Abstract
High-entropy alloys (HEAs) (or multi-principal component alloys) have been mentioned in different studies because they have good corrosion resistance and mechanical properties, but their biocidal ability is still little explored. The microstructural, magnetic, corrosion, and biocidal behavior of as-cast AlxCoCrCuFe high-entropy alloys with x = 0.5 and 0.9 were analyzed using scanning electron microscopy, energy-dispersive X-ray spectroscopy, magnetization measurements as a function of the applied field, X-ray diffraction patterns, electrochemical essays, and antimicrobial analyses. The results indicated that a higher concentration of Al reduces the saturation magnetization, the corrosion potential, as well as the survival time of the bacterium Escherichia coli on the surface of the studied alloys. The dendritic microstructure was also refined with the increase in the concentration of Al and a higher number of Cu-rich precipitate was observed on their surface.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
High-entropy alloys (HEAs) (or multi-principal component alloys) are materials composed by five or more elements with similar molar fractions, that is, they do not have a principal component [1]. Due to the increase in their configurational entropy, HEAs can present a lower number of phases than that expected for traditional alloys. This leads to interactions that normally would not exist among the alloying elements, modifying the properties of the material. The promising characteristics of these materials are linked to their mechanical properties and corrosion resistance [1]. Some researchers have also shown the HEAs ability to resist microorganism-induced corrosion (MIC) [2,3,4]. This type of corrosive damage generates significant losses to industries that operate in susceptible environments to microorganism growth [5]. It is still necessary to highlight the importance of research focused on antimicrobial materials due to the recent SARS-CoV2 pandemic [6, 7], since applying or making surfaces of such materials can reduce transmission via fomites [8]. Metallic copper, its ions in solution, as well as copper-based alloys have been, empirically, used for medical applications by ancient people in many regions around the world [7]. The survival of pathogenic microorganisms on surfaces of metallic Cu and Cu-based alloys was assessed in different studies [7,8,9,10,11], but in most cases, the Cu concentration was higher than 60 wt.% or around it. Li et al. [12] studied the antiviral properties of the CuFeCrCoNi and Al0.4CuFeCrCoNi high-entropy alloys with lower Cu content, showing over 99.99% of efficiency under influenza H1N1 virus and enterovirus after 24 h in contact. These results were related to the interaction of copper ions, hydroxyl radicals, and microorganisms, revealing new possibilities for the application of high-entropy alloys as biocidal materials.
Considering the evaluated compositions, no information was found in the literature about the biocidal characteristics of AlxCoCrCuFe multi-principal element alloys. Ivchenko reported that the microstructure of as-cast AlCrCoCuFeNi alloy is made up of dendrites that can be decomposed into many phases with different morphologies and chemical compositions. This alloy was also susceptible to heat treatment [13]. Jones et al. evaluated the Al0.5CoCrCuFeNi multi-principal element alloy and they verified that the configurational complexity was not enough to stabilize a solid solution phase against enthalpic effects [14]. Another report by Jones et al. [15] verified that the configurational entropy was not dominant over enthalpy for the contribution of Gibbs energy in the Al0.5CoCrCuFeNi alloy. Data from the literature for the Al0.5CoCrCuFeNi multi-principal element alloy also exhibited that the B2 phase, with solvus temperature around 1248 K, is quickly formed in this composition [16]. Furthermore, precipitates of a Cu-rich phase were formed in the dendrites, while a CrCoFe-rich phase was precipitated in the interdendritic region of the Al0.5CrCoCuFeNi alloy [17]. Thus, the possibility of Cu-rich precipitate formation with the variation of the Al concentration appears as an efficient tool to improve the biocidal capacity of high-entropy alloys containing Cu, due to its higher availability to microorganisms.
In this sense, the present study aimed to evaluate the microstructural, magnetic, and biocidal behavior of as-cast AlxCoCrCuFe high-entropy alloys with copper content around 20 at.% (25 wt.%), as well as the effects of change in Al concentration on these properties, including the formation of Cu-rich precipitates.
