Abstract
Novel crystalline and thermally stable chitosan-N-acetylisatin (Chs-NAI) polymer derivatives were synthesized by chitosan modification. These polymers were formed by insertion of β-cyclodextrin (βCD) and do** with zinc oxide nanoparticles (ZnO NPs) yielding pseudopolyrotaxane (Chs-NAI/βCD), Chs-NAI/ZnO-NPs, and Chs-NAI/βCD/ZnO NP composites. The chemical structure and morphology of the newly prepared polymers were investigated by FTIR, XRD, and SEM analyses. Also, the optical properties and thermal stability analysis of the synthesized polymers were examined. These results showed that the use of β-CD or ZnO NPs to modify the polymer (Chs-NAI) dramatically improves the thermal stability and optical properties of the synthesized polymer. The weight losses of the synthesized polymers are 66%, 56%, 48%, and 59% in Chs-NAI, Chs-NAI/βCD, Chs-NAI/ZnO, and Chs-NAI/βCD/ZnO, respectively. For mineral deposition, Chs-NAI/βCD/ZnO was presoaked with a PO43− containing solution before being immersed in a Ca2+ containing solution. The properties of Chs-NAI/βCD/ZnO nanoparticles/calcium phosphate materials were examined to confirm the deposition of the calcium phosphate on the Chs-NAI/βCD/ZnO nanoparticles surface using different techniques. Based on the results of the tests on the Vero cell lines, Chs-NAI/βCD/ZnO NPs)/calcium phosphate demonstrated very mild cytotoxicity. In addition, the feasibility of a wide variety of hybrid was assessed with an MTT test, and it was determined that they were noncytotoxic under a variety of hybrid concentrations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
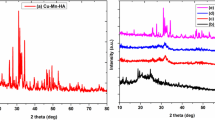
At present, the use of composite scaffolds in tissue engineering for bone repair and regeneration has exploded [1]. It has the potential to restore damaged or lost tissue function while integrating with host tissue. This is attributed to the rise in osteoporosis-related clinical bone disorders such as tumors and infections, as well as trauma-induced bone loss. Numerous studies are being conducted to determine how to regenerate bone defects [2,3,4,5]. Natural polymers are widely used in various fields such as medicine [6], aerospace [7], packaging [8, 9], electronics [10], automobiles [11], foods [12], photophysics [ Chitosan with a deacetylation degree greater than 85%, -CD, ZnO, isatin, dipotassium hydrogen phosphate (K2HPO4), and calcium chloride (CaCl2) were acquired from the Merck Company in Germany. Aldrich, Milwaukee, Wisconsin, and the USA supplied ZnO NPs (> 50 nm, > 97% pure) as well as glacial acetic acid, dimethylformamide (DMF), and acetic anhydride. Without additional purification, all compounds were utilized as received. Scheme 2 shows the flowchart of preparation of Chs-NAI/β-CD/ZnO nanoparticle/calcium phosphate materials. Heating under reflux a mixture of isatin (7 gm) and acetic anhydride (50 ml) at 100 °C for 6 h. The corresponding product precipitated out of the hot solution, cooled, filtered, and crystallized from ethanol with few drops of petroleum ether 40/60 to yield N-acetyl isatin in 95% yield. The chitosan powder (1.0 g) was stirred at room temperature for 8 h, with 50 ml of glacial acetic acid (1% v/v) until a clear solution was obtained, and then a dropwise addition of NAI (0.2 g in 10 ml MeOH) was made. After this, the temperature was raised to 100 °C for 1 h, as shown in Scheme 3. Petri dishes were coated with the Chs-NAI polymer hydrogel and the solvent was evaporated at room temperature. In accordance with Scheme 4, the Chs-NAI polymer (1.0 g) and β-CD (2.0 g) were mixed in DMF (25 ml) and agitated for a period of 30 h at room temperature. Chs-NAI/β-CD was obtained in the form of a pale-yellow powder after the precipitate that had been formed was filtered out under a vacuum and dried at room temperature. At room temperature after 30 h, a combination of Chs-NAI polymer (1.0 g) and ZnO NPs (0.10 g) in DMF (25 ml) was swirled, as shown in Scheme 5. After placing the produced hydrogel in a petri dish and allowing it to dry at room temperature, Chs-NAI/ZnO NPs were formed in the form of green crystals. At room temperature for 8 h, a mixture of Chs-NAI polymer (1.0 g), ZnO nanoparticles (0.10 g), and DMF (25 ml) was swirled. Then, 2.0 g of β-CD dissolved in 25 ml of DMF was added while the mixture was stirred at room temperature for 48 h, Scheme 6. Chs-NAI/β-CD/ZnO NP composite was obtained in the form of a brown crystal after the generated hydrogel was placed into a Petri plate, where it was then allowed to dry at room temperature. Dipotassium hydrogen phosphate (K2HPO4) and calcium chloride (CaCl2) were dispersed in 100 ml of water each, providing 200 mM solutions of both precursors, which were used in the mineralization reaction. In the following experiments, Chs-NAI/β-CD/ZnO NPs (0.6 g) were suspended either at 2 mg/ml in calcium chloride solution. To this, the required amount of (100 mM) K2HPO4 was added drop wise with stirring. Adjust the solutions pH at 8 to 37 °C then leave over night. A milky white polymer slurry was obtained indicating the formation of hydroxyapatite crystals. The samples were centrifuged for 30 minutes at 3000 rpm, and then freeze-dried. The structure of the produced derivatives was studied using Fourier-transformation infrared (FTIR)spectroscopy at room temperature using an infrared spectrometer (Jasco Model 4100 – Japan) in the wavenumber region of 4000–400 cm1. The phase structure and crystallite size of the products were determined by XRD measurements taken at room temperature with a powder diffractometer (Brucker D8 Advance, Germany) equipped with a Cu K radiation source, = 1.5406 and 2 in the range (5–80°). Scanning electron microscope SEM (JEOL SEM model JSM – 5500 – Japan) with accelerated voltage 20 kV was used to study the morphological structures of the produced derivatives. Transmission electron microscopy (TEM) images were captured using a JEOL JEM-2100 electron microscope at 100 k magnification and a 120 kV acceleration voltage. The thermal stability of the synthesized derivatives was measured using a TGA (SDT Q600 V20.9 Build 20) with a 5 °C/min heating rate up to 400 °C and a 5 ml/min nitrogen gas flow. A data collecting and handling mechanism is built into the thermal analyzer (TA-50WSI). The optical characteristics of the produced polymers were determined using UV–visible spectroscopy. The UV–visible spectra were obtained using a UV–visible spectrophotometer (PG Instruments, model T80, UK) and quartz cells with a path length of 1 cm with wavelengths ranging from 200 to 800 nm. To modify the baseline, DMF was utilized as a blank. The in vitro cytotoxicity test was performed using a colorimetric MTT technique on normal cell lines (kidney cells (Vero)) according to Mosmann (1983) [44]. Assays were carried out on MTT monolayer sheets grown on 96-well tissue culture plates incubated at 37 °C for 24 h with 1 × 105 cells/ml (100 l/well). After 5 min of shaking at 150 rpm, the plates were incubated for 1 day at 37 °C, 5% CO2. Ditch the media (dry plate on paper towels to remove filtrate if required). Formazan (MTT metabolic product) was resuspended in DMSO 200 μl and 5-min shake table to carefully combine the formazan and solvent. Read optical density at 560 nm and subtract background at 620 nm. Optical density should be directly correlated with cell quantity. To dissect the phase structure and its crystallite size of Chs, Chs-NAI polymer, pseudopolyrotaxane polymer, Chs-NAI/ZnO NPs, and Chs-NAI/β-CD/ZnO NP composite, XRD analysis has been fulfilled (Fig. 1). XRD spectra were documented at 25 °C in the range 5–80°. The distinctive diffraction peaks of chitosan were detected at 2θ = 8.6° and 20° approving its semi-crystalline nature [39]. XRD analysis showed the preparation of α-ketoamide (Chs- NAI polymer) via the changing of amino group nature. The difference in the crystal size and crystallinity of chitosan as indicated by disappearance peaks in 17.6°, 30.8°, and appearance of a slight peak in 11.5°. Also, the crystallinity values of chitosan and the formed Chs-NAI polymer showed 57.7 and 42.9%, respectively. The crystallinity value of Chs-NAI decreased because of the creation of α-ketoamide and cleavage of hydrogen bonds [40]. The XRD pattern of pseudopolyrotaxane polymer showed sharp diffraction angle at 20.3° and the crystallinity