Abstract
Introduction
Persons with Parkinson’s disease (PwPD) suffer from motor and non-motor symptoms which significantly affect their quality of life (QoL), and the QoL of their care partners (CP). Tandem cycling reduces PwPD motor symptoms; however, no studies have examined other benefits or included PwPD CP. We conducted an 8-week community virtual reality (VR) tandem cycling intervention to assess the feasibility and efficacy for PwPD and their CP (i.e., PD dyads). We hypothesized that dyadic tandem cycling would improve (1) PwPD motor and non-motor symptoms and (2) dimensions of PD dyads’ QoL and physiologic health.
Methods
Ten PD dyads were recruited to complete 8 weeks of progressive intensity, bi-weekly tandem cycling. At pre- and post-testing, PwPD were assessed using the Movement Disorder Society–Unified Parkinson’s Disease Rating Scale-III (MDS-UPDRS-III), functional gait assessment (FGA), and 10-m gait speed test. PD dyads also completed emotional and cognitive status questionnaires [e.g., Geriatric Depression Scale-Short Form (GDS-SF)], and wore BodyGuard 2 heart rate (HR) monitors for 48 h to assess surrogate measures of heart rate variability. Statistical analyses were conducted using Student’s t tests with significance set at p ≤ 0.05.
Results
Eight PD dyads and one PwPD completed the intervention. Retention of PwPD (90%) and CP (80%) was adequate, and PD dyad adherence ranged from 91.67 to 97.91%. PwPD demonstrated significant clinical improvements in MDS-UPDRS-III scores (− 7.38, p < 0.01), FGA scores (+ 3.50, p < 0.01), and 10-m gait speed times (+ 0.27 m/s, p < 0.01), in addition to significant self-reported improvements in mobility (− 13.61, p = 0.02), fatigue (− 5.99, p = 0.02), and social participation (+ 4.69, p < 0.01). CP depressive symptoms significantly decreased (− 0.88, p = 0.02), and PD dyads shared a significant increase in root mean square of the successive differences (RMSSD; p = 0.04).
Conclusion
Our pilot study demonstrated feasibility and multiple areas of efficacy supporting further investigation of community VR tandem cycling as a therapeutic intervention for PD dyads.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Tandem cycling has been demonstrated to reduce persons with Parkinson’s disease (PwPD) motor symptoms; however, no studies have examined other benefits or included PwPD care partners (CP), who are equally affected by their partners’ disease. |
We hypothesized an 8-week dyadic tandem cycling program for PwPD and their CP (i.e., PD dyads) would be feasible, and would improve (1) PwPD motor and non-motor symptoms and (2) dimensions of PD dyads’ quality of life (QoL) and physiologic health. |
What was learned from the study? |
Dyadic VR tandem cycling significantly improved PwPD clinical disease burden, in addition to self-reported mobility, fatigue, and social participation. |
CP depression and PD dyad parasympathetic nervous system function significantly improved following the tandem cycling intervention. |
Our results suggest an 8-week community VR tandem cycling program is a feasible and safe intervention for improving multiple dimensions of QoL and physical health for partners affected by PD. |
Introduction
Parkinson's disease (PD) is a progressive neurodegenerative disorder that affects approximately 8.5 million individuals globally and is projected to double in prevalence by 2050 [1, 2]. Persons with PD (PwPD) experience hallmark motor (i.e., tremors, rigidity, and bradykinesia) and non-motor symptoms (i.e., cognitive impairment, depression, and anxiety) which significantly impact their quality of life (QoL) [3]. Currently, there is no cure for PD, and pharmacologic therapies are only capable of alleviating motor and select non-motor symptoms to a certain degree [3].
Exercise has been identified as an effective intervention for improving motor function and reducing disability in PwPD [4]. Various exercise modalities such as independent stationary cycling, treadmill walking, and strength training have been examined; however, a hallmark study by Ridgel et al. suggested that tandem cycling may be a more effective exercise modality for PwPD [5]. Tandem cycling involves two participants simultaneously cycling on a single-frame bicycle with two seats connected by a single drivetrain. Due to the structure of these bicycles, participants must synchronize pedaling with one another, creating an additional component of forced exercise which requires greater work than independent stationary cycling, and is postulated to confer greater motor benefits for PwPD [5]. Subsequent studies have further supported this theory by demonstrating that tandem cycling not only reduced the severity of PwPD motor symptoms but also improved mobility, muscular strength, and cardiovascular fitness [6, 7]. McGough et al. also demonstrated the feasibility of conducting such an intervention over 10 weeks, and even integrated healthy community-dwelling adult volunteers to share the cycle with the PwPD [7], unlike previous studies which utilized personal trainers [5] and physical educators [6].
Care partners (CP) of PwPD experience tremendous burden due to their role as caregivers, and recent reviews have identified negative changes in physical health, depression, and even increased risk of all-cause mortality [8, 9]. Dyadic exercise interventions engaging both care recipients and CP have been identified as a means to reduce CP burden and improve well-being across other neurodegenerative diseases [10,11,12]. However, no such interventions have been conducted for PwPD and their CP.
As such, we designed a novel 8-week community-based indoor tandem cycling exercise program for PwPD and their CP (i.e., PD dyads). We aimed to examine the feasibility and efficacy of such an intervention to improve (1) PD-related motor and non-motor symptoms, and (2) the QoL and physiologic health of PwPD and their CP, whom we allowed to share the tandem cycle. Unlike any PD exercise study to date, we also integrated a virtual reality (VR) component which is postulated to induce greater physiologic and psychologic benefits compared to regular exercise modalities [13]. By including PD dyads, we anticipated our intervention would confer physical, psychological and therapeutic benefits to both individuals.
Methods
Participant Recruitment
Clinical neurologists at the Prisma Health Neuroscience Associates Movement Disorders clinic identified a total of ten PD dyads for recruitment and subsequent inclusion screening. To be included, PwPD must have been ≥ 30 years of age with a diagnosis of idiopathic, levodopa-responsive PD consistent with the UK Brain Bank Criteria, and have had a Hoehn & Yahr (H&Y) stage score ≤ 3. Exclusion criteria included the participant having (1) an atypical Parkinsonian syndrome or secondary parkinsonism, (2) a history of psychosis or hallucinations within the previous 12 months, and (3) a history of regular exercise behavior. CP of PwPD must have been formal or informal caregivers, spouses, family members, or friends of PwPD and ≥ 18 years of age. All participants must have achieved a MoCA score ≥ 18, and no participants could have had a medical or surgical condition that would impair consent, participation, or completion of the study. The identified PD dyads were offered participation in the study. If both the PwPD and the CP demonstrated interest, they underwent education about the study, provided written informed consent, were screened, and then were scheduled for baseline testing. Prisma Health Institutional Review Board approval was attained prior to beginning the study (Institutional Review Number Pro00110626). This study was conducted in accordance with the Helsinki Declaration of 1964 and its later amendments.
Pre-intervention Assessment
One week before the exercise intervention, PD dyads attended a baseline pre-testing session at the Movement Disorders clinic (Fig. 1, Week 1). All PwPD were asked to take their medications at consistently scheduled times to ideally be tested in the ON medication state. PwPD completed the following battery of assessments to identify the severity of their motor symptoms and PD-related QoL. All scales and questionnaires used had institutional approval, were open access, or did not require a license for clinical use.