Materials and Methods
The Al0.5CoCrCuFe and Al0.9CoCrCuFe multi-principal component alloys were prepared from materials with a purity higher than 99.5%, using an electric arc furnace. The produced ingots were cut into pieces suitable for obtaining images by optical (OM) and scanning electron microscopy (SEM), as well as for biological assays. Details about the adopted procedures for melting, cutting, and preparing samples can be found in reference [18]. An Olympus optical microscope and a Quanta 650FEG scanning electron microscope coupled with energy-dispersive X-ray spectroscopy (EDS) and with a backscattered electrons detector were used for this study. The area percentage of the interdendritic region was quantified using the free software ImageJ. A D8 Discover diffractometer with Cu Kα radiation was utilized to define the X-ray diffraction patterns (XRD) from bulk samples. A PPMS 9 Evercool Quantum Design was used to monitor the magnetization change with the magnetic field (M vs. H) at room temperature in samples with a mass of about 2 mg. An Autolab PGSTAT302N Metrohm potentiostat/galvanostat, with a conventional three electrodes cell containing the Ag/AgCl reference electrode, platinum wire as a counter-electrode, and the sample as a working electrode, monitored the potentiodynamic polarization measurements from − 350 mV versus open circuit potential (OCP) up to 1000 mV versus reference electrode with a scan rate of 1 mV s −1. Previously, the OCP was recorded for 24 h. These assays were performed in a solution of NaCl 1.0 mol L−1 with a pH ~ 6.3 at room temperature. The experiments were carried out in quadruplicate and the solution was not de-aerated. Antimicrobial tests or biocide activity of the alloys were determined by the adaptation of protocol described by ISO 22196/2011, based on the recovery of viable cells after direct contact between the microorganism and the surface of the alloy. Escherichia coli (ATCC 8739) was applied as a model pathogen for the biocide experiments. The bacterial culture was cultured in Luria Bertani medium (Invitrogen™) at 308 K for 16 h, followed by its inoculation as a microbial suspension (108), on samples of AlxCoCrCuFe alloys. After time 0 h (immediately after the contact) and 24 h, at 308 K, the experiment was monitored to verify the recovery of viable cells.
Results and Discussion
Figure 1 shows the scanning electron and optical micrographs obtained for the Al0.5CoCrCuFe and Al0.9CoCrCuFe alloys. From optical micrographs in Fig. 1a and c, it was possible to notice that both alloys presented dendritic microstructure, with well-defined interdendritic regions. Dendrites were formed during the cooling, mainly by the fact that on fast cooling a part of the liquid can be undercooled and rapidly solidify from an initial nucleus, growing with high surface energy. This is common in as-cast material and can lead to a difference in concentration that can form two different liquids that solidify at slightly different temperatures or it can lead to the formation of an intermetallic compound in the interdendritic region.
In OM images (Fig. 1a and c) it was possible to see that the increase in aluminum content seems to refine the dendritic microstructure and increase its fraction (58% of the area in Fig. 1a and 64% of the area in Fig. 1c). This was an interesting result since light elements like Al tends to segregate and disturb the simple solid solution formed in HEAs. In this case, the increment in Al seems to stabilize the solid solution. The scanning electron micrographs in Fig. 1b and d showed details of the interdendritic and dendritic microstructure. In SEM images of Fig. 1b and d, it was possible to notice the presence of small precipitates on the dendritic region of the alloys and that their amount was increased with Al content. EDS data indicated that they are Cu-rich precipitates, with reduced Al fraction and other minor alloying elements. This was an interesting fact because even with the increase in precipitates amount with Al content, the dendrites appear to be thicker, indicating that possibly this precipitation reaction occurred after the alloy solidification, and also it did not interfere with dendrites growth in the studied alloys.
This is an interesting finding since Cu and Al have a certain chemical affinity. To better understand we can resort to the mixing enthalpy of the elements in the alloys, which is related to the chemical interaction between these elements. As one can notice in Table 1, Cu and Al show a chemical affinity (ΔHmix < 0), in a matter of fact, Cu only presents a chemical affinity to Al. So, at first glance, it is intriguing that the increase in Al increases the amount of segregated Cu.
However, in a deep evaluation, we can notice that Al presents a higher interaction with Cr (− 10 kJ/mol), Fe (− 11 kJ/mol), and Co (− 19 kJ/mol) than with Cu (− 1 kJ/mol). This leads to the fact that when the Al fraction is increased, it is easier for the elements to bond with the Al and reject the Cu atoms.