value was 40.1% and the crystal size increased on the cost of the crystallinity value (crystal size values of Chs-NAI and pseudopolyrotaxane polymer were 2.4 and 5.2 nm, respectively). The insertion of ZnO NPs to the Chs-NAI polymer gave a different pattern in XRD analysis. A broad peak in the blind polymer became a broader peak with less intensity and some sharp peaks appeared at 12°, 21°, 22°, and 37.9°. Moreover, the crystal size of Chs-NAI/ZnO NPs (4.6 nm) increased in the same vein with the crystallinity value (48.3%); this difference indicates the interaction between the ZnO NP composite and Chs-NAI polymer. Also, Chs-NAI/β-CD/ZnO NP composite XRD result showed broad peaks at 14°, 14.8°, 15.2°, 17.3°, and 21.6°, higher crystal size 6.3 nm and lower crystallinity value 40.2%. Using ZnO NPs in our study, due to its high stability, high ultraviolet absorption efficiency, environmental friendly feature and low cost, also have potential applications in different fields such as emission displayers, gas sensors, ultraviolet light emitters, piezoelectric devices, dye-sensitized solar cells, and photo catalysts [41]. The increase in crystal size, decrease in crystallinity values, and the alteration in XRD spectra of Chs and other polymers showed the synthesis of these polymers. FTIR spectra were used to clarify the formation of the synthesized polymers. Figure 2A showed the differences absorbance bands between the Chs, NSA, and Chs-NAI polymer. The -OH group characteristic peak in Chs appeared at 3334 cm−1, which appears superimposed to the N–H stretching band and the hydrocarbon bond C-H appeared at 2978 cm−1. In addition, the peaks of –C = O amide and –NH2 were observed at 1657 cm−1 and 1598 cm−1. The spectrum of Chs-NAI polymer showed CO–NH (which attached with acetyl group) band at 1630 cm−1 and-NH appeared at 3392 cm−1. Additionally, α-CO and CO–NH were appeared at 1696 and 1587 cm−1, respectively [42]; such results established the formation Chs-NAI. The changes in absorbance peaks of Chs, NAI, and Chs-NAI polymer are summarized in Table 1.
The absorption band of the hydroxyl group is slightly higher and more intensity than the Chs-NAI. Moreover, the symmetric stretching OH and aliphatic CH- were shifted to higher frequencies compared to those in Chs-NAI polymer. On the other hand, the ν[CO–CO–NH] and ν[CH2-O] bending vibrations were displacement to lower wavenumbers at 1020 and 1150 cm−1, respectively. Also, the ν[C-O] was shifted to higher frequency at 1680 cm−1 and ν [CO–NH] was shifted to lower frequency at 1243 cm−1. This result can be explained as follows: the incorporating of the Chs-NAI chain through the cavity of the cyclodextrin rings leads to increase the frequencies [43], while the reducing in wavenumbers is due to the creation of van der Waals [44] forces and hydrogen bonds between the (OH) groups of β-CD macrocycles and (CO) groups of Chs and NAS molecules, Fig. 2B. The variations between absorbance bands of original β-CD, Chs-NAI polymer, and pseudopolyrotaxane polymer are indicated in Table 2. The Chs-NAI/ZnO NP composite FTIR spectrum revealed a peak at 3550 cm−1, which corresponds to the unique stretching of hydroxyl (OH) groups. The intensity of the aliphatic CH was somewhat higher than that of the pure Chs-NAI polymer. The absorption band at 1600 cm−1 is associated with the C = O stretching vibration [45]. Because of the coupling between the Chs-NAI polymer mix chains and the ZnO NPs, the absorption bands at 1595 and 1470 cm−1 correspond to bending (CO–NH) group and stretching vibration of (CH2-OH) group, respectively. The peak at 1150 cm−1 was attributed to stretching CO–CO–NH, which was reduced in frequency and intensity. These findings strongly support the do** of polymer blend into the bores of ZnO NPs, as shown in Fig. 2B. Table 3 summarizes the differences in absorption peaks of Chs-NAI and Chs-NAI/ZnO NP polymers. Figure 2B shows the FTIR spectrum of Chs-NAI/β-CD/ZnO NP composite which clarify a broad absorption peak of –OH group at 3450 cm−1 with higher intensity. The aliphatic CH groups that appeared at 2920 cm−1 was shifted with an increased in intensity. The absorption peak