Abstract
Expression of inhibitory designer receptors exclusively activated by designer drugs (DREADDs) on excitatory hippocampal neurons in the hippocampus represents a potential new therapeutic strategy for drug-resistant epilepsy. To overcome the limitations of the commonly used DREADD agonist clozapine, we investigated the efficacy of the novel DREADD ligand JHU37160 in chemogenetic seizure suppression in the intrahippocampal kainic acid (IHKA) mouse model for temporal lobe epilepsy (TLE). In addition, seizure-suppressing effects of chemogenetics were compared to the commonly used anti-epileptic drug (AED), levetiracetam (LEV). Therefore, an adeno-associated viral vector was injected in the sclerotic hippocampus of IHKA mice to induce expression of a tagged inhibitory DREADD hM4Di or only a tag (control) specifically in excitatory neurons using the CamKIIα promoter. Subsequently, animals were treated with LEV (800 mg/kg), clozapine (0.1 mg/kg), and DREADD ligand JHU37160 (0.1 mg/kg) and the effect on spontaneous seizures was investigated. Clozapine and JHU37160-mediated chemogenetic treatment both suppressed seizures in DREADD-expressing IHKA mice. Clozapine treatment suppressed seizures up to 34 h after treatment, and JHU37160 effects lasted for 26 h after injection. Moreover, both compounds reduced the length of seizures that did occur after treatment up to 28 h and 18 h after clozapine and JHU37160, respectively. No seizure-suppressing effects were found in control animals using these ligands. Chemogenetic seizure treatment suppressed seizures during the first 30 min after injection, and seizures remained suppressed during 8 h following treatment. Chemogenetics thus outperformed effects of levetiracetam (p < 0.001), which suppressed seizure frequency with a maximum of 55 ± 9% for up to 1.5 h (p < 0.05). Only chemogenetic and not levetiracetam treatment affected the length of seizures after treatment (p < 0.001). These results show that the chemogenetic therapeutic strategy with either clozapine or JHU37160 effectively suppresses spontaneous seizures in the IHKA mouse model, confirming JHU37160 as an effective DREADD ligand. Moreover, chemogenetic therapy outperforms the effects of levetiracetam, indicating its potential to suppress drug-resistant seizures.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Restoring the disturbed balance between excitation and inhibition is the main goal of most anti-epileptic drug (AED) treatments [1]. Commonly used AEDs fail to exclusively modulate neurons in the epileptic network but have more widespread effects in the brain and body, thereby frequently causing severe side effects. Moreover, AEDs are ineffective in about 30% of patients [1]. Optogenetics and chemogenetics are experimental techniques that allow modulation of specific cell types and hold promise to lead to new anti-epileptic treatments with improved target specificity and reduced therapy-related side effects [2,3,4]. Chemogenetics allow long-term suppression of a specific brain area or network without the need for implantation of any device. This technique involves in vivo gene therapy to induce expression of a designer receptors exclusively activated by designer drugs (DREADDs) by neurons in the ictogenic zone. The most commonly used DREADDs are hM3Dq and hM4Di (human muscarinic M4 Gi-coupled DREADD receptor). These receptors are derived from the human Gq-coupled M3 and Gi-coupled M4 muscarinic receptors and mediate neuronal activation or inhibition, resulting in increased or decreased neurotransmitter release, respectively [5, 6]. As a result of genetic engineering, the modified DREADD receptors no longer bind acetylcholine but demonstrate a very high affinity for specific drugs. These drugs can be administered at subclinical doses to selectively target DREADD-expressing neurons. DREADD expression can be limited to a specific subtype of neurons using selective promoters (e.g., Ca2+/calmodulin-dependent protein kinase II alpha (CamKIIα) promoter for glutamatergic neurons) [5, 6]. Chemogenetic inhibition of excitatory neurons in the seizure focus has been shown to strongly reduce the number of epileptic seizures in various preclinical models for epilepsy [7,8,9,10,11,12]. In the intrahippocampal kainic acid (IHKA) mouse model, hippocampal seizures can be completely suppressed after clozapine N-oxide (CNO)- and clozapine-mediated hM4Di activation [7, 8]. Here, we evaluated JHU37160 (abbreviated as JHU in the remaining of this paper) as an alternative to clozapine since it is reported to have higher potency and DREADD occupancy compared to clozapine, which has an unfavorable side effect profile [13, 14]. Compared to our previous study [7], the protocol was modified in that (1) we injected a lower titer of viral vector (E + 11 instead of E + 13 genome copies/ml) to avoid toxicity [15] and (2) the time between induction of TLE and viral vector injection was increased from 2 to 10 weeks to evaluate the effectiveness of chemogenetic treatment in a more chronic disease stage. Moreover, we compared this potential new therapeutic approach with the standard anti-epileptic drug, levetiracetam, which is frequently used in treatment of drug-resistant epilepsy and has been reported to suppress seizures in IHKA mice and thus serves as a good comparison to this novel therapy [16,17,18].
