Abstract
The management of Parkinson’s disease (PD) continues to evolve with advancements in non-oral levodopa-based therapies aiming to provide continuous drug delivery (CDD). Such therapies address the challenges posed by the emergence of motor fluctuations, dyskinesias, and non-motor fluctuations (NMF) associated with oral levodopa administration and contributing to define the advanced stage of PD. The key focus of this review is placed on subcutaneous foslevodopa/foscarbidopa (Foslevodopa/foscarbidopa) infusion, showcasing its recent clinical availability and efficacy in providing continuous levodopa delivery. While providing an overview of the other non-oral levodopa-based CDD systems, such as intrajejunal levodopa–carbidopa infusion and levodopa–entacapone–carbidopa infusion, we highlight the current promising evidence for Foslevodopa/foscarbidopa to improve, for example, “on time” without troublesome dyskinesia and reducing “off time” in people with advanced PD. Additionally, Foslevodopa/foscarbidopa demonstrates potential in managing early morning off periods, sleep quality and other motor and non-motor symptoms. Moreover, other non-oral CDD options such as ND0612 and DIZ102/DIZ101 are discussed, with focus on their pharmacokinetics/pharmacodynamics, efficacy, and safety profiles. While these advancements present new therapeutic avenues, long-term observational studies are warranted to elucidate their impact on existing PD therapies. Overall, this review provides insights into the evolving landscape of non-oral CDD therapies and offers a pragmatic approach for their integration into clinical practice.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Subcutaneous delivery of levodopa providing continuous 24 h levodopa has been a huge clinical and pharmacological challenge to develop but is now available for clinical use. |
Successful pivotal trial data of the novel subcutaneous foslevodopa/foscarbidpa, as well as subcutaneous levodopa/carbidopa using a different approach by Neuroderm (dual syringes and dual infusion sites) has been published. |
A new form of intrajejunal infusion with levodopa–carbidopa–entacpaone gel is also now in clinical use in many European countries and globally. |
Currently foslevdopa/foscarbidopa is registered by licensing authorities and post hoc analysis suggests significant benefit not only on motor function and motor complications of Parkinson’s disease (PD) but also early morning akinesia and sleep as well as nocturia. Monotherapy is also possible in over 30% of cases. |
Skin reaction with subcutaneous foslevodopa/foscarbidoopa as well as levodopa/carbidopa remain a concern but with scrupulous hygiene, tolerability rates are reasonable. |
Introduction
The Need for Non-oral Levodopa-Based Therapies
Levodopa remains the best symptomatic treatment for Parkinson’s disease (PD) since the 1960s and has gained widespread use, enabling a significant reduction in dosing and peripheral side effects with concomitant use of peripheral decarboxylase inhibitors [1]. However, motor fluctuations, dyskinesias, and non-motor fluctuations (NMF) emerge over time and pose significant clinical challenges [2].
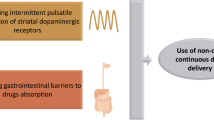
Fluctuating plasma levels of levodopa led to pulsatile stimulation of basal ganglia pathways, resulting in dose-dependent peak and patterns during the day as well as in hypo-dopaminergic states at night [3, 4]. Symptomatic treatment typically begins with three doses per day but, as a result of levodopa’s short half-life, more frequent dosing becomes necessary and attempts to reduce interdose intervals often prove inadequate beyond 4–5 doses/day, due to compliance issues and advancing PD. This can lead to disabling “no on” or “delayed on” periods [5]. To address these challenges, oral therapies including monoamine oxidase inhibitors (MAO), third-generation catechol-O-methyl transferase (COMT) inhibitors such as opicapone, and multi-receptor-active drugs like safinamide have been developed. Longer-acting oral levodopa formulations have been developed to provide continuous drug delivery (CDD) [6]. However, these have not been widely adopted because of various issues, including ongoing blocks to oral delivery (Fig. 1). Non-oral levodopa-based CDD strategies have, therefore, been a major focus for research and clinical trials for effective symptomatic management of PD in moderate-to-advanced stages [7, 8].
A key point for develo** CDD, which is effective for 24 h, as opposed to daytime-only therapies, is the fact that sleep dysfunction affects up to 90% of patients with PD, with issues like nocturnal akinesia and early morning off (EMO) periods posing significant challenges [9]. EMO periods, common in patients treated with conventional oral levodopa, are often overlooked and may be associated with disabling non-motor symptoms [10]. Providing continuous, 24-h delivery of levodopa in fluctuating patients with PD, therefore, remains appealing, as it mitigates the risk of dopamine agonist-related side effects, it provides physiological continuous delivery of dopaminergic stimulation and, additionally, may effectively manage EMO period [7].
