Abstract
Key message
Quercus rubra half sib progenies (N = 93) expressed high levels of variance for both growth and stem form traits in three locations in Indiana, USA at age 11 or 12. Height, diameter, and volume were measured and sweep, branch angle, forking, and branch retention were rated using a (+ / −) system. Families selected for volume showed no unfavorable increases in sweep or branch angle and only a slight increase in branch retention.
Context
Northern red oak (Quercus rubra L.) is the most planted hardwood in the central USA. Red oak seeds from sources with improved growth and form are unavailable in the Central Hardwood Region, in part because the absence of agreed methods for stem form evaluation diminishes the effectiveness of selection.
Aims
To identify red oak families improved for growth and to determine if a simple + / − rating system could identify red oak families with improved form.
Methods
We evaluated 93 open-pollinated families of 11 or 12-year-old red oak growing in three sites in central Indiana (USA) for height, diameter, volume, and four traits rated as + / − : sweep, branch angle, forking, and branch retention.
Results
Family × location effects were significant for all quantitative traits but not for any binary traits; differences among families were significant for all traits at all sites. Heritabilities for most traits were high. Selection of the top 20 families for volume at each site resulted in no change in population means for sweep or branch angle and only a small increase in the retention of large limbs.
Conclusion
A + / − rating system to evaluate hardwood form can help breeders deliver improved red oak to landowners when more complex systems are impractical.
Similar content being viewed by others
1 Introduction
Northern red oak (Quercus rubra L; hereafter simply “red oak”) is a valuable commercial timber species that is popular among landowners because of the diversity of purposes for which it is useful, including: wildlife, urban parks, restoration plantings, riparian areas, reclaimed surface minelands, and carbon sequestration (Huang et al. 2004; Jensen and Löf 2017; Johnson et al. 2019). Red oak is a common species in much of the Central Hardwood Region of the USA, and it often maintains its abundance in landscapes because it regenerates from stump sprouts where conditions are favorable (Dey et al. 2008a, 2017), and from frequent, copious acorn production. Seedling shade tolerance also contributes to enhanced rates of survival following partial harvests (Huang et al. 2004). Red oak tolerates a wide range of soils and topography, so artificial regeneration is the primary means for reforesting highly disturbed sites, areas where red oak has failed to regenerate naturally, or areas where land conversion is desired, including old fields (Dey et al. 2008a). For these reasons and more, red oak is the most planted hardwood tree in the central USA (Pike et al. 2018), and its importance as a planted species is likely to increase as a strategy to offset climate change because red oak is considered highly adaptable (Peters et al. 2020) and shows weak population clines (Leites et al. 2019).
The Indiana State Nursery sells about 140,000 red oak seedlings each year (Hawkins, Pers. Comm). Although Indiana landowners have the option to purchase red oak seedlings from phenotypically superior parents grafted into a seed orchard (versus wild sources) they are often unwilling to pay the cost differential, possibly because the potential benefits from increased growth have not been documented. In addition, some landowners may be reluctant to pay extra for red oak seedlings because stumpage prices for high quality red oak logs can be considerably lower than some sympatric species such as black walnut (Juglans nigra), depending upon the year and local market conditions.
Breeding may be able to improve the value of red oak because it exhibits high levels of genetic and phenotypic variation (Schlarbaum and Bagley 1981; Schwartzman and Gerhold 1991). Red oak improvement is constrained, however, by the species’ reproductive biology; its seeds (acorns) are recalcitrant and their availability is periodic. Acorns have been stored over multiple years — albeit with a significant loss in viability — for the establishment of new progeny tests (Bonner 1973; Connor 2004). In practice, however, progeny tests are established using acorns derived from the current year. Thus, red oak progeny tests are often unbalanced.
Few tree improvement programs in North America focus on Quercus species and other fine hardwood species for which growth rates are often secondary to log quality in importance (Kerr and Morgan 2006). Hurdles to implementing tree improvement for temperate trees include long rotation times, episodic seed production, and unfavorable correlations between growth and form (Pang 2014; Savill et al. 2005; Victory et al. 2004). Efforts to improve stem form, forking, or branch angle may produce superior logs because these traits tend to exhibit heritability high enough that rapid gains are possible (Weng et al. 2017). Stem quality traits such as straightness are typically rated visually (Alcorn et al. 2007; Kerr and Morgan 2006), so subjective elements can bias results (Cooper and Ferguson 1981), and there is no widely accepted system for rating immature hardwood tree form (Balandier 1997; Cornelius and Mesen 1997; Hai et al. 2008; Jensen and Löf 2017). As a result, selection efficiency for the improvement of hardwoods for their most valuable traits (i.e., stem quality) is reduced by biases inherent to visual rating systems, and a lack of consistent methodology needed to establish long-term correlations between juvenile and mature assessments. The absence of established criteria for evaluating fine hardwood tree species diminishes the value of improvement programs (Beineke 1983; MacDonald et al. 2009; Stringer et al. 1995; White et al. 2007; Wu et al. 2008).
We developed a binary stem-rating scale that is relatively simple to describe, apply, and replicate. Our purpose was to score multiple stem quality associated traits, to reduce human bias in ratings, and to simplify and improve selection of red oak progenies for stem form. Our first objective for this research was to assess the genetic parameters for these binary traits. Second, we wanted to demonstrate whether seedlings from the Indiana State Seed Orchard showed improved growth as compared to checks, and third, we wanted to determine if stem volume and stem form can be improved simultaneously in red oak, with the goal of improving the seed orchards that serve the nursery sector in the Central Hardwood Region of the USA.