Considering the Gibbs mixing energy (given in Eq. 1) as a spontaneity factor for the formation of a solid solution it is possible to notice that as the mixing enthalpy is constant, the contribution of entropy depends on the temperature. So, at high temperatures the entropy contribution can be significant and stabilize the Cu in the dendrites; however, as the temperature decreases the enthalpy contribution is prevalent and the Cu is no longer stable in the dendrite, precipitating onto those.
We can further expand Eq. 1 by rewriting the entropy using the Boltzmann equation and the enthalpy using the Miedema model, as given in Eqs. 2 and 3.
The calculated enthalpy and entropy for the studied alloys are presented in Table 2. As expected, the addition of Al stabilizes the solution decreasing the mixing enthalpy.
In order to evaluate the compositional difference in observed microstructures, some SEM images were obtained using backscattered electrons. These ones can be seen in Fig. 2. It was possible to notice that the dendritic and interdendritic regions were indeed chemically different and precipitates on the dendritic region were observed, as also seen in Fig. 1b and d. To assess this different composition, it was conducted point analyses by energy-dispersive X-ray spectroscopy (EDS) in the regions denoted by the numbers 1 and 2 in Fig. 2. The resulting spectra can be seen in Fig. 3.
From the spectra presented in Fig. 3, it is possible to obtain the semiquantitative determination of the alloying elements, as shown in Table 3. In both alloys, the interdendritic (region 1) is mainly composed by Cu and Al, while the dendritic (region 2) is composed mainly of Fe, Co, and Cr. However, with the increase in Al content, the fraction of it in region 2 is raised more than in region 1. This difference can be seen in the EDS composition maps exhibited in Fig. 4.
Maps of EDS composition for the Al0.5CoCrCuFe (top) and Al0.9CoCrCuFe (bottom) alloys in the region of Fig. 2a and b
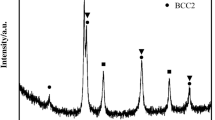
In order to understand the structural information behind the discussed chemical difference, X-ray diffraction patterns for the studied alloys were obtained and can be seen in Fig. 5a. It was possible to notice that both alloys presented similar diffraction peaks; however slight changes in position and intensity were also verified, as shown in the inserted graph in Fig. 5a. These diffraction peaks were consistent with two solid solutions, one of them the face-centered disordered solid solution with Space Group Fm-3m (ICSD 606,883 [20]), henceforth called SSfcc, and the other a body-centered disordered solid solution with Space Group.
Im-3m (ICSD 102,751 [20]), henceforth called SSbcc. The presence of two phases in alloys with five components indicates a dominant effect of entropic factors concerning enthalpic ones. These findings were in agreement with the dendritic region, SSbcc, and interdendritic regions SSfcc shown in Figs. 1, 2, and 4. It is also important to verify that the peak intensity from SSbcc increased when Al was added to the alloy. This is expected since a reduction has been seen in the interdendritic region (see Fig. 1a and c). The second effect of Al addition to the alloy is the Cu and Al content increase and the Co, Cr, and Fe decrease in the dendritic region. This effect was compatible with the increase in the quantity of Cu-rich precipitates observed in the SEM images in Fig. 1b and d.
The main alloying elements forming the dendritic region of materials are Fe, Co, and Cr. These elements are often associated with the appearance of magnetic properties in different types of materials. Thus, if the variation proposed above is consistent, a change in the saturation magnetization of the material can be verified with the increase in the Al content. Thus, to confirm the description presented magnetization measurements with applied field were performed at 300 K, as seen in Fig. 5b.
In Fig. 5b it was possible to notice that the saturation magnetization decreased with the increase in Al content. This result is in agreement with the decrease in the relative fraction of Fe, Co, and Cr in the dendritic region and suggests a direct relationship of these elements with the magnetic effect observed in the alloys. Considering that the dendritic region is associated with the ferromagnetic behavior in these alloys, the reduction in the saturation magnetization (Ms) when Al is added may be related to the substitution of Co, Cr, and Fe by Cu and Al atoms. Therefore, even with a higher relative fraction of Cu in the dendritic region with increasing Al content, this copper is less available than that segregated in the interdendritic region.