at 1570 cm−1 specified to carbonyl stretching vibration, and CO–NH group appeared at 1265 cm−1 with high intensity. The absorption peaks at 1120 and 995 cm−1 according to the stretching vibration of (CH2–OH) group and stretching (CO–CO–NH) became more intense and shifted to lower wavenumbers. The absorption peak differences of Chs-NAI/ZnO NP composite, pseudopolyrotaxane, and Chs-NAI/β-CD/ZnO NP polymer are summarized in Table 4. These differences (shift and increase in the intensity) show the strong interaction among these specific groups in the polymer blend of pseudopolyrotaxane and ZnO NPs. SEM images were taken to study the surface morphology of chitosan, Chs-NAI polymer, βCD, pseudopolyrotaxan polymer, ZnO NPs, Chs-NAI/ZnO NPs, NAS, and Chs-NAI/βCD/ZnO NP composite (Fig. 3a–h). These images show a clear difference between them and the surface shape was altered during the reaction due to the tough nature of the chitosan surface [46]. The Chs-NAI polymer was exhibited as irregular amorphous slides (Fig. 3b). As a result of the incorporation of Chs-NAI polymer into the β-CD, the morphology of β-CD and pseudopolyrotaxane polymer was further modified (Fig. 3d). Pseudopolyrotaxane polymer showed a slightly divided and rough structure with increased in particle crystal size. SEM images of Chs-NAI/ZnO NP composite showed scaly and slightly smooth slides without any pores. ZnO NPs are integrated into the pores of Chs-NAI polymer to form the soft surface of the composite (Fig. 3f). The surface morphology of pseudopolyrotaxane polymer was changed in Chs-NAI/β-CD/ZnO NP composite (Fig. 3g). It has become irregular and aggregated into Chs-NAI/β-CD/ZnO NP composites. Moreover, the particle crystal size decreased compared to the pseudopolyrotaxane polymer (Fig. 3).
Polymers produced should be tested for optical properties before use. As a result, UV–visible spectroscopy of Chs-NAI polymer, Chs-NAI/ZnO NP composite, Chs-NAI/β-CD, and Chs-NAI β-CD/ZnO NP composite was recorded at 25 °C in the range of 200–800 nm (Fig. 4). Because of the presence of –NH–CO, the UV–visible spectra of Chs-NAI polymer revealed a large peak at 290–309 nm. Unusually, adding β-CD and ZnO NPs to Chs-NAI polymer resulted in slight blue shifts in the characteristic peak, from 306 to 291 and 298 nm, respectively. A weak peak at 304 nm was seen after the addition of β-CD and ZnO NPs to Chs-NAI in a one-pot process. Furthermore, Tauc’s method [47] and UV–visible absorption spectra were used to calculate the energy gap energy of the polymers. Chs-NAI polymer, Chs-NAI β-CD, Chs-NAI/ZnO NP composite, and Chs-NAI β-CD/ZnO NP composite had Eg values of 4.35, 4.19, 3.98, and 4.09 eV, respectively. These results supported the development of Chs-NAI, Chs-NAI/ZnO NP composite, Chs-NAI β-CD, and Chs-NAI β-CD/ZnO NP composite. Thermal stability and heat resistance of the polymers were investigated by gravimetric analysis. Figure 5 shows the TGA curves of Chs-NAI, Chs-NAI/ZnO NP composite, Chs-NAI/β-CD, and Chs-NAI/β-CD/ZnO NP composite. In general, this figure shows that the thermal stability of the Chs-NAI/β-CD polymer is inferior compared to the pure Chs-NAI polymer whereas the weight loss at 390 °C is 66% and 56%, respectively. Moreover, the merge of ZnO NPs improved the heat resistance of the formed Chs-NAI/ZnO composite (weight loss at 390 °C is 48%). Moreover, the weight loss of the Chs-NAI/β-CD/ZnO NP composite decreased by 59% at the same temperature compared with the Chs-NAI/β-CD polymer, owing to the addition of ZnO NPs increasing the stability of the polymer. Depending on the results in Fig. 5, the order of thermal stability of the four samples is Chs-NAI/ZnO NPs ˃ Chs-NAI/β-CD/ZnO NPs ˃ Chs-NAI ˃ Chs-NAI/β-CD. The mineralized composite is prepared by in situ precipitation of HA-like crystals on Chs-NAI/β-CD/ZnO NPs at a controlled pH. The morphological and chemical characteristics of the mineralized composite were examined. The morphological and chemical characteristics of the mineralized composite were examined. The SEM, EDX, FTIR, and XRD analyses of the mineralized composite are shown in Fig. 6A, B, C, and D. Upon precipitation, the calcium phosphate