2 Materials and methods
The Hardwood Tree Improvement and Regeneration Center (HTIRC) is a research work unit of the USDA Forest Service Northern Research Station, located at Purdue University in north-central Indiana, USA. HTIRC acquired open-pollinated seeds from 93 red oak families for a multi-site progeny test. The seeds were collected from seed orchards at the Vallonia and Jasper Pulaski Nurseries, operated by the State of Indiana (Coggeshall 1993), and from other locations across Indiana. In some cases, half-sib cohorts collected from ramets of the same genotype growing in different seed orchards were treated as unique half-sib families for the purposes of analysis. All seed orchard trees were grafted selections of wild trees, or trees growing in plantations, that were phenotypically superior in stem form and/or volume compared to neighbors. The checklot consisted of a pooled seedlot acquired by the Indiana State Tree Nursery at Vallonia from commercial seed collectors.
Progeny tests were established in 2007 (sites 115, 117) and 2008 (site 119) on two sites in two locations in Tippecanoe County in central Indiana (Fig. 1) (Woeste et al 2021). Sites 115 and 119 were adjacent at the Purdue University Martell Forest (Lat/Long 40.44, -87.03); the third site (117) was located at the Throckmorton Purdue Agricultural Center (40.27, -86.88) (Table 1). All three plantations were established on former agricultural fields, primarily on Miami Series soils considered well-suited to tree growth, with a site index from 75 to 90 for red oak (Soil Survey Staff 2020). Red oak is a component of the native, second-growth forests adjacent to each planting site. All three sites were planted with 1 + 0 bareroot seedlings in a randomized complete block design with two progeny per family per block in single-tree plots at 2.4-m spacing with 20, 28, and 25 blocks at sites 115, 117, and 119, respectively. Alternate rows of the plantations were planted with 1 + 0 bur oak (Q. macrocarpa) as a spacer and at row ends as a border; bur oaks were removed at year 10 except the row end border trees, increasing spacing to 2.4 × 4.8 m. All seedlings were germinated and grown at the Vallonia Nursery. Including the checklot of pooled sources from commercial vendors which was counted as a single family, we analyzed the growth of 60, 47, and 62 seedling families at locations 115, 117, and 119, respectively (Table 1; Fig. 1); a slightly larger total number of seedlings were planted but five families contained too few seedlings to evaluate. In total, 93 families were tested, but some families were planted at only one or two sites (Fig. 1); 23 families were common across all three sites. A small number of trees that could not be identified to a family were also excluded from analysis (Tables 2 and 3).
Tree height was measured to the nearest decimeter with a hypsometer (Haglof®). Diameter at 1.4 m (DBH) was measured in mm after 11 growing seasons (sites 115, 117) and 10 growing seasons (site 119). Stem volume was calculated using the formula [(diameter)2 *height]. This equation does not include taper and is used to provide a rough estimate of overall tree size. Stem quality for each tree was assessed using four binary (+ / − or 0/1) traits, including stem sweep (presence/absence), branch angle (average angle of branches below 3.7 m measured adaxially) greater than ( +) or less than ( −) 45°, presence or absence of three or more branches with basal diameter greater than 2.5 cm on the bottom 3.7 m of the main stem (hereafter referred to as branch-retention), and presence or absence of a 3.7 m log without a fork (defined as a lateral branch at least 1/3 the diameter of the tree connecting to the main stem, herein referred to as forking). We used a 3.7 m log for evaluation as it represents the standard log length for commercial timber sales in Indiana. A genotype showing an off-center deviation of more than 1/3 of the stem diameter in the bottom 3.7 m of stem or deviation > ¼ stem diameter in two or more planes was scored as showing sweep. Trees with sweep, forks, and excessively acute branch angles were considered undesirable in terms of form, as were trees with large branch attachments (diameter at insertion).
2.1 Statistical analysis
For analysis, binary traits were expressed as the probability an individual in a family presented mean branch angles < 45°, more than three branches 2.5 cm in diameter, showed sweep, or a log with a fork. All quantitative traits (height, diameter, volume) were normally distributed at each site, so no transformations were needed, and traits scored as probabilities showed frequencies near enough to 50% that they did not require arcsin transformation. A linear mixed model was used to detect genotype by environment interactions with site and blocks (within sites) set as fixed effects (SAS, version 6, 2018), all other terms were set as random effects with this model.
where Yijkl is the observed value in each Site (S), Block (B), Family (F), and plot with eijkl as the pooled error [assumed to be NID(0,variance)]. For binary traits, a generalized linear mixed model (Proc Glimmix) was used, with a logit function. For each trait, favorable attribute (stem sweep and forking are absent, wide branch angles, and few lateral branches) were scored as logit (event = 1). Quantitative traits were analyzed with Linear Mixed model (Proc mixed). We compared pseudo loglikelihoods (binary traits) with Chi-square to compare models with and without random effects to assess the significance of each factor for binary traits. We set all factors as fixed effects to compare genotype by environment interactions with Wald’s test for diameters, heights and volumes. We obtain Best Linear Unbiased Predictors (BLUPS) and estimated variance components for heritability estimates (below) using Eq. (1).