Literature data [12]. As the multi-principal element alloys studied here are composed of five elements, we cannot reason that only copper is being oxidized, but the biocidal effect observed must be due mainly to the ions of this element [12]. Although the alloys have a high number of components, the experimental data obtained indicated the formation of a Cu-rich phase at the interdendritic region while the dendritic region is rich in Cr, Fe, Co, and Al (Fig. 4). It is known that this configuration allows the arising of galvanic effects between the dendritic and interdendritic regions. As the relative fraction of Cr in the dendrites is greater than 15at.%, their corrosion resistance is higher, making the Cu-rich region the anode of this galvanic cell. This allows the preferential corrosion of the Cu-rich solution and the formation of copper ions [21]. This mechanism may be responsible for providing copper ions for the inactivation of bacteria on the surface of the alloys. In addition, it has been reported that the survival of Escherichia coli began to decline when the copper ion concentration reached 400 mmol L−1 and came to zero when the Cu concentration reached 600 mmol L−1 [30, 31]. Therefore, the results seem to be related to the microstructure and corrosion behavior of the materials. The images in Fig. 4 showed that, regardless of the Al concentration, copper was deposited preferentially in the interdendritic region of the alloys, but in the Al0.9CoCrCuFe alloy this field seems to be reduced while a higher quantity of Cu-rich precipitates was verified in the dendritic region, as seen in Fig. 2b. Polarization tests showed that the alloy with the highest concentration of Al has a less noble corrosion potential, but its corrosion current density was also lower. This suggests that the observed biocidal effects may be closely related to the corrosion rate of the material and the preferential oxidation of copper. The Al0.9CoCrCuFe alloy exhibited the best results in action against Escherichia coli. It is important to mention that the corrosion current density is directly proportional to the corrosion rate of the material.
The fact that the unpolished alloys showed more interesting results in higher concentrations of bacteria may be due to the contribution of the metallic matrix, surface oxide layer, and corrosion rate. In the case of polished samples, the most significant contribution should be from the metallic matrix. The variation observed as a function of the bacteria concentration was due to the Cu+/Cu2+ ions concentration available to interact with the bacteria. As the time of contact with the metallic matrix was reduced, a lower content of Cu+/Cu2+ ions was produced when compared to the bacteria concentration, and only the number of bacteria proportional to this concentration of Cu+/Cu2+ ions can be inactivated at 0 h. This is remarkable in the unpolished material because the passive layer of the Al0.9CoCuCrFe alloy is a little more protective and the copper is better distributed throughout the matrix, which could also interfere with the availability of Cu ions. Hence, there is a relationship between the biocidal ability of the studied alloys and their microstructure and corrosion behavior.
Conclusions
The increase in the concentration of Al promoted the refinement of the dendrites and the interdendritic regions of the Al0.5CoCrCuFe and Al0.9CoCrCuFe alloys. Both studied alloys exhibited precipitates on the dendritic region that were related to an fcc solid solution, the same phase found in the interdendritic region. The alloy with higher Al content presented lower saturation magnetization and corrosion potential, but also exhibited a lower corrosion current density. These results were related to the biocidal ability of the alloys and the material with lower corrosion current density showed good performance under the bacterial strain Escherichia coli ATCC 8739.
