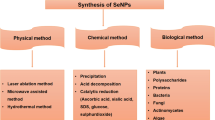
Abstract
Evidence shows that nanoparticles exert lower toxicity, improved targeting, and enhanced bioactivity, and provide versatile means to control the release profile of the encapsulated moiety. Among different NPs, inorganic nanoparticles (Ag, Au, Ce, Fe, Se, Te, Zn, etc.) possess a considerable place owing to their unique bioactivities in nanoforms. Selenium, an essential trace element, played a vital role in the growth and development of living organisms. It has attracted great interest as a therapeutic factor without significant adverse effects in medicine at recommended dose. Selenium nanoparticles can be fabricated by physical, biological, and chemical approaches. The biosynthesis of nanoparticles is shown an advance compared to other procedures, because it is environmentally friendly, relatively reproducible, easily accessible, biodegradable, and often results in more stable materials. The effect of size, shape, and synthesis methods on their applications in biological systems investigated by several studies. This review focused on the procedures for the synthesis of selenium nanoparticles, in particular the biogenesis of selenium nanoparticles and their biomedical characteristics, such as antibacterial, antiviral, antifungal, and antiparasitic properties. Eventually, a comprehensive future perspective of selenium nanoparticles was also presented.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Selenium (Se) was first introduced in 1817 by the Swedish chemist Jons Jacob Berzelius (Skalickova et al. 2017). As a semi-metal element, this chalcogen represented both properties of non-metal and metal element (Burk 1994, Mehdi et al. 2013). Six isotopes of this chalcogen exist together in nature. The mass numbers of its isotopes are almost 74, 76, 77, 78, 80, and 82 (Mehdi et al. 2013). Se resembles sulfur in atomic size, ionization potentials, main oxidation states, and bond energies (Mehdi et al. 2013). This element is stable and does not oxidize at average temperatures. When Se burns, it creates a blue flame, selenium dioxide, and unsightly smell. Se can combine with several elements, such as fluorine, hydrogen, phosphorus, chlorine, bromine, etc. (Burk 1994). Se is present in both form of organic and inorganic. The main organic form is selenocysteine, selenomethionine, while the most abundant inorganic forms are selenite (SeO32−), selenate (SeO42−), selenide (Se2−), and elemental Se (Mehdi et al. 2013). Selenite is the most toxic form of Se (Wadhwani et al. 2016).
Se is a trace element which is essential for human, meaning that they cannot produce it, and they need to obtain it from their diet (Wadhwani et al. 2016; Skalickova et al. 2017). The biological functions of Se caused by the incidence of selenocysteine amino acid in proteins. Proteins that incorporate selenocysteine in their polypeptide chain are named selenoproteins and they are involved in all progenitors of life (eukaryote, archaea, and bacteria). About 100 selenoproteins have been found in mammalian, the most crucial of which are the antioxidant enzymes of glutathione peroxidase, iodothyronine deiodinases, selenoprotein H, selenoprotein K, thioredoxin-glutathione reductase, methionine sulfoxide reductase B1, selenophosphate synthetase, and thioredoxin reductase (Kielczykowska et al. 2018) where Se supplied as their cofactor (Wadhwani et al. 2016). It is feasible for Se to use for the prevention of different diseases containing cystic fibrosis, muscular dystrophy, cardiovascular disease (Weekley and Harris 2013; Skalickova et al. 2017), Alzheimer, leishmaniasis, and cancer (Chaudhary et al. 2014).
Nanotechnology is a cutting-edge field interdisciplinary related to chemistry, fundamental physics, biology, medicine, and material science (Narayanan and Sakthivel 2010). The initial concept of nanotechnology is production of materials of different types at nanoscale level that it presented by Richard Feynman in 1959. Nanoparticles are a broad class of materials that have one dimension less than 100 nm at least (Khan et al. 2019). Nanoparticles usually were synthesized by two approaches including bottom–up and top–down (Wang and ** nanoselenium, produced by green synthesis. IET Nanobiotechnol 11(4):426–432" href="/article/10.1007/s13205-023-03476-4#ref-CR14" id="ref-link-section-d136543381e797">2017) which are shown in Table 1 are among other bacterial strains able to synthesize Se NPs. Pseudomonas alcaliphila and Klebsiella pneumoniae also utilized to synthesize Se NPs by a suitable yield (Husen and Siddiqi 2014). Gerrard et al. (1974) observed Se deposits on the cell wall and cell membrane of Escherichia coli by electron microscopy. They have indicated that the bacteria can reduce sodium selenite to elemental Se.
Synthesis by fungi
Five fungal strains have reported as bioresources for the synthesis of Se NPs, including Aspergillus terreus (Zare et al. 2013), Alternaria alternate (Sarkar et al. 2011), Lentinula edodes (Vetchinkina et al. 2013), Fusarium sp., and Trichoderma reesei (Wadhwani et al. 2016) which are summarized in Table 1. Fungi have many benefits for NP synthesis compared to other microbes and plants. The tolerance of fungal mycelial mesh toward flow pressure, agitation, and other conditions in bioreactors or other chambers compared with plant materials and bacteria is one of these benefits (Narayanan and Sakthivel 2010; Alghuthaymi et al. 2015). In addition, most fungi represent a high resistance toward metals as well as intracellular metal uptake capabilities (Alghuthaymi et al. 2015). They are fastidious grow, easy to handle (Narayanan and Sakthivel 2010), a high wall-binding capability (Alghuthaymi et al. 2015), and easy for fabrication (Narayanan and Sakthivel 2010). Zhang et al. (2019) announced the resistance of fungus Mariannaea sp. HJ to selenate ions during growth phase which might ascribe to the capacity of Se NPs synthesis. Liang et al. (2019) investigated the synthesis of Se NPs by several different fungal genera (Aureobasidium pullulans, Trichoderma harzianum, Mortierella humilis, and Phoma glomerata) through growth on selenium-containing media (1 mM). Filamentous fungi are capable of extracellular and intracellular synthesis of Se NPs, and they have some advantages over bacteria and other unicellular organisms due to easier bioprocessing and biomass handling (Liang et al. 2019).
Plant-assisted synthesis of Se NPs
Plant-assisted fabrication of Se NPs mostly performed by reduction of selenate to selenite in the presence of plant extracts containing alcohols, phenols, proteins, flavonoids amines, and aldehydes. Low dosages of Se can stimulate the growth of the plants, whereas high dosages of it can cause damage to plants. In addition, biogenic Se NPs demonstrated less toxicity as compared to NPs synthesized via chemical methods (Husen and Siddiqi 2014). As for green synthesis, Se NPs synthesized by plant extracts, such as extract of Vitis vinifera (Sharma et al. 2014), Allium sativum (Ezhuthupurakkal et al. 2017), Terminalia arjuna leaf (Prasad and Selvaraj 2014), Clausena dentata plant leaf (Sowndarya et al. 2017), Undaria pinnatifida polysaccharide (Chen et al. 2008a, b), and lemon leaf (Prasad et al. 2013) (Table 1). The synthesis of NPs by plant extracts has more benefits than other biological procedures, because it is cheap and does not need any special conditions (Ramamurthy et al. 2013). Biofabrication of Se NPs by plant extracts led to the formation of Se NPs with different size. For example, application of Vitis vinifera plant led to the formation of Se NPs with the average size of 3–18 nm, while lemon leaf extract fabricated Se NPs with the size range of 60–80 nm. Phenolic groups present in lignin plants usually serves as reducing agent and oxidation of phenol to ketone has occurred during the redox process. Nevertheless, the plant extract additionally comprises fairly substantial amount of reducing sugars. Hence, they also act as reducing agents and formation of Se nanoballs (Husen and Siddiqi 2014).
Synthesis by compounds of animal origin
Propolis or bee glue is a natural substance collected using honey bees from various plant flowers, and due to its biological features, such as antibacterial, antiviral, and antifungal potentials, antitumor, anti-inflammatory, and antiulcer, it obtained a significant attention from investigators (Shubharani et al. 2019). Various reports showed that propolis is composed of different bioactive compounds (such as flavanones, flavones, alcohols, aldehydes, esters, aromatic acids, etc.) that can synthesize NPS by metal ion reduction to their elements (Kumar et al. 2014; Hatami et al. 2020). Shubharani et al. (2019) investigated the synthesis of Se NPs by hydroalcoholic extract of bee propolis (with size ranged of 52.9–118 nm). They revealed the antioxidant, antibacterial, and antifungal activity of Se NPs biosynthesized with ethanol extract of bee propolis (Shubharani et al. 2019). Se NPs biosynthesized by bee propolis hydroalcoholic extract with the size range of 50–60 nm and six different heating methods, namely, microwave, conventional heating (mild heating by heater and stirrer), ultrasonication, self-assembling, hydrothermal, and UV irradiation (Hatami et al. 2020). Hatami et al. (2020) demonstrated that bee propolis extract, due to the presence of natural reducing and stabilizing agents, had potential application in the biosynthesis of Se NPs. Wali (2019) biosynthesized Se NPs by propolis aqueous extract, and they investigated their biological activity, such as body weight, blood Se-binding protein amount, food intake, catalase, and liver enzymes activities. The body weight and food intake in the Se NPs-treated group significantly increased than the control and selenite-treated groups (p ≤ 0.05) during 2 weeks of the tests (Wali 2019). The rat group treated with Se NPs exhibited a significant enhancement in Se-binding proteins and catalase associated with normal values of liver enzymes. Therefore, the novel propolis-mediated Se NPs enhanced the availability of the Se to its binding proteins and decrease its toxicity than inorganic selenite (Wali 2019).
Biological applications of Se NPs
Se NPs have wide application in biomedical fields. Se NPs have found biological applications as antibacterial, antifungal, antioxidant, and anticancer agents, etc. (Fig. 3) (Hariharan et al. 2012; Torres et al. 2012; Yang et al. 2012; Yazdi et al. 2012; Forootanfar et al. 2014; Wadhwani et al. 2016).
