Abstract
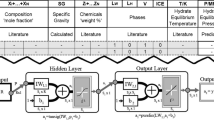
To estimate the surface tension of liquid hydrocarbon mixtures as an essential thermophysical property, artificial neural networks (AANs) are used. To develop the AAN model, 25 binary mixtures containing 560 data points in a wide range of temperatures (287.81–343.15 K) and at atmospheric pressure were considered. The performances of two neural networks including feed-forward (FFNN) and cascade neural network (CNN) are compared with different input variables. For both cases, the Levenberg–Marquardt optimization method is used to optimize the weights and biases of the proposed structures with tansig and pureline transfer functions in the hidden and output layers, respectively. It was found that the CNN with the structure of 5-9-1 and input variables of temperature (T), mole fraction (x), molecular weights of both compounds (MW), and mixture critical temperature (Tc-mix) is the optimum model with the average absolute relative deviation (AARD%) = 1.33 and correlation coefficient (R2) = 0.992. The most important feature of the proposed model is its ability to differentiate between isomers and correctly predict their binary surface tensions.











Similar content being viewed by others
Abbreviations
- MW:
-
Molecular weight: gr/mol
- \(P\mathrm{c}\) :
-
Critical pressure bar
- P C-mix :
-
Critical pressure of mixture bar
- T :
-
Temperature K
- \(T\mathrm{c}\) :
-
Critical temperature K
- T C-mix :
-
Critical temperature of mixture K
- T r :
-
Reduced temperature
- T nbr :
-
Reduced normal boiling point
- \(V\mathrm{c}\) :
-
Critical volume cm3/mol
- V C-mix :
-
Critical volume of mixture cm3/mol
- Z C :
-
Critical compressibility factor
- Z C-mix :
-
Critical compressibility factor of mixture
- s :
-
Specific gravity gr/cm3
- x :
-
Mole fraction
- µr :
-
Reduced dipole moment
- ω :
-
Acentric factor
- ω mix :
-
Acentric factor of mixture
- AAN:
-
Artificial neural network
- AARD:
-
Average absolute relative deviation
- CNN:
-
Cascade neural network
- F:
-
Transfer function
- FFNN:
-
Feed-forward neural network
- \({I}_{\mathrm{mix}}\) :
-
Properties of mixtures
- MLP:
-
Multilayer perceptron
- R 2 :
-
Correlation coefficient
- W :
-
Weight
- X :
-
Input variable
- b :
-
Bias
- \({n}_{j}\) :
-
Predicted property
References
G. Pazuki, M. Nikookar, L. Sahranavard, Petroleum Sci. Technol. 29, 2384 (2011)
A. Roosta, B. Sadeghi, Chem. Eng. Commun. 203, 1349 (2016)
B. Carey, L. Scriven, H. Davis, AIChE J. 24, 1076 (1978)
C. Miqueu, B. Mendiboure, A. Graciaa, J. Lachaise, Fluid Phase Equilib. 207, 225 (2003)
A. Mulero, I. Cachadiña, D. Bautista, J. Phys. Chem. Ref. Data 50, 023104 (2021)
A. Mulero, I. Cachadiña, L. Cardona, J. Valderrama, Industr. Eng. Chem. Res. 61, 3457 (2022)
