Abstract
Oral medical wastewater with heavy metal ions (such as plumbum, Pb2+) is regarded as the main pollutant produced in the oral cavity diagnosis, and the treatment process can pose a serious threat to human health. The removal of Pb2+ from oral medical wastewater facing major difficulties and challenges. Therefore, it is of great significance to take effective measures to remove Pb2+ by using effective methods. A new activated three-dimensional framework carbon (3D AFC), regarded as the main material to remove Pb2+ in the oral medical wastewater, has been fabricated successfully. In this experiment, the effects of 3D AFC absorbing Pb2+ under different conditions (including solid-to-liquid ratio, pH, ionic strength, contact time, and initial concentration, etc.) were discussed. And the result revealed that the adsorption kinetics process of Pb2+ on 3D AFC conformed to the pseudo-second-order model and the adsorption isotherm conformed to the Freundlich model. Under the condition that pH = 5.5 and T = 298 k, the calculated maximum adsorption capacity of 3D AFC for Pb2+ was 270.88 mg/g. In practical application, it has strong adsorption ability for Pb2+ in oral medical wastewater. Thus, 3D AFC shows promise for Pb2+ remove and recovery applications because of high adsorption capacity for Pb2+ in oral medical wastewater due to its high specific surface area, outstanding three-dimensional network structure.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Oral medical wastewater containing a large amount of heavy metal ions will be generated in the process of oral diagnosis and treatment (including the removal of old prosthesis, tooth preparation, prosthesis trial wearing and the use of some metal instruments), such as cobalt ion (Co2+), chromium ion (Cr2+), molybdenum ion (Mo2+), silicon ion (Si2+), cadmium ion (Cd2+), and plumbum ion (Pb2+). If the oral medical wastewater containing these heavy metal ions was discharged into the groundwater system through the water system of the dental chair without any special treatment, it will not only increase the difficulty of water purification, but also threat to human health, cause many diseases, such as anemia, kidney dysfunction, brain tissue damage and even death (Shilu et al. 2000). Pb2+, one of the main heavy metal ion pollutants in oral medical wastewater, has the concentration as high as 4–20 mg/g (Bhattacharjee et al. 2003), far higher than the limit of Pb2+concentration in the water released by the World Health Organization (0.01 mg/L) (Rotimi et al. 2011). Therefore, it is of great significance to take effective measures to remove Pb2+ in oral medical wastewater.
Currently, various methods can be adopted to remove Pb2+ in oral medical wastewater, including ion exchange (Pember et al. 2016), liquid–liquid extraction (Soniya et al. 2015), membrane filtration (Li et al. 2016), biosorption (Oguntimein et al. 2015), electrodialysis (Deghles et al. 2016), electrocoagulation (Ghanbari et al. 2015), and adsorption technology (Zhu et al. 2018; Ghasemi et al. 2018). However, most of them have some disadvantages, such as high operating cost, low processing efficiency, and the possibility of secondary pollution like toxic products (Zhu et al. 2018), thus limiting their applications in practice. Adsorption technology, however, has become one of the most popular and promising method due to the properties of low cost, high adsorption efficiency, and convenient operation (Zhu et al. 2018). For instance, porous hydroxyapatite-carbon composite material (Zhu et al. 2018) and a new Sawdust/MNP/PEI nanocomposite material (Ghasemi et al. 2018) have been successfully used for the removal of Pb2+ in aqueous solution.