Methods
Animals
Male C57BL/6 Hsd mice were obtained from Envigo (The Netherlands). All animals were housed in a room with a fixed 12-h light/dark cycle (lights on between 6 AM and 6 PM) with controlled temperature (20–24 °C) and humidity (40–60%). Food and water were provided ad libitum. During EEG recordings, mice were housed individually in transparent cages positioned next to each other to allow social interaction. Approval for the study protocol was granted by the Animal Experimental Ethical Committee of Ghent University (ECD 16/31), and treatments and care were in compliance with the ARRIVE (Animal Research: Reporting of In Vivo Experiments) and EU Directive 2010/63/EU guidelines. All animals were 8 weeks old at the onset of the experiments. A detailed overview about treatments and groups of animals is provided as Supplementary Fig. S1.
Intrahippocampal Kainic Acid Injection
Mice (n = 15) were anesthetized with isoflurane (5% for induction, 2% for maintenance) and fixed in the stereotactic frame. A midline incision is made in the skin above the skull, and sutures (bregma/lambda) are visualized. A burr hole is made above the right hippocampus (coordinates: −2.0 mm anteroposterior (AP) and + 1.5 mm mediolateral (ML) relative to bregma). Kainic acid (KA) (200 ng in 50 nl saline, Bio-Techne) was injected in the dorsal hippocampus (−1.8 mm dorsoventral (DV) to dura) using a Hamilton Neuros syringe (point style 4) mounted on a pump for automatic injection (Stoelting Quintessential Stereotaxic Injector, Stoelting Co.) at a rate of 100 nl/min. After injection, the needle was left in place for 5 min and slowly removed and the skin was closed to finish the surgery as soon as possible. During surgery, body temperature was maintained at 37 °C by a thermoregulated heating pad. Mice developed status epilepticus and had behavioral seizures following surgery as a consequence of the KA injection.
Recombinant Adeno-Associated Viral Vector Injection and Intrahippocampal EEG Electrode Implantation
At least 10 weeks after KA injection, mice were again anesthetized to inject the viral vector (recombinant adeno-associated viral vector (rAAV)) and implant EEG electrodes. For intracerebral injection, the same procedure was followed as during the IHKA injection. Again, a burr hole was made above the right hippocampus (coordinates: −2.0 mm AP and + 1.5 mm ML relative to bregma) and a Hamilton Neuros syringe (point style 4) mounted on the pump for automated injection (Stoelting Quintessential Stereotaxic Injector, Stoelting Co.) was lowered in the dorsal hippocampus (−1.8 mm DV to dura). The viral vector containing the DREADD sequence under control of the CamKIIα promoter tagged with a hemagglutinin (HA) tag was injected in the dorsal hippocampus of the animals in the DREADD group (500 nl at rate of 100 nl/min, rAAV2/7-CamKIIα-hM4D(Gi)-HA, DREADD group, n = 9, titer: 2.6E + 11 genome copies/ml). The CamKIIα promoter provides a high selectivity (90–95%) of the expression in excitatory neurons [19, 20]. In the control group, a rAAV only containing an mCherry tag under control of the CamKIIα promoter was similarly infused (500 nl, rate of 100 nl/min, rAAV2/7-CamKIIα-mCherry, control group, n = 5, titer: 2.1E-11 genome copies/ml) at the KA injection site. Next, five additional burr holes were made. Two were used for placement of epidural screw electrodes (custom made by attaching an insulated copper wire to an anchor screw): one over the left parietal cortex (to record cortical EEG) and one over the right frontal cortex (which was used as a recording ground/reference). Three additional screws were placed over the parietal cortex (two right and one left) to provide anchoring. Lastly, a stainless steel bipolar depth recording electrode (200 µm tip separation, custom made by twisting two stainless steel wires, California fine wire 70 µm bare diameter) was implanted in the right hippocampus (same AP/ML coordinates as rAAV injection, 2 mm DV to dura) and electrodes were attached to a connector and fixed to the skull with acrylic cement to make a head cap. During surgery, body temperature was maintained at 37 °C by a thermoregulated heating pad. Post-surgery animals were treated with meloxicam (1 mg/kg) to manage pain.
EEG Recording and Treatments
Two weeks after viral vector injection and electrode implantation, mice were connected to the EEG setup for continuous recording. This setup consists of a head stage with unity gain preamplifier (custom made) connected to a 6-channel cable and a commutator (SL6C 6-channel commutator, Plastics One), allowing the animals to move freely. EEG signals were high-pass filtered at 0.15 Hz and amplified 512 × (custom made). An analog–digital convertor (NiDAQ card, National Instruments, USB-6259) was used to digitize EEG at a sampling rate of 2 kHz (16-bit resolution, ± 10 V input range), and the output was stored on a PC for offline analysis. The EEG traces were plotted (Matlab, MathWorks) and visually evaluated for epileptic activity. Electrographic seizures were defined as a repetitive pattern of complex, high-amplitude EEG spikes lasting at least 7 s at hippocampal electrodes. Consecutive seizures are separated by at least 7 s; otherwise, they were counted as one. Electrographic seizures were rarely generalized and were thus typically observed in the hippocampal EEG channels only and not at the cortical scalp electrode. During a typical seizure, no clear behavioral alteration was observed. One animal of the DREADD group did not display clear electrographic seizures and was excluded from the experiment. The other animals were treated with clozapine (Cloz; 0.01 mg/ml, 0.3% DMSO in saline, 0.1 mg/kg i.p., Tocris Bioscience) in a crossover design with levetiracetam (LEV; 100 mg/ml, 800 mg/kg, i.p., Keppra) followed by treatment with JHU37160 (JHU; 0.01 mg/ml in saline, 0.1 mg/kg i.p., Hello Bio). Two additional control animals were treated with levetiracetam after which they were used in another experiment. One DREADD animal died after the crossover study during a period not related to injections and did not receive JHU treatment. All injections took place at 2 PM, and between treatments, there was a washout period of 7 days. An overview of the experimental protocol and treatment schedule can be found in Fig. S1.