Ethical Approval
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Non-oral Levodopa-Based Continuous Drug Delivery Systems
In this review, we focus on the recent release and clinical availability of subcutaneous foslevodopa/foscarbidopa (Foslevodopa/foscarbidopa) preparation for advanced PD, in addition to a short discussion on other similar available or soon-to-be available products. After the first trials in Sweden, levodopa infusion delivered through a gastrojejunostomy came into clinical practice in 2004 [11, 12]. Currently, several non-oral levodopa-based treatment options are in clinical use or in development as below:
-
Intrajejunal levodopa–carbidopa infusion (IJLI, in clinical use globally)
-
Intrajejunal levodopa–entacapone–carbidopa infusion (in clinical use in some European countries)
-
Subcutaneous Foslevodopa/foscarbidopa infusion (in clinical use after recent release in Europe, UK and Japan)
-
Subcutaneous levodopa–carbidopa infusion (ND0612, in development)
-
Intravenous levodopa infusion (in development)
While we will focus principally on Foslevodopa/foscarbidopa, we will also present data related to the other clinical options, either in development or available shortly.
Intrajejunal Levodopa/Carbidopa Infusion
Extensive literature is available about the use of a continuous infusion of levodopa/carbidopa (20 + 5 mg/ml respectively) intestinal gel (LCIG) using a percutaneous endoscopic gastrojejunostomy (PEG-J) for advanced PD. Controlled trials and open-label studies consistently demonstrated LCIG’s ability to maintain stable levodopa plasma levels, reducing “off” periods and improving “on” time without troublesome dyskinesias in patients with advanced PD [13,14,15,16]. Multiple studies, following on from the findings of Honig et al., have consistently demonstrated a reduction in various non-motor symptoms in advanced PD using the PD Non-Motor Symptoms Scale (NMSS) as an outcome measure [15, 17, 18].
Up to 31.4% of patients can be maintained on LCIG and levodopa monotherapy in advanced PD at 12 months’ follow-up, 33.4% at 36 months, offering a simplified treatment approach, especially for those with comorbidities [15]. However, side effects such as abdominal surgery-related complications and device issues are common in the first 3 months, with long-term risks including weight loss and axonal polyneuropathy, requiring careful monitoring of certain vitamins (vitamin B12, B6 and folic acid) [19]. In terms of efficacy, open-label multicentre comparative data (Euroinf studies) suggest equivalent motor and superior non-motor effect with IJLI at 6 months’ follow-up [20]. Cost-effectiveness analyses suggest LCIG may offer incremental benefits over standard care, with an estimated incremental cost-effectiveness ratio of £23,649/quality-adjusted life-year [21].
Intrajejunal Levodopa–Carbidopa–Entacapone Infusion
Levodopa/entacapone/carbidopa intestinal gel (LECIG), branded as Lecigon (other brand names include Lecigimon in Belgium), is a fixed-dose combination of levodopa–carbidopa–entacapone (available in doses of 20 + 5 + 20 mg/ml respectively) in a gel form. Approved by the Swedish Medical Product Agency (MPA) in 2018 and now patented in the USA, LECIG is utilised in European countries (Sweden, Germany, and Finland) and awaits UK regulatory approval. The Crono LECIG pump, which is smaller and lighter than the IJLI CADD Legacy pump (230 vs 500 g), has shown increased patient preference [22], although some patients discontinued LECIG treatment, primarily as a result of diarrhoea, in the single-centre study reported from Sweden [22–24] (Table 1). Studies demonstrate a comparable pharmacokinetics profile to LCIG with increased levodopa exposure [23]. Limited by small sample sizes, safety and efficacy conclusions are uncertain and long-term studies with large multicentre sample sizes are needed. A retrospective study from Romania suggests LECIG significantly reduces off time and moderate to severe dyskinesias [25]. However, the interpretation of this study is limited by a retrospective design with lack of robust outcome measures, quality of life, and non-motor measurements, as well as by the presence of patchy details of tolerability, length of follow-up, and side effects [25]. Another single-centre study by Viljaharju et al. from Finland suggests a higher early discontinuation rate of LECIG (19 patients completed 6 months’ follow-up from baseline cohort of 30) and the conclusion that the smaller size of the pump was the only advantage of the using LECIG [26]. Large-scale trials, including the planned multicentre ELEGANCE registry study, are needed to fully assess benefits and address gaps in data.
Subcutaneous Foslevodopa/Foscarbidopa Infusion
Levodopa is the gold standard for treating PD and continuous 24-h infusion of levodopa remains a key unmet need for patients and clinicians. This approach, from pharmacological, pathophysiological, and clinical perspectives appears to be a reasonable approach for addressing both motor symptoms and key levodopa-responsive non-motor symptoms, ideally round the clock to provide daytime and night-time cover [27]. Although attempted in the 1980s, logistical challenges and systemic volume overload led to the abandonment of this method [11]. Subsequently, Abbvie developed ABBV-951, known as Foslevodopa/foscarbidopa, enabling continuous subcutaneous infusion of levodopa/carbidopa prodrug which converts to levodopa and carbidopa in the body. The higher solubility of Foslevodopa/foscarbidopa allows the delivery of the amount of levodopa used by patients with advanced Parkinson’s in much smaller volumes compared to LCIG [27]. The system is specifically designed as a continuous infusion over 24 h via subcutaneous delivery, using a smaller and lighter pump compared to LCIG pump [25, 26, 28].