Antibacterial activity
Bacterial proliferation is a global concerning and rising problem which led to significant damage in various industries. However, a universal solution for limiting bacterial colonization is not discovered yet. Thus, the new approaches for controlling bacterial activity are required, and nanoparticles’ synthesis is a great alternative (Díez-Pascual 2018). Se NPs synthesized from a biological resource (by Saccharomyces cerevisiae, 30–100 nm size) had shown considerable antimicrobial activity against pathogenic microbes causing nosocomial infection (Hariharan et al. 2012). These pathogenic microbes include Escherichia coli, Staphylococcus aureus, Salmonella typhimurium, Bacillus subtilis, Pseudomonas aeruginosa, and Klebsiella pneumonia which were isolated from hospitals, in India (Hariharan et al. 2012). Se NPs had a high potential broad-spectrum antimicrobial activity (Yip et al. 2014; Stevanović et al. 2015; Wang et al. 2015, 2016; Chung et al. 2016; Huang et al. 2016; Wadhwani et al. 2016; Ionin et al. 2017; Tan et al. 2018). Se NPs can inhibit the growth of both Gram-positive and Gram-negative bacteria (Hariharan et al. 2012). The antimicrobial activity of Se NPs depends on the synthesis method. Piacenza et al. (2017) investigated the antimicrobial effect of two different chemically synthesized Se NP (L‐cysteine and ascorbic acid as the reducing agent, size 99.8 nm and 170.5 nm, respectively) compared to spherical biogenic Se nanostructures produced by Bacillus mycoides SelTE01 (size 160 nm) on Staphylococcus aureus and Pseudomonas aeruginosa biofilms grown onto hydroxyapatite-coated clinical surfaces and devices (Table 2). The antimicrobial activity of the chemically synthesized Se NPs was observed at concentration of 2.5 mg/mL, while the biogenic Se NPs were effective at concentration of 0.078 mg/mL.
Staphylococcus aureus causes 25% of all ventilator-associated pneumonia infections. Polyvinyl chloride coated with Se NPs produced by chemical method (size 80–200 nm) may effectively reduce bacterial adherence and proliferation on rat dermal fibroblasts in vitro (Ramos and Webster 2012). In addition, Se NPs synthesized through chemical (using the reduction of sodium selenite by glutathione, size 74.3 nm), and biological (by Enterococcus faecalis, size range of 29–195 nm) methods exhibited negative effects on the growth and the percentage of live bacteria (Tran and Webster 2011; Shoeibi and Mashreghi 2017; Verma 2017). In another study, the antimicrobial activity of biogenic Se NPs synthesized by the extract of ginger (size 100–150 nm) was evaluated against six bacterial species of Klebsiella sp., Pseudomonas sp., Serratia sp., Proteus sp., S. aureus, and Escherichia coli (Menon et al. 2019). Se NPs showed significant antibacterial activity against Proteus sp. (Menon et al. 2019). The MIC of Se NPs versus Proteus sp. was about 250 μg/mL (Menon et al. 2019). The Se NPs biosynthesized by Ralstonia eutropha (size 40–120 nm) demonstrated great antimicrobial activity (Srivastava and Mukhopadhyay 2015).The concentrations of 100 µg/ml, 100 µg/ml, 100 µg/ml, and 250 µg/ml of Se NPs inhibited (99%) the growth of Streptococcus pyogenes, Pseudomonas aeruginosa, Staphylococcus aureus, and Escherichia coli, respectively (Srivastava and Mukhopadhyay 2015). Se NPs synthesized by Enterococcus faecalis (size 29–195 nm) can employ as anti-staphylococcal to effectively prevent the Staphylococcus aureus infections (Shoeibi and Mashreghi 2017). The antibacterial activity of biogenic Se NPs synthesized by fresh guava leaves (with particle size of 30–50 nm) evaluated against Enterococcus faecalis in several groups [group I: distilled water (control), group II: Se NPs (1 mg/ml), group III: calcium hydroxide (1 mg/ml), group IV: 2% chlorhexidine gluconate, group V: 5.25% sodium hypochlorite] using disk diffusion method. The mean zone of inhibition was higher at different concentrations of Se NPs (10–40 μl of Se NPs with concentration of 1 mg/ml was 11.33, 16.50, 21.00, and, 28.50 mm, respectively) followed by sodium hypochlorite (14.67 mm), chlorhexidine gluconate (13.00 mm), and calcium hydroxide (6.83 mm). Guava leaves extract and sodium selenite (as precursor salt) did not show any zone of inhibition which proposes that the antimicrobial activity was only owing to the interaction of Se NPs with the bacterial cell, and not owing to any additional entities that were employed during the synthesis process (Miglani and Tani-Ishii 2021). The disintegration of the bacterial cell wall using the adhesion of NPs is the most common mechanism of antibacterial activity (Escobar-Ramírez et al. 2021). The conjugating quercetin and acetylcholine to the surface of Se NPs adhere to the cell wall, causing damage to the bacterial membrane cell and thereby reaching a significant synergistic effect to prohibit methicillin-resistant Staphylococcus aureus (Huang et al. 2016). Huang et al. (2016) reported that the synergistic effects of quercetin and acetylcholine increase the antibacterial properties of Se NPs. The result of various studies exhibited bacteria treated with Se NPs have a cellular contraction and take an irregular shape compared to a control group of untreated bacteria (Huang et al. 2016, 2019; Nguyen et al. 2017; Chandramohan et al. 2019; Escobar-Ramírez et al. 2021). Pseudomonas aeruginosa, Escherichia coli, Vibrio parahemolyticus, and Bacillus subtilis treated with bio-Se NPs demonstrated holes and pits on the surface, while in Staphylococcus aureus treated with bio-Se NPs, some membranes were found to be flattened, wrinkled, and surrounded by cytoplasm, that displayed the leakage of intracellular content (Zhang et al. 2021).
Antibiofilm activity
Bacterial biofilm is a large aggregation of bacterial cells that lives as communities in which these cells stick to each other and often also to a surface. Biofilm is considered as one of the main reasons for chronic infections (Jamal et al. 2018). The studies reported the efficacy of the NPs in decrease biofilm formation, eliminating biofilm, and kee** the antibacterial features even after aging (Díez-Pascual 2018). Biogenic Se NPs produced by Stenotrophomonas maltophilia SeITE02 (size 221.1 nm, 345.2 nm and 357.1 nm after 6, 24, and 48 h, respectively) have represented the ability to disrupt microbial biofilms (Zonaro et al. 2015). Biogenic Se NPs synthesized via Bacillus mycoides SelTE01 (size 160 nm) displayed the same effective antibiofilm activity against both S. aureus and P. aeruginosa strains at 0.313 mg/ml and 0.078 mg/ml, respectively (Piacenza et al. 2017). The antibiofilm activity of biogenic Se NPs (produced by Bacillus sp. MSh-1, size 80–220 nm) against common biofilm-forming clinically isolates of S. aureus, P. mirabilis, and P. aeruginosa was evaluated by Shakibaie et al. (2015a, b). The biogenic Se NPs was first produced by Bacillus sp. MSh-1 and characterized to individual and spherical nano structure with size range of 80–220 nm (Shakibaie et al. 2010). The results of antibiofilm activity revealed that biogenic Se NPs reduced biofilm formation of P. aeruginosa, S. aureus, and P. mirabilis to 34.3%, 42%, and 53.4%, respectively, compared to that of controls (Shakibaie et al. 2015a, b; Tan et al. 2018).
Miglani and Tani-Ishii (2021) evaluated the antibiofilm efficacy of biogenic Se NPs produced using guava leaves (with the size range of 30–50 nm) against Enterococcus faecalis. The mean percentage decrease in growth of biofilms in different test groups compared to control was higher in Se NPs, sodium hypochlorite, chlorhexidine gluconate, and calcium hydroxide, respectively (Miglani and Tani-Ishii 2021). They were reported that Se NPs inhibited 65% growth of the biofilms. The ability of these groups for inhibition of biofilm formation by Enterococcus faecalis was investigated using counting the viable bacteria within the biofilm. The percentage of viable cells at 24 h was 21.38% in the presence of Se NPs, followed by sodium hypochlorite (27.09%), chlorhexidine gluconate (30.03%), and calcium hydroxide (72.20%), compared to control (89.06%) (Miglani and Tani-Ishii 2021). The mean percentage reduction of carbohydrates and protein contents of biofilm compared to control was the lowest in calcium hydroxide followed by chlorhexidine gluconate, sodium hypochlorite, and Se NPs (Miglani and Tani-Ishii 2021). The antibiofilm mechanism of Se NPs is after adhesion to the biofilm, NPs could penetrate into the pathogen and disrupt the microbial cell well via replacing with sulfur, which induces 50% suppression of Candida albicans biofilm at low Se NPs’ concentration (Lin et al. 2021).
Antiviral activity
Viral infection considered as a health threat worldwide. Notwithstanding, antiviral drugs that prevent replication of viruses, but many viruses, for example, human immunodeficiency virus, mutate readily and generate resistant strains that they have no effective drugs (Kawai and Akira 2006). The antiviral properties of nanoparticles extensively studied in the past decades. These studies, at least, are divided into two categories. The first category is associated with modified NPs with various organic molecules. The functionalized NPs can be effective on the virus because of interactions among the molecules-receptors and molecules-functionalizer at the virus surface. The second category is related to the antiviral activity of 'pure' (non-functionalized) NPs (Lysenko et al. 2018). It has been shown that Se deficiency plays a critical role in susceptibility to viral infections. Se NPs antiviral activity has its advantages, including less toxicity and excellent activity. The protective effects of supplemental Se explained in mice infected by the H1N1 influenza virus. The mortality of the H1N1 virus-infected Se-deficient mice was three times more than those animals receiving Na2SeO3 with a dose of 0.5 mg Se⋅Kg−1. Mice with low serum Se concentrations demonstrated a significant reduction in lower levels of IFN-γ and TNF-α and body weight (BW) (Yu et al. 2011). The administration of Se NPs is an efficient approach to improve the immune response in the body.