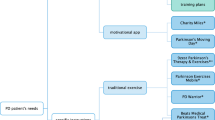
Community virtual reality (VR) tandem cycling program timeline. Participants provided written consent for inclusion of media images. PwPD Persons with Parkinson’s disease, MDS-UPDRS-III Movement Disorder Society-Unified Parkinson’s Disease Rating Scale-III Motor Examination, PDQ-39 Parkinson’s Disease Questionnaire-39, FGA Functional Gait Assessment, CP Care Partners, PROMIS-29 Patient-Reported Outcomes Measurement Information System, GDS-SF Geriatric Depression Scale-Short Form, GAD-7 Generalized Anxiety Disorder-7, MoCA Montreal Cognitive Assessment, BRS Brief Resiliency Scale, RDAS Revised Dyadic Adjustment Scale, HR heart rate
Movement Disorder Society-Unified Parkinson’s Disease Rating Scale-III Motor Examination (MDS-UPDRS-III)
The MDS-UPDRS-III is a motor subscale utilized in the clinical assessment of PD severity consisting of 18 items rated on a Likert scale of 0 (normal) to 4 (severe) [14]. Assessments were conducted by clinical neurologists specialized in movement disorders and video recorded for later score verification by co-author EUM. Total scores indicated mild (≤ 32), moderate (33–58), or severe (≥ 59) motor impairment [15].
Functional Gait Assessment (FGA)
The FGA is an ambulatory assessment evaluating gait, postural stability, and fall risk using 10 items, each rated on a Likert scale of 0 (severe impairment) to 3 (normal) [16]. All assessments were initially scored live and later verified with video recordings by co-authors AS and RR. For older adults with PD (H&Y stage score 1–4), a total FGA score < 15 is considered a high fall risk [17]. As such, higher FGA scores indicate lower fall risk and better mobility.
10-m Gait Speed Test
The 10-m gait speed test is a clinical assessment to further evaluate gait in PwPD by timing ambulation at a comfortable but fast pace over a flat surface [18]. Participants’ speeds were recorded live and later verified via video recordings by AS and RR.
Parkinson’s Disease Questionnaire-39 (PDQ-39)
The PDQ-39 is a self-reported survey of PD-related QoL consisting of 39 items rated on a Likert scale of 0 (never) to 4 (always) [19]. Questionnaire items correlate to one of eight sub-dimensions and sum to yield a summary index (SI). Lower PDQ-39 total SI and sub-dimension scores reflect better QoL.
Both PwPD and their CP individually completed the following battery of questionnaires to assess general QoL, mood (i.e., depression and anxiety), cognition, resiliency, and relationship quality.
Patient-Reported Outcomes Measurement Information System (PROMIS-29)
The PROMIS-29 Profile v2.0 is a self-reported, 29-item questionnaire assessing general QoL across seven sub-dimensions; each sub-dimension consists of 4 items rated on a 5-point Likert scale (0–5) [20]. Higher scores are associated with more of the respective sub-dimensions being measured.
Geriatric Depression Scale-Short Form (GDS-SF)
The GDS-SF is a self-reported screening tool for depression in older adults consisting of 15 items rated on a dichotomous ‘yes’ or ‘no’ scale [21]. Total points are summed to yield a final score indicating normal (GDS score < 5), mild (GDS score between 5 and 8), moderate (GDS score 9–11), or severe (GDS score > 12) depression.
Generalized Anxiety Disorder-7 (GAD-7)
The GAD-7 is a self-reported screening questionnaire for anxiety in adults consisting of seven items scored on a Likert scale of 0 (not at all) to 3 (nearly every day) [22]. Total scores are summed to indicate minimal (GAD-7 score < 5), mild (GAD-7 score between 5 and 9), moderate (GAD-7 score between 10 and 14), or severe (GAD-7 score over 15) anxiety.
Montreal Cognitive Assessment (MoCA)
The MoCA (version 7.1) is a brief screening tool used to assess cognitive function in adults via six domains [23, 24]. Clinical research staff administered the MoCA to participants and scored assessments immediately following administration. Cognition was classified as normal (MoCA score ≥ 26), mild impairment (MCI; MoCA score between 18 and 25), moderate impairment (MoCA score between 10 and 17), or severe impairment (MoCA score < 10).
Brief Resiliency Scale (BRS)
The BRS is a self-reported questionnaire assessing participants’ ability to respond and recover from stressors [25]. Six items are scored on a 5-point Likert scale (strongly disagree to strongly agree), and participants’ mean scores indicated low (BRS score < 3.00), normal (BRS score between 3.00 and 4.30), or high (BRS score > 4.30) resiliency.
Revised Dyadic Adjustment Scale (RDAS)
The RDAS is a self-reported questionnaire measuring dyadic relationship quality using 14 items rated on a 5–6-point Likert scale [26]. Items correlated to one of three dimensions and were summed to yield sub-dimension and total scores indicating relationship distress (RDAS score < 48) or non-distress (RDAS score > 48). PD dyads who were not spouses did not complete the RDAS.
Baseline 48-h Heart Rate Variability (HRV)
A recent review by Laborde et al. was utilized to structure the assessment of HRV throughout our intervention [27]. Before beginning the cycling intervention, PD dyads’ heart rate (HR) data was collected to assess baseline HRV [28]. Participants placed BodyGuard2 HR monitors (Firstbeat Technologies, Jyväskylä, Finland) on 72 h before the first cycling session and were instructed to leave them on continuously for the following 48 h with the exception of any activities that would expose the HR monitors to water, such as showering or swimming. Participants were provided logs to document periods of exercise and sleep over the interval to aid in HRV analysis post-intervention.
VR Tandem Cycling Protocol
PD dyads participated in indoor VR tandem cycling on a stationary and balance-supported tandem bicycle 2 days/week (with 1 consistent day of rest in between sessions) for 8 weeks. Each tandem bicycle was connected to a Computrainer ergometer (Velotron Dynafit Pro; Racermate, Seattle, WA, USA). All ergometers were synced to the ErgVideo Virtual Reality Cycling software (ErgVideo, Ottawa, Ontario, Canada), and television screens visible to all participants streamed pre-selected scenic bicycle routes mirroring the intensity of each session to simulate real outdoor cycling (Fig. 1, Weeks 2–9). A professional cycling coach with over 20 years of experience in training Olympic and para-Olympic athletes as well as retired wounded military warriors created the cycling training regimen and supervised all PD dyads during each exercise session. A circulating team of two to three clinical research personnel was also present to assist in providing supervision and ensuring participant safety during the cycling sessions; this team included a lead Ph.D. exercise physiologist, two M.D. clinical neurologists experienced in movement disorders, and six second-year medical students. During each session, PD dyads completed a 10-min warm-up period at 45% functional threshold power (FTP), followed by 20–50 min of alternating, moderate-to-vigorous cycling, and a 5-min cool-down period. Sessions progressed in duration and intensity each week, reaching 50–65% FTP in Weeks 2–3, 60–75% FTP in Weeks 4–5, and 60–90% FTP in Weeks 6–8, consistent with predetermined ErgVideo settings for each session. The Borg Rating of Perceived Exertion, which gauges participant effort on a scale of 6 (no effort) to 20 (maximal effort), was utilized to monitor and ensure PD dyads were cycling at the desired FTP together [29]. Each session was approximately 60 min in total duration, and all PD dyads were required to complete at least 80% of the total cycling sessions for data to be used in analysis.
Post-intervention HRV and Assessment
Forty-eight hours following the final cycling session, PD dyads were instructed to place the FirstBeat HR monitors on for another 48-h interval to assess for post-intervention changes in HRV. Participants were asked to log hours of sleep and exercise as they previously did. The following week, PD dyads returned to the Movement Disorders clinic to repeat the pre-testing assessment battery and complete the study (Fig. 1, Week 10).
