Abstract
Background
Coronavirus disease 2019 (COVID-19) is responsible for a wide variety of multi-system clinical features. Facial nerve palsy (FNP) is identified as one of the neurological complications of the virus. This work aims to systematically review the clinical picture, laboratory/imaging findings, treatment options, and prognostic factors of FNP in COVID-19 patients.
Methods
Using six online databases, a search was conducted to include all articles with patients infected with COVID-19 and presenting with unilateral or bilateral FNP. Screening for eligibility and data extraction were done by three and four independent reviewers, respectively. Descriptive analyses and data visualizations were done using Google Sheets. Survival analysis and Kaplan–Meier plotting were done by R software.
Results
The data from 22 studies included 32 patients who were infected with COVID-19 and presented with clinical features of FNP. Fourteen patients were male while 18 were female. FNP affected 29 patients unilaterally and 3 patients bilaterally. The imaging findings confirmed that complications of FNP were COVID-19 related. Additionally, antivirals combined with steroids had the lowest median time (21, IQR = 8) to clinical improvement compared to steroid-only (30, IQR = 15) and antiviral-only (33, IQR = 3.5) treatments.
Conclusion
This study has shown a potential correlation between the increased incidence of FNP and COVID-19. We have also found that combining antivirals with steroids may have better outcomes in patients with FNP and COVID-19 although the evidence to support this claim is not strong enough. Further studies are required to assess the extent of linkage between the two conditions and how to properly manage FNP when encountered in COVID-19 patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Since its outbreak on the 31st December of 2019, coronavirus disease 2019 (COVID-19) has infected more than 66 million people till the end of November 2020 globally [1]. Although the reported number of cases is massive, many disease mappers believe that the actual burden of this pandemic is higher than anticipated [2]. This is because up to 80% of infected cases are asymptomatic, and 20% of them will remain asymptomatic disease propagators [3] till testing negative [4]. Moreover, the high rate of transmission (R naught) makes it hard to contain the viral spread [5,6,7,8].
It takes nearly 5.2 days for the virus to start manifesting. Common symptoms include fever, cough with or without sputum production, sore throat, sneezing, headache, and/or diarrhea [5, 7]. Other symptoms that require immediate care and hospitalization include shortness of breath, bluish discoloration of membranes, persistent chest pain, and new confusion [7]. Atypical, non-respiratory manifestations of COVID-19 include a fruitful of central and peripheral nervous system conditions, such as olfaction and gustation dysfunction, encephalitis, Guillain–Barre syndrome, stroke, neuralgia, epilepsy, and ataxia [9]. Based on recent data published, facial nerve palsy (FNP) rates can be correlated to infection with SARS-CoV-2 [10,11,12,13]. One study estimated that the incidence increased from 1.3% in 2019 to 3.5% in 2020 [14].
The pathogenesis of idiopathic FNP is not fully understood to this day. Some researchers found some degree of disrupted blood supply to the nerve through the vasa nervorum. This process can be related to the development of microthrombi, idiopathic vasospasms, or edema [15,16,17,18]. During the COVID-19 pandemic, there is also a possibility of direct viral invasion or autoimmune reaction (e.g., Guillain–Barré syndrome) to be the triggers of FNP [19, 20]. The direct invasion hypothesis is suitable for SARS-CoV-2 due to its high selectivity to the angiotensin-converting enzyme 2 (ACE2) receptor [20, 21]. This specific receptor is found on different tissue cell membranes, including the vascular endothelial cells [22] and brainstem glial cells [23], making neurological complications of COVID-19 more logical [21].
The true impact of COVID-19 on the incidence of FNP is yet to be discovered. In this paper, we aim to represent the clinical picture, laboratory/imaging findings, treatment options, and prognosis of FNP in cases suffering from concomitant COVID-19.
Methods
Protocol development and registration
Guided by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement [24], the protocol was registered on the International Prospective Register of Systematic Reviews (PROSPERO) with ID number CRD42021226404 [25]. Online Resource 1 provides the updated results as a PRISMA checklist. As a systematic review, the manuscript does not contain clinical studies or patient data.