The actinobacterial synthesized Se NPs (size 10–250 nm) displayed good antiviral activity against type-1 dengue virus. This activity increased with increasing doses, and at the same time, the decrease in viral growth documented. Se NPs maximally inhibited the viral growth at 700 ppm (Ramya et al. 2015). Yinghua et al. (Li et al. 2020; Liu et al. 2021; Martinez et al. 2021).
Antifungal activity
The frequency and variety of invasive fungal infections have significantly increased over the last decades (Van Thiel et al. 2012), and these infections considered a major medical concern in the second half of the twentieth century. Fungal infections are hard to manage due to they tend to become chronic, difficult to diagnose, and hard to root out with antifungal drugs (Casadevall 2018). Guisbiers et al. (2017) reported the first synthesis of Se NPs by femtosecond pulsed laser ablation (size 50–400 nm) in deionized water. These pure nanoparticles used to inhibit the formation of Candida albicans biofilms then Se NPs easily adhere on the biofilm, penetrate to pathogen agents, and therefore harm the cell structure by substituting with sulfur (Guisbiers et al. 2017).
The biogenic Se NPs (synthesized by Ralstonia eutropha, size 40–120 nm) at concentration of 500 µg/ml inhibited the growth of fungi Aspergillus clavatus (Srivastava and Mukhopadhyay 2015). Shahverdi et al. (2010), Wadhwani et al. (2016) evaluated antifungal effect of biogenic Se NPs (produced via Bacillus thuringiensis, average size between 50 and 200 nm) against strains of Malassezia and Aspergillus which are two important clinical fungal genera. Se NPs displayed antifungal activity by inhibition spore germination, and these inhibited dermatophytes like Malassezia sympodialis and Malassezia furfur. Shakibaie et al. (2015a, b) evaluated the antifungal activity of biogenic Se NPs produced by Bacillus sp. Msh-1 versus Candida albicans and Aspergillus fumigatus. Se NPs demonstrated suitable antifungal activity, and yeast cells were more sensitive than mold cells. The concentrations of 70 µg/ml and 100 µg/ml of Se NPs inhibited the growth of C. albicans and A. fumigatus, respectively. The antimicrobial mechanism of NPs reported DNA damage and cell wall disruption. NPs electrostatically interconnect via the cell wall or cell membrane, inducing microbial cell wall destruction (El-Saadony et al. 2021). Consequently, large molecules transmit through the microbial cell membrane and disrupt DNA, finally causing cell death (El-Saadony et al. 2021).
Antiparasitic activity
Parasites are a group of pathogens that are more detrimental to humans than bacteria, and they usually induce chronic infection (Sun et al. 2007). Biogenic Se NPs (biosynthesized by Bacillus sp. MSh-1, size 80–220 nm) showed a significant scolicidal activity versus Echinococcus granulosus, which is the agent of cystic hydatid disease. Se NPs can use as therapeutic agents against visceral and cutaneous leishmaniasis, and the results reported to kill amastigotes and promastigotes of Leishmania major and Leishmania infantum. The 50% inhibitory concentrations (IC50) of Se NPs ranging from 1 to 25 μg/ml (Mahmoudvand et al. 2014a, b; Wadhwani et al. 2016). Beheshti et al. (2013) evaluated the antiparasitic effectiveness of biogenic Se NPs produced by Bacillus sp. MSh-1 (size 80–220 nm), against Leishmania major in vivo and in vitro. The obtained results displayed the highest toxicity occurred after 72 h against both amastigote and promastigote forms of Leishmania major and the IC50 of the Se NPs reported to be 4.4 ± 0.6 μg ml−1 and 1.62 ± 0.6 μg ml−1, respectively (Beheshti et al. 2013). Similar outcomes of the antileishmanial property of biosynthesized Se NPs (produced by Bacillus sp. MSh-1, size 80–220 nm) showed in vitro effect against L. infantum (Soflaei et al. 2012) and L. tropica (Mahmoudvand et al. 2014a, b). Soflaei et al. (2012) investigated the antileishmanial activities of SeO2 compared to biogenic Se NPs (produced by Bacillus sp. MSh-1, size 80–220 nm) in both amastigotes and promastigotes of L. infantum. The results demonstrated a dose-dependent antileishmanial activity for both compounds; although Se NP displayed a greater effect on promastigotes than SeO2. In another study, Shakibaie et al. (2020) evaluated prophylactic effects of Se NPs against acute toxoplasmosis caused by a single-celled parasite called Toxoplasma gondii in the mice. In this experiment, the rate of mortality from an experimental group (receiving Se NPs) compared to a control group (mice did not receive Se NPs) at doses of 10 mg/kg was 100% 10 days after receiving Se NPs. The results of the current study demonstrated the significant efficacy of Se NPs with no important toxicity for curing acute toxoplasmosis in the mice model. Furthermore, Keyhani et al. (2020a, b) demonstrated that the mean number of brain–tissue cysts of Toxoplasma gondii was remarkably (p < 0.001) reduced in mice treated with Se NPs (biosynthesized using Bacillus sp. MSh-1) in a dose-dependent response compared to the control group. The mRNA levels of IFN-γ, TNF-α, IL-12, and inducible nitric oxide synthase were notably raised in mice orally treated with Se NPs (10 mg/kg) compared with the control subgroup (p < 0.05). No significant variation (p > 0.05) was shown in clinical biochemical factors among the mice in experimental subgroups (treated with Se NPs) compared to control subgroups (Keyhani et al. 2020a, b). In addition, another study showed that biogenic Se NPs (synthesized by Bacillus sp. MSh-1) in concentrations of 2.5–10 mg/kg for 2 weeks were able to prevent severe symptoms of toxoplasmosis in mice. These results demonstrated the prophylactic properties of Se NPs with no significant toxicity against chronic toxoplasma gondii infection (Keyhani et al. 2020a, b). Se NPs biosynthesized using Bacillus sp. could induce apoptosis of Leishmania major via DNA fragmentation (Zhang et al. 2021).
Antioxidant activity
Oxidation is a chemical reaction that generates free radicals, the cause chain reactions that can harm the cells of organisms (Brewer 2011). Most often, the best solution to prevent these harmful effects is addition of antioxidants. Antioxidants are compounds that inhibit oxidation and prevent free radical formation (Brewer 2011). The selenium compounds selenomethionine, selenocystine, and methyl-selenocysteine can play a significant role in minimizing the free radical concentration to prevent the oxidative damage of DNA in both in vitro and in vivo conditions (Battin et al. 2011). Forootanfar et al. (2014) investigated the antioxidant and cytotoxic effect of biogenic Se NPs produced by marine bacterial strain Bacillus sp. MSh-1. The results displayed that at a similar concentration of 200 μg/mL, SeO2 and Se NPs represented a scavenging activity of 13.2 ± 3.1% and 23.1 ± 3.4%, respectively. Nevertheless, reducing power assay showed higher electron-donating activity of SeO2 compared to Se NPs.
Antioxidant activity of Se NPs is dependent on the nanoparticle size; smaller Se NPs have greater activity, and this activity of stabilized Se NPs displayed high activity when compared to selenite and Se NPs without stabilization (Torres et al. 2012). Stabilized biologically produced Se NPs with a size of less than 100 nm can be used as a food additive with antioxidant effects (Torres et al. 2012). In another study, the moderate antioxidant activity of biogenic Se NPs (fabricated by ginger extract, size 100–150 nm) confirmed by comparing the inhibition percentage of 2,2-diphenyl-1-hydrazine (DPPH) radical formation with that of ascorbic acid (Menon et al. 2019). Shinde and Desai (2022) synthesized Se NPs coated with methionine and folic acid by chemical precipitation approach with size of 50 nm and evaluated its total antioxidant activity in terms of scavenging of DPPH free radicals. An amount of 10 μg/mL of such coated Se NPs could inhibit 41% of DPPH, demonstrating its scavenger function at the lowest concentration (Shinde and Desai 2022).
**a et al. (2022) indicated that chitosan-stabilized Se NPs have high immunomodulation activity. They reported that immunomodulation activity was correlated to antioxidant activity and lipid metabolism. The chitosan-stabilized Se NPs demonstrated more broad-spectrum impacts on the immune system than an exogenous antioxidant Trolox®. Se NPs has a significant effect on the glutathione system through the promotion of activities and presumably synthesis of selenoenzymes. The biological functions increased using Se NP were almost equal in the healthy and disease individuals that transcriptome analysis on the kidneys and liver and serum proteomics analysis were used to identify molecular pathways. Nevertheless, Se NPs modulates the immune system in various paths, depending on the host condition; in the healthy condition, uptaken Se NPs decline ROS formation to inhibit inflammation and decrease oxidative stress, but Se NPs increase ROS generation during disease conditions. The SOD and NFkβ played an important role in switch changing impact of Se NPs when individuals are under disease, exhibiting the close association between immune and redox regulation (**a et al. 2022).