Pharmacokinetics
Pharmacokinetics/pharmacodynamics studies in healthy subjects having ABBV-951 administered subcutaneously showed the pharmacokinetic profile of Foslevodopa/foscarbidopa to be similar to LCIG infusion data with steady levodopa levels achieved 2 h after administration [29]. Rosebraugh et al. also showed that Foslevodopa/foscarbidopa subcutaneous infusion reduced fluctuations in plasma levodopa levels, provided equivalent levodopa levels to LCIG infusion over the 16-h interval and, additionally, maintained those levels throughout the night-time period providing 24 h cover.
Clinical Trials
The pivotal licensing trial with Foslevodopa/foscarbidopa was a 12-week randomised, double-blind, double-dummy, active-controlled study (NCT04380142) and enrolled 174 patients, with 141 randomised to continuous subcutaneous infusion of Foslevodopa/foscarbidopa plus oral placebo capsules (n = 74) or oral immediate-release levodopa/carbidopa plus outcome measure with Foslevodopa/foscarbidopa showing a significantly greater increase in on time without troublesome dyskinesia (“good on”) of 1.75 h (p = 0.0083) and a significantly greater reduction in “off time” (− 1.79 h, p = 0.0054) [30]. Key secondary efficacy measures also included MDS-UPDRS part 2, although there were no significant differences between Foslevodopa/foscarbidopa and oral levodopa/carbidopa [30]. The authors note that there was a numerical improvement in MDS-UPDRS 2 favouring Foslevodopa/foscarbidopa and statistically the unadjusted magnitude of this trend was considered clinically meaningful for this patient group. The study also confirmed that the Foslevodopa/foscarbidopa drug device is also able to adjust dosing and deliver a range of therapeutically relevant doses of foslevodopa (approximately 600–4250 mg/day levodopa equivalents) for personalising treatment. Aldred et al. reported a 52-week, open-label international phase 3 registrational trial (NCT03781167) evaluating safety/tolerability as well as long-term efficacy of 24-h/day subcutaneous Foslevodopa/foscarbidopa in advanced PD [31]. Out of 244 enrolled subjects, 137 completed treatment and, at the 52-week follow-up, “on time” without troublesome dyskinesia and “off time” remained significantly improved from baseline. There was also a concomitant improvement in quality of life (QoL), while motor complications were noted at week 1 after initiation of Foslevodopa/foscarbidopa and sustained throughout the 52-week treatment period. In the UK, the National Institute of Health and Care Excellence (NICE) has issued guidance [32] for the use of Foslevodopa/foscarbidopa recommending it as an option for treating advanced levodopa-responsive Parkinson’s in adults whose symptoms include severe motor fluctuations and hyperkinesia or dyskinesia, when available medicines are not working well enough or if they cannot have apomorphine or deep brain stimulation, or these treatments no longer control symptoms effectively. Elsewhere in Europe, the indications vary. The warnings and precautions for use are generic for levodopa and therefore the same for Foslevodopa/foscarbidopa.
Early Morning Off and Sleep
As previously mentioned, EMO periods, often also termed “early morning akinesia”, pose a significant challenge in PD treatment, affecting over 60% of levodopa-treated patients, with more than 80% experiencing severe non-motor fluctuations [10]. Foslevodopa/foscarbidopa has shown promise in reducing EM akinesia, with Aldred et al. reporting a drop from 77.7% (129 patients) at baseline to 27.8% (25 patients) at week 52 in patients experiencing EMO, accompanied by improvements in sleep quality and QoL [31]. At week 12, patients who received Foslevodopa/foscarbidopa reached good “on time” after waking nearly three times faster (28.9 vs 82.9 min; p = 0.004) and more patients who received Foslevodopa/foscarbidopa woke up in good “on” state than patients who received oral immediate-release levodopa/carbidopa (83.3% vs 39.7%). Temporal patterns showed that greatest improvements and differences were reported in the first hours after waking. Chaudhuri et al. found that improved sleep with Foslevodopa/foscarbidopa correlated positively with reductions in “off” time and improvements in motor experiences of daily living and QoL, potentially due to successful management of EMO [33]. In addition, there was also a significant positive correlation between improvement in Parkinson’s Disease Sleep Scale 2 (PDSS-2) scores and change to week 26 in MDS-UPDRS part II scores [33]. This data, therefore, further suggests that improved sleep with Foslevodopa/foscarbidopa was associated with improved QoL and “off” time and could be consequent to successful management of EMO. Data also suggests that patients with advanced PD on Foslevodopa/foscarbidopa experience good “on time” within 30 min on waking in the morning [34].
Other benefits of Foslevodopa/foscarbidopa have also been reported mainly in abstracts so far, and are indicated in Fig. 2, where we have summarised the constellation of effects and other possible efficacy measures related to the clinical use of Foslevodopa/foscarbidopa. These include nocturia, freezing of gait, benefits in early advanced PD, cost–benefit analysis, monotherapy and safety.