Search strategy
In November 2020, we searched six electronic databases for possible inclusion. In PubMed, we used this search term: [(facial nerve) OR (seventh cranial nerve) OR (facial paralysis) OR (bell’s palsy) OR (facial palsy)] AND (SARS OR MERS OR coronavirus OR coronaviruses OR HCoV OR nCoV OR Cov OR Cov2 OR COVID OR COVID19 OR corona OR coronaviridae). The term was edited to suit the other databases (Scopus, Web of Science, Google Scholar, Virtual Health Library VHL, and Ovid). Online Resource 2 provides the search terms used for each database and the yielded number of articles.
Manual search
Before summarizing the data from the included studies, another PubMed search, in 2021, was done to identify newer articles, if available. Additionally, two manual searches were conducted to trace the references and citations of the included studies using PubMed, Google Scholar, and Scopus to identify any other relevant studies.
Eligibility criteria
The inclusion criteria were applied as follows: any study reporting positive COVID-19 and facial nerve palsy regardless of race, age, sex, language, socioeconomic status, ethnicity, geographical area/place, and publication date. Non-extractable data, duplications, unreliable or incomplete reports, conference proceedings, commentaries, editorial, discussions, books, or book chapters were excluded. Overlapped data sets were also excluded after extensive assessment and revisions. Finally, studies reporting FNP as part of a syndrome like Guillain–Barré syndrome and Melkersson–Rosenthal syndrome were not included.
Article selection
Titles and abstracts were firstly screened for inclusion using the above-mentioned criteria by three independent reviewers. Then, the full texts were obtained and screened with the same procedure. Through discussion, any disagreement was resolved. In case the three reviewers did not reach a consensus, a senior reviewer helped in solving the dispute.
Data extraction
Using Google Sheets, a pilot extraction sheet was developed using the two most relevant articles included. The data extraction process was held in a proximate fashion to the screening steps where four independent authors extracted the data, compared the results at the end, and solved any differences by discussing them. Further conflicts were resolved by a senior researcher. The extracted data included the study referencing form (first author’s last name, year of publication, and country of investigation), basic study characteristics (publishing journal, type of study, and peer-review status), and patients’ data (age, sex, comorbidities, medical history, COVID-19 status, side of the affected nerve, other nerves affected besides cranial nerve VII, time of symptom resolution, and the follow-up duration). Data about investigations were also extracted and discussed if available; cerebrospinal fluid (CSF), magnetic resonance imaging (MRI), and computerized tomography (CT) scan.
Quality assessment
Quality assessment of all included studies was performed in the same manner as data extraction. The quality of the included studies was weighed using the Joanna Briggs Institute Critical Appraisal Checklist for Case Reports [26].
Statistical analysis
Basic descriptive statistical analyses and data visualizations were done using Google Sheets. The data was presented in crude and relative numbers using percentages. As for the survival analysis and Kaplan–Meier plotting, they were done using R software version 4.1.1 [27] with the help of the “prodlim” package [28]. Survival analysis was presented in median and interquartile range (IQR).
Results
Results of the literature search
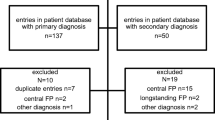
The literature search yielded a sum of 274 studies. Using EndNote X8, 96 duplicates were identified and removed. Only 25 articles of 178 were included after screening titles and abstracts for eligibility. After reading the full texts meticulously and obtaining 10 papers by manual search, we included 22 final papers in our qualitative synthesis. The PRISMA flow diagram of the literature search and screening is illustrated in Fig. 1.
Study characteristics
From the 22 included studies, we extracted the data of 32 FNP patients who were infected with COVID-19. Among the patients, 14 were male and 18 were female. Each patient had been diagnosed with COVD-19 and had experienced either unilateral or bilateral FNP. The mean age was 31.9 years. Two patients were pregnant and primigravida [10, 29]. Three other patients had cranial polyneuropathy [17, 30, 31]. The average number of days for follow-up was 27 ± 9.3 and 26.3 ± 13 (mean ± standard deviation) for COVID-19 and FNP status, respectively. The baseline characteristics of each patient are summarized in Table 1. Combined patient baseline characteristics are summarized in Table 2.
