Abstract
Honeybees form aerial mating aggregations, which consist of many males (drones) from colonies nesting in an area. Dispersal from natal colonies underlies the formation of drone congregations, but little is known about the effect of dispersal and dispersal-related traits on the structure and characteristics of these congregations. This study measured the body size and age of drones in congregation sites at different distances from the colonies to evaluate the potential factors limiting dispersal distance. The relationship between these factors and flight abilities was also assessed using a flight mill to understand how these affect the congregations via dispersal. The size of trapped drones increased, the proportion of young drones (< 10 days old) decreased with distance from the colonies, and the size was correlated with flight time and velocity. The results indicate that body size and age of drones may affect the structure and characteristics of mating congregations via dispersal.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Dispersal is defined broadly as movement of individuals with potential consequences for gene flow (Ronce 2007), which is a key process sha** the structure and characteristics of populations or groups of individuals and influencing the likelihood of inbreeding and intensity of mate competition with relatives (Pusey and Wolf 1996; Bowler and Benton 2005; Ronce 2007). Dispersal is a multi-causal process that is affected by various ecological constraints, such as habitat quality, population density, and kin interactions, as well as individual phenotypes, such as morphology and endocrine function (Dufty et al. 2002; Bowler and Benton 2005; Clobert et al. 2009). Body size is a fundamental life-history trait that affects an individual’s movement and dispersal capacity (Greenleaf et al. 2007). Body size is also correlated with many other facets, such as metabolism, fecundity, superiority of resources, and mate competition (Thornhill and Alcock 1983; Honěk 1993; Blanckenhorn 2000). Thus, the multiple facets of dispersal-related traits may indirectly affect the structure and characteristics of populations or groups of individuals via dispersal, as proposed in the context of dispersal syndrome; Dispersal syndrome refers to the covariation of dispersal and traits, i.e., a suite of traits correlated with dispersal (Clobert et al. 2009; Stevens et al. 2014).
Social Hymenoptera insects are susceptible to inbreeding and genetic drift because of their small effective population size resulting from limited reproductive individuals (queens). In addition, haplodiploidy causes a decrease in the gene copies (allele) of males in the population’s gene pool, resulting in a smaller effective population size, which is three-fourths of diploid under an equal sex ratio (Hedrick and Parker 1997). Furthermore, in the case of complementary sex determination systems (where sex determines at a single locus), the increased frequency of homozygous genotypes with inbreeding causes diploid males to emerge from fertilised eggs that usually develop into females, i.e. queens and workers (Cook 1993). This causes indirect death of females and not only limits colony growth but also reduces effective population size due to decreasing the number of females (Hedrick and Parker 1997; Zayed 2004, 2009). These characteristics imply the need for effective outbreeding.
Eusocial Hymenoptera insects, such as ants and bees, form temporal male aggregations for mating (Ayasse et al. 2001; Boomsma et al. 2005). The congregation consists of males from colonies nesting in an area (Baudry et al. 1998; Mueller et al. 2012; Beaurepaire et al. 2014; dos Santos et al. 2016), and has a potentially important effect on decreasing the probability of inbreeding (Paxton 2005). Thus, understanding the factors and processes that affect the structure and characteristics of male congregations is crucial. In the mating systems, most eusocial hymenopteran males, including honeybees, cannot independently survive away from colonies (Winston 1987; Hakala et al. 2019), so the underlying process for forming mating congregations is the dispersal of males from natal colonies.
Honeybees (Apis mellifera L.) aggregate and mate at specific aerial mating locations called drone congregation areas (DCAs) (Zmarlicki and Morse 1963; Ruttner 1966). DCAs are found at several locations within the range where males (drones) are capable of flight, and have unique landscape characteristics that orient drones toward these places (Galindo-Cardona et al. 2012; Koeniger et al. 2014; Hayashi and Satoh 2021). Meanwhile, observation using X-band radar on a uniform landscape has shown that drones form flyways that contain many individuals and that the flyways run parallel to prominent landscape features such as the tree line. In this case, many drones aggregate at the intersection of flyways, where interaction between individuals is likely to occur, but they can also aggregate within the flyways (Loper et al. 1992). Thus, although it is controversial (since drones’ congregation does not necessarily occur only in distinct, defined places), the formation of aggregations in their mating is universal.