Nocturia
Nocturia is common in PD and often disrupts sleep. Chaudhuri et al. conducted post hoc analyses of data from the 12-week pivotal trial (NCT04380142) and the 52-week open-label safety study (NCT03781167) of Foslevodopa/foscarbidopa in patients with PD, investigating changes in nocturia, measured with the PDSS-2, over time [33]. Results indicate a significant improvement in nocturia symptoms with Foslevodopa/foscarbidopa compared to oral levodopa at week 12 (p < 0.01). In the open-label study, there were significant reductions in nocturia scores at weeks 6, 13, 26, and 52 compared to baseline (p < 0.001 for all comparisons). Bladder function in PD may involve dopamine D1 receptor activity, and the D1 effect of Foslevodopa/foscarbidopa combined with sustained overnight stimulation may be the underlying mechanism, warranting further investigation.
Freezing of Gait
Odin et al. performed a post hoc analysis of single items related to gait and freezing [Movement Disorders Society-Unified Parkinson’s Disease Rating Scale (MDS-UPDRS) items 2.12, 2.13. 3.10–3.13] from Foslevodopa/foscarbidopa registration trials and showed that patients in the Foslevodopa/foscarbidopa arm achieved significant improvements from baseline to week 12 in single items for walking and balance, freezing, and gait compared to oral levodopa therapy in the double-blind trials. In the open-label analysis, significant improvements were observed from baseline in walking and balance and freezing at weeks 13 and 52 although gait, postural stability, and posture worsened vs baseline at week 52. Further work in relation to gait and freezing and use of Foslevodopa/foscarbidopa is therefore warranted [35].
Early Advanced PD
Antonini et al. reported post hoc analysis of efficacy of Foslevodopa/foscarbidopa in the earlier stage of advanced PD which they defined as age ≤ 65 years, Hohen and Yahr stage ≤ 2 (in “on” state) and time since motor fluctuations of ≤ 3 years [36]. Patients with an earlier stage of advanced PD and later stages reported similar improvements in motor fluctuations and QoL following treatment with Foslevodopa/foscarbidopa.
Monotherapy with Foslevodopa/Foscarbidopa
Aldred et al. reported data on concomitant dopaminergic medication use in 244 patients on Foslevodopa/foscarbidopa. In patients with advanced PD who were initiated on Foslevodopa/foscarbidopa treatment, over 30% could achieve monotherapy by week 52 [31]. During this period overall, there was a 10.3% reduction in the number of patients taking ≥ 3 PD medications and the levodopa equivalent daily dose of Foslevodopa/foscarbidopa remained stable over time (range 1621.9–1847.0 mg/day) [29].
Safety
In the pivotal study comparing Foslevodopa/foscarbidopa with oral levodopa, adverse events were reported in 85% of Foslevodopa/foscarbidopa participants and in 63% of oral levodopa–carbidopa participants [30]. Serious adverse events occurred at similar rates between the groups. The main side effects in the Foslevodopa/foscarbidopa group were related to infusion site issues, including erythema (27%), local site pain (26%), cellulitis (19%), and skin oedema (12%) [31]. It is also to be noted that there were no reports of polyneuropathy in this study. Most adverse events were mild or moderate in severity and non-serious. Serious adverse events were noted in 9% of the Foslevodopa/foscarbidopa arm, with hallucinations and psychosis reported in 15%. The percentage of patient who discontinued Foslevodopa/foscarbidopa in the open-label phase as a result of hallucinations was 4.1%. Notably, hallucinations and psychosis were observed in the 12-week trial, and it is possible that this was related to the trial regime which did not allow any night-time dose modifications [30]. In real-life practice with commercially available pumps, dosing adjustments can be made, potentially alleviating these effects. Nurse support could also help prevent and manage side effects, which is crucial in the real-world use of Foslevodopa/foscarbidopa. These issues have been discussed in a recent paper addressing practical tips on initiation and maintenance of Foslevodopa/foscarbidopa [37]. In the 12-month, open-label, phase 3 study, serious adverse events were reported in 25.8% of patients, including infusion site cellulitis (4.1%) and infusion site abscess (3.3%) [31]. The majority of adverse events were nonserious, mild, or moderate in severity and resolved with appropriate care over time [31].
ND0612 (Subcutaneous Levodopa/Carbidopa)
ND0612, developed by NeuroDerm, is another subcutaneous form of levodopa but, unlike Foslevodopa/foscarbidopa, it is a liquid formulation of levodopa/carbidopa providing delivery through a continuous subcutaneous infusion pump system using dual syringes and dual infusion sites as opposed to one for Foslevodopa/foscarbidopa. The pivotal trial data has recently been published and ND0612 remains under review by the US Food and Drug Administration (FDA).