Clinical features
FNP symptoms appeared at a median of 7 and a half days after the onset of COVID-19 symptoms. 16 patients experienced FNP on the right side, while 13 patients had it on the left side. Three patients experienced facial nerve palsy bilaterally. According to the House–Brackmann grading system reported, facial nerve damage resulted in mild (grade 2) dysfunction in five patients, moderate (grade 3) dysfunction in four patients, moderately severe (grade 4) in two patients, severe (grade 5) in one patient, and total paralysis (grade 6) in one patient. Four patients presented with drooling, fifteen with ophthalmoplegia, four with facial asymmetry at rest, three with facial numbness, one with tingling in the face, one with jaw pain, nine with facial droop, nine with frontalis muscle involvement, one with decreased sensation, nine with labial commissure deviation, seventeen with facial weakness, but none of the patients presented with parotid swelling nor hyperacusis. Tables 3 and 4 summarize the clinical and laboratory features of COVID-19 and FNP, respectively, in the included patients. Fever was the most encountered symptom of COVID-19 in the population, while facial weakness and ophthalmoplegia represented most of the FNP symptomatology. Figures 2 and 3 provide the frequency of the symptoms or signs with which the patients presented.
Investigation findings
Tests of patients’ CSF, in addition to serology, brain MRIs, electroneuromyography, and cerebral vascular imaging were conducted. From the CSF, it was concluded that very few patients exhibited high cell counts, protein levels, glucose levels, or pressure. Tests were not positive in any patients for human immunodeficiency virus (HIV) or herpes simplex viruses 1 and 2 (HSV-1 and HSV-2). Brain MRIs showed facial nerve enhancement in 11 patients. Electroneuromyography showed decreased compound muscle action potential in one patient (Table 4). Chest CT also showed ground-glass opacities in five patients.
Treatment and prognosis
Prednisone and prednisolone were administered to 14 and 6 patients, respectively, while the rest of the patients were given either ciclesonide, dexamethasone, betamethasone, or methylprednisolone. Moreover, only around a third of the patients (37.5%) received antiviral therapy, where acyclovir, valacyclovir, remdesivir, and favipiravir were provided to three, three, two, and one patients, respectively. Only one patient [12] of the patients who received antiviral therapy did not have concomitant corticosteroid administration. For COVID-19, 11 patients recovered while 1 patient had minimal improvement and 1 patient had definite improvement. Seventeen patients were not reported. For FNP, 20 patients recovered, there was one patient with no improvement and 5 patients who had only minimal improvement with 3 patients for definite improvement. Three patients were not reported (Table 4). Using survival analysis and Kaplan–Meier plotting for the treatment modalities, the median days for improvement for the combination (antiviral + steroids), steroid-only, and antiviral-only were (21, IQR = 8), 30 (IQR = 15), 33 (IQR = 3.5), respectively (Fig. 4).
Quality assessment
In terms of quality assessment, 7 were good, 14 were fair, and one was poor. Online Resource 3 provides the quality assessment of all the included studies.
Discussion
By systematically reviewing the literature for patients suffering from FNP and COVID-19, we were able to identify a potential correlation between the occurrence of FNP and COVID-19. From 22 studies, we were able to extract data from 32 patients.
As FNP pathogenesis can be linked to microthrombi formation, direct viral invasion, or autoimmune reactions [15,16,17,18], the hypercoagulability or direct neural invasion of SARS-CoV-2 can be blamed for this increased incidence of FNP. Multiple studies have indicated that patients with COVID-19 had an absence of SARS-CoV-2 viral ribonucleic acid (RNA) in their CSF analyses [17]. Due to the absence of the viral RNA in the CSF, it is possible to rule out direct viral neurotropism as a cause for neurological manifestations [32]. Instead, there is more evidence to suggest that the FNP can be a result of the activation of the angiotensin-converting enzyme (ACE2) receptors in the nervous system. Once SARS-CoV-2 binds to the ACE2 receptors within the central nervous system, it will induce a release of cytokines that can cause negative effects. The SARS-CoV-2 can enter the central nervous system via mucosa or viremia. This can cause peripheral neuropathies in the facial nerve and olfactory nerve, leading to FNP and anosmia [33]. The cytokine release can lead to immune-mediated inflammatory responses such as ischemia of vasa nervorum and demyelination at the site of the facial nerve [17]. Further research is required to confirm the pathogenesis between FNP and COVID-19.