Individual heritabilities, which describe the percent of all variation that is additive, were calculated from variances obtained in Eq. (1) using the standard assumptions of open-pollination in which the family variance accounts for one quarter of the additive genetic variation (Eq. (2)), which is a generally a valid assumption in red oak (Schwartzman and Gerhold 1991). Family mean heritability, which describes the percent of variation attributed to genotype (additive and non-additive), was calculated for each trait (Eq. (3)).
where VF, VS*F, VF(B), and Verror is the variance component for family, site by family interaction, block within family, and residual error, respectively. In Eq. (3), s is the number of sites, sb is the number of trees per plot and sbn is the number of trees per family per site estimated using Type III ANOVA. A logit distribution was used for binary traits with residual variance set to (π^2)/3 (3.289) (Nakagawa and Schielzeth 2010). Terms with variance equal to zero were removed from the model to estimate variance components, i.e., for branch retention [(family*block(site))], branch angle, and stem sweep [(site*family)]. Standard errors were calculated using the Delta method (Lynch and Walsh 1998). Heritability for forking was estimated across two sites only (115, 119) because family variance was zero at site 117 (Table 2).
A linear mixed model was used to generate breeding values and genetic gains for each site, with block and family terms only, set as random effects. We obtained the Best Linear Unbiased Predictors (BLUPS) for each family and multiplied by 2 to generate parental breeding values (BV), where:
For each quantitative variable, the site mean was added to the BV, and genetic gain was expressed as a percent relative to the checklot for each trait at each site. The breeding values for stem form traits were multiplied by 2, then converted to percent probabilities (%prob) using an inverse link function:
The percent probabilities reflect the probability of an undesirable phenotype. The breeding values (volume) were plotted against the percent probabilities (stem form traits) to assess the growth vs stem-form tradeoffs across all families. Twenty families with the highest BV for volume were identified at each site, and the stem quality percent probabilities (branch angle, sweep, branch retention, forking), for the selected populations were averaged to measure growth-form trade-offs in the selected population.
3 Results
Survival across all sites exceeded 90%, so competition was similar at the three sites. Sites differed significantly for the quantitative traits but did not differ significantly for the four binary traits (Table 3). Genotype by environment interactions (G × E) were significant for all traits except sweep and branch retention (Table 3), and rank shifts for family mean volume were considerable (Appendix Fig. 3). Differences among families were significant for all traits at all sites. Percent probabilities for occurrence of the binary traits varied widely among families (Table 4). All heritabilities differed from zero by a margin greater than the estimated standard error (Table 2). Heritabilility values for quality-related traits (both on an individual tree and family basis) were moderate to high, but generally highest for branch angle, followed by stem sweep and branch retention and lowest for forking. Heritabilities were higher for diameter and volume than height, but all differed from zero by a margin greater than the estimated standard error.
The difference between the checklot and the progeny test populations was greatest for volume; ranging at the three test sites between 32 and 66%. The difference between the test populations and the checklot was greater for diameter than for height at all sites. At all three sites, about half of the trees showed stem sweep, but the presence of sweep was uncommon in some families (Table 4). In general, sweep was independent of volume, and some high-volume families showed little sweep. We selected the 20 best families (selected population) based on volume at each site (20/60 in site 115, 20/47 in site 117, 20/62 in site 119) with the goal of calculating expected change in other studied traits from a seed orchard comprised of the parents of the selected families (backward selection). Despite significant G × E for volume (Table 3), all of the 20 top families for volume at site 115 were also among the top 20 at one other site (most were shared with site 117 the non-adjacent site, four with site 119), and 13 of the top 20 families at site 117 were among the top 20 at another site. Twenty-three families were common to all three sites, but no single family was in the top 20% for volume at all three sites (not shown). The selected population at each site averaged at least 67% more volume than the checklot (Table 5). The selected population with largest volume at each site showed a slightly lower probability of stem sweep compared to the overall population at site 115 (44 versus 47%), a slightly increased probability of sweep at site 117 (52 versus 50%), and there was no difference at site 119 (50%) (Table 5). Branch angle of the families in the top 20% for volume was slightly better (more horizontal) than the branch angle of the entire population at each site (Table 5, Fig. 2), although in general, branch angle was independent of volume. Families in the selected population were somewhat more likely to have large-diameter lateral branches in the most valuable part of the stem, i.e., the lowest 3.7-m section of the butt-log (Fig. 2). This (disadvantageous) trade-off between volume and frequency of large-diameter branches in the butt log ranged from an increase of 7% (site 119) to 12% (site 115) in the probability of having more than three large branches.
