Abstract
Traumatic brain injuries (TBIs) are associated with high morbidity and mortality due to both the original insult as well as the destructive biological response that follows. Medical management aims to slow or even halt secondary neurological injury while simultaneously laying the groundwork for recovery. Statins are one class of medications that is showing increased promise in the management of TBI. Used extensively in cardiovascular disease, these drugs were originally developed as competitive inhibitors within the cholesterol production pipeline. They are now used in diverse disease states due to their pleiotropic effects on other biological processes such as inflammation and angiogenesis. Preclinical studies, retrospective reviews, and randomized clinical trials have shown a variety of benefits in the management of TBI, but to date, no large-scale randomized clinical trial has been performed. Despite this limitation, statins’ early promise and well-tolerated side effect profile make them a promising new tool in the management of TBIs. More bench and clinical studies are needed to delineate proper treatment regimens as well as understand their true potential.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Traumatic brain injury (TBI) is a leading health problem in both develo** and high-income countries. Over 50 million TBIs occur internationally each year, causing one-third of injury-related deaths and costing 0.5% of worldwide GDP [1]. TBI-related disability has an incidence of approximately 4 million people in the USA. This disease disproportionally affects the young, with peaks in the adolescent and the elderly stages of life [2]. These numbers underestimate the true impact of TBIs, with an order of magnitude more going unreported and unnoticed [2, 3].
Traumatic brain injury is a complex heterogeneous pathology with a wide clinical impact, ranging from asymptomatic to a neurologically devastating disease. Severe TBI has been estimated to have a mortality rate of almost 40%. Those who survive are often debilitated with severe physical, emotional, and economic burdens [4]. Nearly half of TBI survivors develop depression and later in life suffer from dementia at five times the average rate [5]. Even mild TBIs can have long-term impacts including increased risk of dementia, seizures [6], functional limitations, disability, mood disorders [7], and reduced quality of life [8].
Crucial to the management of TBI is recognition that it is not an acute condition, but a chronic and evolving disease process. The “second hit” model posits that damage continues even after the original injury, as swelling and inflammation sets in. Additionally, the recovery process is a complex, poorly understood process dependent on the resha** or reformation of the damaged neural networks [9].
The standard of care for TBI management is a constantly changing field. In the acute period, surgical intervention such as placement of a ventriculostomy or craniectomy can have a significant impact on morbidity and mortality [10, 11]. However, the vast majority of cases are managed conservatively [10]. In addition to long-term support including physical and occupational therapy, many different medical interventions have been tried. Aside from antiepileptics prevention of early-onset seizures [12] and multimodal therapy for intracranial pressure control [13], no other regimen has been codified in the management of TBI sequelae [14, 15].
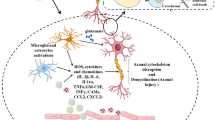
This large divide between a clear clinical need and a lack of solution has driven an enormous amount of research into potential treatments. Clinical trials have investigated a wide variety of known medications [15], such as magnesium [16] and cyclosporine-A [17]. One class of medications receiving increased interest is statins. Statins classically impact physiology as inhibitors of the enzyme 3-hydroxy-3-methyl-glutaryl-coenzyme A reductase (HMG-CoA reductase), the rate-controlled step within the mevalonate pathway. This interrupts the metabolic chain reaction that eventually produces cholesterol and other organic isoprenoid derivates such as steroids and vitamins. However, further investigations have revealed many alternative impacts of statins, including stimulating angiogenesis, anti-inflammatory effects, and influence of neural circuit formation (Fig. 1) [18].
History
In the late twentieth century, researchers began to elucidate the role of cholesterol in cardiovascular disease and looked for ways to control cholesterol levels. The first statin was actually a byproduct of antibiotic research. Inspired by the discovery of penicillin, researchers were culturing fungi at a large scale to find new compounds. Mevastatin, also known as compactin, was isolated in 1971 from the fungus Penicillium citrinum by researchers looking for an enzyme that might target microbes that depended on sterols or other isoprenoids [19, 20]. Lovastatin was isolated by Merck in 1978 from Aspergillus terreus and in 1987 became the first statin to be approved by the FDA. The incredible results of early trials [21] caused a wave of public interest in statins, leading to the development of many alternative compounds. In the early 2000s, blockbuster drugs such as simvastatin, pravastatin, and atorvastatin had an average annual cost of nearly $25 billion in the USA alone [22].