Drones from approximately 10–100 unrelated colonies aggregate and fly within DCAs to find queens with which to mate (Baudry et al. 1998; Jaffé et al. 2010; Moritz et al. 2013). The genetic structure and size of the congregations vary spatially and temporally (Taylor and Rowell 1988; Kraus et al. 2005; Jaffé et al. 2009; Utaipanon et al. 2019). Variations in drones’ flight abilities, which is affected by their body size, would be a predominant proximate cause limiting dispersal distance and pattern (Gençer and Firatli 2005; Hrassnigg et al. 2005). Furthermore, drones with better flight abilities have a selective advantage for mating with queens because they must stay until queens visit DCAs and can quickly reach the queens before their competitors (Coelho 1996; Koeniger and Koeniger 2000; Koeniger et al. 2005a). Therefore, the body size of drones may not only limit their dispersal to DCAs, but may also influence competitive traits that affect male-male competition in congregations via dispersal.
This study evaluated the effect of body size on dispersal by trap** drones at DCAs at different distances from the colonies, and also tested for a correlation between body size and flight abilities to determine if body size affects dispersal distances and the structure and characteristics of mating congregations. A previous study has shown that the homing range of drones varies with age, suggesting that their flight range likewise varies because their survival strongly depends on homing to the colony when they fail to mate with queens (Hayashi et al. 2022). Thus, this study also evaluated the effect of age on congregation via dispersal.
2 Material and methods
2.1 Drone rearing
Honeybee (Apis mellifera L.) colonies were reared on a farm at the Tokyo University of Agriculture and Technology (TUAT). To obtain the experimental drones, empty drone combs were introduced into the queenright colonies. The honeybee queen laid unfertilised eggs that developed into adult drones in the cells of the drone combs. The combs were removed from the colonies 10 days after the cells were sealed and placed in an incubator at 33 °C in the dark. To obtain samples of known age and colony, the thorax and abdomen of the emerged drones were paint-marked within 24 h after emergence. In experiment 1, drones derived from queens of seven different colonies were used. In experiment 2, drones derived from queens of two different colonies were used. The marked drones were introduced into queenright colonies (> 32,000 workers).
2.2 Experiment 1: Observation of drone size and age in DCAs
The study period was 40 days between late June and the end of July. Experimental drones were introduced into three natal or foster colonies for 51 days, starting from 23 days before the experiment. On average, the number of drones introduced into the colonies per day was 320 ± 45 (mean ± standard error) and in total, 17,958 drones were introduced. Drones were captured at three DCA locations: approximately 200, 500, and 1000 m away from the colonies in the south and southeast direction (Figure 1). Locations of DCAs were previously surveyed by presenting queen-sized black wood pieces applied with 9-oxo-2-decenoic acid (9-ODA) to a height of 10 m; 9-ODA is a queen pheromone that attracts drones (Gary 1962). Identified DCA locations within the study area were relatively open-landed, low elevation and high solar irradiance (Hayashi and Satoh 2021). The places where drones aggregate in the study area were limited, and the distances between the DCAs used in this study and other identified DCAs were over 250 m away.
Map of study site locations. The three DCA locations where drones were trapped are shown by filled circle. Location of colonies where experimental drones were introduced is shown by star symbols. Tall plants, including trees and crops, and structures are shown by shaded and open boxes, respectively. This map is based on data from the Geospatial Information Authority of Japan (GIAJ; Basic Geospatial Information; Perimeter line of structures) and Japan Aerospace Exploration Agency (JAXA; High Resolution Land Use Land Cover diagram).
Drone trap** in DCAs was conducted for 1–1.5 h per day, a total of 14 days, with 4–5 days at each location. Drones were trapped by raising a glass fibre pole with a Taylor trap 9 m above the ground (Taylor 1984). Several queen-sized black wood pieces were hung inside the trap, and 500 µg of 9-ODA was applied to one each of the top and bottom pieces. The drones were captured between 13:30 and 15:45. The ages of the trapped drones were recorded based on the marked colour, and their body sizes were measured in the laboratory. The distance between the wing bases of drones, which is referred to as intertegular (IT) span, was measured as an index of their body size (Cane 1987; Greenleaf et al. 2007). The measurements were obtained using a light microscope (cellSens Standard Olympus software version 1.6, Olympus Optical Co., Tokyo, Japan).
2.3 Experiment 2: Evaluation of the relationship between body size or age and flight ability
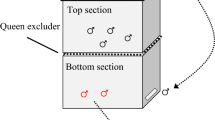
Experimental drones were introduced into foster colonies of two-story hives with a horizontal queen excluder separating the upper and lower hives (Hayashi et al. 2022). Drones were collected from the upper and lower groups 9–12 days after emergence and used in the flight experiment (n = 74). Differences between collected groups were not considered because no effects on flight parameters were found (flight time, distance, and velocity; GLMM: P > 0.52).