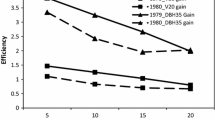
Genetic correlations between half-sib family mean volume and branch traits in three red oak plantations, probabilities are expressed as likelihood a member of a half sib family retained three or more branches with diameter greater than 2.5 cm on the bottom 3.7 m of the main stem (panel A, C, E) or that the mean adaxial branch angle of all branches on the bottom 3.7 m of the main stem was greater than 45° (panels B, D, F). Site 115 (panels A, B), Site 117 (C, D), Site 119 (panels E, F)
4 Discussion
Red oak is a desirable species for a variety of planting types across the eastern USA, but tree improvement efforts in this species have been relatively scant (Cech 1971; Mackay 1993; Struve and McKeand 1993), even though there is high potential for gains, and high-end markets for quality logs. We evaluated 93 open-pollinated families in a progeny test after 10/11 years of growth to determine phenotypic variance, heritabilities, and potential to improve seven traits affecting the value of red oak. Although form and branch-related traits can be highly variable within species, they are also plastic to light competition and wind, and exhibit growth rate versus stem quality trade-offs (MacFarlane and Kane 2017). Although many of the female parent trees of the test populations were within a seed orchard comprised of grafted clones of phenotypically superior parents, mass selection based on phenotype does not always result in detectable improvement in this species (Stringer et al. 1995). We observed significant differences among families for nearly all the traits. Phenotypic variance for volume was especially high (Table 1). The seed orchard parents of the progeny families in this study were derived from a wide range of latitudes, so some families with higher volume may have gained a growth advantage from earlier leaf flush (Baliuckas and Pliura 2003).
Although red oak growth is responsive to site factors including intraspecific competition (Burkardt et al. 2019), genetic factors related to source are often more important drivers of adaptational phenotypic variance than site factors (Swaim et al. 2016), as was shown in Q. robur (George et al. 2020). The spatial and ecological scale at which adaptational differences among Q. rubra families is expressed is not well documented, although environmental clines for red oak may be weak (Leites et al. 2019). Our test sites were all located in one county of central Indiana; each appeared typical of red oak afforestation sites with respect to soil type, soil depth, moisture, and slope, so we were surprised to observe large rank shifts among families (Appendix Fig. 3). Significant site by family effects (Table 3) indicated considerable variation among families for growth traits across the three sites, although about 15 families excelled for volume at two of the three sites. The importance of the observed site × family effects may have been inflated by unbalance in the experimental design. There were some apparent differences among the planted sites, but it was not obvious to us which, if any, were important drivers of the significant site by family effects observed. Edaphic factors seem most likely, although soil types at every site are considered well suited for red oak. Sites 115 and 119, which are adjacent, differed somewhat as to soil type. Both included Rockfield and Starks/Fincastle soils, but site 115 also included a large area of Rainsville soils which are generally better than Starks/Fincastle. Because the plantation sites were all former agricultural fields, subsurface and drainage effects might have affected site quality and contributed to growth interactions. Site 119 is flatter than 115 and 117; site 117 was the most rolling and it was planted in three separate (but nearly adjacent) compartments. Trees at site 115 and 117 were one year older than those at 119; age difference might result in site effects, but it is difficult to understand how a year difference in age could have generated large site × family interaction effects. It is possible and even likely that half-sib families harvested in different years from the same mother trees did not sample the same pollen pools (Alexander and Woeste 2017). In those cases, male effects may partly explain half-sib family rank shifts between the adjacent sites 115 and 119, which were planted a year apart. But we also observed that in two instances distinct half-sib cohorts from the same genotype (seeds from the same genotype growing at different seed orchards) both entered the top 20% for volume. In other words, those genotypes were superior for volume at site 115 irrespective of the male effect. Given the large interaction effects we observed in nearby sites planted in subsequent years, predicting an effective match of parents to untested sites will be a challenge. High site × family effects are probably best managed by maintaining high levels of genetic diversity in seed orchard parents. In fact, the parents used in this study were intentionally selected to represent as many of the 92 counties in Indiana as possible. Research into factors that influence site × family productivity of red oak is needed.
We did not observe significant site × family effects for branch retention and effects were zero for sweep and branch angle, which likely indicates that these traits were less plastic than tree size traits. Spacing can affect traits such as branch angle and branch size in Eucalypts (Alcorn et al. 2007) and Q. robur (Malinauskas 2007), but all three sites were planted at the same spacing, so we could not determine the effect of spacing on heritability or variability in any of the traits we studied.
The selected population (based on volume) consisted of 20 families at each of the three sites (115, 117, 119), with 23 families in common across all three sites. Tradeoffs between volume and branch retention were consistent at all sites, a genetic correlation that is likely caused by pleiotropy in which genes that control stem size also control branch size. The absence of correlation between stem volume and other traits (stem sweep, branch angle) indicated that these traits were inherited independently from volume. This finding reveals that breeders may use independent culling (Zobel and Talbert 1984) to select families that have the highest volume and best stem form (stem sweep, branch angle) in these populations, recognizing that selection for volume may include trees that retain large lateral branches. Breeders may opt to omit or ignore families with a tendency to retain large branches or opt for silvicultural approaches to suppress or aggressively excise large branches on young trees to improve future log value.
The ideal stem form for red oak depends on its intended commercial use, which can involve complicated trade-offs. Acute branch angles (erect limbs) are associated with smaller branches, larger knot area, and less volume growth; but limbs with higher branch angles (more horizontal) may produce permanent defects in logs if they fail to self-prune or fail to occlude when they self-prune. In red oak, successful occlusion of branch scars was associated with fast growth and limbs smaller than ~ 3 cm (Nicolescu et al. 2013). In our study populations, high stem volume was associated with the presence of more large limbs on the lower stem. Genetic correlations among traits such as branch number and canopy size, and their relationship to log value, remain important areas for research, not just for red oak but for other commercially important hardwood species as well.