Pharmacokinetics
In a series of six phase I and II studies involving healthy volunteers and fluctuating patients with PD, LeWitt et al. demonstrated that levodopa concentration of 60 mg/ml combined with carbidopa 7.5 mg/ml, complemented with oral levodopa/carbidopa, exhibited an acceptable profile for continuous 24-h administration in patients with PD [38]. On the basis of this finding, NeuroDerm developed a drug-device combination designed to continuously deliver liquid levodopa/carbidopa (60/7.5 mg/ml) via subcutaneous infusion in clinical studies.
Poewe et al. reported the BeyoND study, a 12-month open-label safety study with ND0612, demonstrating significant improvement in on time without troublesome dyskinesias (− 1.3 (1.7) change from baseline) but no significant changes in “off” time or “good on” time [39]. No major differences in safety between the 16- and 24-h regimens were noted [40].
Espay et al. recently reported the results of the BouNDless trial (NCT04006210), a randomised, active-controlled, double-blind, double-dummy trial designed to assess the efficacy, safety, and tolerability of ND0612 compared to oral levodopa/carbidopa in patients with PD with motor fluctuations [41]. Patients underwent a 12-week trial followed by two sequential open-label periods for optimization of treatment. Of 259 subjects with PD, 128 were randomly assigned to subcutaneous ND0612 and 131 to oral levodopa–carbidopa with a 94% completion rate [41]. ND0612 improved “on time” without troublesome dyskinesia compared to oral levodopa/carbidopa by 1.72 h (95% CI 1.08–2.36, p < 0.0001). There were nine prespecified hierarchical outcomes, and significant treatment differences favouring subcutaneous ND0612 were reported for four, namely daily “off time” [− 1.40 h (95% CI − 1.99 to − 0.80)], MDS-UPDRS part II scores, PGIC, and Clinical Global Impression of Change (CGI-C).
Safety
Initial studies revealed significant side effect issues, leading to protocol revisions and temporary trial suspensions in certain countries. Consent withdrawal (19.6%) and adverse events (17.3%) were the leading causes of study discontinuation. Following a protocol revision and retraining, the discontinuation rate decreased from 49% to 29%. In the trial by Espay et al., 7% of participants discontinued because of infusion-site reactions, and 9% reported infusion-site infections (cellulitis) [41]. Notably, patients with severe PD (Hoehn and Yahr stage ≥ 4) or severe disabling dyskinesia were excluded from the study. PDSS-2 data did not show significant changes, and there is a lack of overall non-motor or early morning akinesia-related data. A long-term, open-label, follow-up study is underway. Practical issues with ND0612 include the use of two syringes and needles, as well as a maximum daily dose limited to 720 mg.
Subcutaneous DIZ102 and Intravenous DIZ101
DIZ102, branded as Infudopa SubC™, is a concentrated acidic levodopa/carbidopa solution (8:1) developed in Sweden, intended for outpatient treatment via subcutaneous infusion using a portable twin pump for continuous mixing before infusion [42]. It is designed for use as monotherapy with levodopa, has a shelf life of 36 months in the refrigerator and at least 3 months at room temperature, requiring two subcutaneous infusion catheters. The intravenous DIZ101 has the same levodopa/carbidopa concentration, delivered intravenously through an indwelling vein catheter in the arm [42].
In a recent single-centre pharmacokinetic crossover study involving 18 evaluable subjects with PD, DIZ101 demonstrated plasma levels of levodopa comparable to those obtained by LCIG, with 100% bioavailability with subcutaneous administration compared to 80% with IJLI [42]. DIZ102 displayed similar pharmacokinetic properties to DIZ101, with the fastest increase in plasma levodopa levels after infusion [42].
Skin-related side effects were absent or mild, and DIZ101 compared favourably with high doses of oral/intestinal levodopa, providing rapid onset of action. DIZ101 can be stored for up to 1 year at room temperature, with potentially low rates of skin reactions/infections due to its low pH. However, the large volume of infusion raises concerns about volume overload, and there is no data on long-term tolerability. The 16-h infusions lack data on sleep or non-motor aspects of PD.
A Potential Clinical Pathway of Care
In Fig. 3 we propose a pragmatic evidence-based and clinical experience-based flowchart to aid how the different treatment strategies that are currently available (as such, Neuroderm, DIZ101 and DZ102 are not considered) can be used in the clinic. These options could be seen as an addition to the New European Academy of Neurology/Movement Disorder Society European Section Guideline for Invasive Therapies in Parkinson’s Disease for further use [43]. In the flowchart of Fig. 3 we have considered several options, including the potential use of Foslevodopa/foscarbidopa therapy for non-motor fluctuations (NMF) as a whole, based on the current evidence and clinical experience [43]. In the flowchart, we have suggested that NMF that accompany motor fluctuations, such as pain, anxiety and low mood, can also be effectively managed by using Foslevodopa/foscarbidopa. This is also evident from clinical experience of using other levodopa-based infusion therapies, although specific studies need to be performed. In this flowchart, we also consider the option of infusion therapy in older patients (> 75 years of age) with the option of home nursing support, if needed.