A flight mill was used to evaluate the flight abilities of the drones. A 19-mm-long pin was attached to the dorsal part of the drones’ thorax using instant glue; they were tethered to the tip of the arm of 19.5 cm radius in a roundabout. First, their energy reserves were depleted by stimulating flight until wing movement weakened. Flight was then stimulated by removing a ball of paper that had been used to restrict them (Gmeinbauer and Crailsheim 1993). Second, the drones were weighed, fed 10 µL of 2 M glucose solution using a Hamilton syringe (701 RN, Hamilton Co.), then weighed again to check the feeding amount. After feeding, the drones’ movements were restricted again with the paper ball, and they were allowed to rest. The drones were stimulated to fly again 5 min after feeding; the flight time and number of rotations in the roundabout were measured. The flight velocity was calculated based on the flight time and rotation number. Flight observations were conducted at 25 °C under a 60-W light bulb. The roundabout was surrounded by a strip of black and white at an interval of 2.5 cm to provide the drones with a homogenous the visual environment during flight. The IT span of the drones was measured after flight observation.
2.4 Statistical analysis
2.4.1 Experiment 1
All analyses were performed using R, version 3.5.0 (R Core Team 2018). The relationships of the size and age of drones with the distance of DCAs from the colonies were analysed using generalised linear mixed models (GLMM, R package ‘lme4’) with Gaussian and Poisson error structures, respectively. The age and IT span of the trapped drones were incorporated into each model as the response variables. The distances of DCAs from the colonies were scaled to mean = 0 and standard deviation (SD) = 1 to avoid convergence problems in the models and were incorporated into models as explanatory variables. The IDs of the natal colonies of trapped drones were incorporated into all models as random terms to consider the variance in the colonies of the trapped drones. The statistical significance of the variables in the models was determined using the likelihood ratio test (α = 0.05). Furthermore, to detect a trend in the age of drones in DCAs that the variance of the observed age might mask, age was categorised into old and young groups based on the mean age of drones in the DCAs. The categorical variable was treated as binary data with 1 for young and 0 for old, and a similar analysis was conducted using GLMMs with binomial error structures. To better understand the age structure of drones in DCAs, the difference in age variance between DCAs was evaluated using the F-test, and P values were corrected using the Bonferroni method. To obtain a comparable variance index, the coefficient of variation (CV) for the age of the drones was calculated by dividing the SD by the mean for each DCA.
2.4.2 Experiment 2
The effects of body size and age on the three flight parameters were analysed using GLMMs with a Gaussian error structure. Prior to the analysis, three flight parameters and IT span variables were log-transformed to enhance normality and stabilise the error structures. Flight parameters were incorporated as response variables in each statistical model. The IT span and age of the drones were incorporated into the models as explanatory variables; the IDs of the natal colonies were incorporated into the models as random terms.
3 Results
3.1 Experiment 1: Observation of drone sizes and ages in DCAs
A total of 107, 79, and 29 drones were trapped and marked at DCAs 200, 500, and 1000 m away from the colonies, respectively. A summary of the IT span and the age of drones in each DCA is shown in Table I, and details of the age and natal colony structures are summarised in Supplementary Tables 1 and 2. The IT span of the drones in DCAs was 4.270 ± 0.166 mm (mean ± SD), and significantly increased with the distance of the DCAs from the colonies (GLMM: P < 0.001, Figure 2, Table II). The age of the drones in DCAs was 8.9 ± 3.3 days (mean ± SD), and ranged from 5 to 38 days. No significant correlation was found between the age of the drones and the distance of the DCAs (GLMM: P = 0.125, Figure 3, Table II). Furthermore, the proportion of young drones (< 10 days) decreased with the distance of the DCAs from the colonies (GLMM: P < 0.001, Figure 4, Table II). The CV of age on DCAs 200, 500, and 1000 m away from the colonies were 0.324, 0.457, and 0.262, respectively. The age variance of DCA at 500 m was significantly larger than that at 200 and 1000 m (200 vs. 500 m: F = 0.420, P < 0.001; 500 vs 1000 m: F = 3.071, P = 0.004), and the variance between DCAs at 200 and 1000 m was not significantly different (F = 1.288, P > 0.05).
Box-plot representing intertegular (IT) spans of trapped drones at three DCAs with different distances from the colonies. [Sample size in each DCA: n = 107, n = 79, n = 29 for 200, 500, and 1000 m, respectively; see Table I for details]. Drones’ IT span was significantly correlated with the distance of DCAs from the colonies (GLMM: P < 0.001).
Box-plot representing ages (days) of trapped drones at three DCAs with different distances from the colonies. [Sample size in each DCA: n = 107, n = 79, n = 29 for 200, 500 and 1000 m, respectively; see Table I for details]. Drones’ age was not correlated with the distance of DCAs from the colonies (GLMM: P = 0.125).
Bar-plot representing the proportion of young drones (< 10 days old) at each DCA, categorised based on the mean age of drones on three DCAs. [Number of young and old drones in each DCA: (200 m: n = 93, n = 14 for young and old drones, respectively), (500 m: n = 57, n = 22), (1000 m: n = 13, n = 16)]. The proportion of young drones was correlated with the distance of DCAs from the colonies (GLMM: P < 0.001).