We found that reducing quality scores to binary traits made them relatively easy to assess and describe, and their use produced relatively high heritability estimates. We suggest that in the absence of a well-established, reliable quantitative method for evaluating stem straightness, a simpler, binary evaluation system such as the one described here minimizes inherent bias and may be better than typical rating systems (e.g., 1 to 4) for species like red oak (Sohn and Goddard 1979). In addition to easing the consistency and repeatability of assessments, binary traits bypass the complexity of multi-categorical analyses, and assumptions for a logit function are fairly easy to meet. Use of + / − phenotypes is a limiting case (categories are reduced to two) that is uncommon in tree breeding except in rating survival; animal breeders have given it considerable attention (Moghadam et al. 2005). The choice of numbers of categories and the method of analysis should be based on the presumed relationship between the phenotypic categories and the underlying trait of interest, which is likely continuous (Dempster and Lerner 1950). Lopes et al. (2000) make clear that it is the underlying trait that the breeder wishes to improve, and that the correspondence between the heritability of the underlying trait and the heritability based on a binary estimate was highest when trait incidence is near 50% or when heritability is low (< 0.3). If a trait is uncommon, Falconer and Mackay (1996) suggest selection based on mean liability of a family, rather than individual mass selection.
A polychotomous rating scale is often preferred to a dichotomous one if it can better represent the underlying trait distribution, but when traits are rated polychotomously, “the problem of assigning scores to response categories is not a trivial one” (Gianola and Foulley 1983); skew is a particular problem (Gianola and Foulley 1983), so scaling can help intervals correspond to the underlying normal distribution (Snell 1964). Aside from analytical difficulties, it is obvious that a tree rated 2 is not half as good as one rated 4 nor does it have half the value; in fact, one may be acceptable while the other isn’t. For these reasons, animal breeders sometimes adjust categories using a vector of weights (Lopes et al. 2000). When considering number of categories, there is a trade-off with time and repeatability. Less obvious, perhaps, is that many categories of unacceptable phenotypes add nothing to selection. Meuwissen et al. (1995) suggest breaking the acceptable category into subcategories, and that distinctions near the threshold are most informative.
Our heritability estimates for red oak growth traits were higher than those reported in studies of Q. alba in Indiana (Huang et al. 2016) and like those reported for Q. robur (Bogdan et al. 2004). Our data shows there is high genetic variance for growth in red oak, and that growth traits measured in the present study are moderately heritable, so there is potential for gains from selection. Of course, volume gains can be maximized by proper management (Dey et al. 2017; Jensen and Löf 2017), and even in some cases by pruning to improve form (Balandier 1997; Kerr and Morgan 2006). Many of the parent trees that produced the open-pollinated seedling families used for this study were grafted ramets of trees with better than average bole quality and growth rates, and they represented a wide range of sites in Indiana and other states in the Central Hardwood Region (Coggeshall 1987). Based on the three sites we studied, some red oak parents at the existing seed orchard have outstanding potential for inclusion in an improved seed orchard. Backward selection and propagation of a clonal red oak seed orchard is complicated by lack of protocols for rooting and by delayed rootstock incompatibility but grafted red oak seed orchards have been successfully developed (Coggeshall 1993). Forward selection and conversion of the seedling progeny trials into seed orchards are also a possibility; approximately 50% of the parents in the grafted seed orchards that produced the seedlings for this study were forward selected from 10- to 50-year-old plantations.
In theory, improvements in red oak bole quality have the potential to benefit landowners, but there is little published data regarding the genetic regulation of, or potential to improve, quality traits such as branch angle or sweep (Irgens-Moller 1955). Plantations of the type described here have value for future studies of management and climate adaptation, since the locations of the parent trees are known, but the overall value of a program for red oak improvement depends on the willingness of landowners to pay for improved stock and to plant red oak (as opposed to relying on natural regeneration). Communication of the results of this study to landowners and consulting foresters who plant trees should provide the incentives they need to justify investment in improved planting stock. To increase the demand for improved versus wild-collected stock from nurseries, it will be useful to develop better models relating sources, stock quality, site types, and management to growth and survival (Dey et al. 2008a). Demand for improved stock can be supported by economic models relating improvement in quality traits such as sweep or branch architecture to landowner value. But whatever the growth benefits or economic returns gained by planting improved red oak, deer browse remains a serious impediment to planting red oak seedlings in many locations (Owings et al. 2017). Where browse pressure is high, landowners may need to exclude deer with fences if they want red oak regeneration of any kind, as fencing combined with silvicultural treatments improved red oak regeneration (Frank et al. 2018; Redick et al. 2020). Improved red oak could make fences a worthwhile investment if improvements in site quality result in reduced years to rotation or improved log quality at harvest (Löf et al. 2010).
The seed orchards that produced the seedlings we studied were propagated with an eye toward maximizing the genetic diversity of the seeds, and without regard to preserving local or regional adaptation. Because seedlings from the red oak seed orchards at Vallonia, Indiana are sold to landowners everywhere in the state (9.4 million ha, five provisional seed zones; Pike et al. 2020), the goal was to ensure that some seedlings would grow well irrespective of the specific conditions at any site. One possible consequence of that strategy is that the red oak seedlings from the seed orchard will not be well-adapted to any site. On the other hand, if the three sites we planted are typical, site × family interactions can be expected for growth traits, and high genetic variance in the seed orchard should assure that at least some seedlings will grow well at any particular site. Since the number of trees/ha at maturity/harvest for red oak can be as low as 50 (Dey et al. 2008b), down from 1600 or more at establishment, the risk of planting seedlings from a few (potentially) maladapted families may be acceptable versus the potential benefit of faster-growing and better formed crop trees from the families that are adapted to a particular site.