Regarding diagnostics, there were a high number of patients with COVID-19 presenting with neurological manifestations. Other than these neurological issues, these patients were asymptomatic for COVID-19. Since the patients presented asymptomatically, physicians need to be aware of the possible strong correlation between FNP and COVID-19. In the CSF polymerase chain reaction (PCR) analysis of the patients, the cell count and protein levels were normal. There was no CSF evidence of SARS-CoV-2 or any other infections such as Epstein–Barr virus (EBV) and HSV. In one case report, the patient presented with serological evidence of coinfection with EBV and SARS-CoV-2 but lacked evidence of these infections in the CSF PCR [31]. EBV can lead to bilateral nerve palsy and in this case, there is a possibility that this can be linked to COVID-19 [34]. The effect of coinfections and FNP is an avenue for future evaluation.
Twenty-three patients out of the 32 included in the review received different steroids as a controlling measure of FNP. Although the exact correlation between steroid usage and FNP is unknown, there were some improvements in FNP when steroids were used. The steroids used include prednisone, methylprednisolone, prednisolone, ciclesonide, dexamethasone, or betamethasone. Using these medications, 20 patients had clinical improvement or recovered completely from FNP, while 1 patient failed to show any recovery [11]. Additionally, three people were not reported [32, 35, 36]. Specifically, glucocorticoids were recommended for the treatment of Bell’s palsy, but glucocorticoids can be contraindicated in COVID-19. For short-term COVID-19 cases, glucocorticoids can help decrease inflammation and a reduction in the need for mechanical ventilation. Despite the benefits, glucocorticoid usage can lead to delayed viral clearance and an increased risk of secondary infections [37]. Additionally, in our paper, we found that combining steroids with antivirals can decrease the time till the improvement of FNP using a Kaplan–Meier plot. Since COVID-19 is relatively new, further research is required to understand the correlation between different treatment options such as corticosteroids. Once that correlation has been established, continuing research must be conducted to analyze effective FNP treatments in concordance with COVID-19 treatments.
COVID-19 has been reported to have caused multiple, devastating non-respiratory complications in the infected population [4, 5, 8, 13]. The incidence of different nervous system inflammatory conditions, whether central or peripheral, has been widely reported in the literature to be increasing in the era of COVID-19 [14]. For instance, patients are more likely to experience anosmia or hyposmia when infected by SARS-CoV-2 than other pathogens belonging to the coronavirus family [2, 19]. Therefore, it is of importance for physicians to suspect COVID-19 when encountering etiologically unexplained neurological manifestations.