Anticancer activity
Cancer or malignancy of cells is one of the health problems around the world (Chaudhary et al. 2014). The six common traits considered hallmarks of cancer cells that including replicative immortality, proliferative signaling, evasion of growth suppressors, invasion and metastasis, angiogenesis, and resistance to cell death (Chaudhary et al. 2014; Nazir et al. 2014). Different diagnostic procedures, such as computed tomography (CT), biosensing, and magnetic resonance imaging (MRI), have been developed in the early detection of cancer (Chen et al. 2008a, b; Muthu and Singh 2009; Janib et al. 2010). Current cancer therapies usually destroy normal cells and therefore represent significant lethal activities and unavoidable side effects. The exceptional capacity of bionanomaterials also opens a novel way for cancer therapy. Se NPs are one of the essential elements with broad pharmacological actions, intrinsic non-toxicity, and significant physiological functions (Wang et al. 2005; Chen et al. 2008a, b; Li et al. 2011; Shi et al. 2011; Duntas 2012; Wang and Webster 2012). Results from human clinical and preclinical experiments (Maiyo and Singh 2017) showed that different forms of Se NPs (produced by fenugreek extract with size of 50–150 nm, ZnS coated quercetin/CdSe with size of 10 nm, single chains lentinan-coated Se NPs with average size of 25 nm, Se NPs functionalized using a novel polysaccharide extracted from Dictyophora indusiata with size of 89 nm, novel selenium-substituted hydroxyapatite nanoparticles with size of 160–200 nm, respectively) represented significant anticancer activity (Yang et al. 2012, 2017; Ramamurthy et al. 2013; Jia et al. 2015; Liao et al. 2015, 2016; Yanhua et al. 2016). These compounds can be also used in the diagnosis, treatment, and chemotherapy of cancer and also as drug carriers (Ip 1998; Maiyo and Singh 2017; Tan et al. 2018). Se NPs can inhibit the growth of cancer cells by inducing cell cycle arrest at S phase which mediated with deregulation of the eIF3 (elongation factor 3) protein complex (Hosnedlova et al. 2018). Cell membrane plays a vital role in inducing the cell cycle arrest and Se NPs -induced toxicity in cancer cells, respectively (Pi et al. 2013). Nonetheless, there are different anticancer mechanisms of Se that include three broad categories of (i) chromatin binding and modification, (ii) ROS production and (iii) thiol modification (El-Bayoumy and Sinha 2005; Weekley and Harris 2013; Maiyo and Singh 2017). Yazdi et al. (2012) evaluated the immunostimulatory effect of biogenic Se NPs (fabricated by Lactobacillus plantarum with size of 250 nm) on the 4T1-induced breast cancer tumors. The results of tumor growth measurement displayed a considerable decrease in the growth rate of tumor in the test mice when compared to the control group. They attributed such effect to enhancement of cellular immunity and promotion of Th1 immune responses after oral administration of Se NPs.
One way to increase the anticancer activity of nanoparticles and prevention of Se NPs aggregation is the conjugation of Se NPs to organic molecules or drugs (Li et al. 2011; Ramamurthy et al. 2013; Rezvanfar et al. 2013; Vekariya et al. 2013; Ahmad et al. 2015). Functionalization of Se NPs with Spirulina polysaccharides (synthesized with chemical reduction method using ascorbic acid by diameter ranging from 20 to 50 nm) inhibited the growth of tumor via inducing apoptosis (Yang et al. 2012). These conjugates also help to specific interactions between carbohydrates and lectins present on the cell surface for targeted delivery of Se NPs to cancer cells (Yang et al. 2012; Wadhwani et al. 2016). Se NPs (synthesized using GSH solution, with average size of 12.4 nm) also demonstrated suppression of prostate LNCaP cancer cells growth in vitro through caspase-mediated apoptosis (Kong et al. 2011; Tan et al. 2018). Furthermore, the results showed when nanorod elemental Se NPs (produced by Lactobacillus brevis) administered orally to BALB/c mice bearing 4T1 breast cancer, tumor-related volume was decreased (Yazdi et al. 2015). They also demonstrated that levels of TGF-β in these mice decreased in comparison to the control groups (Yazdi et al. 2015). In contrast, levels of cellular immunomodulatory components (for example, IL-2, IL-12, granzyme B, and IFN-γ) significantly increased in mice treated with both Se NPs and crude antigens of 4T1 cells (Yazdi et al. 2015; Tan et al. 2018). The anticancer mechanisms of Se NPs are not yet fully understood, but the most typical reported pathways are internalization of the NPs, stimulation of autophagy, regulation of the reactive oxygen species generation, and activation of the intrinsic apoptotic pathway (Lin et al. 2021; Spyridopoulou et al. 2021a, b). However, the main mechanism of anticancer activity Se NPs was reported apoptosis in different studies. Se NPs have been demonstrated to cause caspase-mediated apoptosis in various cancer cells, such as melanoma, hepatocarcinoma, cervical, prostate, and breast cancer cells (Li et al. 2019; Alkhudhayri et al. 2020; Spyridopoulou et al. 2021a, b).
Anti-inflammatory
Inflammation is an immune system’s defensive response that can be triggered by different factors, involving pathogens, toxic compounds, and damaged cells. The severe and prolonged inflammatory response may involve in progression of many diseases, including cardiovascular diseases, diabetes, cancer, and autoimmune diseases (Liu et al. 2019). Several studies have reported that Se possesses anti-inflammatory properties, and limiting factors including toxicity and bioavailability. El-Ghazaly et al. (2017) evaluated the anti-inflammatory activity of Se NPs (synthesized with a chemical reduction method using SeO2 with size of 13.4 nm) on inflammation induced in irradiated rats (were exposed to 6 Gy gamma irradiation). They are used the carrageenan-induced paw edema model and were measured paw volume and nociceptive threshold. Se NPs were administered in an oral dose of 2.55 mg/kg. Se NPs reduced the paw edema in irradiated and non-irradiated rats, but it did not change the nociceptive threshold of the both (El-Ghazaly et al. 2017).
The anti-inflammatory effect of Se NPs (folic acid protected/modified selenium nanoparticles with size of 70 nm) was investigated and displayed that Se NPs localized intracellularly in lysosomes and mitochondria of MCF-7 cell (breast cancer cell line) and changed membrane biomechanical properties of MCF-7 cells via disturbing membrane molecules (such as CD44 molecules) and F-actin (Pi et al. 2013). The main mechanism of Se NPs was inducing intracellular toxicity in cancer cells, and it can induce apoptosis and necrosis of these cells (Pi et al. 2013). Furthermore, Se NPs caused down-regulation and disorganization of F-actin, thus, remarkably reducing Young’s modulus and adhesion force of MCF-7 cells (Pi et al. 2013). Ren et al. (2019) evaluated the anti-inflammatory activity of Se NPs (Se NPs dispersed in 1% p-coumaric acid with average size of 40 nm) against complete Freund’s adjuvant induced rheumatoid arthritis rat model. Their results showed that the symptoms of RA animals which treated with Se NPs (500 μg/Kg body weight) were significantly decreased (p < 0.001). They reported some significant decline of antioxidant levels of enzymes, such as superoxide dismutase, catalase, and glutathione peroxidase activities and reduced glutathione, but increased of thiobarbituric acid reactive substances (TBARS) level in RA rats compared to control rats (Ren et al. 2019). Therefore, cyclooxygenase-2 activity, various antioxidant enzyme activities, TBARS, and inflammatory cytokines (TNF-a, MCP-1IL-1b, and IL-6) were significantly reverted to normal levels in treated animals with Se NPs (500 μg/kg) compared with non-treated RA rats.
Se NPs in plant biology
Ragavan et al. (2017) investigated the effect of Se NPs (synthesized by ascorbic acid, size of 50–150 nm) on growth, biochemical characteristics, and yield of cluster bean Cyamopsis tetragonoloba. Pot culture of cluster bean treatment with different amounts of Se NPs including 0, 100, 200, 300, 400, and 500 mg was performed and growth biochemical characteristics and yield measured at the end of 60 days (Ragavan et al. 2017). The germination percentage were 100%, 90%, 80%, 90%, 100%, and 100%, respectively. Root length, leaf area, and fresh and dry weight were higher in treatment groups containing 200 mg of Se NPs. The shoot length was lower (12.01 cm) and higher (21.8 cm) in 500 mg and 100 mg treatment group, respectively (Ragavan et al. 2017). Also, the carotenoids, anthocyanin, chlorophyll a, chlorophyll b, and total chlorophyll, protein, L-proline, free amino acids, and leaf nitrate were higher in 400 mg treatment group (Ragavan et al. 2017). The vigor index was higher in 400 mg treatment group. Among the groups, the yield of the cluster bean was lowest in untreated and highest in 400 mg treatment group.
Se NPs in animal husbandry
Some studies have displayed that addition of Se NPs to livestock feed can improve the quality of the tissue and meat and growth requirements (Rajendran 2013). The result showed that average daily gain and final body weight increased in bucks supplemented by different Se forms (sodium selenite, elemental nano-Se or selenized yeast) compared to un-supplemented control groups (Shi et al. 2011). Radwan et al. (2015) reported about adding Se NPs (synthesized using chemical reduction method with size of 80 nm) and sodium selenite to laying hens’ nutrition egg production and they observed that feed conversion ratio improved in the nano-selenium groups. Furthermore, in egg yolk of hens receiving Se NPs, lower malondialdehyde content and higher activity of glutathione peroxidase were found (Konkol and Wojnarowski 2018). Se NPs led to the better fatty acid profile in eggs by reducing the ratio of saturated to unsaturated fatty acids and also significantly increased the level of HDL fraction and decreased the level of total cholesterol and total lipids in plasma and egg yolk of laying hens (Konkol and Wojnarowski 2018).
Se represented a positive impact on the normal reproductive capacity of spermatozoa, normal testicular development, spermatogenesis, and spermatozoa motility, and also incorporated into the mitochondrial protein (Boitani and Puglisi 2009; Sarkar et al. 2015). The two most critical regulating proteins necessary in spermatogenesis are phospholipid hydroperoxide, glutathione peroxidase, and selenoprotein P, which are responsible for carrying Se to the testis (Olson et al. 2005; Imai et al. 2009). Some papers displayed that the supplementation of nano-selenium with 0.3 mg/kg body weight in male boar goats significantly led to better semen glutathione peroxidase activity, testicular Se level, and ATPase activity as compared to control/un-supplemented group (Shi et al. 2010). The results indicated that Se NPs’ (provided by Shanghai Stone Nano-Technology Port Co. Ltd., China, with sizes of 60–80 nm) addition increased testicular and semen glutathione peroxidase activities, and testis Se content, and play an essential role in protecting the membrane system integrity. Therefore, Se NPs seem to be able to increase male reproductive capacity more than the other form of elemental Se (Shi et al. 2010).
Conclusion
Fabrication of selenium nanostructures using biological resources, such as microbial strains, herbal extracts, and biological macromolecules, have gained attention during the last decades. Lots of biomedical characteristics, such as antioxidant, cytotoxic, antimicrobial, antiparasitic, anticancer, and immunomodulator activity, compared to that of bulk selenium launched it as a valuable material.
Data availability
Not applicable.