Activated carbon, with large specific surface area (250–3000 m2/g), high stability, especially no degradation risk in aqueous solution, has been acknowledged as one of the most popular and widely used adsorbent in the treatment of polluted wastewater in the world. There have been many reports discussing the application of activated carbon adsorbents in the removal of Pb2+, but the adsorption capacity varies. For example, the activated carbon before functionalization has the limited adsorption capacity for Pb2+, being 20.3 mg/g only (Asuquo et al. 2017). The MOS2@Kaolin type activated carbon adsorption material (Yuan et al. 2020) greatly improved the adsorption capacity of Pb2+. A new type of protonated graphite carbon nitride and acid activated montmorillonite (g-C3N4/Mt) composite material was fabricated by Wan et al. (Wan et al. 2019), and the adsorption capacity of Pb2+ was 124.2 mg/g. A dual-ecological pistachio wood-derived activated carbon prepared by a two-stage process (PWAC-2) had an adsorption capacity of 190.2 mg/g for Pb2+ (Sajjadi et al. 2019). In a word, though the activated carbon adsorption materials are fabricated by different methods, they all possess good adsorption capacity for Pb2+. However, the removal of Pb2+ from oral medical wastewater has not been studied. And these activated carbon adsorption materials reported have more or less disadvantages, such as demanding preparation conditions, complicated fabricating processes, and existing difficulty in mass production, hence limiting the applications in practice. In our previous experiments, a new method for the large-scale preparation of three-dimensional framework carbon (3D FC) by directly calcining the sodium citrate without any additional carbon source, template or catalyst was reported in clean energy field, and this method could making the whole process simple and inexpensive (Yang et al. 2018). But, the 3D FC prepared by this method do not contain oxygen-containing functional groups, and it is great significance to evaluate whether the activated 3D FC (3D AFC) can be used for the removal of Pb2+ from oral medical wastewater.
So, in this study, for the first time to explore the 3D AFC to the removal of Pb2+ in oral medical wastewater and discuss the influencing factors like ionic strength, pH value, contact time and solid–liquid ratio on the adsorption of Pb2+ on the 3D AFC surface.
Materials and methods
Material
Sulfuric acid (98%) and nitric acid (70%) were purchased from Sinopharm Chemical Reagent Network. Pb(NO3)2 was purchased from Aladdin. And the pH meter model was pHS-3C (Shanghai, China). The Ultraviolet and visible spectrophotometry (UV–VIS) adsorption spectrometer model was UV-1800, MAPADA (Shanghai, China).
The fabrication and activation treatment of 3D FC
The fabrication of 3D FC was referred to the methods previously reported by our research group (Yang et al. 2018). The specific experimental steps were as follows: The sodium citrate was calcinated at 1000 °C for 1 h (The heating rate was 5 °C/min) under the protection of nitrogen. And the black product obtained from the above steps was cleaned alternately with deionized water and ethanol at 80 °C until the residual sodium, salt and impurities were removed. Finally, it was dried at 100 °C, hence obtaining the 3D FC. The activation steps of 3D FC were as follows: 2.0 g 3D FC sample was weighed and mixed in sulfuric acid (98%) and nitric acid (70%) with a volume ratio of 1:3, and then it was poured into the reactor. The sample was kept at 100 °C for 2 h. After cooling to room temperature, it was fully cleaned with a large amount of deionized water until pH value reached to 7. When the drying process was completed, the activated 3D FC was obtained, marking as 3D AFC.
Batch adsorption experiment
The 1 g/L Pb2+ standard solution was fabricated using Pb(NO3)2 as the raw material, and all the batch adsorption experiments were completed in polyethylene centrifuge tubes. HCl or NaOH solutions with different concentrations (0.001, 0.01, 0.1, 1.0, and 2.0 mol/L) were selected to adjust the pH value of the solution. 0.0020 g 3D AFC (m/V = 0.4 g/L) was added into the 10 mL polyethylene centrifuge tube and pH value was adjusted to 5.5, ensuring that the total volume of each sample was 5 mL by adding deionized water. Meanwhile, the sample was placed in a thermostatic oscillator for 12 h and filtered. Next, the filtered supernatant was taken and the concentration of Pb2+ ion was determined by flamless atomic absorption spectrophotometry (FAAS). Finally, the effects of solid–liquid ratio, contact time, pH value, ionic strength and initial concentration on the adsorption behavior of Pb2+ on 3D AFC surface were evaluated. The adsorption percentage and adsorption capacity of Pb2+ were calculated according to formulas (1) and (2).
In the equations, C0 and Ce represent the initial and equilibrium concentrations (mg/L) of Pb2+ in aqueous phase, respectively. m and V designate the weight (g) of the 3D AFC and the solution volume (L), respectively.