5 Conclusion
Red oak half-sib families aged 10 or 11 years derived from the seed orchard at the Indiana State Tree Nursery in Vallonia, Indiana, USA which contains grafted, select parents showed high genetic and phenotypic variance for growth and stem form traits, and dramatic (> 50%) improvement in volume growth over nursery stock from wild-collected seeds at three test sites. Additional breeding is expected to produce large initial gains in volume and stem form traits, including sweep, branch retention, branch angle, and possibly forking. Seedlings of trees selected for higher volume are not expected to show sweep, high branch angles, or forking at a higher frequency than seedlings from wild-collected seeds, although they may have a slightly higher probability of retaining large lower limbs.
We concluded that a binary scoring system to rate stem form traits (+ / −) showed several advantages; it was easy for field staff to apply, less biased by user than previous multi-category rating methods, and it was far simpler to analyze than methods based on quantitative measures without loss of power. High levels of family × site interactions were observed for growth traits despite the superficial similarity of the test sites and conditions, reinforcing the need for better knowledge of how site factors and red oak genetic sources interact. Landowner investment in improved red oak seedlings can be rewarded with a considerable improvement in growth without sacrifice of form if trees receive protection from herbivory.
Data and materials availability
The dataset generated during and/or analyzed during the current study are available in the data repository of the Hardwood Tree Improvement and Regeneration Center (HTIRC): Forest Service Research Data Archive. https://doi.org/10.2737/RDS-2021-0059
Code availability
Code for analysis of the data described in the current study is not currently archived due to database limitations but are available by request from the Director of HTIRC, Department of Forestry and Natural Resources, Purdue University, 715 W. State Street, West Lafayette, IN, 47,907; or from the author Carolyn.c.pike@usda.gov.
References
Alcorn PJ, Pyttel P, Bauhus J, Smith RGB, Thomas D, James R, Nicotra A (2007) Effects of initial planting density on branch development in 4-year-old plantation grown Eucalyptus pilularis and Eucalyptus cloeziana trees. For Ecol Man 252(1–3):41–51
Alexander L, Woeste K (2017) Pollen gene flow, male reproductive success, and genetic correlations among offspring in a northern red oak (Quercus rubra L.) seed orchard. PloS One 12(2):e0171598
Balandier P (1997) A method to evaluate needs and efficiency of formative pruning of fast-growing broad-leaved trees and results of an annual pruning. Can J For Res 27(6):809–816
Baliuckas V, Pliura A (2003) Genetic variation and phenotypic plasticity of Quercus robur populations and open-pollinated families in Lithuania. Scand J Res 18(4):305–319
Beineke WF (1983) The genetic improvement of black walnut for timber production. In Plant breeding reviews (pp. 236–266). https://doi.org/10.1007/978-1-4684-8896-8_8
Bogdan S, Katičić-Trupčević I, Kajba D (2004) Genetic variation in growth traits in a Quercus robur L. open-pollinated progeny test of the Slavonian provenance. Silvae Genetica 53(1–6):198–201
Bonner FT (1973) Storing red oak acorns. Tree Planters Notes 24(3):12–13
Burkardt K, Annighöfer P, Seidel D, Ammer C, Vor T (2019) Intraspecific competition affects crown and stem characteristics of non-native Quercus rubra L. stands in Germany. Forests 10(10):846
Cech FC (1971) Tree improvement research in oak species. In Oak Symposium Proceedings. USDA Forest Service, Northeastern Forest Experiment Station 55–59
Coggeshall MV (1987) New approaches to northern red oak improvement in Indiana. In: Guries, R.P. (ed) Proceedings 5th North Central Tree Improvement Conference. 17–19 August 1983 Columbus, OH. North Central Tree Improvement Association 221–227
Coggeshall MV (1993) Oak tree improvement in Indiana. In Annales des Sciences Forestières Vol 50 No. Supplement:416s-419s EDP Sciences
Connor K (2004) Storing acorns. Native Plants J Summer Fall 5(2):160–166
Cooper DT, Ferguson RB (1981) Evaluation of bole straightness in cottonwood using visual scores. USDA Forest Research Note SO-277. New Orleans: Southern Forest Experiment Station 3
Cornelius JP, Mesen JF (1997) Provenance and family variation in growth rate, stem straightness, and foliar mineral concentration in Vochysia guatemalensis. Can J For Res 27(7):1103–1109
Dempster ER, Lerner IM (1950) Heritability of threshold characters. Genetics 35:212–236
Dey DC, Jacobs D, McNabb K, Miller G, Baldwin V, Foster G (2008a) Artificial regeneration of major oak (Quercus) species in the eastern United States—a review of the literature. For Sci 54(1):77–106
Dey DC, Miller GW, Kabrick JM (2008b) Sustaining northern red oak forests: managing oak from regeneration to canopy dominance in mature stands. United States Department of Agriculture Forest Service General Technical Report PNW, 733, p. 91