3.2 Experiment 2: Evaluation of the relationship between body size or age and flight ability
The drone flight time using the flight mill was 612 ± 150 s (mean ± SD). The flight time was negatively correlated with IT span (GLMM: P < 0.05, Figure 5, Table III). The flight velocity was 1.35 ± 0.37 ms−1 (mean ± SD), and positively correlated with IT-span (GLMM: P < 0.05, Table III). Flight distances were 807 ± 240 m (mean ± SD) and not significantly correlated with IT span (GLMM: P = 0.866, Table III). None of the flight parameters was significantly correlated with the age of the drones (GLMM; P > 0.05, Table III).
Relationship between log-transformed flight parameters and the intertegular (IT) span of drones. The panel shows flight time a, flight distance b, and flight velocity c. Regression lines on the panels are based on estimation obtained from simple variables models derived using the IT span of drones [(GLMM: (flight time; y = 8.661 − 1.591x), (flight distance; y = 6.469 + 0.114x), (flight velocity; y = − 2.110 + 1.650x)]. Significant (α = 0.05) and non-significant lines for correlation are shown by solid and dashed lines, respectively.
4 Discussion
The effects of body size (IT span) and age on the structure of honeybee drones’ congregation for mating via dispersal were evaluated. Most studies have reported that larger drones have higher mating and reproductive success, suggesting that this is caused by differences in competitive traits based on size, such as mobility and the number of spermatozoa (Coelho 1996; Berg et al. 1997; Schlüns et al. 2003; Koeniger et al. 2005a; Couvillon et al. 2010; Gençer and Kahya 2020). This study found that trapped drones’ body size increased with the distance of DCAs from the colonies and was also correlated with flight abilities (time and velocity) that are linked to dispersal capacity and male-male competition within DCAs (Figures 2 and 5). Thus, multiple facets of body size can influence the structure and characteristics of the congregation via dispersal. Furthermore, the age structure of drones in DCAs varied based on the distance of DCAs from the colonies (Figure 4), but it was not explained by differences in their flight abilities within the observed age (Table II). Thus, the age of drones is likely to influence the congregation through a process that is different from that of size. This study provides insight into factors and processes limiting the components and structure of drones’ congregation in mating, which affects the likelihood of inbreeding and competition between male relatives.
Higher energy is needed for long-distance flights, and the absolute energy demands are predicted to be higher for larger individuals (Grula et al. 2021). A previous study showed that small drones raised in worker cells can take longer flight times and travel greater distances than drones developed in drone cells (Hrassnigg et al. 2005). This study showed that larger drones have shorter flight times; however, flight distances were not correlated with body size (Figure 5). The difference in correlation between flight distances and body size might be caused by the narrow range of body sizes of the drones used here because they were restricted to individuals developed in drone cells. Thus, effect of size on flight distances was ambiguous, but the observed negative correlation of flight time with size indicates that larger drones generally consume more energy per unit time for flight. This implies a similar effect of body size on flight, as observed when the difference in the size distribution of the drones used for comparison is clear (Hrassnigg et al. 2005), and can limit flight distances and times. In this context, drone dispersal distances may decrease with body size, contrasting with the observed relationship between dispersal and body size (Figure 2). Meanwhile, the larger drones showed higher velocity (Figure 5), indicating that they have greater propulsion during flight, which is likely to explain a function of the increased dispersal distances and body size. Flight activities are often affected by external factors, such as wind (Loper et al. 1992); therefore, drones must have stable and powerful flights to reach DCAs that are distant from a colony. Furthermore, body size may involve an increase in the capacity of energy loading for flight, and a higher flight force allows for holding more energy content in crop by reducing the cost per mass. This would have a significant influence, even if the increase in the loading amount is small because drones load highly concentrated nectar as fuel (Hayashi et al. 2016). Thus, the differences based on size are most likely a proximate mechanism for increased dispersal distances.