Statistics
The number of seizures and seizure length was determined per 30-min bins during 8 h before each treatment (baseline) and up to 54 h post treatment. Given the long-lasting effects of DREADD activation, 2-h bins were used to evaluate the seizure-suppressing effects of clozapine and JHU. A within-subject comparison was performed for the seizure-suppressing effects of levetiracetam and clozapine as those compounds were administered in a crossover design. For JHU and clozapine treatments, no within-subject comparison was done as JHU was injected after the clozapine/levetiracetam crossover (overview of treatment schedule can be found in Supplementary Fig. S1). Effects of clozapine and JHU treatments were evaluated by constructing 4 random-effects models with either the number of seizures per hour or average seizure length as dependent variable, subject ID as random factor, and time as fixed factors (SPSS statistics). The ante-dependence first-order and the first-order autoregressive moving average (ARMA1.1) models were selected as covariance structures for the 2 models comparing the number of seizures and the 2 models regarding seizure length, respectively. To compare the effects of clozapine and levetiracetam, two random-effects models were similarly constructed with either the number of seizures per hour or average seizure length as dependent variable, subject ID as random factor and treatment, and time and treatment by time as fixed factors (SPSS statistics), and the first-order autoregressive moving average model (ARMA1.1) was selected as covariance structure. All data are represented as mean ± standard error of the mean (SEM), and a Bonferroni correction was applied. A p value of < 0.05 was required for rejection of the null hypothesis. GraphPad software (v6-8), Windows PowerPoint 365, ImageJ, and Inkscape were used to create the artwork.
Immunohistochemistry
Mice were transcardially perfused with phosphate-buffered saline (PBS) and a 4% paraformaldehyde in 0.1 M phosphate buffer solution. Coronal brain sections were made using a cryostat (Leica) after cryoprotection with a 30% sucrose solution and snap freezing in ice-cold isopentane. After two washing steps (2 × 5 min, dH2O), sections were kept in 0.5% and 1% H2O2 (dissolved in dH2O) for 30 min and 60 min, respectively. Next, sections were permeabilized and blocked in 0.2% Triton X-100/0.4% fish skin gelatin (= blocking buffer, dissolved in PBS) for 1 h followed by the primary antibody solution (rat anti-HA tag, Roche 11,867,423,001, 1:1000) in which they were kept overnight (4 °C). Next, sections were washed twice in blocking buffer for 10 min and transferred to secondary antibody solution for 1 h (donkey anti-rat Ig-G Alexa Fluor 488, Thermo Fisher A-21208, 1:1000). After two PBS wash steps (2 × 5 min), nuclei were stained with DAPI (4′,6‐diamidino‐2‐fenylindool, 1 µg/ml, Sigma‐Aldrich, 1 min) and sections were mounted on glass slides. Slides were scanned with a fluorescent microscope (Panoramic 250, 3D Histech, 40 × magnification) and automatically stitched. Exposure times were set using the negative control (slice which was not incubated with primary antibody solution but with only blocking buffer during that staining step), and this exposure time remained constant during the entire scanning process.
Results
Treatment with DREADD Agonists Clozapine and JHU37160
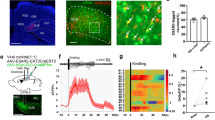
During the baseline period, mice had on average 21 ± 1 seizures of 42 ± 5-s duration per h (example of an EEG trace and seizure in Fig. 1). A strong and robust seizure suppression was observed after injection of JHU and clozapine in DREADD-expressing mice (Fig. 2). During the first hours after injection, the number of seizures was almost completely suppressed, and seizures that did occur were shorter in duration. Thereafter, both gradually increased again. In case of clozapine treatment, the number of seizures per hour was no longer different from baseline up to 40 h after injection (with exception of a 2-h period between 34 and 36 h after injection). Effects on the average length of seizures lasted up to 28 h after injection. For JHU, the decrease in the number of seizures was no longer significant 26 h after injection, and effects on seizure length lasted 18 h. For clozapine and JHU, a significant rebound effect was present with an increased number of seizures compared to baseline between 48 and 52 h and between 42 and 50 h post injection, respectively. For JHU, seizures also were significantly longer during the 40–44-h period after injection. In control animals, the number of seizures and seizure lengths seem to be unchanged after both JHU treatment (animals had 26 and 15 seizures per h of 45 s and 78 s during the 4-h baseline recordings which remained comparable during the first 4 h after the JHU treatment when animals had 22 and 13 seizures per h of 56 s and 83 s in control animals 1 and 2, respectively) and clozapine treatment (24 and 15 seizures per h of 52 s and 78 s during baseline, 27 and 17 seizures per h of 48 s and 75 s after clozapine treatment for control animals 1 and 2, respectively).