Potential algorithm for clinical use of available advanced therapies treatment options. PD Parkinson’s disease, APD advanced PD, PKG Personal KinetiGraph, HY Hohen & Yahr, NMF non-motor fluctuations, MRgFUS MRI-guided focused ultrasound, DBS deep brain stimulation, STN subthalamic nucleus, GPi globus pallidus internus, RLS restless legs syndrome, IJLI intrajejunal levodopa infusion, LECIG levodopa–entacapone–carbidopa intestinal gel, ? indicates possible consideration of the technique if locally available
Conclusions
For over a decade, advanced therapies for PD were limited to surgical and infusion therapies. The motor and non-motor effects of these therapies have been well established and compared in studies like Euroinf 2 [20]. However, since the late 2010s, the landscape has changed with the introduction of intrajejunal LECIG delivered via a smaller pump system, as well as the recent approval of Foslevodopa/foscarbidopa. This marks the first clinically approved use of subcutaneous levodopa, presenting new therapeutic challenges.
The emergence of new treatment options prompts questions about their impact on existing therapies. Definitive answers require long-term observational studies comparing LCIG and Foslevodopa/foscarbidopa as well as LCIG versus LECIG trials as was done in the Euroinf studies. Skin hygiene is crucial for maintaining Foslevodopa/foscarbidopa therapy, while patient preference for smaller pump sizes may favour LECIG. Despite these advancements, there remains a role for IJLI and apomorphine infusion, especially for managing night-time sleep dysfunction, as demonstrated in the APOMORPHEE trial [44] or as a rescue option for predictable off periods. Patients may not tolerate entacapone infusion contained in LECIG and IJLI may be the preferred option while dyskinesias may also favour use of LCIG. Data from the BouNDless trial for ND0612 show acceptable tolerability, but its long-term efficacy, sleep benefits, and early morning effects remain uncertain, leaving its position in advanced PD care pathways unclear at present.
Data Availability
This a review of published data, all data discussed are in the public domain.
References
Papavasiliou PS, Cotzias GC, Düby SE, Steck AJ, Fehling C, Bell MA. Levodopa in Parkinsonism: potentiation of central effects with a peripheral inhibitor. N Engl J Med. 1972;286:8–14.
Ray Chaudhuri K, Poewe W, Brooks D. Motor and nonmotor complications of levodopa: phenomenology, risk factors, and imaging features. Mov Disord. 2018;33:909–19.
Stocchi F, Jenner P, Obeso JA. When do levodopa motor fluctuations first appear in Parkinson’s disease? Eur Neurol. 2010;63:257–66.
Kuoppamäki M, Korpela K, Marttila R, et al. Comparison of pharmacokinetic profile of levodopa throughout the day between levodopa/carbidopa/entacapone and levodopa/carbidopa when administered four or five times daily. Eur J Clin Pharmacol. 2009;65:443–55.
Leta V, Klingelhoefer L, Longardner K, et al. Gastrointestinal barriers to levodopa transport and absorption in Parkinson’s disease. Eur J Neurol. 2023;30:1465–80.
Lees A, Tolosa E, Stocchi F, et al. Optimizing levodopa therapy, when and how? Perspectives on the importance of delivery and the potential for an early combination approach. Expert Rev Neurother. 2023;23:15–24.
Urso D, Chaudhuri KR, Qamar MA, Jenner P. Improving the delivery of levodopa in Parkinson’s disease: a review of approved and emerging therapies. CNS Drugs. 2020;34:1149–63.
Olanow CW, Obeso JA, Stocchi F. Continuous dopamine-receptor treatment of Parkinson’s disease: scientific rationale and clinical implications. Lancet Neurol. 2006;5:677–87.
Falup-Pecurariu C, Diaconu Ş. Twenty-three—sleep dysfunction in Parkinson’s disease. In: Chaudhuri KR, Titova N, editors. International review of neurobiology. Cambridge: Academic; 2017. p. 719–42.
Rizos A, Martinez-Martin P, Odin P, et al. Characterizing motor and non-motor aspects of early-morning off periods in Parkinson’s disease: an international multicenter study. Parkinsonism Relat Disord. 2014;20:1231–5.
Quinn N, Marsden CD, Parkes JD. Complicated response fluctuations in Parkinson’s disease: response to intravenous infusion of levodopa. Lancet. 1982;320:412–5.
Nyholm D, Nilsson Remahl AI, Dizdar N, et al. Duodenal levodopa infusion monotherapy vs oral polypharmacy in advanced Parkinson disease. Neurology. 2005;64:216–23.
Olanow CW, Kieburtz K, Odin P, et al. Continuous intrajejunal infusion of levodopa–carbidopa intestinal gel for patients with advanced Parkinson’s disease: a randomised, controlled, double-blind, double-dummy study. Lancet Neurol. 2014;13:141–9.