In the pre-copulation process, a honeybee drone needs the ability to reach and locate mating places (DCAs) and detect queens, suggesting the presence of selection when drones aggregate at the mating place (Jaffé and Moritz 2010). Empirical studies have shown that lower wing size asymmetry, which is known to be associated with higher flight performance in fruit flies (Markow and Ricker 1992), is found in drones caught from DCAs compared with those from colonies (Jaffé and Moritz 2010). The number of visiting drones in DCAs also decreases with the distance of the source colony (Taylor and Rowell 1988; Koeniger et al. 2014). This finding was also observed in the present study (Table I). One hypothesis for the increased body size with the distance of DCAs is that it results in a selection during drone aggregation to DCAs (based on the distance from the natal colony). This affects the congregation’s structure and is likely to have an important function in avoiding inbreeding, competition between relatives, and deleterious factors by dispersal. For example, inbred and Varroa-infected drones with lower flight abilities are likely to be selected during distant flights (Moritz 1988; Duay et al. 2002; Yañez et al. 2012), and more related individuals would be included in DCAs that are near to the colonies (Koeniger et al. 2005b). Particularly, inbreeding in honeybees causes the production of diploid drones due to the increasing frequency of homozygotes at the sex determination locus, and also negatively affects wing symmetry, homeostasis, and foraging in worker bees (Brückner 1978). In addition, honeybees are extremely polyandrous, and queens mate with multiple drones, resulting in high performance and productivity of colonies via increased intra-colonial genetic diversity (Mattila and Seeley 2007; Mattila et al. 2012), but inbreeding causes loss of diversity. Thus, selection related to dispersal and aggregation toward DCAs may indirectly affect mate selection (Jaffé and Moritz 2010), and the potentially high requirement for dispersal may explain the evolution of honeybee drones’ large body size that allows greater and stable dispersal. Our results are based on observations using colonies located at similar sites, but feral Apis colonies have been reported to have clumped distribution in habitats (Oldroyd et al. 1995, 2000; Wattanachaiyingcharoen et al. 2008). Thus, selection via dispersal and aggregation could be one of the underlying principles determining the structure of drone congregations and their dispersal patterns, although it might be limited when colonies are clumped.
A study using harmonic radar reported that drones take long-distance flights for mating and begin as early as the second flight (Woodgate et al. 2021). They can visit several DCAs within a single flight, which suggests that drones require fewer systematic searching processes to locate DCAs (Woodgate et al. 2021). This is supported by our results, in which the mean age of drones in DCAs coincided with the timing for their initiation of flight (Witherelli 1971; Reyes et al. 2019). Meanwhile, DCAs that drones are capable of visiting or locating may vary with age. A study on homing distance toward the hive, which is often used as a proxy for their flight range, showed that the homing distance increases with age (Hayashi et al. 2022). In this study, no significant correlation between age and distance of DCAs from the colonies was found, but the proportion of young drones (< 10 days old) decreased with the distance of DCAs (Figure 4). The lack of a direct correlation between age and distance would be because of the large variance in age. Specifically, approximately 70–80% of the marked drones near DCAs (200 and 500 m) consisted of young drones, and the remaining were a small number of considerably old drones (max. 38 days old). This might reflect the relative ease of accessing DCAs near the colonies for the older drones with lower abilities that are unsuccessful in mating until that time, and also a decreased performance with ageing. Ageing accompanies familiarity with sites via flight experience and various physiological changes in the nervous system and motor activity (Fahrbach et al. 1997; Page and Peng 2001; Hrassnigg and Crailsheim 2005; Degen et al. 2015); thus, factors causing the difference cannot be elucidated here. Nevertheless, an interesting possibility is that the dispersal distance of young drones is limited and can affect the structure of congregations. The drone components for ejaculation and viability of spermatozoa change with age (Locke and Peng 1993; Baer et al. 2012; Stürup et al. 2013; Metz and Tarpy 2019), and these differences may affect the fecundity of queens and the intensity of post-copulatory male-male sperm competition until stored in the spermathecae of queens (den Boer et al. 2010; Gençer and Kahya 2020) via mating with a group of drones that is different in age structure.
Besides dispersal, the observed size and age structure in DCAs is likely affected by various aspects such as the observation methodology, season, and mortality. For example, the trapped drones were collected for measurement of their size at any time, although many drones were introduced into colonies over the study period, so older drones may be present at a low probability. In addition, our study was conducted from late spring to summer, and the longevity of drones is shorter in summer than in spring and autumn (Fukuda and Ohtani 1977; Reyes et al. 2019). Furthermore, honeybee drones undergo age-independent deaths such as predation during flight (Rueppell et al. 2005). Particularly, the number of the trapped drones compared with introduced ones into the colonies was considerably small, which would need to be noted because the observed pattern in DCAs might also be affected by the selection of individuals via potential factors causing mortality. Therefore, further studies are needed to understand the effect of age and body size on the dispersal process and structure of congregations.
5 Conclusion
The results of this study suggest that the body size and age of drones are potential factors limiting the dispersal distance. Given that body size and age are related to competitive traits during mating, these dispersal-related factors could influence the structure and characteristics of drones in DCAs via dispersal.
Data availability
The datasets are available on reasonable request.
Code availability
Not applicable.