Y.-X. Zuo, E.H. Stenby, Fluid Phase Equilib. 132, 139 (1997)
A. Jouyban, W.E. Acree Jr., J. Mol. Liq. 323, 115054 (2021)
L. Chunxi, W. Wenchuan, W. Zihao, Fluid Phase Equilib. 175, 185 (2000)
M.S.C. Santos, J.C.R. Reis, Fluid Phase Equilib. 423, 172 (2016)
E.S. Bezerra, J.M. Santos, M.L. Paredes, Fluid Phase Equilib. 288, 55 (2010)
I. Egry, Int. J. Thermophys. 26, 931 (2005)
A. Jouyban, A. Fathi-Azarbayjani, W.E. Acree, Chem. Pharmaceutical Bull. 52, 1219 (2004)
G.M. Wilson, J. Am. Chem. Soc. 86, 127 (1964)
A. Rafati, A. Bagheri, M. Najafi, J. Chem. Thermodyn. 43, 248 (2011)
A. Rafati, A. Bagheri, A. Khanchi, E. Ghasemian, M. Najafi, J. Colloid Interface Sci. 355, 252 (2011)
E.G. Lemraski, J. Mol. Liq. 203, 52 (2015)
V. Vinš, B. Planková, J. Hrubý, Int. J. Thermophys. 34, 792 (2013)
J.M. Garrido, A. Mejía, M.M. Pineiro, F.J. Blas, E.A. Müller, AIChE J. 62, 1781 (2016)
E.A. Müller, A. Mejía, Fluid Phase Equilib. 282, 68 (2009)
L.I. Rolo, A.I. Caço, A.J. Queimada, I.M. Marrucho, J.A. Coutinho, J. Chem. Eng. Data 47, 1442 (2002)
H. Lin, Y.-Y. Duan, Q. Min, Fluid Phase Equilib. 254, 75 (2007)
H. Lin, Y. Duan, J. Zhang, Int. J. Thermophys. 29, 423 (2008)
G. Di Nicola, M. Pierantozzi, Int. J. Refrig 36, 562 (2013)
D.M. Himmelblau, Korean J. Chem. Eng. 17, 373 (2000)
D.M. Himmelblau, Ind. Eng. Chem. Res. 47, 5782 (2008)
S. Curteanu, H. Cartwright, J. Chemom. 25, 527 (2011)
C.B. Carvalho, E.P. Carvalho, M.A. Ravagnani, Brazilian J. Chem. Eng. 37, 729 (2020)
Á. Mulero, I. Cachadiña, J.O. Valderrama, Fluid Phase Equilib. 451, 60 (2017)
A. Roosta, P. Setoodeh, A. Jahanmiri, Industr. Eng. Chem. Res. 51, 561 (2012)
M. Lashkarbolooki, M. Bayat, Chem. Eng. Res. Des. 137, 154 (2018)
K. Movagharnejad, F. Zareei, B. Mehdizadeh, S. Salahi, M. Lashkenari, Petroleum Sci. Technol. 33, 1008 (2015)
H. Parhizgar, M.R. Dehghani, A. Khazaei, M. Dalirian, Industr. Eng. Chem. Res. 51, 2775 (2012)
M. Nabipour, Fluid Phase Equilib. 456, 151 (2018)
G. Bakeri, M. Delavar, M. Soleimani Lashkenari, J. Oil, Gas Petrochem. Technol. 2, 14 (2015)
H.A. Ojaki, M. Lashkarbolooki, K. Movagharnejad, Colloids Surfaces A: Physicochem. Eng. Aspects 590, 124474 (2020)
M. Lashkarbolooki, Sep. Sci. Technol. 52, 1454 (2017)
R.L. Schmidt, J.C. Randall, H.L. Clever, J. Phys. Chem. 70, 3912 (1996)
D. Papaioannou, C. Panayiotou, J. Colloid Interface Sci. 130, 432 (1989)
S. Suri, V. Ramakrishna, J. Phys. Chem. 72, 3073 (1968)
R. Tahery, J. Chem. Thermodyn. 106, 95 (2017)
D. Gómez-Díaz, J.C. Mejuto, J.M. Navaza, A. Rodríguez-Álvarez, J. Chem. Eng. Data 47, 872 (2002)
H. Kahl, T. Wadewitz, J. Winkelmann, J. Chem. Eng. Data 48, 1500 (2003)
Z. Li, B.C.-Y. Lu, Chem. Eng. Sci. 56, 6977 (2001)
S. Pezhhanfar, M. Zarei, J. Iran. Chem. Soc. 19, 231 (2022)