Dey DC, Kabrick JM, Schweitzer CJ (2017) Silviculture to restore oak savannas and woodlands. J for 115(3):202–211
Falconer DS, Mackay TFC (1996) Introduction to Quantitative Genetics, 4th edn. New York: Longman Scientific and Technical
Frank GS, Rathfon RA, Saunders MR (2018) Ten-year responses of underplanted northern red oak to silvicultural treatments, herbivore exclusion, and fertilization. Forests 9(9):571
George JP, Theroux-Rancourt G, Rungwattana K, Scheffknecht S, Momirovic N, Neuhauser L, Weißenbacher L, Watzinger A, Hietz P (2020) Assessing adaptive and plastic responses in growth and functional traits in a 10‐year‐old common garden experiment with pedunculate oak (Quercus robur L.) suggests that directional selection can drive climatic adaptation. Evol Appl 13(9):2422–2438
Gianola D, Foulley JL (1983) Sire evaluation for ordered categorical data with a threshold model. Genetiques Sel Evol (Paris) 15:201–224
Hai PH, Jansson G, Harwood C, Hannrup B, Thinh HH (2008) Genetic variation in growth, stem straightness and branch thickness in clonal trials of Acacia auriculiformis at three contrasting sites in Vietnam. For Ecol Manag 255(1):56–167
Huang C, Bates R, Kronrad G, Cheng S (2004) Economic analyses of sequestering carbon in Loblolly Pine, Cherrybark Oak, and Northern Red Oak in the United States. Environ Manag 33:S187–S199
Huang Y, Zhang H, Rogers S, Coggeshall M, Woeste KE (2016) White oak growth after 23 years in a three-site provenance/progeny trial on a latitudinal gradient in Indiana. For Sci 61(1):99–106
Irgens-Moller H (1955) Forest-tree genetics research: Quercus L. Econ Bot 9(1):53
Jensen AM, Löf M (2017) Effects of interspecific competition from surrounding vegetation on mortality, growth and stem development in young oaks (Quercus robur). For Ecol Manag 392:176–183
Johnson PS, Shifley SR, Rogers R, Dey DC, Kabrick JM (2019) The ecology and silviculture of oaks. Cabi
Kerr G, Morgan G (2006) Does formative pruning improve the form of broadleaved trees? Can J for Res 36(1):132–141
Leites LP, Rehfeldt GE, Steiner KC (2019) Adaptation to climate in five eastern North America broadleaf deciduous species: growth clines and evidence of the growth-cold tolerance trade-off. Perspect Plant Ecol Evol Syst 37(January):64–72. https://doi.org/10.1016/j.ppees.2019.02.002
Löf M, Bergquist J, Brunet J, Karlsson M, Welander NT (2010) Conversion of Norway spruce stands to broadleaved woodland-regeneration systems, fencing and performance of planted seedlings. Ecol Bull 1:65–174
Lopes UV, Huber DA, White TL (2000) Comparison of methods for prediction of genetic gain from mass selection on binary threshold traits. Silvae Genetica 49(1):50–56
Lynch M, Walsh B (1998) Genetics and Analysis of Quantitative Characters. Sunderland: Sinauer. Pp 980
MacDonald E, Mochan S, Connolly T (2009) Validation of a stem straightness scoring system for Sitka spruce (Picea sitchensis (Bong.) Carr.). Forestry 82(4):419–429
MacFarlane DW, Kane B (2017) Neighbor effects on tree architecture: functional trade-offs balancing crown competitiveness with wind resistance. Funct Ecol 31(8):1624–1636
MacKay J (1993) Seed source selection and genetic improvement of red oak (Quercus rubra L) in Québec. Annales des Sciences Forestières Vol 50, No Supplement, pp 420s-424s EDP Sciences
Malinauskas A (2007) Influence of initial density of oak (Quercus robur L.) plantations to stem quality. Baltic Forestry 13(1):83–88
Meuwissen THE, Engel B, Van der Werf JHJ (1995) Maximizing selection efficiency for categorical traits. J Anim Sci 73(7):1933–1939
Moghadam HK, McMillan I, Chambers JR, Julian RJ, Tranchant CC (2005) Heritability of sudden death syndrome and its associated correlations to ascites and body weight in broilers. British Poultry Sci 46(1):54–57
Nakagawa S, Schielzeth H (2010) Repeatability for Gaussian and non-Gaussian data: a practical guide for biologists. Biol Rev 85:935–956. https://doi.org/10.1111/j.1469-185X.2010.00141.x
Nicolescu VN, Sandi M, Păun M (2013) Occlusion of pruning wounds on northern red oak (Quercus rubra) trees in Romania. Scand J for Res 28(4):340–345
Owings CF, Jacobs DF, Shields JM, Saunders MR, Jenkins MA (2017) Individual and interactive effects of white-tailed deer and an exotic shrub on artificial and natural regeneration in mixed hardwood forests. AoB Plants 9(4)
Pang K (2014) “Crown ideotypes for genetically improved black walnut (Juglans nigra L.) clones under an intensive management regime in Indiana, USA” (2014) Open Access Dissertations 1050 https://docs.lib.purdue.edu/open_access_dissertations/1050 (Last accessed 1/14/2021)
Peters MP, Prasad AM, Matthews SN, & Iverson LR (2020) Climate change tree atlas, Version 4. Delaware: U.S. Forest Service, Northern Research Station and Northern Institute of Applied Climate Science. https://www.nrs.fs.fed.us/atlas