This paper encourages physicians to pay close attention to patients presenting with sole FNP, since the etiology of this morbidity may be related to an asymptomatic infection with COVID-19. However, the authors could not identify a way of analyzing the probable incidence of FNP caused by the virus. Another limitation of this study is that the included papers were essentially descriptive of the patients included within. Due to the urgent need for information about the virus, physicians are unable to conduct analytical studies, which are required to detect dissimilarities in the prevalence of FNP in infected and non-infected cohorts. Also, some papers with ethical issues may pass unnoticed. We have included the study published by Elkhouly and Kaplan at first [38] but excluded it once it got retracted, and we removed it from the qualitative synthesis once we saw the retraction notice [39]. Besides, 25 patients can be considered an insufficient sample size required to confirm precise correlations between COVID-19 and FNP. The insufficient sample size could be related to the low incidence of FNP and COVID-19 due to asymptomatic patients. For example, a study in Italy noted that there was a marked increase in the incidence of FNP from 2019 (before COVID-19) to 2020 (once COVID-19 began) [40]. Since patients with different defining characteristics such as age or gender were compared, this can be another potential limitation. This can cause a false sense of precision due to the unknown effects of the defining characteristics on the potential correlation between FNP and COVID-19. Finally, some papers have based the COVID-19 diagnosis on only the clinical findings, serological testing, and/or CT scan findings without the confirmation of a PCR test [35, 41, 42]. This was widely exercised at the beginning of the pandemic due to the scarcity of PCR tests, the overwhelming number of new cases, or the high cost of a PCR test, especially in low-to-middle income countries [43]. This systematic review is one of the first reviews performed to establish an important correlation between FNP and COVID-19. Due to the small sample size, the different types of antivirals used, and the insufficient details about the treatment (e.g., when the medications were started), this systematic review does not provide comparative data regarding the treatment modalities that can be used in managing FNP. In future studies, increasing the sample size and conducting a methodical analytical investigation can confirm the correlation between COVID-19 and FNP.
Conclusion
COVID-19 has been linked to an unlimited number of neurological symptoms. In this study, we performed an online literature search of 6 databases, yielding 22 studies reporting 32 patients who suffered from FNP and COVID-19 simultaneously. After comparing various case studies and reports, it is safe to assume that there is a potential correlation between the occurrence of FNP and COVID-19. Moreover, starting a combined antiviral-steroid treatment regimen may decrease the time needed for improvement although the evidence is not strong enough. However, further studies are warranted to measure the correlation between FNP and COVID-19 and to examine the best treatment modality of FNP in the context of COVID-19.
Clinical correlation
As the pandemic continues, it is crucial to test for and promptly treat SARS-CoV-2 when addressing a patient presenting with FNP. Starting with a combined regimen of antivirals and steroids may improve the clinical manifestations more rapidly.
Availability of data and material
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Code availability
Not applicable.
Abbreviations
- ACE2:
-
Angiotensin-converting enzyme 2
- COVID-19:
-
Coronavirus disease 2019
- CSF:
-
Cerebrospinal fluid
- CT:
-
Computed tomography
- EBV:
-
Epstein–Barr virus
- FNP:
-
Facial nerve palsy
- HIV:
-
Human immunodeficiency virus
- HSV:
-
Herpes simplex virus
- IQR:
-
Interquartile range
- MRI:
-
Magnetic resonance imaging
- PCR:
-
Polymerase chain reaction
- PRISMA:
-
Preferred Reporting Items for Systematic Reviews and Meta-Analysis
- PROSPERO:
-
International Prospective Register of Systematic Reviews
- RNA:
-
Ribonucleic acid
References
World Health Organization: WHO Coronavirus Disease (COVID-19) Dashboard (2020). https://covid19.who.int/table. Accessed 28 Nov 2020
Meng H, **ong R, He R, Lin W, Hao B, Zhang L et al (2020) CT imaging and clinical course of asymptomatic cases with COVID-19 pneumonia at admission in Wuhan. J Infect 81(1):e33–e39. https://doi.org/10.1016/j.**f.2020.04.004
