persons. Front Immunol. 2019;10:785. https://doi.org/10.3389/fimmu.2019.00785.
Chai JC, Deik AA, Hua S, et al. Association of lipidomic profiles with progression of carotid artery atherosclerosis in HIV infection. JAMA Cardiol. 2019;4(12):1239–49. https://doi.org/10.1001/jamacardio.2019.4025.
Jiang XC, Paultre F, Pearson TA, et al. Plasma sphingomyelin level as a risk factor for coronary artery disease. Arterioscler Thromb Vasc Biol. 2000;20(12):2614–8. https://doi.org/10.1161/01.atv.20.12.2614.
Tarasov K, Ekroos K, Suoniemi M, et al. Molecular lipids identify cardiovascular risk and are efficiently lowered by simvastatin and PCSK9 deficiency. J Clin Endocrinol Metab. 2014;99(1):E45-52. https://doi.org/10.1210/jc.2013-2559.
Zhao W, Wang X, Deik AA, et al. Elevated plasma ceramides are associated with antiretroviral therapy use and progression of carotid artery atherosclerosis in HIV infection. Circulation. 2019;139(17):2003–11. https://doi.org/10.1161/circulationaha.118.037487.
Lao D, Liu R, Liang J. Study on plasma metabolomics for HIV/AIDS patients treated by HAART based on LC/MS-MS. Front Pharmacol. 2022;13: 885386. https://doi.org/10.3389/fphar.2022.885386.
Wu Y, Liu Y, Gulbins E, Grassmé H. The anti-infectious role of sphingosine in microbial diseases. Cells. 2021. https://doi.org/10.3390/cells10051105.
Greig SL, Deeks ED. Elvitegravir/cobicistat/emtricitabine/tenofovir alafenamide: a review in HIV-1 infection. Drugs. 2016;76(9):957–68. https://doi.org/10.1007/s40265-016-0586-z.
Xu L, Liu H, Murray BP, et al. Cobicistat (GS-9350): a potent and selective inhibitor of human CYP3A as a novel pharmacoenhancer. ACS Med Chem Lett. 2010;1(5):209–13. https://doi.org/10.1021/ml1000257.
Köfeler HC, Ahrends R, Baker ES, et al. Recommendations for good practice in MS-based lipidomics. J Lipid Res. 2021;62: 100138. https://doi.org/10.1016/j.jlr.2021.100138.
Park JH, Lee MH, Shim JS, et al. Effects of age, sex, and menopausal status on blood cholesterol profile in the Korean population. Korean Circ J. 2015;45(2):141–8. https://doi.org/10.4070/kcj.2015.45.2.141.
Scully EP. Sex differences in HIV infection. Curr HIV/AIDS Rep. 2018;15(2):136–46. https://doi.org/10.1007/s11904-018-0383-2.
Acknowledgements
The authors would like to thank staff on the study team and all volunteers.
Funding
This work was supported by the National Key R and D Program of China [grant number 2022YFC2305202] and the medical and health research youth innovation project of Zhejiang province [2023RC164]. The journal’s rapid service fee was funded by the National Key R and D Program of China [grant number 2022YFC2305202].
Author information
Authors and Affiliations
Contributions
Biao Zhu contributed to the conception and design of the study. Zhikai Wan, Junwei Su, Xueling Zhu, and **ang Liu contributed to the acquisition and analyses of data. Zhikai Wan contributed to drafting the text, preparing the tables and figures. Yongzheng Guo, Dairong **ang, **aotang Zhou, **aorong Peng, Ran Tao, Qing Cao, Guan**g Lang, and Ying Huang contributed to sample collection and sample management. All authors read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
Zhikai Wan, Junwei Su, Xueling Zhu, **ang Liu, Yongzheng Guo, Dairong **ang, **aotang Zhou, **aorong Peng, Ran Tao, Qing Cao, Guan**g Lang, Ying Huang, and Biao Zhu declare declare there are no conflicts of interest with respect to this research study and paper.
Ethical Approval
This study was performed in line with the principles of the Declaration of Helsinki. The trial protocol was granted by the Ethics Committee of The First Affiliated Hospital, Zhejiang University School of Medicine (Approval Number: ITT20220125B-R1). Informed consent was obtained from all individual participants included in the study. This study was registered with ClinicalTrials.gov (NCT06019273).
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Wan, Z., Su, J., Zhu, X. et al. Distinct Lipidomic Profiles between People Living with HIV Treated with E/C/F/TAF or B/F/TAF: An Open-Label Prospective Cohort Study. Infect Dis Ther 13, 727–744 (2024). https://doi.org/10.1007/s40121-024-00943-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40121-024-00943-0