Outcome Measures
As a pilot feasibility study, our primary outcome measures included the following indicators of feasibility: participant retention, intervention adherence, safety, and efficacy [30]. Participant retention was evaluated by measuring the percentage of PwPD and CP who completed the 8-week intervention. Intervention adherence was evaluated by measuring the percentage of cycling sessions PD dyads attended and completed. Safety was measured by the number and type of adverse effects that occurred during the exercise intervention, including physical injuries, falls, and medical emergencies that may occur during the tandem cycling exercise sessions. Measures assessing QoL, mood, cognition, resiliency, relationship quality, and HRV were selected to assess the efficacy of our intervention for PD dyads. Measures assessing the clinical severity and mobility of PwPD were also selected to evaluate disease-specific changes post-intervention.
Analysis of Functional Assessments and Psychometric Questionnaires
Post-intervention changes in measures of efficacy were examined using a within-subjects research design. The means of total scores and sub-dimension scores (if available) for each metric were derived for PwPD and CP; PwPD 10-m gait speeds were averaged. Mean changes between pre-testing and post-testing scores were reported as mean differences (MD). A test for normality was performed and assessed prior to statistical analyses by visual examination of histograms. Paired t tests were then conducted using R (version 4.3.1) with significance set at p ≤ 0.05 [31]. All p values are reported unadjusted. The full dataset and R code created to perform all statistical analyses can be provided upon request to the corresponding author. All pre-testing means (Pre-M), post-testing means (Post-M), and MD are reported in the tables with the standard error of the mean (SE), confidence intervals (CI), and p values (p).
HRV Analysis
Firstbeat BodyGuard2 HR monitor data were analyzed using the time–domain method and the Kubios HRV Premium software (version 3.5.0). Automatic noise detection was set to medium and beat correction threshold was set to automatic. Each participant’s total 48-h HR recordings were split into two 24-h recordings, and the following indices were extracted using Kubios: mean HR (bpm), mean respiratory rate (RR; ms), root mean square of successive RR interval differences (RMSSD), relative number of successive RR interval pairs that differ more than 50 ms (pNN50), and standard deviation of normal-to-normal RR intervals (SDNN). RMSSD and pNN50 are both well-established variables for the measure of parasympathetic nervous system (PNS) function and activity [32, 33]. Conversely, SDNN is a well-established measure of complete autonomic nervous system (ANS) function, including the PNS and sympathetic nervous system (SNS) [30, 31]. As such, these indices were used to assess post-intervention changes in HRV. The means of each variable were obtained between the two 24-h recordings for each participant. As described above, MD for each indice were acquired, and paired t tests were conducted using R (version 4.3.1) with statistical significance set at p ≤ 0.05 [31].
Results
Participant Retention, Adherence and Safety
A total of ten PD dyads were initially recruited for the intervention. Eight PD dyads were married couples, one PD dyad was a parent and child, and one PD dyad was close friends. Three waves of the study were conducted with 2–3 PD dyads participating at a time. One PD dyad was lost to follow-up during Wave 1 Week 1 of the intervention due to their CP drop** from the study. During Wave 2 Week 5, one CP was lost to follow-up due to unforeseen travel obligations. However, since the PwPD could not identify another CP and was still motivated to continue, the research team decided to allow clinical staff to serve as substitute tandem cycling partners so that the PwPD could continue to participate. This protocol deviation was justified since the participant completed over half of the intervention with their original CP before requiring a substitute, and they requested to continue participating in the intervention for their health. Following three waves, eight PD dyads and one additional PwPD completed the study, resulting in 90% retention of PwPD and 80% retention of CP. Dyadic adherence to exercise sessions per wave of the intervention was as follows: 91.67% (Wave 1), 93.75% (Wave 2; including substitute cycling partners), and 97.91% (Wave 3). No adverse events occurred during any waves of the exercise intervention.
Participant Demographics and PwPD Clinical Metrics
All demographic data are summarized in Table 1. Across the PwPD, 44% were female and 55% were male. On average (SD), PwPD were 70.22 years old (5.8) and had been diagnosed with PD for 1.54 years (1) before the start of the study. The average H&Y score for PwPD before the intervention was 2.22 (0.36) and PwPD were on an average of 163.89–655.56 mg of carbidopa-levodopa (71.93–287.71) per day. There were no medication changes reported during the study. Across CP, all were informal, spousal caregivers. CP were 62% female and 37% male, with an average age of 68.50 years old (7.39). PwPD and CP each had an average of 14.56–14.57 years of schooling (1.94–2.51).
MDS-UPDRS-III
Mean total MDS-UPDRS-III scores significantly decreased post-intervention, indicating an overall decrease in motor disability (Table 2). Individual changes in MDS-UPDRS-III score for each PwPD can be visualized in Fig. 2a.
Individual functional changes in persons with Parkinson’s disease (PwPD). Each PwPD is represented by a color coordinated ID. Changes in Movement Disorders Society-Unified Parkinson’s Disease Rating Scale-III (MDS-UPDRS-III) scores (a), Functional Gait Assessment (FGA) scores (b), and the 10-m gait speed test (c) from pre-testing to post-testing can be visualized for each participant. Raw differences in scores and 10-m gait speed times are represented in corresponding boxes
FGA
Mean total FGA scores significantly increased following the study (Table 2). Pre-intervention, two out of nine PwPD were considered at high risk of a fall; however, all nine PwPD were not considered to be at a high fall risk following the intervention. Individual changes in FGA score reflecting these improvements can be visualized in Fig. 2b.
10-m Gait Speed Test
One PwPD was removed from the analysis due to an invalid post-test assessment; upon video recording review, the participant did not follow the designated walking path and subsequently ambulated a longer distance. The remaining eight PwPD demonstrated a significant increase in mean 10-m gait speed post-intervention (Table 2). The increase in individual PwPD gait speeds ranged from + 0.06 to + 0.53 m/s (Fig. 2c).
PDQ-39
Total SI PDQ-39 scores improved post-intervention, although the MD did not achieve statistical significance (Table 2). Among the eight sub-dimensions assessed (Table 2), PwPD reported a significant improvement in the mobility dimension. Although no other sub-dimension changes achieved statistical significance, PwPD also reported improvements in emotional well-being, cognition, social support, communication, activities of daily living, and bodily discomfort. Interestingly, PwPD reported greater stigma after the completion of the intervention.
PROMIS-29
PwPD QoL assessed using the PROMIS-29 showed improvements across all seven dimensions post-intervention (Table 2). However, PwPD only demonstrated significant improvements in fatigue and the ability to participate in social roles and activities. Although CP also demonstrated improvements in physical function, depression, fatigue, and social participation, these improvements were not statistically significant. No improvements were identified in CP anxiety, sleep disturbance, or pain interference (Table 3).
GDS-SF
PwPD’s mean total GDS-SF score decreased post-intervention, although this decrease was not statistically significant (Table 2). CP’s mean total GDS-SF score did however significantly decrease post-intervention (Table 3). Changes in PwPD and CP GDS-SF score classifications are demonstrated in Table 4.
GAD-7
No statistically significant changes in mean total GAD-7 scores were observed post-intervention, although PwPD mean total score increased (Table 2) and CP mean total score decreased (Table 3). Changes in PwPD and CP GAD-7 score classifications are demonstrated in Table 4. The post-intervention changes in PwPD classifications were the result of three PwPD moving from minimal to mild anxiety, and two moving from moderate and mild anxiety to minimal anxiety.
MoCA
Post-intervention, PwPD mean total MoCA score increased from 25.12 to 25.67, although this increase was not statistically significant (Table 2). On sub-domain analysis, PwPD showed statistically insignificant increases in delayed recall, language, naming, and visuospatial/executive abilities. Interestingly, PwPD showed a statistically significant decrease in attention post-intervention. PwPD orientation abilities also decreased; however, this decrease was not statistically significant. PwPD abstraction abilities were unchanged. Regarding cognitive classification (Table 4), PwPD post-intervention changes were due to one PwPD moving from normal cognition to MCI, and two moving from MCI to normal cognition.