References
Ahmad MS, Yasser MM, Sholkamy EN, Ali AM, Mehanni MM (2015) Anticancer activity of biostabilized selenium nanorods synthesized by Streptomyces bikiniensis strain Ess_amA-1. Int J Nanomed 10:3389–3401
Alagesan V, Venugopal S (2019) Green synthesis of selenium nanoparticle using leaves extract of Withania somnifera and its biological applications and photocatalytic activities. Bionanoscience 9(1):105–116
Alam H, Khatoon N, Khan MA, Husain SA, Saravanan M, Sardar M (2019) Synthesis of selenium nanoparticles using probiotic bacteria Lactobacillus acidophilus and their enhanced antimicrobial activity against resistant bacteria. J Cluster Sci 31(5):1003–11
Alghuthaymi MA, Almoammar H, Rai M, Said-Galiev E, Abd-Elsalam KA (2015) Myconanoparticles: synthesis and their role in phytopathogens management. Biotechnol Biotechnol Equip 29(2):221–236
Alkhudhayri AA, Wahab R, Siddiqui MA, Ahmad J (2020) Selenium nanoparticles induce cytotoxicity and apoptosis in human breast cancer (MCF-7) and liver (HEPG2) cell lines. Nanosci Nanotechnol Lett 12(3):324–330
Baig N, Kammakakam I, Falath W (2021) Nanomaterials: a review of synthesis methods, properties, recent progress, and challenges. Mater Adv 2(6):1821–1871
Bartůněk V, Junková J, Šuman J, Kolářová K, Rimpelová S, Ulbrich P, Sofer Z (2015) Preparation of amorphous antimicrobial selenium nanoparticles stabilized by odor suppressing surfactant polysorbate 20. Mater Lett 152:207–209
Battin EE, Zimmerman MT, Ramoutar RR, Quarles CE, Brumaghim JL (2011) Preventing metal-mediated oxidative DNA damage with selenium compounds. Metallomics 3(5):503–512
Beheshti N, Soflaei S, Shakibaie M, Yazdi MH, Ghaffarifar F, Dalimi A, Shahverdi AR (2013) Efficacy of biogenic selenium nanoparticles against Leishmania major: in vitro and in vivo studies. J Trace Elem Med Biol 27(3):203–207
Boitani C, Puglisi R (2009) Selenium, a key element in spermatogenesis and male fertility. Adv Exp Med Biol 636:65–73
Brewer M (2011) Natural antioxidants: sources, compounds, mechanisms of action, and potential applications. Compr Rev Food Sci Food Safety 10(4):221–247
Burk RF (1994) Selenium in biology and human health. Springer, New York. https://doi.org/10.1007/978-1-4612-2592-8
Casadevall A (2018) Fungal diseases in the 21st century: the near and far horizons. Pathog Immun 3(2):183–196
Cavalu S, Prokisch J, Laslo V, Vicas S (2017) Preparation, structural characterisation and release study of novel hybrid microspheres entrap** nanoselenium, produced by green synthesis. IET Nanobiotechnol 11(4):426–432
Chandramohan S, Sundar K, Muthukumaran A (2019) Reducing agents influence the shapes of selenium nanoparticles (SeNPs) and subsequently their antibacterial and antioxidant activity. Mater Res Express 6(8):0850–0852
Chaudhary S, Umar A, Mehta SK (2014) Surface functionalized selenium nanoparticles for biomedical applications. J Biomed Nanotechnol 10(10):3004–3042
Chen PC, Mwakwari SC, Oyelere AK (2008a) Gold nanoparticles: from nanomedicine to nanosensing. Nanotechnol Sci Appl 1:45–65
Chen T, Wong YS, Zheng W, Bai Y, Huang L (2008b) Selenium nanoparticles fabricated in Undaria pinnatifida polysaccharide solutions induce mitochondria-mediated apoptosis in A375 human melanoma cells. Colloids Surf B Biointerfaces 67(1):26–31
Chen W, Li Y, Yang S, Yue L, Jiang Q, **a W (2015) Synthesis and antioxidant properties of chitosan and carboxymethyl chitosan-stabilized selenium nanoparticles. Carbohydr Polym 132:574–581
Chung S, Ercan B, Roy AK, Webster TJ (2016) Addition of Selenium nanoparticles to electrospun silk scaffold improves the mammalian cell activity while reducing bacterial growth. Front Physiol 7:297–297
Cittrarasu V, Kaliannan D, Dharman K, Maluventhen V, Easwaran M, Liu WC, Balasubramanian B, Arumugam M (2021) Green synthesis of selenium nanoparticles mediated from Ceropegia bulbosa Roxb extract and its cytotoxicity, antimicrobial, mosquitocidal and photocatalytic activities. Sci Rep 11(1):1–15
Cremonini E, Zonaro E, Donini M, Lampis S, Boaretti M, Dusi S, Melotti P, Lleo MM, Vallini G (2016) Biogenic selenium nanoparticles: characterization, antimicrobial activity and effects on human dendritic cells and fibroblasts. Microb Biotechnol 9(6):758–771
Dhand C, Dwivedi N, Loh XJ, Jie Ying AN, Verma NK, Beuerman RW, Lakshminarayanan R, Ramakrishna S (2015) Methods and strategies for the synthesis of diverse nanoparticles and their applications: a comprehensive overview. RSC Adv 5(127):105003–105037
Díez-Pascual AM (2018) Antibacterial activity of nanomaterials. Nanomaterials (Basel) 8(6):359
Diko CS, Zhang H, Lian S, Fan S, Li Z, Qu Y (2020) Optimal synthesis conditions and characterization of selenium nanoparticles in Trichoderma sp WL-Go culture broth. Mater Chem Phys 246:122583
Długosz O, Banach M (2020) Inorganic nanoparticle synthesis in flow reactors–applications and future directions. React Chem Eng 5(9):1619–1641
Duntas LH (2012) The evolving role of selenium in the treatment of Graves ‘ disease and ophthalmopathy. J Thyroid Res 2012:736161
Dwivedi C, Shah CP, Singh K, Kumar M, Bajaj PN (2011a) An organic acid-induced synthesis and characterization of selenium nanoparticles. J Nanotechnol 2011:1–6
El-Bayoumy K, Sinha R (2005) Molecular chemoprevention by selenium: a genomic approach. Mutat Res 591(1–2):224–236
El-Ghazaly MA, Fadel N, Rashed E, El-Batal A, Kenawy SA (2017) Anti-inflammatory effect of selenium nanoparticles on the inflammation induced in irradiated rats. Can J Physiol Pharmacol 95(2):101–110
El-Saadony MT, Saad AM, Najjar AA, Alzahrani SO, Alkhatib FM, Shafi ME, Selem E, Desoky E-SM, Fouda SE, El-Tahan AM (2021) The use of biological selenium nanoparticles to suppress Triticum aestivum L. crown and root rot diseases induced by Fusarium species and improve yield under drought and heat stress. Saudi J Biol Sci 28(8):4461–4471
Escobar-Ramírez MC, Castañeda-Ovando A, Pérez-Escalante E, Rodríguez-Serrano GM, Ramírez-Moreno E, Quintero-Lira A, Contreras-López E, Añorve-Morga J, Jaimez-Ordaz J, González-Olivares LG (2021) Antimicrobial activity of Se-nanoparticles from bacterial biotransformation. Fermentation 7(3):130
Estevam EC, Griffin S, Nasim MJ, Denezhkin P, Schneider R, Lilischkis R, Dominguez-Alvarez E, Witek K, Latacz G, Keck C, Schafer KH, Kiec-Kononowicz K, Handzlik J, Jacob C (2017) Natural selenium particles from Staphylococcus carnosus: Hazards or particles with particular promise? J Hazard Mater 324(Pt A):22–30
Eszenyi P, Sztrik A, Babka B, Joe P (2011) Elemental, Nano-sized (100–500 nm) selenium production by probiotic lactic acid bacteria. Int J Biosci Biochem Bioinform 1(2):148
Ezhuthupurakkal PB, Polaki LR, Suyavaran A, Subastri A, Sujatha V, Thirunavukkarasu C (2017) Selenium nanoparticles synthesized in aqueous extract of Allium sativum perturbs the structural integrity of Calf thymus DNA through intercalation and groove binding. Mater Sci Eng C Mater Biol Appl 74:597–608
Forootanfar HB, Zare H, Fasihi-Bam S, Amirpour-Rostami A, Ameri M, Shakibaie M, Nami M (2013) Biosynthesis and characterization of selenium nanoparticles produced by terrestrial actinomycete Streptomyces microflavus strain FSHJ31. J Microbiol Biotechnol 3(2):47–53
Forootanfar H, Adeli-Sardou M, Nikkhoo M, Mehrabani M, Amir-Heidari B, Shahverdi AR, Shakibaie M (2014) Antioxidant and cytotoxic effect of biologically synthesized selenium nanoparticles in comparison to selenium dioxide. J Trace Elem Med Biol 28(1):75–79
Gera T, Nagy E, Smausz T, Budai J, Ajtai T, Kun-Szabó F, Homik Z, Kopniczky J, Bozóki Z, Szabó-Révész P (2020) Application of pulsed laser ablation (PLA) for the size reduction of non-steroidal anti-inflammatory drugs (NSAIDs). Sci Rep 10(1):1–13
Gerrard TL, Telford JN, Williams HH (1974) Detection of selenium deposits in Escherichia coli by electron microscopy. J Bacteriol 119(3):1057–1060
Guisbiers G, Wang Q, Khachatryan E, Arellano-Jimenez MJ, Webster TJ, Larese-Casanova P, Nash KL (2014) Antibacterial selenium nanoparticles produced by UV/VIS/NIR pulsed nanosecond laser ablation in liquids. Laser Phys Lett 12(1):016003
Guisbiers G, Lara HH, Mendoza-Cruz R, Naranjo G, Vincent BA, Peralta XG, Nash KL (2017) Inhibition of Candida albicans biofilm by pure selenium nanoparticles synthesized by pulsed laser ablation in liquids. Nanomedicine 13(3):1095–1103
Haque R (2007) Human intestinal parasites. J Health Popul Nutr 25(4):387–391