phases tended to organize into spherical aggregates of uniform size, which were tightly packed within the Chs-NAI/β-CD/ZnO NP matrix. As a result, the EDX tests reveal the preparation of stoichiometric hydroxyapatite crystalline phases (Ca/P 1.76), which is consistent with previous findings [48, 49]. In order to further investigate the microstructural properties of calcium phosphates dispersed in the matrix. The FTIR analyses were evaluated. According to Fig. 6C, the phosphate group absorption peaks were found in the spectra of Chs-NAI/β-CD/ZnO nanoparticles. These peaks, located between 1050 and 1160 cm−1 (P–O3 mode), 560 cm−1 (P–O4 mode), and 899 cm−1 (P–O1 mode) are assigned to different vibration modes of the PO43− group, confirmed that the mineralization process had taken place. Figure 6D depicts the XRD spectra of the hybrid hydrogels before and during the precipitation of calcium phosphate materials, which exhibited diffraction patterns with hydroxyapatite-typical signals at 2θ = 26°, 31.5°, 37.9°, 42.5°, and 45°. Transmission electron microscope (TEM) was used to show the interior structure of biomimetic calcium phosphate. Figure 7A shows needle-shaped mineralized nanocrystals. Based on the image, the particle’s size is estimated to be between 20 and 50 nm. Selected area electron diffraction was used to evaluate the crystallinity of mineralized calcium phosphate (SAED). The mineralized calcium phosphate pattern is shown in Fig. 7B. It has well-resolved ring patterns, indicating crystalline compositions. Also, the mineralized calcium phosphate electron diffraction displays hydroxy apatite-crystal diffraction. The highest non-toxic dose of Chs-NAI/β-CD/ZnO nanoparticles/calcium phosphate was determined using Vero cells as a test subject (MNTD). In Fig. 8, the viability of Vero cells was greater than 95% at concentrations of 31.2, 62.5, and 125 μg/ml for Chs-NAI/β-CD/ZnO nanoparticles/calcium phosphate hybrid. When concentrations exceeding 125 μg/ml, cytotoxicity against the Vero cell line was detected; these values are considered the maximal feasible hazardous dose. The 50% cytotoxic concentration (CC50) of the hybrid materials was around 500 μg/ml, as demonstrated by these results. In this study, it was used that Chs polymer to produce Chs-NAI polymers by different methods. Thus, the (Chs-NAI) polymer was added to β-CD and ZnO NPs to afford the pseudopolyrotaxane inclusion complex and the Chs-NAI/ZnO NP composite, respectively, in an appropriate and easy manner that was both cost-effective and ecologically benign. A one-pot four-component reaction of Chs, NAI, ZnO NPs, and β-CD gave Chs-NAI/β-CD/ZnO NP composite. These polymers were established by XRD and FT-IR spectra, whereas SEM analysis designated remarkable morphological variations between them. Furthermore, by do** Chs-NAI polymer with ZnO nanoparticles, the thermal stability of Chs-NAI polymer was improved. For concentrations of Chs-NAI/β-CD/ZnO nanoparticles/calcium phosphate of 31.2, 62.5, and 125 μg/ml, Vero cells showed greater than 95% feasibility. When the concentrations exceeded 125 μg/ml, they were observed to be cytotoxic against the Vero cell line, considering these concentrations to be the maximum viable toxic dose.2 Materials and methods
2.1 Materials
2.2 Synthesis of N-acetyl isatin
2.3 Synthesis of Chs-NAI polymer
2.4 Preparation of pseudopolyrotaxane (Chs-NAI/β-CD)
2.5 Synthesis of Chs-NAI/ZnO NP composite
2.6 Synthesis of Chs-NAI/β-CD/ZnO NP composite
2.7 Mineralization reactions
2.8 Characterizations
2.8.1 Fourier-transformation infrared (FTIR)
2.8.2 X-ray diffraction (XRD)
2.8.3 Scanning electron microscope (SEM)
2.8.4 Transmission electron microscopy (TEM)
2.8.5 Thermogravinmetric analysis (TGA)
2.8.6 UV–visible spectroscopy
2.9 Cytotoxicity study
3 Results and discussion
3.1 XRD analysis
3.2 Fourier-transformation infrared spectroscopy (FTIR)
3.3 SEM analysis
3.4 Optical properties
3.5 Thermal analysis
3.6 Characterization of Chs-NAI/β-CD/ZnO NPs/calcium phosphate
3.7 Effect of Chs-NAI/CD/ZnO nanoparticles/calcium phosphate materials on the cell viability
4 Conclusion
Data availability
All data is contained within the manuscript.