Rothe C, Schunk M, Sothmann P, Bretzel G, Froeschl G, Wallrauch C et al (2020) Transmission of 2019-nCoV infection from an asymptomatic contact in Germany. N Engl J Med 382(10):970–971. https://doi.org/10.1056/NEJMc2001468
Buitrago-Garcia D, Egli-Gany D, Counotte MJ, Hossmann S, Imeri H, Ipekci AM et al (2020) Occurrence and transmission potential of asymptomatic and presymptomatic SARS-CoV-2 infections: a living systematic review and meta-analysis. PLoS Med 17(9):e1003346. https://doi.org/10.1371/journal.pmed.1003346
Giwa AL, Desai A, Duca A (2020) Novel 2019 coronavirus SARS-CoV-2 (COVID-19): an updated overview for emergency clinicians. Emerg Med Pract 22(5):1–28
Lopez-Villegas A, Maroto-Martin S, Baena-Lopez MA, Garzon-Miralles A, Bautista-Mesa RJ, Peiro S et al (2020) Telemedicine in times of the pandemic produced by COVID-19: implementation of a teleconsultation protocol in a hospital emergency department. Healthcare (Basel) 8(4):357. https://doi.org/10.3390/healthcare8040357
Rothan HA, Byrareddy SN (2020) The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak. J Autoimmun 109:102433. https://doi.org/10.1016/j.jaut.2020.102433
Wu JT, Leung K, Leung GM (2020) Nowcasting and forecasting the potential domestic and international spread of the 2019-nCoV outbreak originating in Wuhan, China: a modelling study. Lancet 395(10225):689–697. https://doi.org/10.1016/S0140-6736(20)30260-9
Abobaker A, Raba AA, Alzwi A (2020) Extrapulmonary and atypical clinical presentations of COVID-19. J Med Virol 92(11):2458–2464. https://doi.org/10.1002/jmv.26157
Figueiredo R, Falcao V, Pinto MJ, Ramalho C (2020) Peripheral facial paralysis as presenting symptom of COVID-19 in a pregnant woman. BMJ Case Rep 13(8):e237146. https://doi.org/10.1136/bcr-2020-237146
Goh Y, Beh DLL, Makmur A, Somani J, Chan ACY (2020) Pearls & Oy-sters: facial nerve palsy in COVID-19 infection. Neurology 95(8):364–367. https://doi.org/10.1212/WNL.0000000000009863
Wan Y, Cao S, Fang Q, Wang M, Huang Y (2020) Coronavirus disease 2019 complicated with Bell’s palsy: a case report. Research Square, Durham. https://doi.org/10.21203/rs.3.rs-23216/v1
Whittaker A, Anson M, Harky A (2020) Neurological manifestations of COVID-19: a systematic review and current update. Acta Neurol Scand 142(1):14–22. https://doi.org/10.1111/ane.13266
Zammit M, Markey A, Webb CA (2020) A rise in facial nerve palsies during the coronavirus disease 2019 pandemic. J Laryngol Otol. https://doi.org/10.1017/S0022215120002121
Duarte-Neto AN, Monteiro RAA, da Silva LFF, Malheiros D, de Oliveira EP, Theodoro-Filho J et al (2020) Pulmonary and systemic involvement in COVID-19 patients assessed with ultrasound-guided minimally invasive autopsy. Histopathology 77(2):186–197. https://doi.org/10.1111/his.14160
Gussen R (1977) Pathogenesis of Bell’s palsy. Retrograde epineurial edema and postedematous fibrous compression neuropathy of the facial nerve. Ann Otol Rhinol Laryngol 86(4):549–558. https://doi.org/10.1177/000348947708600416
Lima MA, Silva MTT, Soares CN, Coutinho R, Oliveira HS, Afonso L et al (2020) Peripheral facial nerve palsy associated with COVID-19. J Neurovirol 26(6):941–944. https://doi.org/10.1007/s13365-020-00912-6
Zhang W, Xu L, Luo T, Wu F, Zhao B, Li X (2020) The etiology of Bell’s palsy: a review. J Neurol 267(7):1896–1905. https://doi.org/10.1007/s00415-019-09282-4
Berlit P, Bosel J, Gahn G, Isenmann S, Meuth SG, Nolte CH et al (2020) “Neurological manifestations of COVID-19” - guideline of the German society of neurology. Neurol Res Pract 2(1):51. https://doi.org/10.1186/s42466-020-00097-7
Hoffmann M, Kleine-Weber H, Schroeder S, Kruger N, Herrler T, Erichsen S et al (2020) SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 181(2):271-280.e8. https://doi.org/10.1016/j.cell.2020.02.052
Alomari SO, Abou-Mrad Z, Bydon A (2020) COVID-19 and the central nervous system. Clin Neurol Neurosurg 198:106116. https://doi.org/10.1016/j.clineuro.2020.106116
Hamming I, Timens W, Bulthuis ML, Lely AT, Navis G, van Goor H (2004) Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol 203(2):631–637. https://doi.org/10.1002/path.1570
**a H, Lazartigues E (2010) Angiotensin-converting enzyme 2: central regulator for cardiovascular function. Curr Hypertens Rep 12(3):170–175. https://doi.org/10.1007/s11906-010-0105-7
Moher D, Liberati A, Tetzlaff J, Altman DG, Group P (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 6(7):e1000097. https://doi.org/10.1371/journal.pmed.1000097
Turki A, Abbas KS, Makram AM, Elmarabea M, Elfert M, Abdalshafy H, et al. (2021) Facial palsy in COVID-19 patients: a systematic review. https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42021226404.