Experimental for Pb2+ selectivity in oral medical wastewater
The oral medical wastewater was extracted randomly from the Hospital of Stomatology, Lanzhou University, check and measure the composition and concentration of the main polluted metal ions, that is, Cd2+ 0.00235 mg/L, Co2+ 2.678 mg/L, Cr2+ 0.00268 mg/L, Pb2+ 0.00835 mg/L, Sr2+ 2.6515 mg/L. And then, a total volume of 5 mL mixed solution was adopted, and the pH value was adjusted to 5.5 at room temperature. 3D AFC material sample of 0.002 g was placed in the sample of the oral medical wastewater, fully oscillated, and fully adsorbed for 48 h. The sample solution was taken to analyze the remained concentrations of each ions.
Characterization
The morphology structure and chemical composition of 3D AFC were characterized by scanning electron microscopy (SEM, Hitachi s4800), X-ray diffraction (XRD, panalytical x'pert pro, Cuk α), and Fourier-transform infrared spectroscopy (FTIR, spectrum 100 Perkin Elmer, USA) before and after Pb2+adsorption. The concentration of Pb2+ in solution before and after the adsorption was measured by UV–Vis adsorption spectrometer (uv-1800, mapada, Shanghai, China).
Results and discussions
Characterization of the 3D AFC before and after Pb2+ adsoprtion
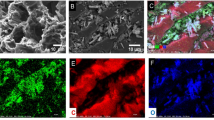
The morphology of 3D AFC was characterized by SEM. As shown in Fig. 1, the SEM results of the fabricated 3D AFC show that the ultra-thin and curved carbon sheets are interconnected to form a porous three-dimensional network structure, and the pore structure size is 50–80 nm. In addition, good three-dimensional pore network structure is conducive to improve the adsorption performance (Cao et al. 2020).
The chemical composition of 3D AFC before and after the adsorption of Pb2+ was characterized by XRD and FTIR (Fig. 2), respectively. In the XRD results (Fig. 2a), a large peak appears at 2θ = 26.3° before the adsorption of Pb2+, corresponding to amorphous carbon (Prabhakar et al. 2020; Zhang et al. 2015). After the adsorption of Pb2+, the peaks at 2θ = 23.2°, 36.1°, 39.5°, 43.2°, 47.5° and 48.6° correspond to the standard diffraction card (jcpds6-0452) (Tartaja et al. 2001), respectively, indicating that surface precipitation might be one of the mechanisms for 3D AFC to adsorb Pb2+ (Farzin et al. 2020; Azouaou et al. 2014). In the FTIR results (Fig. 2b), before the adsorption of Pb2+, a strong broad peak at 3448.53 cm−1 corresponds to the stretching vibration peak of –OH (Azouaou et al. 2014; Ai et al. 2018a, Ai Y, Liu Y, Lan W et al (2018) The effect of pH on the U(VI) sorption on graphene oxide (GO): a theoretical study. Chem Eng J 343:460–466. https://doi.org/10.1016/j.cej.2018.03.027 Ai Y, Liu Y, Lan W, ** J, **ng J, Zou Y et al (2018) The effect of pH on the U(VI) sorption on graphene oxide (GO): a theoretical study. Chem Eng J 343:460–466. https://doi.org/10.1016/j.cej.2018.03.027 Asuquo E, Martin A, Nzerem P et al (2017) Adsorption of Cd(II) and Pb(II) ions from aqueous solutions using mesoporous activated carbon adsorbent: equilibrium, kinetics and characterisation studies. J Environ Chem Eng 5(1):679–98. https://doi.org/10.1016/j.jece.2016.12.043 Ayben K (2003) The effect of various cations and pH on the adsorption of U(VI) on Amberlite IR-118H resin. Appl Radiat Isotopes 58:713–717. https://doi.org/10.1016/S0969-8043(03)00116-7 Azouaou N, Sadaoui Z, Mokaddem H (2014) Adsorption of lead from aqueous solution onto untreated orange barks: equilibrium, kinetics and thermodynamics. E3S Web of Conferences 1:41034. https://doi.org/10.1051/e3sconf/20130141034 Bhattacharjee S, Chakrabarty