Hariharan H, Al-Dhabi NA, Karuppiah P, Rajaram SK (2012) Microbial synthesis of selinium nanocomposite using Saccharomyces cerevisiae and its antimicrobial activity against pathogens causing nosocomial infection. Chalcogenide Lett 9(12):509–515
Hatami R, Javadi A, Jafarizadeh-Malmiri H (2020) Effectiveness of six different methods in green synthesis of selenium nanoparticles using propolis extract: screening and characterization. Green Process Synth 9(1):685–692
Hiffler L, Rakotoambinina B (2020) Selenium and RNA virus interactions: potential implications for SARS-CoV-2 infection (COVID-19). Front Nutr 7:164
Hosnedlova B, Kepinska M, Skalickova S, Fernandez C, Ruttkay-Nedecky B, Peng Q, Baron M, Melcova M, Opatrilova R, Zidkova J, Bjorklund G, Sochor J, Kizek R (2018) Nano-selenium and its nanomedicine applications: a critical review. Int J Nanomed 13:2107–2128
Hou J-Y, Shi-Yun A, Shi W-J (2011) Preparation and characterization of Nano-Se/Silk fibroin colloids. Chem Res Chin Univ 27(1):158–60
Huang X, Chen X, Chen Q, Yu Q, Sun D, Liu J (2016) Investigation of functional selenium nanoparticles as potent antimicrobial agents against superbugs. Acta Biomater 30:397–407
Huang T, Holden JA, Heath DE, O’Brien-Simpson NM, O’Connor AJ (2019) Engineering highly effective antimicrobial selenium nanoparticles through control of particle size. Nanoscale 11(31):14937–14951
Husen A, Siddiqi KS (2014) Plants and microbes assisted selenium nanoparticles: characterization and application. J Nanobiotechnol 12(1):1–10
Imai H, Hakkaku N, Iwamoto R, Suzuki J, Suzuki T, Tajima Y, Konishi K, Minami S, Ichinose S, Ishizaka K (2009) Depletion of selenoprotein GPx4 in spermatocytes causes male infertility in mice. J Biol Chem 284(47):32522–32532
Ionin AA, Ivanova AK, Khmelnitskii RA, Klevkov YV, Kudryashov SI, Levchenko AO, Nastulyavichus AA, Rudenko AA, Saraeva IN, Smirnov NA, Zayarny DA, Gonchukov SA, Tolordava ER (2017) Antibacterial effect of the laser-generated Se nanocoatings on Staphylococcus aureus and Pseudomonas aeruginosa biofilms. Laser Phys Lett 15(1):015604
Ip C (1998) Lessons from basic research in selenium and cancer prevention. J Nutr 128(11):1845–1854
Iravani S, Korbekandi H, Mirmohammadi SV, Zolfaghari B (2014) Synthesis of silver nanoparticles: chemical, physical and biological methods. Res Pharm Sci 9(6):385–406
Jamal M, Ahmad W, Andleeb S, Jalil F, Imran M, Nawaz MA, Hussain T, Ali M, Rafiq M, Kamil MA (2018) Bacterial biofilm and associated infections. J Chin Med Assoc 81(1):7–11
Janib SM, Moses AS, MacKay JA (2010) Imaging and drug delivery using theranostic nanoparticles. Adv Drug Deliv Rev 62(11):1052–1063
Jia X, Liu Q, Zou S, Xu X, Zhang L (2015) Construction of selenium nanoparticles/beta-glucan composites for enhancement of the antitumor activity. Carbohydr Polym 117:434–442
Joseph S, Mathew B (2014) Microwave assisted biosynthesis of silver nanoparticles using the rhizome extract of Alpinia galanga and evaluation of their catalytic and antimicrobial activities. J Nanoparticles 2014:1–9
Kawai T, Akira S (2006) Innate immune recognition of viral infection. Nat Immunol 7(2):131–137
Kessi J, Ramuz M, Wehrli E, Spycher M, Bachofen R (1999) Reduction of selenite and detoxification of elemental selenium by the phototrophic bacterium Rhodospirillum rubrum. Appl Environ Microbiol 65(11):4734–4740
Keyhani A, Shakibaie M, Mahmoudvand H, Jahanbakhsh S, Kareshk AT, Shojaee S, Ziaali N (2020a) Prophylactic activity of biogenic selenium nanoparticles against chronic Toxoplasma gondii infection. Recent Pat Antiinfect Drug Discov 15(1):75–84
Keyhani A, Ziaali N, Shakibaie M, Kareshk AT, Shojaee S, Asadi-Shekaari M, Sepahvand M, Mahmoudvand H (2020b) Biogenic selenium nanoparticles target chronic toxoplasmosis with minimal cytotoxicity in a mouse model. J Med Microbiol 69(1):104–110
Khan I, Saeed K, Khan I (2019) Nanoparticles: properties, applications and toxicities. Arab J Chem 12(7):908–931
Kielczykowska M, Kocot J, Pazdzior M, Musik I (2018) Selenium - a fascinating antioxidant of protective properties. Adv Clin Exp Med 27(2):245–255
Kong L, Yuan Q, Zhu H, Li Y, Guo Q, Wang Q, Bi X, Gao X (2011) The suppression of prostate LNCaP cancer cells growth by Selenium nanoparticles through Akt/Mdm2/AR controlled apoptosis. Biomaterials 32(27):6515–6522
Konkol D, Wojnarowski K (2018) The use of nanominerals in animal nutrition as a way to improve the composition and quality of animal products. J Chem 2018:1–7
Kora AJ, Rastogi L (2016) Biomimetic synthesis of selenium nanoparticles by Pseudomonas aeruginosa ATCC 27853: an approach for conversion of selenite. J Environ Manag 181:231–236
Kumar PSM, Francis AP, Devasena T (2014) Biosynthesized and chemically synthesized titania nanoparticles: comparative analysis of antibacterial activity. J Environ Nanotechnol 3(3):73–81
Langi B, Shah C, Singh K, Chaskar A, Kumar M, Bajaj PN (2010) Ionic liquid-induced synthesis of selenium nanoparticles. Mater Res Bull 45(6):668–671
Li Y, Li X, Wong YS, Chen T, Zhang H, Liu C, Zheng W (2011) The reversal of cisplatin-induced nephrotoxicity by selenium nanoparticles functionalized with 11-mercapto-1-undecanol by inhibition of ROS-mediated apoptosis. Biomaterials 32(34):9068–9076
Li Y, Lin Z, Guo M, Zhao M, **a Y, Wang C, Xu T, Zhu B (2018) Inhibition of H1N1 influenza virus-induced apoptosis by functionalized selenium nanoparticles with amantadine through ROS-mediated AKT signaling pathways. Int J Nanomed 13:2005–2016
Li H, Liu D, Li S, Xue C (2019) Synthesis and cytotoxicity of selenium nanoparticles stabilized by α-D-glucan from Castanea mollissima Blume. Int J Biol Macromol 129:818–826
Liang X, Perez MAM-J, Nwoko KC, Egbers P, Feldmann J, Csetenyi L, Gadd GM (2019) Fungal formation of selenium and tellurium nanoparticles. Appl Microbiol Biotechnol 103(17):7241–7259
Liao W, Yu Z, Lin Z, Lei Z, Ning Z, Regenstein JM, Yang J, Ren J (2015) Biofunctionalization of selenium nanoparticle with Dictyophora Indusiata polysaccharide and its antiproliferative activity through death-receptor and mitochondria-mediated apoptotic pathways. Sci Rep 5:18629–18629
Liao W, Zhang R, Dong C, Yu Z, Ren J (2016) Novel walnut peptide-selenium hybrids with enhanced anticancer synergism: facile synthesis and mechanistic investigation of anticancer activity. Int J Nanomed 11:1305–1321
Lin W, Zhang J, Xu J-F, Pi J (2021) The advancing of selenium nanoparticles against infectious diseases. Front Pharmacol 12:682284
Liu T, Zeng L, Jiang W, Fu Y, Zheng W, Chen T (2015) Rational design of cancer-targeted selenium nanoparticles to antagonize multidrug resistance in cancer cells. Nanomedicine 11(4):947–958
Liu Y, Kim S, Kim YJ, Perumalsamy H, Lee S, Hwang E, Yi TH (2019) Green synthesis of gold nanoparticles using Euphrasia officinalis leaf extract to inhibit lipopolysaccharide-induced inflammation through NF-κB and JAK/STAT pathways in RAW 264.7 macrophages. Int J Nanomed 14:2945–2959
Liu Q, Zhao X, Ma J, Mu Y, Wang Y, Yang S, Wu Y, Wu F, Zhou Y (2021) Selenium (Se) plays a key role in the biological effects of some viruses: implications for COVID-19. Environ Res 196:110984
Lysenko V, Lozovski V, Lokshyn M, Gomeniuk YV, Dorovskih A, Rusinchuk N, Pankivska Y, Povnitsa O, Zagorodnya S, Tertykh V (2018) Nanoparticles as antiviral agents against adenoviruses. Adv Nat Sci: Nanosci Nanotechnol 9(2):025021
Mahmoudvand H, Fasihi Harandi M, Shakibaie M, Aflatoonian MR, ZiaAli N, Makki MS, Jahanbakhsh S (2014a) Scolicidal effects of biogenic selenium nanoparticles against protoscolices of hydatid cysts. Int J Surg 12(5):399–403
Mahmoudvand H, Shakibaie M, Tavakoli R, Jahanbakhsh S, Sharifi I (2014b) In vitro study of leishmanicidal activity of biogenic selenium nanoparticles against Iranian isolate of sensitive and glucantime-resistant Leishmania tropica. Iran J Parasitol 9(4):452–460
Maiyo F, Singh M (2017) Selenium nanoparticles: potential in cancer gene and drug delivery. Nanomedicine (Lond) 12(9):1075–1089
Martinez SS, Huang Y, Acuna L, Laverde E, Trujillo D, Barbieri MA, Tamargo J, Campa A, Baum MK (2021) Role of selenium in viral infections with a major focus on SARS-CoV-2. Int J Mol Sci 23(1):280
Mehdi Y, Hornick JL, Istasse L, Dufrasne I (2013) Selenium in the environment, metabolism and involvement in body functions. Molecules 18(3):3292–3311
Mellinas C, Jimenez A, Garrigos MDC (2019) Microwave-assisted green synthesis and antioxidant activity of selenium nanoparticles using Theobroma cacao L. bean shell extract. Molecules 24(22):4048