Moola S, Munn Z, Tufanaru C, Aromataris E, Sears K, Sfetcu R, et al. (2017) Chapter 7: systematic reviews of etiology and risk. Joanna Briggs institute reviewer’s manual. The Joanna Briggs Institute, Adelaide p 5
R Core Team (2020) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Gerds TA. (2019) prodlim: Product-Limit Estimation for Censored Event History Analysis. R package version 2019.11.13
Kumar V, Narayanan P, Shetty S, Mohammed AP (2021) Lower motor neuron facial palsy in a postnatal mother with COVID-19. BMJ Case Rep 14(3):e240267. https://doi.org/10.1136/bcr-2020-240267
Gogia B, Gil Guevara A, Rai PK, Fang X (2020) A case of COVID-19 with multiple cranial neuropathies. Int J Neurosci. https://doi.org/10.1080/00207454.2020.1869001
Roussel A, Germanaud D, Bouchoucha Y, Ouldali N, Vedrenne-Cloquet M, Castelle M et al (2021) Cranial polyneuropathy as the first manifestation of a severe COVID-19 in a child. Pediatr Blood Cancer 68(3):e28707. https://doi.org/10.1002/pbc.28707
Ribeiro BNF, Marchiori E (2020) Facial palsy as a neurological complication of SARS-CoV-2. Arq Neuropsiquiatr 78(10):667. https://doi.org/10.1590/0004-282X20200127
Homma Y, Watanabe M, Inoue K, Moritaka T (2020) Coronavirus disease-19 pneumonia with facial nerve palsy and olfactory disturbance. Intern Med 59(14):1773–1775. https://doi.org/10.2169/internalmedicine.5014-20
Cabrera Muras A, Carmona-Abellan MM, Collia Fernandez A, Uterga Valiente JM, Anton Mendez L, Garcia-Monco JC (2021) Bilateral facial nerve palsy associated with COVID-19 and Epstein–Barr virus co-infection. Eur J Neurol 28(1):358–360. https://doi.org/10.1111/ene.14561
Karimi-Galougahi M, Yousefi-Koma A, Raygani N, Bakhshayeshkaram M, Haseli S (2021) 18FDG-PET/CT assessment of COVID-19-induced Bell’s palsy. Acad Radiol 28(1):144–145. https://doi.org/10.1016/j.acra.2020.11.001
Mehta S, Mackinnon D, Gupta S (2020) Severe acute respiratory syndrome coronavirus 2 as an atypical cause of Bell’s palsy in a patient experiencing homelessness. CJEM 22(5):608–610. https://doi.org/10.1017/cem.2020.418
van Paassen J, Vos JS, Hoekstra EM, Neumann KMI, Boot PC, Arbous SM (2020) Corticosteroid use in COVID-19 patients: a systematic review and meta-analysis on clinical outcomes. Crit Care 24(1):696. https://doi.org/10.1186/s13054-020-03400-9
Elkhouly A, Kaplan AC (2020) Noteworthy neurological manifestations associated with COVID-19 infection. Cureus 12(7):e8992. https://doi.org/10.7759/cureus.8992
Elkhouly A, Kaplan AC (2021) Retraction: noteworthy neurological manifestations associated With COVID-19 infection. Cureus 13(3):r26. https://doi.org/10.7759/cureus.r26
Codeluppi L, Venturelli F, Rossi J, Fasano A, Toschi G, Pacillo F et al (2021) Facial palsy during the COVID-19 pandemic. Brain and behavior 11(1):e01939. https://doi.org/10.1002/brb3.1939
Decio A, Mazza A, Quadri V, Ronconi MS, Brusadelli C, Ruggeri M et al (2021) Neurological manifestations of COVID-19 in children: a case of facial nerve palsy. Pediatr Neurol 116:59. https://doi.org/10.1016/j.pediatrneurol.2020.12.006