CP mean total MoCA score increased from 25.75 to 26.13 post-intervention, although this increase was also not statistically significant (Table 3). On sub-domain analysis, CP displayed statistically insignificant increases in abstraction, language, attention, and visuospatial/executive abilities. Conversely, CP showed a non-statistically significant decrease in delayed recall. CP orientation and naming abilities remained unchanged. Post-intervention changes in CP cognitive classifications (Table 4) were due to two CP moving from MCI to normal cognition, while one moved from normal cognition to MCI.
BRS
No statistically significant changes in mean BRS scores were observed post-intervention, although PwPD mean score decreased (Table 2) and CP mean score increased (Table 3).
Following the intervention, both PwPD and CP demonstrated variable fluctuations in score classifications (Table 4). For PwD, one shifted from normal to high resiliency, two shifted from high to normal resiliency, and one shifted from normal to low resiliency; of note, these downward changes were due to very minor decreases in total score (∆ = − 0.16–0.19). For CP, two shifted from normal to high resiliency while one shifted from high to normal resiliency (∆ = − 0.50).
RDAS
One PD dyad was not a spousal couple and did not complete the questionnaire. One CP incorrectly completed the questionnaire; as such, their responses were removed from the final analysis, which included seven CP and eight PwPD. Before and after the intervention, all eight dyads’ relationships were classified as non-distress (Table 4). Although no statistically significant changes in sub-dimension scores were noted (Tables 2, 3), both PwPD and CP indicated improvement in cohesion. PwPD also reported increased consensus, although their CP reported decreased consensus. Lastly, PwPD relationship satisfaction decreased, although this change was not reciprocated by their CP.
HRV
No HR data were obtained for one PD dyad due to manual issues placing the HR monitors on during baseline testing. One PwPD also experienced a fall during the 48-h post-testing interval while ambulating on uneven cobblestone (determined not related to the intervention per IRB review). Due to the known effect of significant stress on measures of HRV [34], this participant’s HR data was removed from the analysis. Ultimately, eight PwPD and seven CP had complete HR data sets for both pre- and post-testing, yielding a total of 15 complete HR data sets for analysis. Post-intervention, both PwPD and CP showed a statistically significant improvement in PNS function, as represented by the RMSSD (Tables 2, 3). PD dyads’ pNN50 and SDNN indices also increased, although these increases were not statistically significant.
Discussion
To our knowledge, this study is the first to examine the feasibility and efficacy of a community-based VR tandem cycling intervention in improving dimensions of QoL and physical health for PwPD and their CP. Compared to a previous study [7], our tandem cycling program exhibited similarly high retention, adherence, and safety, offering further support that community-based tandem cycling is a feasible exercise intervention for PwPD. However, our study also demonstrated such an intervention is feasible for CP of PwPD, which has yet to be demonstrated in the literature. As such, the present study introduces a novel exercise intervention which can be emulated by hospital systems, outpatient clinics, or community fitness facilities interested in offering such programming for local individuals managing PD. With regards to potential efficacy, our study demonstrated clinically and statistically significant changes for both PwPD and their CP.
Effects of VR Tandem Cycling on PwPD
Following 8 weeks of bi-weekly VR tandem cycling, PwPD demonstrated statistically significant improvements in functional health, as measured by the MDS-UPDRS-III, FGA, and 10-m gait speed test, suggesting our intervention elicited a marked reduction in overall PD-symptom severity. These results are consistent with the findings of previous stationary cycling interventions [35,36,37,38,39,40] and the two other tandem cycling interventions known to date [6, 7], offering further support for the effectiveness of stationary cycling in improving mobility and fall risk in PwPD. Of note, our study also achieved minimal clinically important differences (MCID) across these metrics; i.e., changes considered significant to the patient. For the MDS-UPDRS-III, all nine PwPD (100%) had at least a MCID (− 2.3 to − 2.7), while four (44.4%) achieved a moderate clinically important difference (between − 4.5 to − 6.7), and three (33.3%) achieved a large clinically important difference (between − 10.7 to − 10.8) [41,42,43]. Four (44.4%) PwPD also achieved a MCID in FGA score (∆ > 4.30), and five (55.5%) exceeded a MCID in 10-m gait speed [44,45,46]. As such, PwPD also recognized an improvement in their PD-related symptoms following our intervention.
Unlike any previous PD tandem cycling study, we also examined changes outside motor function in PwPD. Among these variables, our study elicited the most significant effect on self-reported QoL, as PwPD achieved statistically significant improvements in mobility, fatigue, and ability to participate in social roles and activities, as measured by the PDQ-39 and PROMIS-29. The total change in SI PDQ-39 score also achieved a MCID [47], suggesting PwPD also recognized an improvement in their QoL as it relates to their disease. Decline in social functioning and the ability to fulfill social roles across different relationships is a life-altering, yet clinically under-recognized, burden experienced by patients with chronic illnesses such as PD. A recent review identified that PwPD experience social withdrawal in part due to the physical manifestations of their disease, including voice changes and the unpredictability of symptoms [48]. While fatigue is one of the most common non-motor symptoms associated with PD [49, 50], it has also been demonstrated to further contribute to PwPD social withdrawal [51, 52]. Current treatments for PD, such as carbidopa-levodopa and deep brain stimulation, improve the motor deficits associated with PD [53]; however, they have been found to have little to no efficacy in improving these non-motor symptoms [48, 54, 55]. Interestingly, group dance classes and support sessions are effective therapies for improving social withdrawal [48], but the efficacy of rehabilitation interventions (i.e., aerobic exercise classes) on fatigue remains inconclusive [56,57,58,59]. As such, our study may be the first to demonstrate that community VR tandem cycling is an efficacious treatment for not only improving mobility but also social participation and fatigue in PwPD. It is important to note that PwPD did report worse stigma post-intervention. Although not anticipated, we hypothesize this may be due to the nature of our intervention, which only included PD dyads. Therefore, future tandem cycling interventions may benefit by integrating PD dyads into larger, community-wide interventions to reduce stigma while maintaining the physical and psychosocial benefits identified thus far.
Although no statistically significant changes were observed in PwPD anxiety or depression, our intervention did demonstrate unique clinical changes. Regarding depression, the PwPD with severe depressive symptoms improved to minimal depressive symptoms (∆ GDS-SF score = − 8), the group of PwPD classified as mildly depressed had no worsening of existing symptoms (∆ GDS-SF score = 0), and no PwPD classified as normal reported develo** mild-to-severe depressive symptoms following the study. Although the ability of aerobic exercise interventions to improve depression in PwPD remains inconclusive [60,61,62,63,64,65,66,67,68,69], our findings support the growing theory that exercise instead confers a neuroprotective effect for patients with co-morbid PD and depression [70, 71]. Our slight, yet non-statistically significant decrease in mean GDS-SF score also suggests that improving depressive symptoms in PD may require a longer, more rigorous or multi-dimensional exercise intervention. Regarding anxiety, our study demonstrated mixed clinical effects as three PwPD reported MCID, consistent with worsening anxiety (i.e., an increase in GAD-7 score by 4–7), while two reported MCID consistent with improved anxiety (i.e., a decrease in GAD-7 score by 4–9) following the intervention [72]. To our knowledge, no studies have examined the direct effect of stationary cycling on PwPD anxiety; however, other physical activity interventions have proven efficacious in improving co-morbid PD and anxiety [67, 68, 73, 74]. We hypothesize that the variability observed following our intervention could be due to a myriad of unaccounted individual differences, including episodic changes in motor function (i.e., OFF medication phases), emotional fluctuations, and the degree of dopaminergic neuron degeneration [75].