References
N.G. Jones, J.W. Aveson, A. Bhowmik, B.D. Conduit, H.J. Stone, On the entropic stabilisation of an Al0.5CrFeCoNiCu high entropy alloy. Intermetallics. 54, 148–153 (2014). https://doi.org/10.1016/j.intermet.2014.06.004
E. Zhou, D. Qiao, Y. Yang, D. Xu, Y. Lu, J. Wang, J.A. Smith, H. Li, H. Zhao, P.K. Liaw, F. Wang, A novel Cu-bearing high-entropy alloy with significant antibacterial behavior against corrosive marine biofilms. J. Mater. Sci. Technol. 46, 201–210 (2020). https://doi.org/10.1016/j.jmst.2020.01.039
G. Perumal, H.S. Grewal, M. Pole, L.V.K. Reddy, S. Mukherjee, H. Singh, G. Manivasagam, H.S. Arora, Enhanced biocorrosion resistance and cellular response of a dual-phase high entropy alloy through reduced elemental heterogeneity. ACS Appl. Bio Mater. 3, 1233–1244 (2020). https://doi.org/10.1021/acsabm.9b01127
Y. Lou, C. Dai, W. Chang, H. Qian, L. Huang, C. Du, D. Zhang, Microbiologically influenced corrosion of FeCoCrNiMo01 high-entropy alloys by marine Pseudomonas aeruginosa. Corros. Sci. 165, 108390 (2020). https://doi.org/10.1016/j.corsci.2019.108390
G. Koch, Cost of corrosion. Elsevier Ltd. (2017). https://doi.org/10.1016/B978-0-08-101105-8.00001-2
W.H. Organization, Naming the coronavirus disease (COVID-19) and the virus that causes it, (n.d.). https://www.who.int/emergencies/diseases/novel-coronavirus-2019/technical-guidance/naming-the-coronavirus-disease-(covid-2019)-and-the-virus-that-causes-it Accessed April 6, 2020.
J.R. Scully, The COVID-19 pandemic, Part 1: Can antimicrobial copper-based alloys help suppress infectious transmission of viruses originating from human contact with high-touch surfaces? Corrosion. 76, 523–527 (2020). https://doi.org/10.5006/3568
N. van Doremalen, T. Bushmaker, D.H. Morris, M.G. Holbrook, A. Gamble, B.N. Williamson, A. Tamin, J.L. Harcourt, N.J. Thornburg, S.I. Gerber, J.O. Lloyd-Smith, E. de Wit, V.J. Munster, Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N. Engl. J. Med. 382, 1564–1567 (2020). https://doi.org/10.1056/NEJMc2004973
S.L. Warnes, S.M. Green, H.T. Michels, C.W. Keevil, Biocidal efficacy of copper alloys against pathogenic enterococci involves degradation of genomic and plasmid DNAs. Appl. Environ. Microbiol. 76(16), 5390–5401 (2010). https://doi.org/10.1128/AEM.03050-09
M. Vincent, R.E. Duval, P. Hartemann, M. Engels-Deutsch, Contact killing and antimicrobial properties of copper. J. Appl. Microbiol. 124, 1032–1046 (2017). https://doi.org/10.1111/jam.13681
J.O. Noyce, H. Michels, C.W. Keevil, Use of copper cast alloys to control Escherichia coli O157 cross-contamination during food processing. Appl. Environ. Microbiol. 72(6), 4239–4244 (2006). https://doi.org/10.1128/AEM.02532-05
Z. Li, D. Qiao, Y. Xua, E. Zhoua, C. Yanga, X. Yuana, Lu. Yi**, Gu. Ji-Dong, S. Wolfgang, D. Xua, F. Wanga, Cu-bearing high-entropy alloys with excellent antiviral properties. J. Mate.r Sci. Technol. 84, 59–64 (2021). https://doi.org/10.1016/j.jmst.2020.12.027
M.V. Ivchenko, V.G. Pushin, N. Wanderka, High-entropy equiatomic AlCrFeCoNiCu alloy: Hypotheses and experimental data. Technol. Phys. 59, 211–223 (2014). https://doi.org/10.1134/S1063784214020108
E.J. Pickering, H.J. Stone, N.G. Jones, Fine-scale precipitation in the high-entropy alloy Al 0.5 CrFeCoNiCu. Mater. Sci. Eng. A. 645, 65–71 (2015). https://doi.org/10.1016/j.msea.2015.08.010
N.G. Jones, A. Frezza, H.J. Stone, Phase equilibria of an Al0.5CrFeCoNiCu high entropy alloy. Mater. Sci. Eng. A. 615, 214–221 (2014). https://doi.org/10.1016/j.msea.2014.07.059
N.G. Jones, R. Izzo, P.M. Mignanelli, K.A. Christofidou, H.J. Stone, Phase evolution in an Al0.5CrFeCoNiCu high entropy alloy. Intermetallics. 71, 43–50 (2016). https://doi.org/10.1016/j.intermet.2015.12.001
N.G. Jones, K.A. Christofidou, H.J. Stone, Rapid precipitation in an Al0.5CrFeCoNiCu high entropy alloy. Mater. Sci. Technol. (United Kingdom). 31, 1171–1177 (2015). https://doi.org/10.1179/1743284715Y.0000000004
A. Paganotti, C.V.X. Bessa, L.S. Silva, R.A.G. Silva, Metallic sample preparation for phase transformation analysis. MethodsX. 811, 152029 (2019). https://doi.org/10.1016/j.mex.2019.09.041
M. Ming-xing, W. Zhi-xin, Z. Jia-chen, L. Cun, Z. Cong, Phase structure of multiprincipal component AlCoCuFeMnNi alloy prepared by melting casting. Int. J. New Dev. Eng. Soc. 1, 111–114 (2017). https://doi.org/10.25236/IJNDES.17334
Inorganic Crystal Structure Database – ICSD, (n.d.). https://icsd.fiz-karlsruhe.de/search/basic.xhtml.