Mechanism of Action
Statins were originally discovered for their ability to competitively inhibit HMG-CoA reductase due to their molecular similarity to HMG-CoA (Fig. 2). This competition for the enzyme’s active site allows it to compete with the native substrative and reduce the rate that mevalonate is produced. The lower availability of mevalonate decreases the body’s ability to generate cholesterol (a downstream molecule). This impact is compounded by the liver, which increases the production of LDL receptors to harvest circulating cholesterol, further lowering bloodstream levels [23].
Yet as their use became more widespread, new findings began to suggest that this is not the only action of statins in the body. In fact, a large part of its impact may actually be derived from other sources. For example, simply lowering cholesterol by other means does not have the same benefit, and these drugs have been shown to have a benefit in disease processes not classically associated with elevated lipid levels [24].
More than 20 years since statins were put on the market, new findings demonstrated that many of their health benefits may be through their immunomodulatory impact. For example, they are known to inhibit the inductive effect of interferon-γ on major histocompatibility class II (MHC-II), thereby repressing MHC-II-medicated T-cell activation [25]. They have also been shown to lower C reactive protein (CRP) levels by one-third [26], as well as other inflammatory markers such as inducible nitric oxide synthase (iNOS), tumor necrosis factor alpha (TNF-α), and interleukin-1β (IL-1β) [27]. Other studies have shown a disruption of lipid rafts, preventing the organization of proteins necessary for the activation of immune cells [28]. Natural killer cells have lower cytotoxicity in patients on statins, leading to their use in preventing organ rejection [29]. Other autoimmune disorders that have been treated with statins include multiple sclerosis, rheumatoid arthritis, and osteoporosis [25].
Further investigations have also shown a direct impact on vasculature. Endothelial-dependent flow significantly improves after statin treatment [30]. Statins induce endothelial nitric oxide synthase (eNOS), an enzyme that generates nitric oxide (NO) within vessel walls to promote vascular relaxation and decrease interactions with circulating leukocytes and platelets [31]. They also induce the expression of various genetic profiles involved in the remodeling of both endothelial and smooth muscle cells [24].
Lastly, there may be direct neurological impacts both cholesterol and non-cholesterol mediated. Cholesterol is a major component of neural membranes and is a rate-limiting step in synaptogenesis [32]. Compactin has been shown to promote the maintenance of dendritic and axonal connectivity patterns [33]. Statins can protect neurons from excitotoxic damage, such as NMDA-mediate excitotoxic cell death [34]. Simvastatin in particular has been shown to stimulate the Bcl-2 gene, promoting neuronal survival [35] and attenuating axonal injury [76]. However, not all studies are uniformly positive. Robertson et al. performed a phase II clinical trial and found that atorvastatin for 7 days had no difference in the Rivermead score (a post-concussive assessment) at 3 months post-injury [77]. Notably, as with some of the animal studies, these trials have shown the effective time window to be as long as 24 h post-injury, increasing the clinical utility of statins in real-world situations.
Other studies aimed to correlate these outcomes with physiologic parameters of injury. From the inflammatory perspective, statins were shown to reduce levels of tumor necrosis factor- α (TNF-α), with mixed results on interleukin (IL) levels [67, 68]. Similarly, simvastatin has been shown to lower CRP in severe TBIs requiring ICU admission [69]. Interestingly, even in patients that show long-term benefits, it is difficult to notice any immediate difference on cranial imaging, with similar contusion volumes and rates of expansion [73], though one study showed decreased cortical loss [78].
Lastly, atorvastatin will be tested in a multi-arm, multi-stage adaptive platform trial for the acute treatment of TBI by the TRACK-TBI network. This will be a multi-center, double-blind, placebo-controlled adaptive platform, precision medicine trial conducted under a single multi-arm, multi-stage (MAMS) study with parallel groups. Subjects will be randomized to receive one of four possible treatments, being atorvastatin a study drug. It is expected that the findings of this study will assist in clarifying the potential beneficial effect of statins in the management of TBI (Table 2).
Conclusion
TBI is a significant and growing public health problem. There is no current standard of care regimen in the medical management of TBI. Statins are a well-studied popular class of medication with minimal side effect profile relative to the proposed benefits. Recent research has demonstrated that their benefits are not limited solely to the cardiovascular outcomes. In vitro, animal, retrospective, and randomized control studies have all demonstrated the potent for multimodal impact on both motor and cognitive outcomes. Further work is needed to clarify how to maximize its impact to ensure that its putative and potential benefits are realized.
References
Maas AIR, et al. Traumatic brain injury: integrated approaches to improve prevention, clinical care, and research. Lancet Neurol. 2017;16:987–1048.
Frieden TR, Houry D, Baldwin G. Traumatic Brain injury in the United States: epidemiology and rehabilitation. CDC NIH Rep to Congr. 2015;1–74.