Pike C, Warren J, Coggeshall MV (2018) Trends in production of hardwood tree seedlings across the northeast United States from 2008 to 2016. Tree Planters’ Notes 16(1):18–25
Pike C, Potter K, Berrang P, Crane B, Bagg J, Leites L, Luther T (2020) New seed-collection zones for the Eastern United States: the eastern seed zone forum. J for 118(4):444–451. https://doi.org/10.1093/jofore/fvaa013
Redick CH, McKenna JR, Carlson DE, Jenkins MA, Jacobs DF (2020) Silviculture at establishment of hardwood plantations is relatively ineffective in the presence of deer browsing. For Ecol Manag 474:118339
Savill PS, Fennessy J, Samuel CJA (2005) Approaches in Great Britain and Ireland to the genetic improvement of broadleaved trees. Forestry 78(2):163–173
Schlarbaum SE, Bagley WT (1981) Intraspecific variation in of Quercus rubra L., Northern red oak. Silvae Genet 30(2–3):50–56
Schwartzman J, Gerhold HD (1991) Genetic structure and mating system of Northern Red Oak (Ouercus rubra L.) in Pennsylvania. For Sci 37(5):1376–1389
Snell EJ (1964) A scaling procedure for ordered categorical data. Biometrics 20(3):592–607
Sohn SI, Goddard RE (1979) Influence of infection percent on improvement of fusiform rust resistance in slash pine. Silvae Genet 28:173–180
Soil Survey Staff, Natural Resources Conservation Service, United States Department of Agriculture. Web Soil Survey. Available online at the following link: http://websoilsurvey.sc.egov.usda.gov/. Accessed Aug 18, 2020
Stringer JW, Wagner DB, Schlarbaum SE, Houston DB (1995) An analysis of phenotypic selection in natural stands of northern red oak (Quercus rubra L.). In: Gottschalk KW, Fosbroke SLC, ed. Proceedings, 10th Central Hardwood Forest Conference; 1995 March 5–8; Morgantown, WV: Gen. Tech. Rep. NE-197. Radnor: US Department of Agriculture, Forest Service, Northeastern Forest Experiment Station. Vol 197:226–237
Struve DK, McKeand SE (1993) A means of accelerating red oak genetic tests. Annales des Sciences Forestières. Vol 50 No Supplement:410s-415s. EDP Sciences
Swaim JT, Dey DC, Saunders M, Weigel DR, Thornton CD, Kabrick JM, Jenkins MA (2016) Predicting the height growth of oak species (Quercus) reproduction over a 23-year period following clearcutting. For Ecol Manag 364:101–112
Victory E, Woeste K, Rhodes Jr OE (2004) History of black walnut genetics research in North America, 1 – 8. In: Michler, CH et al., eds. Black walnut in a new century, Proceedings of the 6th Walnut Council Research Symposium; July 25–28, Lafayette, IN. Gen. Tech. Rep. NC-243. U.S.D.A. Forest Service, North Central Research Station, St. Paul
Weng Y, Ford R, Tong Z, Krasowski M (2017) Genetic parameters for bole straightness and branch angle in jack pine estimated using linear and generalized linear mixed models. For Sci 63:111–117
White TL, Adams WT, Neale DB eds (2007) Forest Genetics Cabi
Woeste KE, Pike C, Warren J, Coggeshall M (2021) Northern Red Oak Progeny Trial, HTIRC. Fort Collins: Forest Service Research Data Archive. [dataset]. V1. https://doi.org/10.2737/RDS-2021-0059
Wu HX, Ivkovic M, Gapare WJ, Matheson AC, Baltunis BS, Powell MB, McRae TA (2008) Breeding for wood quality and profit in Pinus radiata: a review of genetic parameter estimates and implications for breeding and deployment. New Zealand J for Sci 38(1):56–87
Zobel B, Talbert J (1984) Applied forest tree improvement. Blackburn Press, Caldwell
Acknowledgements
The authors thank Mr. Austin Albert for assisting with data collection, James McKenna for planting and managing the progeny trials, and the Indian Department of Natural Resources Division of Forestry for maintaining the red oak seed orchard.
Funding
Support was provided by the Van Eck Forest Foundation. This work was funded in part by the U.S. Department of Agriculture Forest Service.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics declaration
The authors declare that they follow the rules of good scientific practice.
Consent for publication
All authors gave their informed consent to this publication and its contents.
Competing interests
The authors declare no competing interests.
Additional information
Handling Editor: John M Lhotka
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contribution of the co-authors
James Warren collected the data and produced figures.
Carolyn Pike conceptualized the study and suggested and performed the analysis, produced tables and wrote the preliminary draft.
Mark Coggeshall established the seed orchard, assisted with editing all subsequent drafts, contributed theoretical approach and conclusions.
Keith Woeste produced figures, wrote and edited intermediate and final drafts.
Rights and permissions
About this article
Cite this article
Woeste, K.E., Pike, C.C., Warren, J.C. et al. Characterization of stem volume and form tradeoffs in a northern red oak (Quercus rubra) breeding population in early stages of selection. Annals of Forest Science 78, 72 (2021). https://doi.org/10.1007/s13595-021-01084-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13595-021-01084-x