Representative example of an EEG trace. A Animals display frequent seizures during baseline (10 min are displayed), and those are suppressed after chemogenetic treatment (10-min EEG recorded during the first hour after treatment is shown). B Zoom on an epileptic seizure recorded during baseline (70 s). C Additional zoom (5 s) on the EEG during a seizure. The red bar indicates injection of 0.1 mg/kg clozapine; H1 and H2 are the 2 hippocampal recording channels, and S indicates the scalp EEG
Treatment with DREADD agonists clozapine and JHU (both 0.1 mg/kg, i.p.) suppressed spontaneous seizures in DREADD-expressing mice. A Clozapine significantly suppressed the number of seizures up to 34 h post injection and B JHU up to 26 h post injection. C Seizures were shorter in duration for 28 h after clozapine and D 18 h after JHU treatment, respectively. During the washout period, a rebound increase in the number of seizures is seen for both treatments (A, B) and in seizure length for JHU treatment (D). None of the compounds changed the number of seizures nor their duration in the two control animals (represented individually in blue lines). *p < 0.05. The arrow indicates the time of administration of clozapine or JHU
Chemogenetic Treatment Compared to the AED Levetiracetam
Seizure suppression was more effective in case of clozapine-mediated chemogenetic treatment than with the commonly used AED levetiracetam (Fig. 3). Administration of a high dose of LEV (800 mg/kg) decreased the number of seizures during the first 30 min and between 1.5 and 2 h after treatment (baseline 11 ± 1 seizures/h of 40 ± 4 s; 0–0.5 h after treatment 5 ± 1 seizures/h of 42 ± 4 s; 1.5–2 h after treatment: 7 ± 1 seizures/h of 37 ± 4 s; reduction in seizure frequency of 55 ± 9% and 36 ± 9%). Three out of twelve animals could be considered as responders to levetiracetam, showing a decrease of at least 75% in seizure frequency during at least one of the four 30-min periods following treatment. Levetiracetam did not show an effect on the length of seizures. Clozapine treatment completely suppressed seizure activity during the first 30 min after treatment in all DREADD mice. During the first 8 h after treatment, the number of seizures remained almost completely suppressed (baseline 10 ± 2 seizures/h; first 8 h after treatment: 1 ± 1 seizures/h) and each 30-min interval of the 8-h post-treatment period was significantly different from baseline. All animals (8/8) were considered responders to chemogenetic treatment. Moreover, seizures that did occur during this period were different from baseline during multiple 30-min intervals (0.5–2 h, 2.5–3 h, and 5–7.5 h after injection). Mixed model analysis revealed a significant interaction between time and treatment (p < 0.001) as well as effects of time (p < 0.001), treatment (p < 0.001) for the number of seizures per hour, and significant differences of treatment (p < 0.001) and time (p < 0.05) for seizure length.
Comparison between the effects of levetiracetam (LEV, in all IHKA mice) and chemogenetic suppression (clozapine in DREADD-expressing IHKA mice). The number of seizures per hour (A) and the seizure length (B) are plotted in 30-min bins for LEV (800 mg/kg) and clozapine (0.1 mg/kg) during 8 h before and 8 h after treatments. Levetiracetam treatment only affected the number of seizures during the first 30 min after treatment and between 1.5 and 2 h post injection (A) and did not affect the length of seizures (B). Chemogenetic suppression with clozapine suppressed the number of seizures during all 30-min bins after treatment (A) as well as seizure length during multiple post-treatment bins (B). Asterisk indicates significant differences between post-treatment intervals and baseline (*p < 0.05, ***p < 0.001). The arrow indicates the administration of clozapine or LEV. No seizures were present during the first 30 min after clozapine treatment; therefore, no statistics could be performed on the seizure length during this bin (B)
DREADD Expression
DREADD expression was visualized by fluorescence immunostaining against the HA tag fused to the receptors. In the ipsilateral hippocampus, the typical histopathological changes due to IHKA injection can be observed [21, 22]. These changes are associated with epileptogenesis (development of spontaneous seizures after IHKA injection) and include loss of CA1 and CA3 pyramidal neurons, loss of interneurons, hippocampal shrinkage (smaller hippocampal volume compared to the contralateral hippocampus due to cell death), and dispersion of the dentate gyrus granule cell layer (making it thicker and less densely organized). DREADD expression is mainly observed in the ipsilateral (injected) hippocampus (fibers and cell bodies, Fig. 4A, B) but also in the contralateral hippocampus (fibers, Fig. 4A, C). Some expression is observed in the retrosplenial cortex (fibers, Fig. 4D), in reticular and midline thalamic nuclei (fibers), and alongside the injection tract (cortex, fibers, and cell bodies). Slices showing expression throughout the AP axis of the hippocampus can be found in Supplementary Fig. S2.