Similar to mood, our findings showed no statistically significant improvements in PwPD cognition post-intervention. In fact, we observed a significant decrease in the attention sub-domain. Previous human and animal studies have mainly described beneficial effects of cycling for cognitive function and neuroprotection in PD [36, 37, 76,77,78,79]. Specific improvements in attention have also been demonstrated in the study by David et al. following a structured resistance training program in PwPD [78]. Although our finding was unexpected and contradictory to the current literature, it remains unclear whether this change is clinically relevant, and what individual factors (such as fluctuations in cognition and cognitive dysfunction subtype) may be related to this change [80, 81]. As exercise is blanketly recommended as a non-pharmacologic treatment for PD-related cognitive dysfunction, our study propagates the need to further elucidate the specific cognitive changes induced by tandem cycling.
Effects of VR Tandem Cycling on CP
Across all variables assessed, one of the most significant findings of our study identified that CP self-reported depressive symptoms significantly decreased post-intervention. Depression is not only the most prevalent neuropsychiatric disorder among CP of PwPD [82] but it has also been directly correlated to CP burden, and PwPD depression, disease severity, disability, and QoL [83,84,85,86,87,88]. As such, alleviating PD CP depression has the potential to simultaneously improve dyadic well-being and PwPD disease burden. Previous studies have demonstrated that dyadic mindfulness interventions [89,90,91] and multidisciplinary rehabilitation programs [92] improve depression in PwPD and their CP. To our knowledge, this study is the first cycling intervention to improve depressive symptoms experienced by PD CP.
With regards to overall CP QoL, anxiety, and cognition, our tandem cycling study did not demonstrate statistically or clinically significant changes. These results may be due to multiple factors. CP of PwPD experience marked anxiety [84, 93] and cognitive dysfunction [94] which have recently been found to be linked [95]. As such, the lack of improvement in both may explain our findings. Further, the current literature has only described cognitive behavioral therapy [91, 96, 97] and patient education programs [98] for alleviating PD CP anxiety; exercise interventions have yet to be examined for improving these dimensions of well-being, making our study’s preliminary findings a starting point for further analysis.
Dyadic Effects Following VR Tandem Cycling
As previous studies have identified that PD dyads influence one another’s well-being and relationship satisfaction [82, 99], we hypothesized that PD dyads completing the tandem cycling intervention together would elicit improvements in resiliency and various relationship dimensions. Our study did not demonstrate statistically significant changes in resiliency or dyadic cohesion, consensus, or satisfaction post-intervention. We postulate that this may be due to individual determinants (i.e., gender, self-efficacy, personality, PwPD age of disease onset, and treatment duration) [100, 101], and the limited duration of our intervention; true changes in multi-dimensional constructs such as resiliency and spousal dynamics may require more time. Interestingly, PD dyads did share a statistically significant physiologic improvement in PNS function as represented by RMSSD, which is a well-established representation of central nervous system control over cardiac function via the vagus nerve [32]. Greater cardiac vagal control is believed to be linked to multiple physiologic benefits, including improved cognitive function, emotional regulation, social participation, and reduced risk of myocardial disease [27, 102]. Older adults experience significant alterations in PNS function with age [103], and PNS dysfunction is the root of many non-motor symptoms experienced by PwPD [104]. Although stationary cycling interventions have been shown to improve cardiac vagal tone [105], studies examining such changes following exercise interventions in PwPD are scarce. To our knowledge, our study is the first to demonstrate statistically significant improvements in PNS function following a tandem cycling exercise intervention in PwPD. Further, this effect was shared by their CP, suggesting that the additional physiologic benefits induced by tandem cycling are dyadic.
Study Limitations
There are limitations to consider regarding the present study. First, participants meeting inclusion criteria were selected from one outpatient practice, and those recruited were motivated to partake in the exercise intervention. The final sample size (nine PwPD and eight CP) was small, and may have been underpowered to observe statistically significant effects in some variables. However, as this study successfully demonstrated feasibility with several areas of efficacy, it serves as a foundation for future studies to determine greater generalizability of dyadic tandem cycling using a larger sample size. Secondly, our intervention did not include a group of dyads unaffected by PD, limiting our ability to compare our PD dyads’ findings. We determined that it was unethical to not offer the intervention to PwPD, as previous literature has already demonstrated that tandem cycling is beneficial. Thirdly, PwPD were asked to regularly take their medications throughout the study to ideally participate while in the ON medication state. Although a potential confounder, PwPD medication adherence was carefully monitored to reduce variability in performance, and no changes in medication timing or dose occurred during the intervention. Finally, the outcomes of our study are limited to a 10-week window and do not encompass the potential long-term results of our intervention. This creates a promising gateway for further exploration of the long-term effectiveness of dyadic tandem cycling in subsequent studies.
Future Directions
Although tandem cycling and the use of exercise as medicine for PwPD is becoming increasingly supported by the literature, best practices and further studies examining effects beyond functional benefits are needed. In addition, the amount of PD literature dedicated to CP or PD dyads remains scarce compared to other neurological disorders. As such, subsequent studies examining the joint effects of dyadic exercise interventions such as tandem cycling on PD dyads are needed to further improve the QoL and disease burden inherent to families affected by PD.
Conclusion
Our study is the first to demonstrate the feasibility and efficacy of a community VR tandem cycling intervention in improving the QoL and physical health of PwPD and their CP. Following 8 weeks of bi-weekly VR tandem cycling, PwPD demonstrated significant improvements in (1) PD disease burden, (2) mobility, (3) fatigue, (4) participation in social roles and activities, and (5) PNS function. These findings not only offer further support for the efficacy of tandem cycling in improving the well-known motor disabilities experienced by PwPD but also identify a potential treatment modality for common non-motor symptoms currently under-treated. This study is also the first to demonstrate the dyadic benefits of tandem cycling for CP of PwPD, who shared the improvement in PNS function and demonstrated a significant improvement in depression following the intervention. As CP are under-recognized yet integral stakeholders in the overall treatment plan for PwPD, our results offer support for dyadic tandem cycling as a therapeutic intervention for PD dyads.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Rocca WA. The burden of Parkinson’s disease: a worldwide perspective. Lancet Neurol. 2008;17(11):928–9.
Parkinson disease: a public health approach. Technical brief. Geneva: World Health Organization; 2022. Licence: CC BY-NC-SA 3.0 IGO.
Poewe W, Seppi K, Tanner CM, et al. Parkinson disease. Nat Rev Dis Primers. 2017;3:17013.
Choi HY, Cho KH, ** C, et al. Exercise therapies for Parkinson's disease: a systematic review and meta-analysis. Parkinson's Dis. 2020;2565320.
Ridgel AL, Vitek JL, Alberts JL. Forced, not voluntary, exercise improves motor function in Parkinson’s disease patients. Neurorehabil Neural Repair. 2009;23(6):600–8.
Segura C, Eraso M, Bonilla J, Mendivil CO, Santiago G, Useche N, et al. Effect of a high-intensity tandem bicycle exercise program on clinical severity, functional magnetic resonance imaging, and plasma biomarkers in Parkinson’s disease. Front Neurol. 2020;11:656.
McGough EL, Robinson CA, Nelson MD, et al. A tandem cycling program: feasibility and physical performance outcomes in people with Parkinson disease. J Neurol Phys Ther. 2016;40(4):223–9.
Martinez-Martin P, Macaulay D, Jalundhwala YJ, et al. The long-term direct and indirect economic burden among Parkinson’s disease caregivers in the United States. Mov Disord. 2019;34(2):236–45.