Menon S, KS SD, Agarwal H, Shanmugam VK (2019) Efficacy of biogenic selenium nanoparticles from an extract of ginger towards evaluation on anti-microbial and anti-oxidant activities. Colloid Interface Sci Commun 29:1–8
Miglani S, Tani-Ishii N (2021) Biosynthesized selenium nanoparticles: characterization, antimicrobial, and antibiofilm activity against Enterococcus faecalis. PeerJ 9:e11653
Muthu MS, Singh S (2009) Targeted nanomedicines: effective treatment modalities for cancer, AIDS and brain disorders. Nanomedicine (Lond) 4(1):105–118
Narayanan KB, Sakthivel N (2010) Biological synthesis of metal nanoparticles by microbes. Adv Colloid Interface Sci 156(1–2):1–13
Nazir S, Hussain T, Ayub A, Rashid U, MacRobert AJ (2014) Nanomaterials in combating cancer: therapeutic applications and developments. Nanomedicine 10(1):19–34
Nguyen TH, Vardhanabhuti B, Lin M, Mustapha A (2017) Antibacterial properties of selenium nanoparticles and their toxicity to Caco-2 cells. Food Control 77:17–24
Olson GE, Winfrey VP, NagDas SK, Hill KE, Burk RF (2005) Selenoprotein P is required for mouse sperm development. Biol Reprod 73(1):201–211
Panahi-Kalamuei M, Salavati-Niasari M, Mostafa Hosseinpour-Mashkani S (2014) Facile microwave synthesis, characterization, and solar cell application of selenium nanoparticles. J Alloys Compd 617:627–632
Pi J, Yang F, ** H, Huang X, Liu R, Yang P, Cai J (2013) Selenium nanoparticles induced membrane biomechanical property changes in MCF-7 cells by disturbing membrane molecules and F-actin. Bioorg Med Chem Lett 23(23):6296–6303
Piacenza E, Presentato A, Zonaro E, Lemire JA, Demeter M, Vallini G, Turner RJ, Lampis S (2017) Antimicrobial activity of biogenically produced spherical Se-nanomaterials embedded in organic material against Pseudomonas aeruginosa and Staphylococcus aureus strains on hydroxyapatite-coated surfaces. Microb Biotechnol 10(4):804–818
Prasad KS, Selvaraj K (2014) Biogenic synthesis of selenium nanoparticles and their effect on As(III)-induced toxicity on human lymphocytes. Biol Trace Elem Res 157(3):275–283
Prasad KS, Patel H, Patel T, Patel K, Selvaraj K (2013) Biosynthesis of Se nanoparticles and its effect on UV-induced DNA damage. Colloids Surf B Biointerfaces 103:261–266
Radwan NL, Eldin TS, El-Zaiat A, Mostafa MA (2015) Effect of dietary nano-selenium supplementation on selenium content and oxidative stability in table eggs and productive performance of laying hens. Int J Poult Sci 14(3):161
Ragavan P, Ananth A, Rajan MR (2017) Impact of Selenium nanoparticles on growth, biochemical characteristics and yield of cluster bean Cyamopsis tetragonoloba. Int J Environ, Agric Biotechnol 2(6):2917–2926
Rajendran D (2013) Application of Nano minerals in animal production system. Res J Biotechnol 8(3):1–3
Ramamurthy C, Sampath KS, Arunkumar P, Kumar MS, Sujatha V, Premkumar K, Thirunavukkarasu C (2013) Green synthesis and characterization of selenium nanoparticles and its augmented cytotoxicity with doxorubicin on cancer cells. Bioprocess Biosyst Eng 36(8):1131–1139
Ramos JF, Webster TJ (2012) Cytotoxicity of selenium nanoparticles in rat dermal fibroblasts. Int J Nanomedicine 7:3907–3914
Ramya S, Shanmugasundaram T, Balagurunathan R (2015) Biomedical potential of actinobacterially synthesized selenium nanoparticles with special reference to anti-biofilm, anti-oxidant, wound healing, cytotoxic and anti-viral activities. J Trace Elem Med Biol 32:30–39
Ren SX, Zhan B, Lin Y, Ma DS, Yan H (2019) Selenium nanoparticles dispersed in phytochemical exert anti-inflammatory activity by modulating catalase, GPx1, and COX-2 gene expression in a rheumatoid arthritis rat model. Med Sci Monit 25:991–1000
Rezvanfar MA, Rezvanfar MA, Shahverdi AR, Ahmadi A, Baeeri M, Mohammadirad A, Abdollahi M (2013) Protection of cisplatin-induced spermatotoxicity, DNA damage and chromatin abnormality by selenium Nano-particles. Toxicol Appl Pharmacol 266(3):356–365
Sarkar J, Dey P, Saha S, Acharya K (2011) Mycosynthesis of selenium nanoparticles. Micro Nano Lett 6(8):599–602
Sarkar B, Bhattacharjee S, Daware A, Tribedi P, Krishnani KK, Minhas PS (2015) Selenium nanoparticles for stress-resilient fish and livestock. Nanoscale Res Lett 10(1):371
Sasidharan S, Balakrishnaraja R (2014) Comparison studies on the synthesis of selenium nanoparticles by various micro-organisms. Int J Pure Appl Biosci 2:112–7
Schütz MB, **ao L, Lehnen T, Fischer T, Mathur S (2018) Microwave-assisted synthesis of nanocrystalline binary and ternary metal oxides. Int Mater Rev 63(6):341–374
Shahverdi AR, Fakhimi A, Mosavat G, Jafari-Fesharaki P, Rezaie S, Rezayat M (2010) Antifungal activity of biogenic selenium nanoparticles. World Appl Sci J 10(8):918–22
Shakibaie M, Khorramizadeh MR, Faramarzi MA, Sabzevari O, Shahverdi AR (2010) Biosynthesis and recovery of selenium nanoparticles and the effects on matrix metalloproteinase-2 expression. Biotechnol Appl Biochem 56(1):7–15
Shakibaie M, Forootanfar H, Golkari Y, Mohammadi-Khorsand T, Shakibaie MR (2015a) Anti-biofilm activity of biogenic selenium nanoparticles and selenium dioxide against clinical isolates of Staphylococcus aureus, Pseudomonas aeruginosa, and Proteus mirabilis. J Trace Elem Med Biol 29:235–241
Shakibaie M, Salari Mohazab N, Ayatollahi Mousavi SA (2015b) Antifungal activity of selenium nanoparticles synthesized by bacillus species Msh-1 Against Aspergillus fumigatus and Candida albicans. Jundishapur J Microbiol 8(9):e26381
Shakibaie M, Ezzatkhah F, Gabal E, Badparva E, Jahanbakhsh S, Mahmoudvand H (2020) Prophylactic effects of biogenic selenium nanoparticles on acute toxoplasmosis: an in vivo study. Ann Med Surg 54:85–88
Shar A, Lakhan M, Wang J, Ahmed M, Alali K, Ahmed R, Ali I, Dayo A (2019) Facile synthesis and characterization of selenium nanoparticles by the hydrothermal approach. Dig J Nanomater Biostruct 14:867–872
Sharma G, Sharma AR, Bhavesh R, Park J, Ganbold B, Nam JS, Lee SS (2014) Biomolecule-mediated synthesis of selenium nanoparticles using dried Vitis vinifera (raisin) extract. Molecules 19(3):2761–2770
Shi L-G, Yang R-J, Yue W-B, Xun W-J, Zhang C-X, Ren Y-S, Shi L, Lei F-L (2010) Effect of elemental nano-selenium on semen quality, glutathione peroxidase activity, and testis ultrastructure in male Boer goats. Anim Reprod Sci 118(2–4):248–254
Shi L, Xun W, Yue W, Zhang C, Ren Y, Shi L, Wang Q, Yang R, Lei F (2011) Effect of sodium selenite, Se-yeast and nano-elemental selenium on growth performance, Se concentration and antioxidant status in growing male goats. Small Rumin Res 96(1):49–52
Shinde V, Desai K (2022) In vitro cytotoxicity, macromolecular interaction and antioxidant potential of dual coated selenium nanoparticles. J Biomed Mater Res Part B: Appl Biomater. 110(6):1400–11
Shoeibi S, Mashreghi M (2017) Biosynthesis of selenium nanoparticles using Enterococcus faecalis and evaluation of their antibacterial activities. J Trace Elem Med Biol 39:135–139
Shubharani R, Mahesh M, Yogananda Murthy V (2019) Biosynthesis and characterization, antioxidant and antimicrobial activities of selenium nanoparticles from ethanol extract of Bee Propolis. J Nanomed Nanotechnol 10(522):2
Singh SC, Mishra SK, Srivastava RK, Gopal R (2010) Optical properties of selenium quantum dots produced with laser irradiation of water suspended Se nanoparticles. J Phys Chem C 114(41):17374–17384
Skalickova S, Milosavljevic V, Cihalova K, Horky P, Richtera L, Adam V (2017) Selenium nanoparticles as a nutritional supplement. Nutrition 33:83–90
Soflaei S, Dalimi A, Abdoli A, Kamali M, Nasiri V, Shakibaie M, Tat M (2012) Anti-leishmanial activities of selenium nanoparticles and selenium dioxide on Leishmania infantum. Comp Clin Pathol 23(1):15–20
Sonkusre P, Singh Cameotra S (2015) Biogenic selenium nanoparticles inhibit Staphylococcus aureus adherence on different surfaces. Colloids Surf B Biointerfaces 136:1051–1057
Sowndarya P, Ramkumar G, Shivakumar MS (2017) Green synthesis of selenium nanoparticles conjugated Clausena dentata plant leaf extract and their insecticidal potential against mosquito vectors. Artif Cells Nanomed Biotechnol 45(8):1490–1495
Spyridopoulou K, Aindelis G, Pappa A, Chlichlia K (2021a) Anticancer activity of biogenic selenium nanoparticles: apoptotic and immunogenic cell death markers in colon cancer cells. Cancers 13(21):5335