DREADD expression. Alterations typical for the intrahippocampal kainic acid mouse model can be observed in the ipsilateral hippocampus: loss of pyramidal neurons in CA1–CA3 regions (left and right CA1 pyramidal cell layers are indicated with a black arrow in A), hippocampal shrinkage (sclerosis), and dentate granule cell dispersion (reorganization and enlargement of the granule cell layer, thickness of left and right granule cell layers are indicated with a black line in A). The HA tag which is coupled to the hM4Di DREADD receptor is mainly localized in the sclerotic hippocampus (fibers and cell bodies, A, B) but also in the contralateral hippocampus (fibers, A and C). Some expression is observed in strongly connected regions such as retrosplenial cortex (fibers, D), reticular and midline thalamic nuclei (fibers), and alongside the injection tract (cortex, fibers, and cell bodies). Some cell bodies are indicated with an arrow. Scale bar = 1 mm
Discussion
Chemogenetic treatment was very effective in suppressing seizures in DREADD-expressing IHKA mice. This was reached using two different DREADD agonists, namely clozapine and JHU37160. In contrast, treatment with the commonly used AED levetiracetam induced only minor seizure-suppressive effects.
Initially, DREADD receptors were designed to be sensitive to the inert molecule CNO. However, it became clear that CNO poorly crosses the blood–brain barrier and that CNO is metabolized to clozapine which passes the blood–brain barrier and activates the DREADD receptors. Therefore, clozapine itself can be used to activate DREADD receptors [7, 23].
However, the use of clozapine in humans is limited by the risk of causing severe neutropenia, a potentially lethal side effect, even when used in the very low doses typical for DREADD activation [24]. Therefore, in view of a possible clinical translation of chemogenetics, several novel DREADD agonists such as compound 21 [25], perlapine [26], deschloroclozapine [27], olanzapine [28], JHU37152 [13], and JHU37160 [13] are currently under investigation. Here, JHU37160 was successfully used as a DREADD ligand in chemogenetic seizure suppression. Administration of the same dose of JHU37160 and clozapine resulted similarly in seizure suppression in DREADD-expressing IHKA mice. This study is the first to use JHU37160 for chemogenetic modulation of the hippocampus and the first time it has been used in chemogenetic seizure suppression. Only few studies have used JHU37160 for chemogenetic modulation of other brain regions targeting the dorsal striatum [13], the ventromedial hypothalamus [29], nucleus accumbens addiction pathways [30, 31], or the orbitofrontal cortex [32]. Our continuous readout of the effect of chemogenetic inhibition is a major advantage to evaluate the timescale of chemogenetic effects, allowing optimization of experimental designs when, similarly as in the two former mentioned studies, only one post-treatment evaluation (such as a behavioral test) is possible. Both compounds have similar affinities for the hM4Di receptor (clozapine-hM4Di Ki = 3.5 ± 0.7 nM [13, 23], JHU37160-hM4Di Ki = 3.6 nM [13]). As JHU37160 has high in vivo potency and potentially fewer off-target effects, this drug might open the path towards clinical translation of chemogenetics [13]. In controls, neither clozapine nor JHU37160 did affect seizure activity. A limitation of our study is that only two control animals were included. However, results were consistent and in line with our previous study [7]. Most importantly to date, no off-target effects of JHU37160 have been reported [13, 29, 30, 32, 33] and JHU37160 did not modulate locomotion or brain metabolic activity at 0.1 mg/kg used in this study [13]. However, in the future, a thorough behavioral analysis during chronic chemogenetic treatment will be necessary to fully evaluate the side effect profile of this novel compound [13]. In this study, only male animals were used as the oestrous cycle has a large impact on the seizure rate of IHKA mice; however, in future experiments, also female subjects will need to be added to take the next steps in the translation of chemogenetics [34].
Next, we compared the effects of chemogenetic treatment with those of the commonly used AED levetiracetam, to evaluate the clinical potential of this novel treatment strategy. A high dose of levetiracetam (800 mg/kg) only slightly reduced the time in seizures during the first 1.5 h after treatment (maximal reduction of 48 ± 11%). These effects are inferior to the results of previous studies evaluating LEV-mediated seizure suppression in IHKA mice, an accepted model for difficult-to-treat partial seizures [17, 18, 35]. Duveau et al. [17] reported a reduction of 95% of seizure activity between 10 and 30 min after 800 mg/kg levetiracetam injection. Klein et al. [18] pointed out that, similar as in humans, there could be striking inter-individual differences between animals in the reaction to AED treatments. Therefore, on an individual basis, animals were considered responders to therapy when achieving a decrease of at least 75% in seizure frequency during at least one of the four 30-min periods following treatment. In the latter study, four out of nine animals were considered responders after 800 mg/kg levetiracetam treatment [18]. In our series, we identified only three mice out of twelve as responders according to this definition. Inducing expression of the DREADD receptors probably does not influence levetiracetam effects, as both DREADD and control animals reacted poorly to levetiracetam treatment. Although unlikely, however, effects of levetiracetam might differ due to viral vector injection in the hippocampus. Levetiracetam is an efficacious and commonly used third-generation AED, but similar to other AEDs, some patients continue to have seizures despite optimal treatment [1, 16]. Here, we show that using chemogenetics was able to suppress levetiracetam-resistant seizures. Levetiracetam is presumed to mainly act on the SV2A protein which is located on synaptic vesicles and functions as a