Hulshoff MJ, Book E, Dahodwala N, Tanner CM, Robertson C, Marras C. Current knowledge on the evolution of care partner burden, needs, and co** in Parkinson’s disease. Mov Disord Clin Pract. 2021;8(4):510–20.
Doyle KL, Toepfer M, Bradfield AF, et al. Systematic review of exercise for caregiver-care recipient dyads: what is best for spousal caregivers-exercising together or not at all? Gerontologist. 2021;61(6):e283–301.
Lamotte G, Shah RC, Lazarov O, Corcos DM. Exercise training for persons with Alzheimer’s disease and caregivers: a review of dyadic exercise interventions. J Mot Behav. 2017;49(4):365–77.
Fakolade A, Cameron J, McKenna O, et al. Physical activity together for people with multiple sclerosis and their care partners: protocol for a feasibility randomized controlled trial of a dyadic intervention. JMIR Res Protoc. 2021;10(6): e18410.
Qian J, McDonough DJ, Gao Z. The effectiveness of virtual reality exercise on individual’s physiological, psychological and rehabilitative outcomes: a systematic review. Int J Environ Res Public Health. 2020;17(11):4133.
Goetz CG, Tilley BC, Shaftman SR, et al. Movement Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): scale presentation and clinimetric testing results. Mov Disord Off J Mov Disord Soc. 2008;23(15):2129–70.
Martínez-Martín P, Rodríguez-Blázquez C, Alvarez M, et al. Parkinson’s disease severity levels and MDS-Unified Parkinson’s Disease Rating Scale. Parkinsonism Relat Disord. 2015;21(1):50–4. https://doi.org/10.1016/j.parkreldis.2014.10.026.
Wrisley DM, Marchetti GF, Kuharsky DK, Whitney SL. Reliability, internal consistency, and validity of data obtained with the functional gait assessment. Phys Ther. 2004;84(10):906–18.
Leddy AL, Crowner BE, Earhart GM. Functional gait assessment and balance evaluation system test: reliability, validity, sensitivity, and specificity for identifying individuals with Parkinson disease who fall. Phys Ther. 2011;91(1):102–13.
Combs SA, Diehl MD, Filip J, Long E. Short-distance walking speed tests in people with Parkinson disease: reliability, responsiveness, and validity. Gait Posture. 2014;39(2):784–8.
Hagell P, Nygren C. The 39 item Parkinson’s disease questionnaire (PDQ-39) revisited: implications for evidence based medicine. J Neurol Neurosurg Psychiatry. 2007;78(11):1191–8.
Hays RD, Spritzer KL, Schalet BD, Cella D. PROMIS®-29 v.20 profile physical and mental health summary scores. Qual Life Res Int J Qual Life Aspects Treat Care Rehabil. 2018;27(7):1885–91.
Sheikh JI, Yesavage JA. Geriatric Depression Scale-Short Form (GDS-Short Form. APA PsycTests. 1986.
Spitzer RL, Kroenke K, Williams JB, Löwe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006;166(10):1092–7.
Nasreddine ZS, Phillips NA, Bédirian V, et al. The Montreal cognitive assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53(4):695–9.
Lam B, Middleton LE, Masellis M, et al. Criterion and convergent validity of the Montreal cognitive assessment with screening and standardized neuropsychological testing. J Am Geriatr Soc. 2013;61(12):2181–5.
Smith BW, Dalen J, Wiggins K, Tooley E, Christopher P, Bernard J. The brief resilience scale: assessing the ability to bounce back. Int J Behav Med. 2008;15(3):194–200.
Busby DM, Christensen C, Crane DR, Larson JH. A revision of the Dyadic Adjustment Scale for use with distressed and nondistressed couples: construct hierarchy and multidimensional scales. J Marital Fam Ther. 1995;21(3):289–308.
Laborde S, Mosley E, Thayer JF. Heart rate variability and cardiac vagal tone in psychophysiological research—recommendations for experiment planning, data analysis, and data reporting. Front Psychol. 2017;8:213.
AHA. Heart rate variability: standards of measurement, physiological interpretation and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Circulation. 1996;93(5):1043–65.
Borg GA. Psychophysical bases of perceived exertion. Med Sci Sports Exerc. 1982;14(5):377–81.
El-Kotob R, Giangregorio LM. Pilot and feasibility studies in exercise, physical activity, or rehabilitation research. Pilot Feasibility Stud. 2018;4(1):1–7.
R Core Team. R: a language and environment for statistical computing. Vienna: R Foundation for Statistical Computing; 2024.
Shaffer F, Ginsberg JP. An overview of heart rate variability metrics and norms. Front Public Health. 2017;5:258.
Berntson GG, Bigger JT Jr, Eckberg DL, et al. Heart rate variability: origins, methods, and interpretive caveats. Psychophysiology. 1997;34(6):623–48.
Kim HG, Cheon EJ, Bai DS, Lee YH, Koo BH. Stress and heart rate variability: a meta-analysis and review of the literature. Psychiatry Investig. 2018;15(3):235–45.
Uygur M, Bellumori M, Knight CA. Effects of a low-resistance, interval bicycling intervention in Parkinson’s disease. Physiother Theory Pract. 2017;33(12):897–904.
Chang HC, Lu CS, Chiou WD, Chen CC, Weng YH, Chang YJ. An 8-week low-intensity progressive cycling training improves motor functions in patients with early-stage parkinson’s disease. J Clin Neurol. 2018;14(2):225–33.
Tollár J, Nagy F, Hortobágyi T. Vastly different exercise programs similarly improve parkinsonian symptoms: a randomized clinical trial. Gerontology. 2019;65(2):120–7.
Miner DG, Chui K. Immediate effects of high-cadence cycling on core outcomes in individuals with Parkinson's disease. J Aging Phys Act. 2023:1–9.
Nadeau A, Lungu O, Duchesne C, et al. A 12-week cycling training regimen improves gait and executive functions concomitantly in people with Parkinson’s disease. Front Hum Neurosci. 2017;10:690.
Linder SM, Baron E, Learman K, et al. An 8-week aerobic cycling intervention elicits improved gait velocity and biomechanics in persons with Parkinson’s disease. Gait Posture. 2022;98:313–5.
Shulman LM, Gruber-Baldini AL, Anderson KE, Fishman PS, Reich SG, Weiner WJ. The clinically important difference on the unified Parkinson’s disease rating scale. Arch Neurol. 2010;67(1):64–70.
Sánchez-Ferro Á, Matarazzo M, Martínez-Martín P, et al. Minimal clinically important difference for UPDRS-III in daily practice. Mov Disord Clin Pract. 2018;5(4):448–50.
Schrag A, Sampaio C, Counsell N, Poewe W. Minimal clinically important change on the unified Parkinson’s disease rating scale. Mov Disord Off J Mov Disord Soc. 2006;21(8):1200–7.
Petersen C, Steffen T, Paly E, Dvorak L, Nelson R. Reliability and minimal detectable change for sit-to-stand tests and the functional gait assessment for individuals with Parkinson disease. J Geriatr Phys Therapy. 2017;40(4):223–6.
Steffen T, Seney M. Test-retest reliability and minimal detectable change on balance and ambulation tests, the 36-item short-form health survey, and the unified Parkinson disease rating scale in people with parkinsonism. Phys Ther. 2008;88(6):733–46.
Hass CJ, Bishop M, Moscovich M, et al. Defining the clinically meaningful difference in gait speed in persons with Parkinson disease. J Neurol Phys Ther. 2014;38(4):233–8.
Horváth K, Aschermann Z, Kovács M, et al. Changes in quality of life in Parkinson’s disease: how large must they be to be relevant? Neuroepidemiology. 2017;48(1–2):1–8.