S, Maity S et al (2003) Removal of lead from contaminated water bodies using sea nodule as an adsorbent. Water Res 37(16):3954–66. https://doi.org/10.1016/S0043-1354(03)00315-4 Cao L, Fan F (2020) Deformation and instability of three-dimensional graphene honeycombs under in-plane compression: atomistic simulations. Extreme Mech Lett 39:100861. https://doi.org/10.1016/j.eml.2020.100861 Chen L, Lu S (2008) Sorption and desorption of radiocobalt on montmorillonite-Effects of pH, ionic strength and fulvic acid. Appl Radiat Isotopes 66(3):288–94. https://doi.org/10.1016/j.apradiso.2007.07.031 Deghles A, Kurt U (2016) Treatment of tannery wastewater by a hybrid electrocoagulation/electrodialysis process. Chem Eng Process 104:43–50. https://doi.org/10.1016/j.cep.2016.02.009 Farzin S et al (2020) Introducing a framework for modeling of drug electrochemical removal from wastewater based on data mining algorithms, scatter interpolation method, and multi criteria decision analysis (DID). J Clean Prod 266:122075. https://doi.org/10.1016/j.jclepro.2020.122075 Ghanbari F, Moradi M (2015) A comparative study of electrocoagulation, electrochemical Fenton, electro-Fenton and peroxi-coagulation for decolorization of real textile wastewater: electrical energy consumption and biodegradability improvement. J Environ Chem Eng 3(1):499–506. https://doi.org/10.1016/j.jece.2014.12.018 Ghasemi A et al (2018) Preparation and characterization of a new Sawdust/MNP/PEI Nanocomposite and its applications for removing Pb (II) ions from aqueous solution. Water Sci Technol 78(12):2469–2480. https://doi.org/10.2166/wst.2018.521 Ho Y (2006) Review of second-order models for adsorption systems. J Hazard Mater 136(3):681–689. https://doi.org/10.1002/chin.200648222 Kilislioglu A (2003) The effect of various cations and pH on the adsorption of U(VI) on Amberlite IR-118H resin. Appl Radiat Isotopes 58(6):713–7. https://doi.org/10.1016/S0969-8043(03)00116-7 Li C, Song C, Tao P et al (2016) Enhanced separation performance of coal-based carbon membranes coupled with an electric field for oily wastewater treatment. Sep Purif Technol 168:47–56. https://doi.org/10.1016/j.seppur.2016.05.020 Li XC et al (2019) Immobilization of phosphorus in sediments by nano zero-valent iron (nZVI) from the view of mineral composition. Sci Total Environ 694:133695. https://doi.org/10.1016/j.scitotenv.2019.133695 Liu X, Sun J, Xu X et al (2019) Adsorption and desorption of U(VI) on different-size graphene oxide. Chem Eng J 360:941–50. https://doi.org/10.1016/j.cej.2018.04.050 Oguntimein GB (2015) Biosorption of dye from textile wastewater effluent onto alkali treated dried sunflower seed hull and design of a batch adsorber. J Environ Chem Eng 3(4):2647–61. https://doi.org/10.1016/j.jece.2015.09.028 Okeola FO (2010) Comparison of Freundlich and Langmuir isotherms for adsorption of methylene blue by agrowaste derived activated carbon. Adv Environ Bio 4(3):329–335 Pember N, Millar GJ, Couperthwaite SJ et al (2016) BDST modelling of sodium ion exchange column behaviour with strong acid cation resin in relation to coal seam water treatment. J Environ Chem Eng 4(2):2216–24. https://doi.org/10.1016/j.jece.2016.04.002 Prabhakar R et al (2020) Use of adsorption-influencing parameters for designing the batch adsorber and neural network-based prediction modelling for the aqueous arsenate removal using combustion synthesised nano-alumina. Environ Sci Pollut Res 27(21):26367–26384. https://doi.org/10.1007/s11356-020-08975-y Rotimi IA, Chuks OP (2011) Pollution effect of food and beverages effluents on the Alaro river in