Feigin VL, et al. Incidence of traumatic brain injury in New Zealand: a population-based study. Lancet Neurol. 2013;12:53–64.
Rosenfeld Jv, et al. Early management of severe traumatic brain injury. Lancet. 2012;380:1088–1098.
Plassman BL, et al. Documented head injury in early adulthood and risk of Alzheimer’s disease and other dementias. Neurology. 2000;55:1158–66.
Mahler B, et al. Unprovoked seizures after traumatic brain injury: a population-based case-control study. Epilepsia. 2015;56:1438–44.
Hart T, et al. Major and minor depression after traumatic brain injury. Arch Phys Med Rehabil. 2011;92:1211–9.
Riggio S, Wong M. Neurobehavioral sequelae of traumatic brain injury. Mount Sinai Journal of Medicine: J Transl Pers Med. 2009;76:163–72.
Rohling ML, Faust ME, Beverly B, Demakis G. Effectiveness of cognitive rehabilitation following acquired brain injury: a meta-analytic re-examination of Cicerone et al.’s (2000, 2005) systematic reviews. Neuropsychology. 2009;23:20–39.
Bullock MR, et al. Surgical management of traumatic brain injury. Neurosurgery. 2006;58:16–24.
Timofeev I, et al. Ventriculostomy for control of raised ICP in acute traumatic brain injury. 2008;99–104. https://doi.org/10.1007/978-3-211-85578-2_20.
Chang BS, Lowenstein DH. Practice parameter: antiepileptic drug prophylaxis in severe traumatic brain injury. Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2003;60:10–16.
Rangel-Castillo L, Gopinath S, Robertson CS. Management of intracranial hypertension. Neurol Clin. 2008;26:521.
Marehbian J, Muehlschlegel S, Edlow BL, Hinson HE, Hwang DY. Medical management of the severe traumatic brain injury patient. Neurocritical Care. 2017;27(3):430–446.
Gruenbaum SE, Zlotnik A, Gruenbaum BF, Hersey D, Bilotta F. Pharmacologic neuroprotection for functional outcomes after traumatic brain injury: a systematic review of the clinical literature. CNS Drugs. 2016;30:791–806.
Temkin NR, et al. Magnesium sulfate for neuroprotection after traumatic brain injury: a randomised controlled trial. Lancet Neurol. 2007;6:29–38.
Lulic D, Burns J, Bae EC, van Loveren H, Borlongan Cv. A review of laboratory and clinical data supporting the safety and efficacy of cyclosporin A in traumatic brain injury. Neurosurgery. 2011;68:1172–1186.
Farooqui AA, Ong WY, Horrocks LA, Chen P, Farooqui T. Comparison of biochemical effects of statins and fish oil in brain: the battle of the titans. Brain Res Rev. 2007;56:443–71.
Steinberg, D. Thematic review series: the pathogenesis of atherosclerosis. An interpretive history of the cholesterol controversy, part V: The discovery of the statins and the end of the controversy. J Lipid Res. 2006;47:1339–1351.
Endo A. The discovery and development of HMG-CoA reductase inhibitors. J Lipid Res. 1992;33:1569–82.
Pedersen TR, et al. Effect of simvastatin on ischemic signs and symptoms in the scandinavian simvastatin survival study (4S). Am J Cardiol. 1998;81:333–5.
Lin SY, et al. Trends in use and expenditures for brand-name statins after introduction of generic statins in the US, 2002–2018. JAMA Netw Open. 2021;4:e2135371–e2135371.
Goldstein JL, Brown MS. Regulation of the mevalonate pathway. Nature. 1990;343:425–30.
Jain MK, Ridker PM. Anti-inflammatory effects of statins: clinical evidence and basic mechanisms. Nat Rev Drug Discover 2005;4(12):977–987.
Kwak B, Mulhaupt F, Myit S, Mach F. Statins as a newly recognized type of immunomodulator. Nat Med. 2000;6.
Ridker PM, Rifai N, Pfeffer MA, Sacks F, Braunwald E. Long-term effects of pravastatin on plasma concentration of C-reactive protein. The Cholesterol and Recurrent Events (CARE) Investigators. Circulation. 1999;100:230–5.
Pannu R, Barbosa E, Singh AK, Singh I. Attenuation of acute inflammatory response by atorvastatin after spinal cord injury in rats. J Neurosci Res. 2005;79:340–50.
Ehrenstein MR, Jury EC, Mauri C. Statins for atherosclerosis — as good as it gets? N Engl J Med. 2005;352:73–5.
Katznelson S, et al. The effect of pravastatin on acute rejection after kidney transplantation–a pilot study. Transplantation. 1996;61:1469–74.