modulator of neurotransmitter release and is known to have a unique profile in preclinical animal models [36,37,38]. Chemogenetic treatment works through a different mechanism, as activation of the Gi signaling cascade induces a potassium-mediated hyperpolarization (through GIRK channel opening) and a direct and indirect reduction of neurotransmitter release [5, 39, 40]. None of the currently available AEDs has a similar mode of action. Only retigabine modulates voltage-gated potassium channels; however, this compound shows marked adverse effects in epileptic mice [18, 41] and humans [42] because it does not only modulate potassium channels in the epileptogenic region in the brain but also at other sites in the body (e.g., heart or smooth muscles of the bladder) and non-epileptic sites in the brain [42]. Chemogenetic therapy only modulates neurons expressing the designer receptors which provides the unique opportunity to use a Gi-mediated inhibition of neuronal activity with minimal induction of systemic side effects. Compared to our previous study, seizure suppression lasted more than twice as long after clozapine treatment (34 h vs 16 h) [7]. This is probably due to study protocol adjustments. First, the mCherry tag coupled to the hM4Di receptor was replaced by a human influenza HA tag, since a C-terminal–linked mCherry tag has been reported to interfere with intracellular trafficking of the receptor (resulting in poor plasma membrane expression) [43]. Secondly, we injected 100 times lower viral vector titer (10^11 genome copies/ml) after demonstrating toxic effects of the high viral titer [7, 15]. Thirdly, we waited longer after the KA lesion to inject viral vector (10 instead of 2 weeks) in order to validate the effectiveness of chemogenetic treatment in a more chronic disease stage [7]. Different effects of these adjustments could have resulted in the prolonged seizure suppression. Firstly, the potentially improved membrane localization of DREADD receptors on the cell membrane could lead to a larger number of receptors to be activated, resulting in stronger activation of the Gi signaling cascade [43]. Secondly, at 10 weeks post SE, there is less KA-induced inflammation which could neutralize AAV particles. This potentially leads to a larger number of affected neurons [44]. Third, the mean age during which animals were treated with clozapine is higher (between 21 and 23 weeks) compared to the previous study (13–16 weeks old). This could influence clozapine pharmacokinetics, leading to a longer-lasting effect of treatment in older subjects. In schizophrenic patients, the same dose of clozapine indeed results in higher plasma levels in older patients [45]. However, effects of chemogenetic suppression are known to last longer than merely the presence of the agonist (the half-life of clozapine in the blood is only about 2 h in mice) possibly due to the long-term modifications in second messenger systems [46,47,48,49]. The CamKIIα0.4 promoter was used to target expression in excitatory neurons with high specificity (90–95% of transduced neurons were non-GABAergic) [19, 20]. The silenced neurons are most likely excitatory dentate granule cells, as many pyramidal cells and mossy cells are lost during epileptogenesis [21, 22]. However, we cannot exclude that a small amount of GABAergic neurons was silenced as well [19, 20]. Similar to our previous study, DREADD expression was observed also in regions strongly connected to the sclerotic hippocampus (in afferent and efferent excitatory neurons), leading to inhibition of a larger part of the seizure network, potentially contributing to the seizure-suppressive effects [7, 50].
After seizure suppression, a rebound effect with higher seizure frequency compared to baseline was observed. This could be due to homeostatic changes at the cellular and/or network level, compensating for the inhibitory effects of chemogenetic therapy. This phenomenon is observed with many anti-seizure therapies, and a gradual discontinuation of therapy might avoid those effects [38].
To conclude, chemogenetic treatment very effectively suppressed seizures in DREADD-expressing IHKA mice. Both clozapine and the novel DREADD agonist JHU37160 could be used for chemogenetic seizure suppression, and neither compound induced effects in control animals. By contrast, treatment with a commonly used AED, levetiracetam, only yielded a very modest effect on seizures. Thus, chemogenetic treatment outperforms standard treatment with levetiracetam, confirming its potential to suppress drug-resistant seizures.
References
Beghi E, Giussani G, Abd-Allah F, et al. Global, regional, and national burden of epilepsy, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol 2019;18:357–375.
Walker MC, Kullmann DM. Optogenetic and chemogenetic therapies for epilepsy. Neuropharmacology. Elsevier Ltd; 2020. p. 107751.
Forcelli PA. Applications of optogenetic and chemogenetic methods to seizure circuits: Where to go next? J Neurosci Res 2017;95:2345–2356.
Urban DJ, Roth BL. DREADDs (Designer Receptors Exclusively Activated by Designer Drugs): Chemogenetic Tools with Therapeutic Utility. Annu Rev Pharmacol Toxicol 2015;55:399–417.
Roth BL. DREADDs for Neuroscientists. Neuron 2016;89:683–694.
Armbruster BN, Li X, Pausch MH, et al. Evolving the lock to fit the key to create a family of G protein-coupled receptors potently activated by an inert ligand. Proc Natl Acad Sci 2007;104:5163–5168.
Desloovere J, Boon P, Larsen LE, et al. Long-term chemogenetic suppression of spontaneous seizures in a mouse model for temporal lobe epilepsy. Epilepsia 2019;60:2314–2324.
Wang Y, Liang J, Chen L, et al. Pharmaco-genetic therapeutics targeting parvalbumin neurons attenuate temporal lobe epilepsy. Neurobiol Dis 2018;117:149–160.
Kätzel D, Nicholson E, Schorge S, et al. Chemical–genetic attenuation of focal neocortical seizures. Nat Commun 2014;5:3847–3855.
Avaliani N, Andersson M, Runegaard AH, et al. DREADDs suppress seizure-like activity in a mouse model of pharmacoresistant epileptic brain tissue. Gene Ther 2016;23:760–766.