References
Hosseinkhani M, Mehrabani D, Karimfar MH et al (2014) Tissue engineered scaffolds in regenerative medicine. World J Plast Surg 3:3–7
Amini AR, Laurencin CT, Nukavarapu SP (2012) Bone tissue engineering: recent advances and challenges. Crit Rev Biomed Eng 40:363–408. https://doi.org/10.1615/CritRevBiomedEng.v40.i5.10
Henkel J, Woodruff MA, Epari DR et al (2013) Bone regeneration based on tissue engineering conceptions-a 21st century perspective. Bone Research 1:216–248
Abouzeid RE, Khiari R, Salama A et al (2020) In situ mineralization of nano-hydroxyapatite on bifunctional cellulose nanofiber/polyvinyl alcohol/sodium alginate hydrogel using 3D printing. Int J Biol Macromol 160:538–547. https://doi.org/10.1016/j.ijbiomac.2020.05.181
Abouzeid RE, Khiari R, Beneventi D, Dufresne A (2018) Biomimetic mineralization of three-dimensional printed alginate/TEMPO-oxidized cellulose nanofibril scaffolds for bone tissue engineering. Biomacromol 19:4442–4452. https://doi.org/10.1021/acs.biomac.8b01325
Teodorescu M, Bercea M (2015) Poly(vinylpyrrolidone) – a versatile polymer for biomedical and beyond medical applications. Polym Plast Technol Eng 54:923–943. https://doi.org/10.1080/03602559.2014.979506
Muhammad A, Rahman MR, Baini R, Bin Bakri MK (2021) Applications of sustainable polymer composites in automobile and aerospace industry. In: Advances in Sustainable Polymer Composites. Woodhead Publishing, pp 185–207
Yanat M, Schroën K (2021) Preparation methods and applications of chitosan nanoparticles; with an outlook toward reinforcement of biodegradable packaging. React Funct Polym 161. https://doi.org/10.1016/j.reactfunctpolym.2021.104849
El-Wakil NA, Hassan EA, Abou-Zeid RE, Dufresne A (2015) Development of wheat gluten/nanocellulose/titanium dioxide nanocomposites for active food packaging. Carbohyd Polym 124:337–346. https://doi.org/10.1016/j.carbpol.2015.01.076
Alghamdi SS, John S, Choudhury NR, Dutta NK (2021) Additive manufacturing of polymer materials: Progress, promise and challenges. Polymers 13:1–39
Bai Y, Zhang H, Shao Y et al (2021) Recent progresses of superhydrophobic coatings in different application fields: An overview. Coatings 11:1–30
Kehinde BA, Chhikara N, Sharma P, et al (2020) Application of polymer nanocomposites in food and bioprocessing industries. In: Handbook of Polymer Nanocomposites for Industrial Applications. Elsevier, pp 201–236. https://doi.org/10.1016/B978-0-12-821497-8.00006-X
**ao M, Sadhanala A, Abdi-Jalebi M et al (2021) Linking glass-transition behavior to photophysical and charge transport properties of high-mobility conjugated polymers. Adv Funct Mater 31. https://doi.org/10.1002/adfm.202007359
Liu Y, Wang Q (2020) Ferroelectric polymers exhibiting negative longitudinal piezoelectric coefficient: progress and prospects. Adv Sci 7(6):1902468. https://doi.org/10.1002/advs.201902468
Salama A, Abou-Zeid RE, El-Sakhawy M, El-Gendy A (2015) Carboxymethyl cellulose/silica hybrids as templates for calcium phosphate biomimetic mineralization. Int J Biol Macromol. https://doi.org/10.1016/j.ijbiomac.2014.11.041
Salama A, Abou-Zeid RE, El-Sakhawy M (2016) Calcium phosphate mineralization controlled by carboxymethyl cellulose-g-polymethacrylic acid. Soft Mater. https://doi.org/10.1080/1539445X.2016.1171781
Kabir E, Kaur R, Lee J et al (2020) Prospects of biopolymer technology as an alternative option for non-degradable plastics and sustainable management of plastic wastes. J Clean Prod 258:120536. https://doi.org/10.1016/j.jclepro.2020.120536
Cywar RM, Rorrer NA, Hoyt CB et al (2022) Bio-based polymers with performance-advantaged properties. Nat Rev Mater 7:83–103
Peter S, Lyczko N, Gopakumar D et al (2021) Chitin and chitosan based composites for energy and environmental applications: a review. Waste Biomass Valorization 12:4777–4804