X. Chen, J. Hu, Y. Liu, F. **ang, Corrosion behavior in nitric acid solution and tensile properties of (CuFeNiMn)1-xCrx HEAs. Met. Mater Int. 27(27), 2230–2238 (2021). https://doi.org/10.1007/s12540-020-00887-3
E. McCafferty, Validation of corrosion rates measured by the Tafel extrapolation method. Corros. Sci. 47, 3202–3215 (2005). https://doi.org/10.1016/j.corsci.2005.05.046
Y. Qiu, M.A. Gibson, H.L. Fraser, N. Birbilis, Corrosion characteristics of high entropy alloys. Mater. Sci. Technol. (United Kingdom). 31, 1235–1243 (2015). https://doi.org/10.1179/1743284715Y.0000000026
C. Onuoha, Z. Russell, G. Kipouros, Z. Farhat, K. Plucknett, The aqueous electrochemical response of TiC–stainless steel cermets. Metals (Basel). 8, 398 (2018). https://doi.org/10.3390/met8060398
E. McCafferty, Introduction to Corrosion Science. Springer, New York, NY, 2010. https://doi.org/10.1007/978-1-4419-0455-3.
M. Pourbaix, Atlas of electrochemical equilibria in aqueous solutions, Second, Pergamon Press and Cebelcor, 1974. http://sunlight.caltech.edu/aic/pourbaix.pdf.
A.C. Harkness, L. Young, High resistance anodic oxide films on aluminium. Can. J. Chem. 44, 2409–2413 (1966). https://doi.org/10.1139/v66-363
A.R. Brooks, C.R. Clayton, K. Doss, Y.C. Lu, On the role of Cr in the passivity of stainless steel. J. Electrochem. Soc. 133, 2459–2464 (1986). https://doi.org/10.1149/1.2108450
T.M. Butler, M.L. Weaver, Oxidation behavior of arc melted AlCoCrFeNi multi-component high-entropy alloys. J. Alloys Compd. 674, 229–244 (2016). https://doi.org/10.1016/j.jallcom.2016.02.257
C.E. Santo, N. Taudte, D.H. Nies, G. Grass, Contribution of copper ion resistance to survival of Escherichia coli on metallic copper surfaces. Appl. Environ. Microbiol. 74(4), 977–986 (2008). https://doi.org/10.1128/AEM.01938-07
M. Rosenberg, H. Vija, A. Kahrul, W. Keevil, A. Ivask, Rapid in situ assessment of Cu-ion mediated effects and antibacterial efficacy of copper surfaces. Sci. Rep. 8, 8172–8184 (2018). https://doi.org/10.1038/s41598-018-26391-8
Acknowledgements
The authors would like to thank FAPESP (grant no. 2022/04262-9) for financial support. This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brasil (CAPES)—Finance Code 001.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Oliveira, B.S., Paganotti, A., Silva, L.S. et al. Effect of Al Content on the Microstructure and Properties of As-Cast AlxCoCrCuFe Alloys. Metallogr. Microstruct. Anal. 11, 864–872 (2022). https://doi.org/10.1007/s13632-022-00913-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13632-022-00913-3