Spyridopoulou K, Tryfonopoulou E, Aindelis G, Ypsilantis P, Sarafidis C, Kalogirou O, Chlichlia K (2021b) Biogenic selenium nanoparticles produced by Lactobacillus casei ATCC 393 inhibit colon cancer cell growth in vitro and in vivo. Nanoscale Adv 3(9):2516–2528
Srivastava N, Mukhopadhyay M (2015) Green synthesis and structural characterization of selenium nanoparticles and assessment of their antimicrobial property. Bioprocess Biosyst Eng 38(9):1723–1730
Stevanović M, Filipović N, Djurdjević J, Lukić M, Milenković M, Boccaccini A (2015) 45S5Bioglass®-based scaffolds coated with selenium nanoparticles or with poly(lactide-co-glycolide)/selenium particles: processing, evaluation and antibacterial activity. Colloids Surf, B 132:208–215
Sun Y, Chen D, Pan Y, Qu W, Hao H, Wang X, Liu Z, **e S (2019) Nanoparticles for antiparasitic drug delivery. Drug Deliv 26(1):1206–1221
Tan VLC, Hinchman A, Williams R, Tran PA, Fox K (2018) Nanostructured biomedical selenium at the biological interface (Review). Biointerphases 13(6):06D301
Torres SK, Campos VL, León CG, Rodríguez-Llamazares SM, Rojas SM, González M, Smith C, Mondaca MA (2012) Biosynthesis of selenium nanoparticles by Pantoea agglomerans and their antioxidant activity. J Nanopart Res 14(11):1–9
Tran PA, Webster TJ (2011) Selenium nanoparticles inhibit Staphylococcus aureus growth. Int J Nanomed 6:1553–1558
Tugarova AV, Mamchenkova PV, Khanadeev VA, Kamnev AA (2020) Selenite reduction by the rhizobacterium Azospirillum brasilense, synthesis of extracellular selenium nanoparticles and their characterisation. New Biotechnol 58:17–24
Van Thiel DH, George M, Moore CM (2012) Fungal infections: their diagnosis and treatment in transplant recipients. Int J Hepatol 2012:106923
Vekariya KK, Kaur J, Tikoo K (2013) Alleviating anastrozole induced bone toxicity by selenium nanoparticles in SD rats. Toxicol Appl Pharmacol 268(2):212–220
Verma P (2017) Minimum Biofilm Eradication Concentration (MBEC) assay of silver and selenium nanoparticles against biofilm forming Staphylococcus aureus. J Med Sci Clin Res. https://doi.org/10.18535/jmscr/v5i4.77
Verma P, Maheshwari SK (2018) Preparation of sliver and selenium nanoparticles and its characterization by dynamic light scattering and scanning electron microscopy. J Microsc Ultrastruct 6(4):182
Vetchinkina E, Loshchinina E, Kursky V, Nikitina V (2013) Reduction of organic and inorganic selenium compounds by the edible medicinal basidiomycete Lentinula edodes and the accumulation of elemental selenium nanoparticles in its mycelium. J Microbiol 51(6):829–835
Wadhwani SA, Shedbalkar UU, Singh R, Chopade BA (2016) Biogenic selenium nanoparticles: current status and future prospects. Appl Microbiol Biotechnol 100(6):2555–2566
Wali AT (2019) Biosynthesis, characterization and bioactivity of selenium nanoparticles synthesized by propolis: 1Ahmed Thamer Wali1 and 2Majida AJ Alqayim. Iraqi J Vet Med 43(1):197–209
Wang Q, Webster TJ (2012) Nanostructured selenium for preventing biofilm formation on polycarbonate medical devices. J Biomed Mater Res A 100(12):3205–3210
Wang Y, **a Y (2004) Bottom-up and top-down approaches to the synthesis of monodispersed spherical colloids of low melting-point metals. Nano Lett 4(10):2047–2050
Wang H, Wei W, Zhang SY, Shen YX, Yue L, Wang NP, Xu SY (2005) Melatonin-selenium nanoparticles inhibit oxidative stress and protect against hepatic injury induced by Bacillus Calmette-Guerin/lipopolysaccharide in mice. J Pineal Res 39(2):156–163
Wang H, Zhang J, Yu H (2007) Elemental selenium at nano size possesses lower toxicity without compromising the fundamental effect on selenoenzymes: comparison with selenomethionine in mice. Free Radical Biol Med 42(10):1524–1533
Wang Q, Larese-Casanova P, Webster TJ (2015) Inhibition of various gram-positive and gram-negative bacteria growth on selenium nanoparticle coated paper towels. Int J Nanomed 10:2885–2894
Wang Q, Mejia Jaramillo A, Pavon JJ, Webster TJ (2016) Red selenium nanoparticles and gray selenium nanorods as antibacterial coatings for PEEK medical devices. J Biomed Mater Res B Appl Biomater 104(7):1352–1358
Wang C, Chen H, Chen D, Zhao M, Lin Z, Guo M, Xu T, Chen Y, Hua L, Lin T (2020) The inhibition of H1N1 influenza virus-induced apoptosis by surface decoration of selenium nanoparticles with β-thujaplicin through reactive oxygen species-mediated AKT and p53 signaling pathways. ACS Omega 5(47):30633–30642
Weekley CM, Harris HH (2013) Which form is that? The importance of selenium speciation and metabolism in the prevention and treatment of disease. Chem Soc Rev 42(23):8870–8894
** G, **ong K, Zhao Q, Zhang R, Zhang H, Qian Y (2006) Nucleation−dissolution−recrystallization: a new growth mechanism for t-selenium nanotubes. Cryst Growth Des 6(2):577–582
**a IF, Kong H-K, Wu MM, Lu Y, Wong K-H, Kwok KW (2022) Selenium Nanoparticles (SeNPs) immunomodulation is more than redox improvement: serum proteomics and transcriptomic analyses. Antioxidants 11(5):964
Yang F, Tang Q, Zhong X, Bai Y, Chen T, Zhang Y, Li Y, Zheng W (2012) Surface decoration by Spirulina polysaccharide enhances the cellular uptake and anticancer efficacy of selenium nanoparticles. Int J Nanomed 7:835–844
Yang X, Zhang W, Zhao Z, Li N, Mou Z, Sun D, Cai Y, Wang W, Lin Y (2017) Quercetin loading CdSe/ZnS nanoparticles as efficient antibacterial and anticancer materials. J Inorg Biochem 167:36–48
Yanhua W, Hao H, Li Y, Zhang S (2016) Selenium-substituted hydroxyapatite nanoparticles and their in vivo antitumor effect on hepatocellular carcinoma. Colloids Surf B Biointerfaces 140:297–306
Yazdi MH, Mahdavi M, Varastehmoradi B, Faramarzi MA, Shahverdi AR (2012) The immunostimulatory effect of biogenic selenium nanoparticles on the 4T1 breast cancer model: an in vivo study. Biol Trace Elem Res 149(1):22–28
Yazdi MH, Mahdavi M, Faghfuri E, Faramarzi MA, Sepehrizadeh Z, Hassan ZM, Gholami M, Shahverdi AR (2015) Th1 immune response induction by biogenic selenium nanoparticles in mice with breast cancer: preliminary vaccine model. Iran J Biotechnol 13(2):1–9
Yin T, Yang L, Liu Y, Zhou X, Sun J, Liu J (2015) Sialic acid (SA)-modified selenium nanoparticles coated with a high blood-brain barrier permeability peptide-B6 peptide for potential use in Alzheimer’s disease. Acta Biomater 25:172–183
Yip J, Liu L, Wong KH, Leung P, Marcus Yuen CW, Cheung M-C (2014) Investigation of antifungal and antibacterial effects of fabric padded with highly stable selenium nanoparticles. J Appl Polym Sci. https://doi.org/10.1002/app.40728
Yu L, Sun L, Nan Y, Zhu LY (2011) Protection from H1N1 influenza virus infections in mice by supplementation with selenium: a comparison with selenium-deficient mice. Biol Trace Elem Res 141(1–3):254–261
Zare B, Babaie S, Setayesh N, Shahverdi AR (2013) Isolation and characterization of a fungus for extracellular synthesis of small selenium nanoparticles. Nanomed J 1(1):13–19
Zhang C, Zhai X, Zhao G, Ren F, Leng X (2015) Synthesis, characterization, and controlled release of selenium nanoparticles stabilized by chitosan of different molecular weights. Carbohydr Polym 134:158–166
Zhang H, Zhou H, Bai J, Li Y, Yang J, Ma Q, Qu Y (2019) Biosynthesis of selenium nanoparticles mediated by fungus Mariannaea sp. HJ and their characterization. Colloids Surf, A 571:9–16
Zhang H, Li Z, Dai C, Wang P, Fan S, Yu B, Qu Y (2021) Antibacterial properties and mechanism of selenium nanoparticles synthesized by Providencia sp. DCX. Environ Res 194:110630
Zonaro E, Lampis S, Turner RJ, Qazi SJ, Vallini G (2015) Biogenic selenium and tellurium nanoparticles synthesized by environmental microbial isolates efficaciously inhibit bacterial planktonic cultures and biofilms. Front Microbiol 6:584
Acknowledgements
We would like to thank the Pharmaceutical Sciences and Cosmetic Products Research Center, Kerman University of Medical Sciences, (Kerman, Iran) for their support (400001192).
Funding
Kerman University of Medical Sciences, 400001192, Mojtaba Shakibaie.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they do not have any conflict of interest in this study.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Satarzadeh, N., Sadeghi Dousari, A., Amirheidari, B. et al. An insight into biofabrication of selenium nanostructures and their biomedical application. 3 Biotech 13, 79 (2023). https://doi.org/10.1007/s13205-023-03476-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13205-023-03476-4