Khajavian M, Vatanpour V, Castro-Muñoz R, Boczkaj G (2022) Chitin and derivative chitosan-based structures — preparation strategies aided by deep eutectic solvents: a review. Carbohydr Polym 275:118702. https://doi.org/10.1016/j.carbpol.2021.118702
Wegner L, Kinoshita A, de Paiva FFG et al (2021) Only carapace or the entire cephalothorax: which is best to obtain chitosan from shrimp fishery waste? J Mater Cycles Waste Manage 23:1831–1837. https://doi.org/10.1007/s10163-021-01254-z
Thiruganasambanthan T, Ilyas RA, Norrrahim MNF et al (2022) Emerging developments on nanocellulose as liquid crystals: a biomimetic approach. Polymers 14:1546. https://doi.org/10.3390/polym14081546
Chopra L, Thakur KK, Chohan JS et al (2022) Comparative drug release investigations for diclofenac sodium drug (DS) by chitosan-based grafted and crosslinked copolymers. Materials 15:2404. https://doi.org/10.3390/ma15072404
Qiu W, Wang W, Liu Y, Fan R (2021) Synthesis of N -indolated amino acids or peptides from 2-alkynylanilines via a dearomatization process. Org Chem Front 8:6869–6873. https://doi.org/10.1039/d1qo01257f
Liu Q, Xu Q, Sun W (2021) Facile preparation of core-shell magnetic organic covalent framework via self-polymerization of two-in-one strategy as a magnetic solid-phase extraction adsorbent for determination of Rhodamine B in food samples. J Chromatogr A 1657. https://doi.org/10.1016/j.chroma.2021.462566
Nurazzi NM, Asyraf MRM, Fatimah Athiyah S et al (2021) A review on mechanical performance of hybrid natural fiber polymer composites for structural applications. Polymers 13(13):2170
Asyraf MRM, Ishak MR, Syamsir A et al (2022) Mechanical properties of oil palm fibre-reinforced polymer composites: a review. J Market Res 17:33–65
Ilyas RA, Aisyah HA, Nordin AH et al (2022) Natural-fiber-reinforced chitosan, chitosan blends and their nanocomposites for various advanced applications. Polymers 14(5):874
Mittal H, Ray SS, Kaith BS et al (2018) Recent progress in the structural modification of chitosan for applications in diversified biomedical fields. Eur Polymer J 109:402–434
Zhao F, Meng N, Sun T et al (2021) Metal-free electrochemical synthesis of α-ketoamides: Via decarboxylative coupling of α-keto acids with isocyanides and water. Organic Chemistry Frontiers 8:6508–6514. https://doi.org/10.1039/d1qo01351c
Obafemi CA, Adegbite OB, Fadare OA et al (2021) Tryptanthrin from microwave-assisted reduction of isatin using solid-state-supported sodium borohydride: DFT calculations, molecular docking and evaluation of its analgesic and anti-inflammatory activity. Heliyon 7. https://doi.org/10.1016/j.heliyon.2020.e05756
Yang SQ, Yao YQ, Chen XC et al (2021) Pd-catalyst containing a hemilabile P, C-hybrid ligand in amino dicarbonylation of aryl halides for synthesis of α-ketoamides. Organometallics 40:1032–1041. https://doi.org/10.1021/acs.organomet.1c00024
Pitti V, SarmaVangala M, Chidara S, Mogili P (2022) Ligand-free Zn-catalyzed double carbonylation of aryl iodides with secondary amines: a simple and efficient approach to access α-ketoamides. Synth Commun 52:212–217. https://doi.org/10.1080/00397911.2021.2012805
Naskar S, Mal K, Maity R, Das I (2021) Recent advances in the synthesis and applications of α-ketothioesters. Adv Synth Catal 363:1160–1184
Singh S, Popuri S, Junaid QM et al (2021) Diversification of α-ketoamides: via transamidation reactions with alkyl and benzyl amines at room temperature. Org Biomol Chem 19:7134–7140. https://doi.org/10.1039/d1ob01021b
Qi X, Tong X, Pan W et al (2021) Recent advances in polysaccharide-based adsorbents for wastewater treatment. J Clean Prod 315:128221
Li Y, Zheng Y, Zhang Y et al (2021) Brown algae carbohydrates: structures, pharmaceutical properties, and research challenges. Marine Drugs 19