K.N. Seneviratne, T.J. Hughes, M.L. Johns, K.N. Marsh, E.F. May, J. Chem. Thermodyn. 111, 173 (2017)
F. Gozalpour, A. Danesh, A.C. Todd, B. Tohidi, Fluid Phase Equilib. 233, 144 (2005)
J. Satherley, D.L. Cooper, D.J. Schiffrin, Fluid Phase Equilib. 456, 193 (2018)
T.M. Koller, T. Klein, C.D. Giraudet, J. Chen, A. Kalantar, G.P. van der Laan, M.H. Rausch, A.P. Fröba, J. Chem. Eng. Data 62, 3319 (2017)
F.D. Lenahan, M. Zikeli, M.H. Rausch, T. Klein, A.P. Fröba, J. Chem. Eng. Data 66, 2264 (2021)
C.L. Yaws, C. Gabbula, Yaws" Handbook of thermodynamic and physical properties of chemical compounds (Knovel, 2003)
R.M. Santana, D.C. Napoleão, S.G. dos Santos Júnior, R.K. Gomes, N.F. de Moraes, L.E. Zaidan, D.R.M. Elihimas, G.E. Nascimento, M.M. Duarte, Chem. Papers 75, 2305 (2021)
E. Davoudi, B. Vaferi, Chem. Eng. Res. Des. 130, 138 (2018)
B. Vaferi, Y. Rahnama, P. Darvishi, A. Toorani, M. Lashkarbolooki, J. Supercr. Fluids 84, 80 (2013)
Á. Mulero, M. Pierantozzi, I. Cachadiña, G. Di Nicola, Fluid Phase Equilib. 449, 28 (2017)
S. Atashrouz, H. Mirshekar, A. Mohaddespour, J. Mol. Liquids 236, 344 (2017)
S. Jalili, A. Jaberi, M. Mahjani, M. Jafarian, J. Iranian Chem. Soc. 5, 669 (2008)
M. Lashkarbolooki, A.Z. Hezave, S. Ayatollahi, Fluid Phase Equilib. 324, 102 (2012)
M.T. Hagan, H.B. Demuth, M. Beale, Neural network design (PWS Publishing Co., 1997)
G. Cybenko, Math. Control Signals Syst. 2, 303 (1989)
K. Levenberg, Q. Appl. Math. 2, 164 (1944)
M.T. Hagan, M.B. Menhaj, IEEE Trans. Neural Networks 5, 989 (1994)
S. Şahin, S. Sevgen, R. Samli, Chem. Papers 73, 1189 (2019)
M. Niknam Shahrak, M. Esfandyari, M. Karimi, J. Iranian Chem. Soc. 16, 11 (2019)
M. Lashkarbolooki, Z.S. Shafipour, A.Z. Hezave, J. Supercr. Fluids 73, 108 (2013)
V. Lam, G. Benson, Can. J. Chem. 48, 3773 (1970)
D. Gómez-Díaz, J.C. Mejuto, J.M. Navaza, J. Chem. Eng. Data 46, 720 (2001)
M. M. N. Seyyed Fatemeh naghibi, (2007).
M. Domínguez-Pérez, E. Rilo, L. Segade, C. Franjo, O. Cabeza, J. Chem. Eng. Data 55, 1317 (2010)
L. Mosteiro, L.M. Casás, J.L. Legido, J. Chem. Thermodyn. 41, 695 (2009)
H. Kahl, T. Wadewitz, J. Winkelmann, J. Chem. Eng. Data 48, 580 (2003)
A.E. Andreatta, R.E. Martini, J.L. Legido, L. Casás, Int. J. Eng. Res. Sci. 5, 51 (2016)
Á. Piñeiro, P. Brocos, A. Amigo, J. Gracia-Fadrique, M. Guadalupe Lemus, Langmuir 17, 4261 (2001)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ojaki, H.A., Lashkarbolooki, M. & Movagharnejad, K. Checking the performance of feed-forward and cascade artificial neural networks for modeling the surface tension of binary hydrocarbon mixtures. J IRAN CHEM SOC 20, 655–667 (2023). https://doi.org/10.1007/s13738-022-02703-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13738-022-02703-8