Zhou QG, Nemes AD, Lee D, et al. Chemogenetic silencing of hippocampal neurons suppresses epileptic neural circuits. J Clin Invest 2019;129:310–323.
Berglind F, Andersson M, Kokaia M. Dynamic interaction of local and transhemispheric networks is necessary for progressive intensification of hippocampal seizures. Sci Rep 2018;8:5669–5683.
Bonaventura J, Eldridge MAG, Hu F, et al. High-potency ligands for DREADD imaging and activation in rodents and monkeys. Nat Commun 2019;10:4627.
Goutaudier R, Coizet V, Carcenac C, et al. Dreadds: The power of the lock, the weakness of the key. favoring the pursuit of specific conditions rather than specific ligands. eNeuro 2019;6:1–5.
Goossens MG, Larsen LE, Vergaelen M, et al. Level of hM4D(Gi) DREADD Expression Determines Inhibitory and Neurotoxic Effects in the Hippocampus. eNeuro. 2021;8:105–121
Glauser T, Ben-Menachem E, Bourgeois B, et al. Updated ILAE evidence review of antiepileptic drug efficacy and effectiveness as initial monotherapy for epileptic seizures and syndromes. Epilepsia 2013;54:551–563.
Duveau V, Pouyatos B, Bressand K, et al. Differential Effects of Antiepileptic Drugs on Focal Seizures in the Intrahippocampal Kainate Mouse Model of Mesial Temporal Lobe Epilepsy. CNS Neurosci Ther 2016;22:497–506.
Klein S, Bankstahl M, Löscher W. Inter-individual variation in the effect of antiepileptic drugs in the intrahippocampal kainate model of mesial temporal lobe epilepsy in mice. Neuropharmacology 2015;90:53–62.
Gerits A, Vancraeyenest P, Vreysen S, et al. Serotype-dependent transduction efficiencies of recombinant adeno-associated viral vectors in monkey neocortex. Neurophotonics 2015;2:031209.
Scheyltjens I, Laramée M-E, Van den Haute C, et al. Evaluation of the expression pattern of rAAV2/1, 2/5, 2/7, 2/8, and 2/9 serotypes with different promoters in the mouse visual cortex. J Comp Neurol 2015;523:2019–2042.
Venceslas D, Corinne R. A Mesiotemporal Lobe Epilepsy Mouse Model. Neurochem Res 2017;42:1919–1925.
Bouilleret V, Ridoux V, Depaulis A, et al. Recurrent seizures and hippocampal sclerosis following intrahippocampal kainate injection in adult mice: Electroencephalography, histopathology and synaptic reorganization similar to mesial temporal lobe epilepsy. Neuroscience 1999;89:717–729.
Gomez JL, Bonaventura J, Lesniak W, et al. Chemogenetics revealed: DREADD occupancy and activation via converted clozapine. Science 2017;357:503–507.
Alvir JMJ, Lieberman JA, Safferman AZ, et al. Clozapine-Induced Agranulocytosis - Incidence and Risk Factors in the United States. N Engl J Med 1993;329:162–167.
Thompson KJ, Khajehali E, Bradley SJ, et al. Dreadd Agonist 21 (C21) Is an Effective Agonist for Muscarnic-Based Dreadds in Vitro and in Vivo. ACS Pharmacol Transl Sci 2018;21:61–72.
Chen X, Choo H, Huang X-P, et al. The First Structure–Activity Relationship Studies for Designer Receptors Exclusively Activated by Designer Drugs. Acs Chem Neurosci 2015;6:476–484.
Nagai Y, Miyakawa N, Takuwa H, et al. Deschloroclozapine, a potent and selective chemogenetic actuator enables rapid neuronal and behavioral modulations in mice and monkeys. Nat Neurosci 2020;23:1157–1167.
Weston M, Kaserer T, Wu A, et al. Olanzapine: A potent agonist at the hM4D(Gi) DREADD amenable to clinical translation of chemogenetics. Sci Adv 2019;5.
Zhang J, Chen D, Sweeney P, et al. An excitatory ventromedial hypothalamus to paraventricular thalamus circuit that suppresses food intake. Nat Commun 2020 111 2020;11:1–14.
Giannotti G, Gong S, Fayette N, et al. Extinction blunts paraventricular thalamic contributions to heroin relapse. Cell Rep 2021;36:109605.
Heinsbroek JA, Giannotti G, Mandel MR, et al. A common limiter circuit for opioid choice and relapse identified in a rodent addiction model. Nat Commun 2021;12:4788.
Costa KM, Sengupta A, Schoenbaum G. The orbitofrontal cortex is necessary for learning to ignore. Curr Biol 2021;31:2652–2657.
Fleury Curado T, Pho H, Freire C, et al. Designer Receptors Exclusively Activated by Designer Drugs Approach to Treatment of Sleep-disordered Breathing. Am J Respir Crit Care Med 2021;203:102–110.
Li J, Leverton LK, Naganatanahalli LM, et al. Seizure burden fluctuates with the female reproductive cycle in a mouse model of chronic temporal lobe epilepsy. Exp Neurol 2020;334:113492.
Bankstahl M, Klein S, Römermann K, et al. Knockout of P-glycoprotein does not alter antiepileptic drug efficacy in the intrahippocampal kainate model of mesial temporal lobe epilepsy in mice. Neuropharmacology 2016;109:183–195.