References
Ayasse M, Paxton RJ, Tengö J (2001) Mating behavior and chemical communication in the order Hymenoptera. Annu Rev Entomol 46:31–78
Baer B, Zareie R, Paynter E, Poland V, Millar AH (2012) Seminal fluid proteins differ in abundance between genetic lineages of honeybees. J Proteomics 75:5646–5653
Baudry E, Solignac M, Garnery L, Gries M, Cornuet J, Koeniger N (1998) Relatedness among honeybees (Apis mellifera) of a drone congregation. Proc R Soc B Biol Sci 265:2009–2014
Beaurepaire AL, Kraus BF, Koeniger G, Koeniger N, Lim H, Moritz RFA (2014) Extensive population admixture on drone congregation areas of the giant honeybee, Apis dorsata (Fabricius, 1793). Ecol Evol 4:4669–4677
Berg S, Koeniger N, Koeniger G, Fuchs S (1997) Body size and reproductive success of drones (Apis mellifera L). Apidologie 28:449–460
Blanckenhorn WU (2000) The evolution of body size: what keeps organisms small? Q Rev Biol 75:385–407
Boomsma JJ, Baer B, Heinze J (2005) The evolution of male traits in social insects. Annu Rev Entomol 50:395–420
Bowler DE, Benton TG (2005) Causes and consequences of animal dispersal strategies: relating individual behaviour to spatial dynamics. Biol Rev 80:205–225
Brückner D (1978) Why are there inbreeding effects in haplo-diploid systems? Evolution 32:456–458
Cane JH (1987) Estimation of bee size using intertegular span (Apoidea). J Kansas Entomol Soc 60:145–147
Clobert J, Le Galliard JF, Cote J, Meylan S, Massot M (2009) Informed dispersal, heterogeneity in animal dispersal syndromes and the dynamics of spatially structured populations. Ecol Lett 12:197–209
Coelho JR (1996) The flight characteristics of drones in relation to mating. BeeScience 4:21–25
Cook JM (1993) Sex determination in the Hymenoptera: a review of models and evidence. Heredity 71:421–435
Couvillon MJ, Hughes WOH, Perez-Sato JA, Martin SJ, Roy GGF, Ratnieks FLW (2010) Sexual selection in honey bees: colony variation and the importance of size in male mating success. Behav Ecol 21:520–525
Degen J, Kirbach A, Reiter L, Lehmann K, Norton P, Storms M, Koblofsky M, Winter S, Georgieva PB, Nguyen H, Chamkhi H, Greggers U, Menzel R (2015) Exploratory behaviour of honeybees during orientation flights. Anim Behav 102:45–57
den Boer SPA, Baer B, Boomsma JJ (2010) Seminal fluid mediates ejaculate competition in social insects. Science 327:1506–1509
dos Santos CF, Imperatriz-Fonseca VL, Arias MC (2016) Relatedness and dispersal distance of eusocial bee males on mating swarms. Entomol Sci 19:245–254
Duay P, De Jong D, Engels W (2002) Decreased flight performance and sperm production in drones of the honeybee (Apis mellifera) slightly infested by Varroa destructor mites during pupal development. Genet Mol Res 1:227–232
Dufty AM, Clobert J, Møller AP (2002) Hormones, developmental plasticity and adaptation. Trends Ecol Evol 17:190–196
Fahrbach SE, Giray T, Farris SM, Robinson GE (1997) Expansion of the neuropil of the mushroom bodies in male honey bees is coincident with initiation of flight. Neurosci Lett 236:135–138
Fukuda H, Ohtani T (1977) Survival and life span of drone honeybees. Res Popul Ecol (kyoto) 19:51–68
Galindo-Cardona A, Monmany AC, Moreno-Jackson R, Rivera-Rivera C, Huertas-Dones C, Caicedo-Quiroga L, Giray T (2012) Landscape analysis of drone congregation areas of the honey bee, Apis mellifera. J Insect Sci 12:1–15
Gary NE (1962) Chemical mating attractants in the queen honey bee. Science 136:773–774
Gençer HV, Firatli Ç (2005) Reproductive and morphological comparisons of drones reared in queenright and laying worker colonies. J Apic Res 44:163–167
Gençer HV, Kahya Y (2020) Sperm competition in honey bees (Apis mellifera L.): the role of body size dimorphism in drones. Apidologie 51:1–17
Gmeinbauer R, Crailsheim K (1993) Glucose utilization during flight of honeybee (Apis mellifera) workers, drones and queens. J Insect Physiol 39:959–967
Greenleaf SS, Williams NM, Winfree R, Kremen C (2007) Bee foraging ranges and their relationship to body size. Oecologia 153:589–596
Grula CC, Rinehart JP, Greenlee KJ, Bowsher JH (2021) Body size allometry impacts flight-related morphology and metabolic rates in the solitary bee Megachile rotundata. J Insect Physiol 133