Ibadan City. Nigeria. B Chem Soc Ethiopia 25(3):347-360(3). https://doi.org/10.4314/bcse.v25i3.68587 Sajjad SK, Iqbal A (2017) Study of graphene oxide structural features for catalytic, antibacterial, gas sensing, and metals decontamination environmental applications. ACS Appl Mater Inter 9:43393–43414. https://doi.org/10.1021/acsami.7b08232 Sajjadi SA, Meknati A, Lima EC et al (2019) A novel route for preparation of chemically activated carbon from pistachio wood for highly efficient Pb(II) sorption. J Environ Manage 236:34–44. https://doi.org/10.1016/j.jenvman.2019.01.087 Shilu TY, Tippawan P (2000) Environmental lead exposure: a public health problem of global dimensions. B World Health Organ 9:1068–1077. https://doi.org/10.1146/annurev.publhealth.21.1.659 Soniya M, Muthuraman G (2015) Comparative study between liquid–liquid extraction and bulk liquid membrane for the removal and recovery of methylene blue from wastewater. J Ind Eng Chem 30:266–73. https://doi.org/10.1016/j.jiec.2015.05.032 Tartaja J, Fernández JF, Villafuerte-Castrejón ME (2001) Preparation of PbTiO3 by seeding-assisted chemical sol-gel. Mater Res Bull 36(3–4):479–486. https://doi.org/10.1016/S0025-5408(01)00543-8 Vijay S et al (2019) Photocatalytic degradation of irgalite violet dye using nickel ferrite nanoparticles. J Water Supply Res T 68(8):666–674. https://doi.org/10.2166/aqua.2019.039 Wan X, Khan MA, Wang F et al (2019) Facile synthesis of protonated g-C3N4 and acid-activated montmorillonite composite with efficient adsorption capacity for PO43-and Pb(II). Chem Eng Res Des 152:95–105. https://doi.org/10.1016/j.cherd.2019.09.019 **e Y, Chen C, Ren X et al (2019) Emerging natural and tailored materials for uranium-contaminated water treatment and environmental remediation. Prog Mater Sci 103:180–234. https://doi.org/10.1016/j.pmatsci.2019.01.005 Yang B, Chen J, Lei S et al (2018) Spontaneous growth of 3D framework carbon from sodium citrate for high Energy-and power-density and long-life sodium-ion hybrid capacitors. Adv Energy Mater 8(10):1702409. https://doi.org/10.1002/aenm.201702409 Yuan W, Kuang J, Yu M et al (2020) Facile preparation of MoS2@Kaolin composite by one-step hydrothermal method for efficient removal of Pb(II). J Hazard Mater. https://doi.org/10.1016/j.jhazmat.2020.124261 Zhang R, Chen C, Li J et al (2015) Investigation of interaction between U(VI) and carbonaceous nanofibers by batch experiments and modeling study. J Colloid Interf Sci 460:237–46. https://doi.org/10.1016/j.jcis.2015.08.073 Zhao D, Gao X, Chen S, **e F, Feng S, Alsaedi A et al (2018) Interaction between U(VI) with sulfhydryl groups functionalized graphene oxides investigated by batch and spectroscopic techniques. J Colloid Interf Sci 524:129–38. https://doi.org/10.1016/j.jcis.2018.04.012 Zhu Y, Jiang Y, Zhu Z et al (2018) Preparation of a porous hydroxyapatite-carbon composite with the bio-template of sugarcane top stems and its use for the Pb(II) removal. J Clean Prod 187:650–61. https://doi.org/10.1016/j.jclepro.2018.03.275References
Funding
We would like to thank the Chinese Stomatological Association Western Stomatology Clinical Research Fund Project (CSA-W2018-07) and National Natural Science Foundation of China (20190431) for their financial supports.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Song, F., Wang, N., Hu, Z. et al. Effective removal of Pb2+ from oral medical wastewater via an activated three-dimensional framework carbon (3D AFC). Appl Water Sci 11, 157 (2021). https://doi.org/10.1007/s13201-021-01486-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13201-021-01486-2