Lynch BA, Lambeng N, Nocka K, et al. The synaptic vesicle is the protein SV2A is the binding site for the antiepileptic drug levetiracetam. Proc Natl Acad Sci U S A 2004;101:9861–9866.
Deshpande LS, DeLorenzo RJ. Mechanisms of levetiracetam in the control of status epilepticus and epilepsy. Front Neurol 2014;5.
Klitgaard H, Matagne A, Gobert J, et al. Evidence for a unique profile of levetiracetam in rodent models of seizures and epilepsy. Eur J Pharmacol 1998;353:191–206.
Wiegert JS, Mahn M, Prigge M, et al. Silencing Neurons: tools, applications, and experimental constraints. Neuron 2017;95:504–529.
Stachniak TJ, Ghosh A, Sternson SM. Chemogenetic synaptic silencing of neural circuits localizes a hypothalamus → midbrain pathway for feeding behavior. Neuron 2014;82:797–808.
Bankstahl M, Bankstahl JP, Löscher W. Pilocarpine-induced epilepsy in mice alters seizure thresholds and the efficacy of antiepileptic drugs in the 6-Hertz psychomotor seizure model. Epilepsy Res 2013;107:205–216.
Clark S, Antell A, Kaufman K. New antiepileptic medication linked to blue discoloration of the skin and eyes. Ther Adv Drug Saf 2015;6:15–19.
Galvan A, Raper J, Hu X, et al. Ultrastructural localization of DREADDs in monkeys. Eur J Neurosci 2019;50:2801–2813.
Zattoni M, Mura ML, Deprez F, et al. Brain infiltration of leukocytes contributes to the pathophysiology of temporal lobe epilepsy. J Neurosci 2011;31:4037–4050.
Thorn CF, Müller DJ, Altman RB, et al. PharmGKB summary: Clozapine pathway, pharmacokinetics. Pharmacogenet Genomics 2018;28:214–222.
Aitchison KJ, Jann MW, Zhao JH, et al. Clozapine pharmacokinetics and pharmacodynamics studied with CYP1A2-null mice. J Psychopharmacol 2000;14:353–359.
Alexander GM, Rogan SC, Abbas AI, et al. Remote control of neuronal activity in transgenic mice expressing evolved G protein-coupled receptors. Neuron 2009;63:27–39.
Guettier J-M, Gautam D, Scarselli M, et al. A chemical-genetic approach to study G protein regulation of β cell function in vivo. Proc Natl Acad Sci 2009;106:19197–19202.
Boender AJ, de Jong JW, Boekhoudt L, et al. Combined Use of the Canine Adenovirus-2 and DREADD-Technology to Activate Specific Neural Pathways In Vivo. PLoS One 2014;9:e95392.
Taymans J-M, Vandenberghe LH, Haute C Van Den, et al. Comparative analysis of adeno-associated viral vector serotypes 1, 2, 5, 7, And 8 in mouse brain. Hum Gene Ther 2007;18:195–206.
Acknowledgements
We would like to thank the viral vector core Leuven that produced the viral vectors.
Required Author Forms
Disclosure forms provided by the authors are available with the online version of this article.
Funding
This work was supported by the Research foundation – Flanders FWO (JD and MGG are PhD fellows of FWO, grant numbers 1S74519N and 1S30017N).
Author information
Authors and Affiliations
Contributions
Conceptualization and methodology: JD, LEL, JD, EC, KV, WW, PB, and RR; Investigation: JD; Formal analysis: JD; Funding acquisition: JD, PB, and RR; Software: WW; Writing of the original draft: JD; Writing which included review and editing: LEL, MGG, JD, EC, KV, and RR.
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
13311_2021_1160_MOESM10_ESM.tif
Supplementary file10 (TIF 2849 KB). Fig. S1 Flowchart of experiments. Overview of different treatments and groups in this study and the number of animals in each group. Dropouts are depicted in grey. One animal died 1 day after IHKA injection, one animal died during implantation surgery and one animal died during washout between clozapine and JHU injection. One animal was excluded from the study because it did not display clear seizures on EEG. Two non-DREADD animals (indicated in orange) received one levetiracetam treatment and were then used in another experiment. IHKA = intrahippocampal kainic acid, AAV = Adeno-associated viral vector, LEV = levetiracetam, Cloz = clozapine, JHU = JHU37160
13311_2021_1160_MOESM11_ESM.tif
Supplementary file11 (TIF 285967 KB). Fig. S2 Overview of DREADD expression on slices throughout the AP axis of the hippocampus. The images on top always display DAPI nuclear stain; middle figures show DREADD expression (by visualizing the coupled HA tag), the lower panel displays the merged image. Figures A-G show slices selected from anterior to posterior throughout the brain axis. Icon created with BioRender.com
Rights and permissions
About this article
Cite this article
Desloovere, J., Boon, P., Larsen, L.E. et al. Chemogenetic Seizure Control with Clozapine and the Novel Ligand JHU37160 Outperforms the Effects of Levetiracetam in the Intrahippocampal Kainic Acid Mouse Model. Neurotherapeutics 19, 342–351 (2022). https://doi.org/10.1007/s13311-021-01160-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13311-021-01160-0