Hakala SM, Perttu S, Helanterä H (2019) Evolution of dispersal in ants (Hymenoptera: Formicidae): a review on the dispersal strategies of sessile superorganisms. Myrmecological News 29:35–55
Hayashi S, Satoh T (2021) Landscape features causing the local congregation of honeybee males (Apis mellifera L.). Ethology 127:582–591
Hayashi M, Nakamura J, Sasaki K, Harano K (2016) Honeybee males use highly concentrated nectar as fuel for mating flights. J Insect Physiol 93:50–55
Hayashi S, Sasaki T, Farkhary SI, Kaneko K, Hosaka Y, Satoh T (2022) Age-related variation of homing range in honeybee males (Apis mellifera). Insectes Soc 69:37–45
Hedrick PW, Parker JD (1997) Evolutionary genetics and genetic variation of haplodiploids and X-linked genes. Annu Rev Ecol Syst 28:55–83
Honek A (1993) Intraspecific variation in body size and fecundity variation intraspecific relationship general. Oikos 66:483–492
Hrassnigg N, Crailsheim K (2005) Differences in drone and worker physiology in honeybees (Apis mellifera). Apidologie 36:255–277
Hrassnigg N, Brodschneider R, Fleischmann PH, Crailsheim K (2005) Unlike nectar foragers, honeybee drones (Apis mellifera) are not able to utilize starch as fuel for flight. Apidologie 36:547–557
Jaffé R, Moritz RFA (2010) Mating flights select for symmetry in honeybee drones (Apis mellifera). Naturwissenschaften 97:337–343
Jaffé R, Dietemann V, Crewe RM, Moritz RFA (2009) Temporal variation in the genetic structure of a drone congregation area: an insight into the population dynamics of wild African honeybees (Apis mellifera scutellata). Mol Ecol 18:1511–1522
Jaffé R, Dietemann V, Allsopp MH, Costa C, Crewe RM, Dall’olio R, de la Rúa P, El-niweiri MAA, Fries I, Kezic N, Meusel MS, Paxton RJ, Shaibi T, Stolle E, Moritz RFA (2010) Estimating the density of honeybee colonies across their natural range to fill the gap in pollinator decline censuses. Conserv Biol 24:583–593
Koeniger N, Koeniger G (2000) Reproductive isolation among species of the genus Apis. Apidologie 31:313–339
Koeniger N, Koeniger G, Gries M, Tingek S (2005a) Drone competition at drone congregation areas in four Apis species. Apidologie 36:211–221
Koeniger N, Koeniger G, Pechhacker H (2005b) The nearer the better? Drones (Apis mellifera) prefer nearer drone congregation areas. Insectes Soc 52:31–35
Koeniger G, Koeniger N, Ellis J, Conner L (2014) Mating biology of honey bees (Apis mellifera). Wicwas Press
Kraus FB, Koeniger N, Tingek S, Moritz RFA (2005) Temporal genetic structure of a drone congregation area of the giant Asian honeybee (Apis dorsata). Naturwissenschaften 92:578–581
Locke SJ, Peng YS (1993) The effects of drone age, semen storage and contamination on semen quality in the honey bee (Apis mellifera). Physiol Entomol 18:144–148
Loper GM, Wolf WW, Taylor ORJ (1992) Honey bee drone flyways and congregation areas: radar observations. J Kansas Entomol Soc 65:223–230
Markow TA, Ricker JP (1992) Male size, developmental stability, and mating success in natural populations of three Drosophila species. Heredity 69:122–127
Mattila HR, Seeley TD (2007) Genetic diversity in honey bee colonies enhances productivity and fitness. Science 317:362–364
Mattila HR, Reeve HK, Smith ML (2012) Promiscuous honey bee queens increase colony productivity by suppressing worker selfishness. Curr Biol 22:2027–2031
Metz BN, Tarpy DR (2019) Reproductive senescence in drones of the honey bee (Apis mellifera). InSects 10:11
Moritz RFA (1988) Biochemical changes during honey bee flight muscle development. Biona Rep 6:51–64
Moritz RFA, Kraus FB, Huth-Schwarz A, Wolf S, Carrillo CAC, Paxton RJ, Vandame R (2013) Number of honeybee colonies in areas with high and low beekee** activity in Southern Mexico. Apidologie 44:113–120
Mueller MY, Moritz RFA, Kraus FB (2012) Outbreeding and lack of temporal genetic structure in a drone congregation of the neotropical stingless bee Scaptotrigona mexicana. Ecol Evol 2:1304–1311
Oldroyd BP, Smolenski A, Lawler S, Estoup A, Crozier R (1995) Colony aggregations in Apis mellifera L. Apidologie 26:119–130
Oldroyd BP, Osborne KE, Mardan M (2000) Colony relatedness in aggregations of Apis dorsata Fabricius (Hymenoptera, Apidae). Insectes Soc 47:94–95