Antonini A, Abbruzzese G, Berardelli A, et al. The TANDEM investigation: efficacy and tolerability of levodopa-carbidopa intestinal gel in (LCIG) advanced Parkinson’s disease patients. J Neural Transm (Vienna). 2020;127:881–91.
Chaudhuri KR, Kovács N, Pontieri FE, et al. Levodopa carbidopa intestinal gel in advanced Parkinson’s disease: DUOGLOBE final 3-year results. J Parkinsons Dis. 2023;13:769–83.
Antonini A, Odin P, Pahwa R, et al. The long-term impact of levodopa/carbidopa intestinal gel on ‘off’-time in patients with advanced Parkinson’s disease: a systematic review. Adv Ther. 2021;38:2854–90.
Fasano A, García-Ramos R, Gurevich T, et al. Levodopa–carbidopa intestinal gel in advanced Parkinson’s disease: long-term results from COSMOS. J Neurol. 2023;270:2765–75.
Honig H, Antonini A, Martinez-Martin P, et al. Intrajejunal levodopa infusion in Parkinson’s disease: a pilot multicenter study of effects on nonmotor symptoms and quality of life. Mov Disord. 2009;24:1468–74.
Leta V, Batzu L, Williams L, et al. Levodopa–carbidopa intestinal gel infusion and long-term tolerability in Parkinson’s: multicentre, international 13-year follow-up data [abstract]. Mov Disord. 2022;37(suppl 2). https://www.mdsabstracts.org/abstract/levodopa-carbidopa-intestinal-gel-infusion-and-long-term-tolerability-in-parkinsons-multicentre-international-13-year-follow-up-data/. Accessed 14 May 2024.
Dafsari HS, Martinez-Martin P, Rizos A, et al. EuroInf 2: Subthalamic stimulation, apomorphine, and levodopa infusion in Parkinson’s disease. Mov Disord. 2019;34:353–65.
Chaudhuri KR, Pickard AS, Alobaidi A, et al. The cost effectiveness of levodopa–carbidopa intestinal gel in the treatment of advanced Parkinson’s disease in England. Pharmacoeconomics. 2022;40:559–74.
Öthman M, Widman E, Nygren I, Nyholm D. Initial experience of the levodopa–entacapone–carbidopa intestinal gel in clinical practice. J Pers Med. 2021;11:254.
Senek M, Nielsen EI, Nyholm D. Levodopa–entacapone–carbidopa intestinal gel in Parkinson’s disease: a randomized crossover study. Mov Disord. 2017;32:283–6.
Nyholm D, Jost WH. Levodopa–entacapone–carbidopa intestinal gel infusion in advanced Parkinson’s disease: real-world experience and practical guidance. Ther Adv Neurol Disord. 2022;15:17562864221108018.
Szász JA, Dulamea AO, Constantin VA, et al. Levodopa–carbidopa–entacapone intestinal gel in advanced parkinson disease: a multicenter real-life experience. Am J Ther. 2024;31:e209–18.
Viljaharju V, Mertsalmi T, Pauls KAM, et al. Levodopa–entacapone–carbidopa intestinal gel treatment in advanced Parkinson’s disease: a single-center study of 30 patients. Mov Disord Clin Pract. 2024;11:159–65.
LeWitt PA, Chaudhuri KR. Unmet needs in Parkinson disease: motor and non-motor. Parkinsonism Relat Disord. 2020;80(Suppl 1):S7-s12.
Rosebraugh M, Liu W, Neenan M, Facheris MF. Foslevodopa/foscarbidopa is well tolerated and maintains stable levodopa and carbidopa exposure following subcutaneous infusion. J Parkinsons Dis. 2021;11:1695–702.
Rosebraugh M, Stodtmann S, Liu W, Facheris MF. Foslevodopa/foscarbidopa subcutaneous infusion maintains equivalent levodopa exposure to levodopa–carbidopa intestinal gel delivered to the jejunum. Parkinsonism Relat Disord. 2022;97:68–72.
Soileau MJ, Aldred J, Budur K, et al. Safety and efficacy of continuous subcutaneous foslevodopa–foscarbidopa in patients with advanced Parkinson’s disease: a randomised, double-blind, active-controlled, phase 3 trial. Lancet Neurol. 2022;21:1099–109.
Aldred J, Freire-Alvarez E, Amelin AV, et al. Continuous subcutaneous foslevodopa/foscarbidopa in Parkinson’s disease: safety and efficacy results from a 12-month, single-arm, open-label, phase 3 study. Neurol Ther. 2023;12:1937–58.
NICE. Foslevodopa-foscarbidopa for treating advanced Parkinson's with motor symptoms. Available at: https://www.nice.org.uk/guidance/ta934/resources/foslevodopafoscarbidopa-for-treating-advanced-parkinsons-with-motor-symptoms-pdf-82615608069829. Accessed 9 June 2024
Chaudhuri KR, Facheris MF, Bergmans B, et al. Improved sleep correlates with improved quality of life and motor symptoms with foslevodopa/foscarbidopa. Mov Disord Clin Pract. 2024. https://doi.org/10.1002/mdc3.14018.