Perepezko K, Hinkle JT, Shepard MD, et al. Social role functioning in Parkinson’s disease: a mixed-methods systematic review. Int J Geriatr Psychiatry. 2019;34(8):1128–38.
Barone P, Antonini A, Colosimo C, et al. The PRIAMO study: a multicenter assessment of nonmotor symptoms and their impact on quality of life in Parkinson’s disease. Mov Disord Off J Mov Disord Soc. 2009;24(11):1641–9.
Friedman JH, Abrantes A, Sweet LH. Fatigue in Parkinson’s disease. Expert Opin Pharmacother. 2011;12(13):1999–2007.
Herlofson K, Larsen JP. The influence of fatigue on health-related quality of life in patients with Parkinson’s disease. Acta Neurol Scand. 2003;107(1):1–6.
Havlikova E, Rosenberger J, Nagyova I, et al. Clinical and psychosocial factors associated with fatigue in patients with Parkinson’s disease. Parkinsonism Relat Disord. 2008;14(3):187–92.
Church FC. Treatment options for motor and non-motor symptoms of Parkinson’s disease. Biomolecules. 2021;11(4):612.
Rahman MM, Uddin MJ, Chowdhury JH, Chowdhury TI. Effect of levodopa and carbidopa on non-motor symptoms and signs of Parkinson’s disease. Mymensingh Med J. 2014;23(1):18–23.
Fabbri M, Coelho M, Guedes LC, et al. Acute response of non-motor symptoms to subthalamic deep brain stimulation in Parkinson’s disease. Parkinsonism Relat Disord. 2017;41:113–7.
Winward C, Sackley C, Meek C, et al. Weekly exercise does not improve fatigue levels in Parkinson’s disease. Mov Disord Off J Mov Disord Soc. 2012;27(1):143–6.
Coe S, Franssen M, Collett J, et al. Physical activity, fatigue, and sleep in people with Parkinson's disease: a secondary per protocol analysis from an intervention trial. Parkinson's Dis. 2018;1517807.
Lin I, Edison B, Mantri S, et al. Triggers and alleviating factors for fatigue in Parkinson’s disease. PLoS ONE. 2021;16(2): e0245285.
Folkerts AK, Nielsen J, Gollan R, et al. physical exercise as a potential treatment for fatigue in Parkinson’s disease? A systematic review and meta-analysis of pharmacological and non-pharmacological interventions. J Parkinsons Dis. 2023;13(5):659–79.
Harper SA, Dowdell BT, Kim JH, Pollock BS, Ridgel AL. Non-motor symptoms after one week of high cadence cycling in Parkinson’s disease. Int J Environ Res Public Health. 2019;16(12):2104.
Burini D, Farabollini B, Iacucci S, et al. A randomised controlled cross-over trial of aerobic training versus Qigong in advanced Parkinson’s disease. Eura Medicophys. 2006;42(3):231–8.
Smania N, Corato E, Tinazzi M, et al. Effect of balance training on postural instability in patients with idiopathic Parkinson’s disease. Neurorehabil Neural Repair. 2010;24(9):826–34.
Khallaf M, Fathy H. Effect of treadmill training on activities of daily living and depression in patients with Parkinson’s disease. Middle East Curr Psychiatry. 2011;18:144–8.
Dashtipour K, Johnson E, Kani C, et al. Effect of exercise on motor and nonmotor symptoms of Parkinson's disease. Parkinson's Dis. 2015;586378.
Lee NY, Lee DK, Song HS. Effect of virtual reality dance exercise on the balance, activities of daily living, and depressive disorder status of Parkinson’s disease patients. J Phys Therapy Sci. 2015;27(1):145–7.
Sajatovic M, Ridgel AL, Walter EM, et al. A randomized trial of individual versus group-format exercise and self-management in individuals with Parkinson’s disease and comorbid depression. Patient Prefer Adher. 2017;11:965–73.
Ferreira RM, Alves WMGDC, de Lima TA, et al. The effect of resistance training on the anxiety symptoms and quality of life in elderly people with Parkinson’s disease: a randomized controlled trial. Arq Neuropsiquiatr. 2018;76(8):499–506.
Slomski A. Yoga for anxiety and depression associated with Parkinson disease. JAMA. 2019;322(4):296.
Li X, Taylor A, Li J, Wang T, Kuang J, et al. Effects of health qigong exercise on depression and anxiety in patients with Parkinson’s disease. Int J Ment Health Promot. 2022;24(6):855–67.
Cheon SM, Chae BK, Sung HR, Lee GC, Kim JW. The efficacy of exercise programs for Parkinson’s disease: Tai Chi versus combined exercise. J Clin Neurol. 2013;9(4):237–43.
Altmann LJ, Stegemöller E, Hazamy AA, et al. Aerobic exercise improves mood, cognition, and language function in Parkinson’s disease: results of a controlled study. J Int Neuropsychol Soc. 2016;22(9):878–89.
Toussaint A, Hüsing P, Gumz A, et al. Sensitivity to change and minimal clinically important difference of the 7-item Generalized Anxiety Disorder Questionnaire (GAD-7). J Affect Disord. 2020;265:395–401.
Kwok JYY, Kwan JCY, Auyeung M, et al. Effects of mindfulness yoga vs stretching and resistance training exercises on anxiety and depression for people with Parkinson disease: a randomized clinical trial. JAMA Neurol. 2019;76(7):755–63.
Abuoaf R, AlKaabi R, Mohamed Saleh A, et al. The effect of physical exercise on anxiety in people with Parkinson’s disease: a systematic review of randomized control trials. NeuroRehabilitation. 2023;52(3):387–402.
Chen JJ, Marsh L. Anxiety in Parkinson’s disease: identification and management. Ther Adv Neurol Disord. 2014;7(1):52–9.
Ridgel AL, Kim CH, Fickes EJ, Muller MD, Alberts JL. Changes in executive function after acute bouts of passive cycling in Parkinson’s disease. J Aging Phys Act. 2011;19(2):87–98. https://doi.org/10.1123/japa.19.2.87.
Duchesne C, Lungu O, Nadeau A, et al. Enhancing both motor and cognitive functioning in Parkinson’s disease: aerobic exercise as a rehabilitative intervention. Brain Cogn. 2015;99:68–77.
David FJ, Robichaud JA, Leurgans SE, et al. Exercise improves cognition in Parkinson’s disease: the PRET-PD randomized, clinical trial. Mov Disord Off J Mov Disord Soc. 2015;30(12):1657–63.
Schirinzi T, Canevelli M, Suppa A, Bologna M, Marsili L. The continuum between neurodegeneration, brain plasticity, and movement: a critical appraisal. Rev Neurosci. 2020;31(7):723–42.
Kehagia AA, Barker RA, Robbins TW. Cognitive impairment in Parkinson’s disease: the dual syndrome hypothesis. Neurodegener Dis. 2013;11(2):79–92.
Fang C, Lv L, Mao S, Dong H, Liu B. Cognition deficits in Parkinson's disease: mechanisms and treatment. Parkinson's Dis. 2020;2076942.
Lee Y, Chiou YJ, Hung CF, et al. A dyadic study of psychological well-being of individuals with Parkinson’s disease and their caregivers. Sci Rep. 2021;11(1):957.
Aarsland D, Larsen JP, Karlsen K, Lim NG, Tandberg E. Mental symptoms in Parkinson’s disease are important contributors to caregiver distress. Int J Geriatr Psychiatry. 1999;14(10):866–74.
Martínez-Martín P, Forjaz MJ, Frades-Payo B, et al. Caregiver burden in Parkinson’s disease. Mov Disord Off J Mov Disord Soc. 2007;22(7):924–1060.
D’Amelio M, Terruso V, Palmeri B, et al. Predictors of caregiver burden in partners of patients with Parkinson’s disease. Neurol Sci. 2009;30:171–4.