Neo WL, Ng JCF, Iyer NG (2021) The great pretender-Bell’s palsy secondary to SARS-CoV-2? Clin Case Rep 9(3):1175–1177. https://doi.org/10.1002/ccr3.3716
da Silva SJR, Silva C, Guarines KM, Mendes RPG, Pardee K, Kohl A et al (2020) Clinical and laboratory diagnosis of SARS-CoV-2, the virus causing COVID-19. ACS Infect Dis 6(9):2319–2336. https://doi.org/10.1021/acsinfecdis.0c00274
Correa DG, da Hygino Cruz Jr LC, Lopes FCR, Rangel CC, de Araujo Henriques Tessarollo AL, Coelho KCG et al (2021) Magnetic resonance imaging features of COVID-19-related cranial nerve lesions. J Neurovirol 27(1):171–177. https://doi.org/10.1007/s13365-020-00934-0
Derollez C, Alberto T, Leroi I, Mackowiak MA, Chen Y (2020) Facial nerve palsy: an atypical clinical manifestation of COVID-19 infection in a family cluster. Eur J Neurol 27(12):2670–2672. https://doi.org/10.1111/ene.14493
Bastola A, Sah R, Nepal G, Gajurel BP, Rajbhandari SK, Chalise BS et al (2021) Bell’s palsy as a possible neurological complication of COVID-19: a case report. Clin Case Rep 9(2):747–750. https://doi.org/10.1002/ccr3.3631
Dahl EH, Mosevoll KA, Cramariuc D, Vedeler CA, Blomberg B (2021) COVID-19 myocarditis and postinfection Bell’s palsy. BMJ Case Rep 14(1):240095. https://doi.org/10.1136/bcr-2020-240095
Casas E, Barbosa A, Rubio-Garcia E, Cebrian J, Diaz-Perez C, de la Fuente E et al (2020) Isolated peripheral facial paralysis in a patient with COVID-19. Rev Neurol 71(1):40–41. https://doi.org/10.33588/rn.7101.2020229
Garcia Ochoa-Fernandez E, Villora-Morcillo N, Taboas-Pereira MA (2021) Peripheral facial palsy in a paediatric patient with no risk factors within the context of infection by SARS-CoV-2. Rev Neurol 72(5):177–178. https://doi.org/10.33588/rn.7205.2020629
Theophanous C, Santoro JD, Itani R (2021) Bell’s palsy in a pediatric patient with hyper IgM syndrome and severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Brain Dev 43(2):357–359. https://doi.org/10.1016/j.braindev.2020.08.017
Zain S, Petropoulou K, Mirchia K, Hussien A, Mirchia K (2021) COVID-19 as a rare cause of facial nerve neuritis in a pediatric patient. Radiol Case Rep 16(6):1400–1404. https://doi.org/10.1016/j.radcr.2021.03.063
Funding
No funding was received for conducting this study.
Author information
Authors and Affiliations
Contributions
KSA developed the idea. KSA and AT performed the literature search. KSA, HA, KSA, MEF, and MEM contributed to eligibility screening. MEF, MEM, NA, HA, and AT contributed to data extraction. All members checked for more articles to include via manual search. AMM, AS, and SC contributed to data analysis, manuscript writing, and the formation of tables and figures. All authors approved the final version under the supervision of NTH.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Turki, A., Abbas, K.S., Makram, A.M. et al. Epidemiology, clinical features, and treatment modalities of facial nerve palsy in COVID-19 patients: a systematic review. Acta Neurol Belg 122, 1419–1432 (2022). https://doi.org/10.1007/s13760-022-02026-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13760-022-02026-8