Pahwa R, Malaty I, Soileau Met al. Stability and predictability of motor symptom control in patients with advanced Parkinson's disease (aPD) receiving continuous subcutaneous foslevodopa/foscarbidopa (LOP/CDP) [abstract]. Abstract 105 Mov Disord. 2023; 38 (suppl 1). In: Presented at the 17th International Congress on Movement Disorders, Copenhagen, 2023.
Odin P, Kimura T, Santos-Garcia D et al. Effect of continuous subcutaneous foslevodopa/foscarbidopa treatment on falls, posture and freezing of gait. Presented at the European Academy of Neurology, 1-4 July 2023, Budapest, Hungary: EPR-281. Eur J Neural. 2023;1:1–837. https://doi.org/10.1111/ene.15944.
Antonini A, Bergmans B, Kern D et al. Improvement in motor symptoms and quality of life in patients with an earlier stage of advanced Parkinson’s disease treated with foslevodopa/foscarbidopa subcutaneous 24 hour infusion [abstract]. Abstract 23 Mov Disord. 2023;38 (suppl 1). Presented at the International Congress of Parkinson’s Disease and Movement Disorders, 27–31 August 2023, Copenhagen, Denmark.
Fung VSC, Aldred J, Arroyo MP, et al. Continuous subcutaneous foslevodopa/foscarbidopa infusion for the treatment of motor fluctuations in Parkinson’s disease: considerations for initiation and maintenance. Clin Park Relat Disord. 2024;10: 100239.
LeWitt PA, Stocchi F, Arkadir D, et al. The pharmacokinetics of continuous subcutaneous levodopa/carbidopa infusion: Findings from the ND0612 clinical development program. Front Neurol. 2022;13:1036068.
Poewe W, Stocchi F, Arkadir D, Ebersbach G, Ellenbogen AL, Giladi N, Isaacson SH, Kieburtz K, LeWitt P, Olanow CW, Simuni T, Thomas A, Zlotogorski A, Adar L, Case R, Oren S, Fuchs Orenbach S, Rosenfeld O, Sasson N, Yardeni T, Espay AJ (2021) Subcutaneous levodopa infusion for parkinson’s disease: 1-year data from the open-label beyond study. Mov Disord 36(11):2687–2692. https://doi.org/10.1002/mds.28758.
Isaacson S, Warren Olanow C, Simuni T, et al. Long-term safety of continuous levodopa/carbidopa infusion with ND0612: results from the ongoing BeyoND study. Poster presented at American Academy of Neurology Annual Meeting; 2022 Apr 2–7; Seattle, WA.
Espay AJ, Stocchi F, Pahwa R, et al. Safety and efficacy of continuous subcutaneous levodopa–carbidopa infusion (ND0612) for Parkinson’s disease with motor fluctuations (BouNDless): a phase 3, randomised, double-blind, double-dummy, multicentre trial. Lancet Neurol. 2024;23(5):465–76.
Bergquist F, Ehrnebo M, Nyholm D, et al. Pharmacokinetics of intravenously (DIZ101), subcutaneously (DIZ102), and intestinally (LCIG) infused levodopa in advanced Parkinson disease. Neurology. 2022;99:e965–76.
Brinker D, Smilowska K, Paschen S, Antonini A, Moro E, Deuschl G. How to use the New European Academy of Neurology/Movement Disorder Society European section guideline for invasive therapies in Parkinson’s disease. Mov Disord Clin Pract. 2024;11:209–19.
De Cock VC, Dodet P, Leu-Semenescu S, et al. Safety and efficacy of subcutaneous night-time only apomorphine infusion to treat insomnia in patients with Parkinson’s disease (APOMORPHEE): a multicentre, randomised, controlled, double-blind crossover study. Lancet Neurol. 2022;21:428–37.
Funding
No funding or sponsorship was received for this study or publication of this article.
Author information
Authors and Affiliations
Contributions
Karolina Poplawska-Domaszewicz, Lucia Batzu and Kallol Ray Chaudhuri all contributed to conception, writing and contributing to all figures and tables and reference integrity. Cristian Falup Pecurariu helped review the text as well as provide expert opinion. All authors contributed to writing the review.
Corresponding author
Ethics declarations
Conflict of Interest
Kallol Ray Chaudhuri has been expert and advisor for Abbvie, Britannia, Stada and Neuroderm (clinical trial). Cristian Falup Pecurariu has been expert and advisor for Abbvie, Britannia and Stada. Karolina Poplawska-Domaszewicz: has been expert and advisor for Abbvie, Britannia and Stada. Lucia Batzu has nothing to disclose.
Ethical Approval
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Poplawska-Domaszewicz, K., Batzu, L., Falup-Pecurariu, C. et al. Subcutaneous Levodopa: A New Engine for the Vintage Molecule. Neurol Ther (2024). https://doi.org/10.1007/s40120-024-00635-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40120-024-00635-4