Anderson TJ, et al. The effect of cholesterol-lowering and antioxidant therapy on endothelium-dependent coronary vasomotion. N Engl J Med. 1995;332:488–93.
Balakumar P, Kathuria S, Taneja G, Kalra S, Mahadevan N. Is targeting eNOS a key mechanistic insight of cardiovascular defensive potentials of statins? J Mol Cell Cardiol. 2012;52:83–92.
Mauch DH, et al. CNS synaptogenesis promoted by glia-derived cholesterol. Science. 2001;294:1354–7.
Fan Q-W, et al. Cholesterol-dependent modulation of dendrite outgrowth and microtubule stability in cultured neurons. J Neurochem. 2002;80:178–90.
Zacco A, et al. 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors protect cortical neurons from excitotoxicity. J Neurosci. 2003;23:11104–11.
Johnson-Anuna LN, et al. Simvastatin protects neurons from cytotoxicity by up-regulating Bcl-2 mRNA and protein. J Neurochem. 2007;101:77–86.
Wu H, Mahmood A, Qu C, **ong Y, Chopp M. Simvastatin attenuates axonal injury after experimental traumatic brain injury and promotes neurite outgrowth of primary cortical neurons. Brain Res. 2012;1486:121–30.
McFarland AJ, et al. Molecular mechanisms underlying the effects of statins in the central nervous system. Int J Mol Sci. 2014;15:20607–20637 15, 20607–20637.
Garcia M, Reinoso R, Sanchez Navarro A, Prous J. Clinical pharmacokinetics of statin. Methods Find Exp Clin Pharmacol. 2003;25:457–481.
Botti RE, Triscari J, Pan HY, Zayat J. Concentrations of pravastatin and lovastatin in cerebrospinal fluid in healthy subjects. Clin Neuropharmacol. 1991;14:256–61.
Johnson-Anuna LN, et al. Chronic administration of statins alters multiple gene expression patterns in mouse cerebral cortex. J Pharmacol Exp Ther. 2005;312:786–93.
Thompson PD, Clarkson P, Karas RH. Statin-associated myopathy. JAMA. 2003;289:1681–90.
Ganga Hv, Slim HB, Thompson PD. A systematic review of statin-induced muscle problems in clinical trials. Am Heart J. 2014;168:6–15.
Russo MW, et al. Spectrum of statin hepatotoxicity: experience of the drug-induced liver injury network. Hepatology. 2014;60:679–86.
Charles EC, Olson KL, Sandhoff BG, McClure DL, Merenich JA. Evaluation of cases of severe statin-related transaminitis within a large health maintenance organization. Am J Med. 2005;118:618–24.
Swerdlow DI, et al. HMG-coenzyme A reductase inhibition, type 2 diabetes, and bodyweight: evidence from genetic analysis and randomised trials. Lancet. 2015;385:351–61.
Riaz H, et al. Meta-analysis of placebo-controlled randomized controlled trials on the prevalence of statin intolerance. Am J Cardiol. 2017;120:774–81.
Mountney A, et al. Simvastatin treatment in traumatic brain injury: operation brain trauma therapy. J Neurotrauma. 2016;33:567–80.
Chen S-F, et al. Lovastatin improves histological and functional outcomes and reduces inflammation after experimental traumatic brain injury. Life Sci. 2007;81:288–98.
Wang H, et al. Simvastatin and atorvastatin improve behavioral outcome, reduce hippocampal degeneration, and improve cerebral blood flow after experimental traumatic brain injury. Exp Neurol. 2007;206:59–69.
Wu H, et al. Induction of angiogenesis and modulation of vascular endothelial growth factor receptor-2 by simvastatin after traumatic brain injury. Neurosurgery. 2011;68:1363–71.
Wang K-W, et al. Simvastatin combined with antioxidant attenuates the cerebral vascular endothelial inflammatory response in a rat traumatic brain injury. Biomed Res Int. 2014;2014:1–6.
Xu X, et al. Anti-inflammatory and immunomodulatory mechanisms of atorvastatin in a murine model of traumatic brain injury. J Neuroinflammation. 2017;14:167.
Lu D, et al. Atorvastatin reduction of intravascular thrombosis, increase in cerebral microvascular patency and integrity, and enhancement of spatial learning in rats subjected to traumatic brain injury. J Neurosurg. 2004;101:813–21.
Lu D, et al. Atorvastatin reduces neurological deficit and increases synaptogenesis, angiogenesis, and neuronal survival in rats subjected to traumatic brain injury. J Neurotrauma. 2004;21:21–32.