Rubin P A, Cecone C, Trotta F, Zanetti M (2021) Mechanosynthesis of β-Cyclodextrin polymers based on natural deep eutectic solvents. ACS Sust Chem Eng 9:14881–14889. https://doi.org/10.1021/acssuschemeng.1c04988
Suvarna P, Chaudhari P, Birangal S et al (2022) Voriconazole–cyclodextrin supramolecular ternary complex-loaded ocular films for management of fungal keratitis. Mol Pharm 19:258–273. https://doi.org/10.1021/acs.molpharmaceut.1c00746
Dardeer HM, El-sisi AA, Emam AA, Hilal NM (2017) Synthesis, application of a novel azo dye and its inclusion complex with beta-cyclodextrin onto polyester fabric. Int J Text Sci 2017:79–87. https://doi.org/10.5923/j.textile.20170603.01
Dardeer HM, Mahgoub MY, Abouzeid RE et al (2022) Novel pseudopolyrotaxane composite based on biopolymers: synthesis, characterization and application in water treatment. Environ Nanotechnol Monit Manag 17. https://doi.org/10.1016/j.enmm.2021.100639
Dardeer HM, Ebnalwaled AA (2019) On improving the spectral response of organic dyes sensitizer based on β-cyclodextrin inclusion complex. Optik 178:197–209. https://doi.org/10.1016/j.ijleo.2018.10.012
Dardeer HM, Assran AS, Al-Hussain SA, Toghan A (2021) Synthesis, spectroscopic and molecular docking studies of novel 10-heterylazo-9-anthrone derivatives as potential antimicrobial and anticancer agents. J Mol Struct 1226. https://doi.org/10.1016/j.molstruc.2020.129359
Mosmann T (1983) Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J Immunol Methods 65:55–63. https://doi.org/10.1016/0022-1759(83)90303-4
Gao S, Liu Y, Jiang J et al (2021) Thiram/hydroxypropyl-β-cyclodextrin inclusion complex electrospun nanofibers for a fast dissolving water-based drug delivery system. Colloids Surf B 201. https://doi.org/10.1016/j.colsurfb.2021.111625
Liu W, Qin Y, Liu S et al (2018) Synthesis, characterization and antifungal efficacy of chitosan derivatives with triple quaternary ammonium groups. Int J Biol Macromol 114:942–949. https://doi.org/10.1016/j.ijbiomac.2018.03.179
Tauc J, Grigorovici R, Vancu A (1966) Optical properties and electronic structure of amorphous germanium. Physica Status Solidi (B) 15:627–637. https://doi.org/10.1002/pssb.19660150224
Salama A, Abou-Zeid RE, Cruz-Maya I, Guarino V (2020) Soy protein hydrolysate grafted cellulose nanofibrils with bioactive signals for bone repair and regeneration. Carbohyd Polym 229. https://doi.org/10.1016/j.carbpol.2019.115472
Salama A, Abou-Zeid RE, El-Sakhawy M (2016) Calcium phosphate mineralization controlled by carboxymethyl cellulose-g-polymethacrylic acid. Soft Mater 14:154–161. https://doi.org/10.1080/1539445X.2016.1171781
Acknowledgements
The authors express their gratitude to the National Research Centre, for the technical support of this work. The authors also thank South Valley University for providing necessary and facilities.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
The experiments were carried out by Hemat M. Dardeer, Ahmed G. Taha, and Ragab Abouzeid. The manuscript was written by all the authors. All authors discussed the results and commented on the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
(i) New chitosan-N-acetylisatin (Chs-NAI) polymer derivatives were prepared.
(ii) The generated polymers were estimated by XRD, FTIR, and SEM analysis.
(iii) Chs-NAI/βCD/ZnO NPs)/calcium phosphate is a promising demonetarizing very mild cytotoxicity.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Dardeer, H.M., Taha, A.G., Abouzeid, R.E. et al. Novel chitosan-acetyl isatin polymer derivatives: synthesis, characterization, and applications in bone tissue engineering. Biomass Conv. Bioref. 14, 12427–12440 (2024). https://doi.org/10.1007/s13399-022-03176-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-022-03176-8