Happe S, Berger K. The association between caregiver burden and sleep disturbances in partners of patients with Parkinson’s disease. Age Ageing. 2002;31(5):349–54.
Whiteley N, Pluim CF, Split M, et al. Prospective predictors of care partner burden and depression in Parkinson’s disease. Int J Geriatr Psychiatry. 2022. https://doi.org/10.1002/gps.5795.
Rashid R, Aamodt WW, Horn S, Dahodwala N. Association of caregiver depression risk with patient outcomes in Parkinson disease. JAMA Netw Open. 2023;6(8): e2327485.
Cash TV, Ekouevi VS, Kilbourn C, et al. Pilot study of a mindfulness-based group intervention for individuals with Parkinson’s disease and their caregivers. Mindfulness. 2016;7:361–71.
Shah-Zamora D, Allen AM, Rardin L, et al. Mindfulness based stress reduction in people with Parkinson’s disease and their care partners. Complement Ther Clin Pract. 2021;43: 101377.
Seritan AL, Iosif AM, Prakash P, Wang SS, Eisendrath S. Online mindfulness-based cognitive therapy for people with Parkinson’s disease and their caregivers: a pilot study. J Technol Behav Sci. 2022;7(3):381–95.
Trend P, Kaye J, Gage H, Owen C, Wade D. Short-term effectiveness of intensive multidisciplinary rehabilitation for people with Parkinson’s disease and their carers. Clin Rehabil. 2002;16(7):717–25.
Macchi ZA, Koljack CE, Miyasaki JM, et al. Patient and caregiver characteristics associated with caregiver burden in Parkinson’s disease: a palliative care approach. Ann Palliat Med. 2020;9(Suppl 1):S24–33.
Allen AP, Curran EA, Duggan Á, et al. A systematic review of the psychobiological burden of informal caregiving for patients with dementia: focus on cognitive and biological markers of chronic stress. Neurosci Biobehav Rev. 2017;73:123–64.
Wang HY, Ren L, Li T, et al. The impact of anxiety on the cognitive function of informal Parkinson’s disease caregiver: evidence from task-based and resting-state fNIRS. Front Psych. 2022;13: 960953.
Secker DL, Brown RG. Cognitive behavioural therapy (CBT) for carers of patients with Parkinson’s disease: a preliminary randomised controlled trial. J Neurol Neurosurg Psychiatry. 2005;76(4):491–7.
Mosley PE, Robinson K, Dissanayaka NN, et al. A pilot trial of cognitive behavioral therapy for caregivers after deep brain stimulation for Parkinson’s disease. J Geriatr Psychiatry Neurol. 2021;34(5):454–65.
A’Campo LE, Wekking EM, Spliethoff-Kamminga NG, Le Cessie S, Roos RA. The benefits of a standardized patient education program for patients with Parkinson’s disease and their caregivers. Parkinsonism Relat Disord. 2010;16(2):89–95.
Karlstedt M, Fereshtehnejad SM, Aarsland D, Lökk J. Determinants of dyadic relationship and its psychosocial impact in patients with Parkinson's disease and their spouses. Parkinson's Dis. 2017;4697052.
Kim SR, Chung SJ, Shin NM, Shin HW, Kim MS, Lee SJ. Resilience in patients with parkinson’s disease. Korean J Adult Nurs. 2010;22(1):60–9.
Southwick SM, Bonanno GA, Masten AS, Panter-Brick C, Yehuda R. Resilience definitions, theory, and challenges: interdisciplinary perspectives. Eur J Psychotraumatol. 2014. https://doi.org/10.3402/ejpt.v5.25338.
Routledge FS, Campbell TS, McFetridge-Durdle J, Bacon SL. Improvements in heart rate variability with exercise therapy. Can J Cardiol. 2010;26(6):303–12.
Verdú E, Ceballos D, Vilches JJ, Navarro X. Influence of aging on peripheral nerve function and regeneration. J Peripher Nerv Syst. 2000;5(4):191–208.
Zesiewicz TA, Baker MJ, Wahba M, Hauser RA. Autonomic nervous system dysfunction in Parkinson’s disease. Curr Treat Options Neurol. 2003;5(2):149–60.
Leicht AS, Allen GD, Hoey AJ. Influence of intensive cycling training on heart rate variability during rest and exercise. Can J Appl Physiol. 2003;28(6):898–909.
Acknowledgements
The authors would like to sincerely thank Jim Cunningham and the participants who brought this intervention to fruition and were instrumental to its success.
Funding
This study was funded by the Prisma Health-Upstate Office of Philanthropy and Partnership (Greenville, SC, USA). This journal’s Rapid Service Fee was funded by the authors.
Author information
Authors and Affiliations
Contributions
Conceptualization: Jennifer L. Trilk, Fredy J. Revilla; Methodology: Jennifer L. Trilk, Fredy J. Revilla, Tracie Mcconnell; Formal analysis and investigation: Alia T. Sadek, Leila Djerdjour, Ryan A. Reyes, Greggory P. Adams, Cara H. Logan, Margaret A. Smith, Sara G. Biddle, Timothy S. Wiles, PhD, Enrique Urrea-Mendoza, MD, Tracie M. McConnell, PhD, Fredy J. Revilla, MD, and Jennifer L. Trilk, PhD; Writing—original draft preparation: Alia T. Sadek, Leila Djerdour, Ryan Reyes, Greggory Adams, Cara Logan, Margaret Smith, Jennifer L. Trilk; Writing—review and editing: Alia T. Sadek, Leila Djerdjour, Ryan A. Reyes, Greggory P. Adams, Cara H. Logan, Margaret A. Smith, Sara G. Biddle, Timothy S. Wiles, PhD, Enrique Urrea-Mendoza, MD, Tracie M. McConnell, PhD, Fredy J. Revilla, MD, and Jennifer L. Trilk, PhD; Funding acquisition: Jennifer L. Trilk, Fredy J. Revilla; Resources: Jennifer L. Trilk, Fredy J. Revilla, Tracie McConnell, Enrique Urrea-Mendoza; Supervision: Jennifer L. Trilk, Fredy J. Revilla.
Corresponding author
Ethics declarations
Conflict of Interest
All authors (Alia T. Sadek, Leila Djerdjour, Ryan A. Reyes, Greggory P. Adams, Cara H. Logan, Margaret A. Smith, Sara G. Biddle, Timothy S. Wiles, PhD, Enrique Urrea-Mendoza, MD, Tracie M. McConnell, PhD, Fredy J. Revilla, MD, and Jennifer L. Trilk, PhD) declare they have no competing interests.
Ethical Approval
Prisma Health Institutional Review Board approval was attained prior to beginning the study (Institutional Review Number Pro00110626). All subjects provided informed consent to participate in the study. No patients were involved in the design of this study or the dissemination of results. This study was conducted in accordance with the Helsinki Declaration of 1964 and its later amendments.
Additional information
Prior Presentations: 2021 USC School of Medicine Greenville Annual Research Symposium (Greenville, SC), 2022 Prisma Health Research Showcase (Greenville, SC), American College of Lifestyle Medicine 2022 Conference (Orlando, FL), 2024 American Academy of Neurology Conference (Denver, CO), 20th Annual SC Upstate Research Symposium (Spartanburg, SC) and 2024 South Carolina Medical Association Annual Meeting (Greenville, SC).
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Sadek, A.T., Djerdjour, L., Reyes, R.A. et al. The Feasibility and Efficacy of a Virtual Reality Tandem Cycling Program for Persons with Parkinson’s Disease and Their Care Partners. Neurol Ther (2024). https://doi.org/10.1007/s40120-024-00636-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40120-024-00636-3