Lu D, et al. Statins increase neurogenesis in the dentate gyrus, reduce delayed neuronal death in the hippocampal CA3 region, and improve spatial learning in rat after traumatic brain injury. J Neurotrauma. 2007;24:1132–46.
Darwish H, Mahmood A, Schallert T, Chopp M, Therrien B. Simvastatin and environmental enrichment effect on recognition and temporal order memory after mild-to-moderate traumatic brain injury. Brain Inj. 2014;28:211–26.
**e C, et al. The effect of simvastatin treatment on proliferation and differentiation of neural stem cells after traumatic brain injury. Brain Res. 2015;1602:1–8.
Mahmood A, et al. Long-term benefits after treatment of traumatic brain injury with simvastatin in rats. Neurosurgery 2009;65:187–91, discussion 191–2.
Mountney A, et al. Intravenous administration of simvastatin improves cognitive outcome following severe traumatic brain injury in rats. J Neurotrauma. 2016;33:1492–500.
Lu D, et al. Delayed thrombosis after traumatic brain injury in rats. J Neurotrauma. 2004;21:1756–66.
Lu D, et al. Atorvastatin reduction of intracranial hematoma volume in rats subjected to controlled cortical impact. J Neurosurg. 2004;101:822–5.
Abrahamson EE, et al. Cerebral blood flow changes after brain injury in human amyloid-beta knock-in mice. J Cereb Blood Flow Metab. 2013;33:826–33.
Khokhar B, et al. Mortality and associated morbidities following traumatic brain injury in older medicare statin users. Journal of Head Trauma Rehabilitation. 2018;33:E68–76.
Mansi IA, English JL, Alvarez CA, Mortensen EM, Pugh MJ. Statins in survivors of traumatic brain injury: a propensity score-matched analysis. Brain Inj. 2020;34:1367–74.
Redelmeier DA, Manzoor F, Thiruchelvam D. Association between statin use and risk of dementia after a concussion. JAMA Neurol. 2019;76:887.
Li M, et al. Beneficial association of angiotensin-converting enzyme inhibitors and statins on the occurrence of possible Alzheimer’s disease after traumatic brain injury. Alzheimers Res Ther. 2020;12:33.
Sánchez-Aguilar M, et al. Effect of rosuvastatin on cytokines after traumatic head injury. J Neurosurg. 2013;118:669–75.
Tapia-Perez JH, et al. Effect of rosuvastatin on amnesia and disorientation after traumatic brain injury (NCT003229758). J Neurotrauma. 2008;25:1011–7.
Naghibi T, Madani S, Mazloomzadeh S, Dobakhti F. Simvastatin’s effects on survival and outcome in traumatic braininjury patients: a comparative study. Turk J Med Sci. 2016;46:1–5.
Soltani F, et al. The effect of low-dose atorvastatin on inflammatory factors in patients with traumatic brain injury: a randomized clinical trial. Arch Neurosci. 2020;7.
Shafiee S, et al. The effect of oral simvastatin on the clinical outcome of patients with severe traumatic brain injury: a randomized clinical trial. Ethiop J Health Sci. 2021;31:807–16.
Lokhandwala A, et al. Preinjury statins are associated with improved survival in patients with traumatic brain injury. J Surg Res. 2020;245:367–72.
Farzanegan GR, Derakhshan N, Khalili H, Ghaffarpasand F, Paydar S. Effects of atorvastatin on brain contusion volume and functional outcome of patients with moderate and severe traumatic brain injury; a randomized double-blind placebo-controlled clinical trial. J Clin Neurosci. 2017;44:143–7.
Schneider EB, et al. Premorbid statin use is associated with improved survival and functional outcomes in older head-injured individuals. J Trauma. 2011;71:815–9.
Neilson SJ, See AAQ, King NKK. Effect of prior statin use on outcome after severe traumatic brain injury in a South-East Asian population. Brain Inj. 2016;30:993–8.
Orlando A, et al. Unintentional discontinuation of statins may increase mortality after traumatic brain injury in elderly patients: a preliminary observation. J Clin Med Res. 2013;5:168–73.
Robertson CS, et al. Phase II clinical trial of atorvastatin in mild traumatic brain injury. J Neurotrauma. 2017;34:1394–401.
Govindarajan KA, et al. Cortical Thickness in mild traumatic brain injury. J Neurotrauma. 2016;33:1809–17.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
None.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Katlowitz, K., Gopinath, S., Cruz Navarro, J. et al. HMG-CoA Reductase Inhibitors for Traumatic Brain Injury. Neurotherapeutics 20, 1538–1545 (2023). https://doi.org/10.1007/s13311-023-01399-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13311-023-01399-9