Page REJ, Peng CYS (2001) Aging and development in social insects with emphasis on the honey bee, Apis mellifera L. Exp Gerontol 36:695–711
Paxton RJ (2005) Male mating behaviour and mating systems of bees: an overview. Apidologie 36:145–156
Pusey A, Wolf M (1996) Inbreeding avoidance in animals. Trends Ecol Evol 11:201–206
R Core Team (2018) The R: project for statistical computing
Reyes M, Crauser D, Prado A, Le Conte Y (2019) Flight activity of honey bee (Apis mellifera) drones. Apidologie 50:669–680
Ronce O (2007) How does it feel to be like a rolling stone? Ten questions about dispersal evolution. Annu Rev Ecol Evol Syst 38:231–253
Rueppell O, Fondrk MK, Page REJ (2005) Biodemographic analysis of male honey bee mortality. Aging Cell 4:13–19
Ruttner F (1966) The life and flight activity of drones. Bee World 47:93–100
Schlüns H, Schlüns EA, van Praagh J, Moritz RFA (2003) Sperm numbers in drone honeybees ( Apis mellifera ) depend on body size. Apidologie 34:577–584
Stevens VM, Whitmee S, Le Galliard JF, Clobert J, Böhning-Gaese K, Bonte D, Brändle M, Matthias Dehling D, Hof C, Trochet A, Baguette M (2014) A comparative analysis of dispersal syndromes in terrestrial and semi-terrestrial animals. Ecol Lett 17:1039–1052
Stürup M, Baer-Imhoof B, Nash DR, Boomsma JJ, Baer B (2013) When every sperm counts: factors affecting male fertility in the honeybee Apis mellifera. Behav Ecol 24:1192–1198
Taylor ORJ (1984) An aerial trap for collecting drone honeybees in congregation areas. J Apic Res 23:18–20
Taylor ORJ, Rowell GA (1988) Drone abundance, queen flight distance, and the neutral mating model for the honey bee, Apis mellifera. In: Needham GR, Page REJ, Delfinado-Baker M, Bowman CE (eds) Africanized honey bees and bee mites. Ellis Horwood, pp 173–183
Thornhill R, Alcock J (1983) The evolution of insect mating systems. Harvard University Press
Utaipanon P, Holmes MJ, Chapman NC, Oldroyd BP (2019) Estimating the density of honey bee (Apis mellifera) colonies using trapped drones: area sampled and drone mating flight distance. Apidologie 50:578–592
Wattanachaiyingcharoen W, Wongsiri S, Oldroyd BP (2008) Aggregations of unrelated Apis florea colonies. Apidologie 39:531–536
Winston ML (1987) The biology of the honey bee. Harvard University Press, Cambridge
Witherelli PC (1971) Duration of flight and of interflight time of drone honey bees, Apis mellifera. Ann Entomol Soc Am 64:609–612
Woodgate JL, Makinson JC, Rossi N, Lim KS, Reynolds AM, Rawlings CJ, Chittka L (2021) Harmonic radar tracking reveals that honeybee drones navigate between multiple aerial leks. iScience 24
Yañez O, Jaffé R, Jarosch A, Fries I, Robin FAM, Robert JP, De Miranda JR (2012) Deformed wing virus and drone mating flights in the honey bee (Apis mellifera): implications for sexual transmission of a major honey bee virus. Apidologie 43:17–30
Zayed A (2004) Effective population size in Hymenoptera with complementary sex determination. Heredity 93:627–630
Zayed A (2009) Bee genetics and conservation. Apidologie 40:237–262
Zmarlicki C, Morse RA (1963) Drone Congregation Areas J Apic Res 2:64–66
Acknowledgements
We thank all the members of Fuchunomori Park and Mr. Sekine for their acceptance of this investigation. We thank K. Kaneko and K. Nomura for their assistance in marking the experimental bees. Finally, we appreciate K. Harano, J. Nakamura, and S. Koyama for their cooperation and valuable suggestions during the early stages of this work.
Author information
Authors and Affiliations
Contributions
Shinya Hayashi prepared the material, collected and analysed the data, and wrote the manuscript. Toshiyuki Satoh contributed to data collection and writing of the manuscript.
Corresponding author
Ethics declarations
Ethics approval
This study was conducted under permission from owner and administrator of the lands.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Manuscript editor: Mathieu Lihoreau
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hayashi, S., Satoh, T. Body size and age of drone honeybees (Apis mellifera) affect the structure and characteristics of mating congregations via dispersal. Apidologie 54, 3 (2023). https://doi.org/10.1007/s13592-022-00983-w
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13592-022-00983-w