Abstract
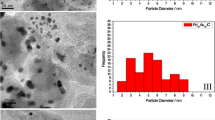
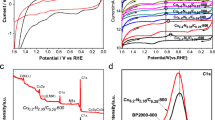
The electro-oxidation of glycerol (EOG) has gained wide attention as an alternative to producing value-added chemicals for glycerol valorization. In this study, a multimetallic electrocatalyst containing copper (Cu), nickel (Ni), tin (Sn), and phosphorus (P) was supported on a carbon catalyzed substrate (CCS) using an electroless deposition technique and evaluated for EOG. The effect of the electroless deposition time (15, 30, and 45 min) was also studied. Characterization of the CuNiSnP/CCS electrocatalyst via X-ray diffraction, scanning electron microscopy, and inductively coupled plasma optical emission spectroscopy revealed the formation of a thin-film morphology containing Cu as the main species on the surface and covering the carbon substrate. The electrochemical performance evaluation showed that the electrocatalyst obtained after 30 min of electroless deposition produced the maximum current density (6.5 mA/cm2). The multimetallic composition of CuNiSnP/CCS provided better reaction performance than related tri- (CuNiP/CCS and NiSnP/CCS), bi- (NiP/CCS), and monometallic (Cu/CCS) composites according to the peak current densities for the forward (if) and backward (ib) oxidation, the if/ib ratio, and the onset potential. Furthermore, CuNiSnP/CCS exhibited more stable and stronger resistance to poisoning. Overall, this study demonstrates the potential of the new electrode material CuNiSnP/CCS as an effective electrocatalyst for EOG.
Graphical Abstract















Similar content being viewed by others
Data Availability
Not applicable.
References
C.A.G. Quispe, C.J.R. Coronado, J.A.C. Jr, Glycerol: Production, consumption, prices, characterization and new trends in combustion. Renew. Sust. Energ. Rev. 27, 475–493 (2013). https://doi.org/10.1016/j.rser.2013.06.017
R.G.D. Silva, S.A. Neto, K.B. Kokoh, A.R.D. Andrade, Electroconversion of glycerol in alkaline medium: From generation of energy to formation of value-added products. J Power Sources 351, 174–182 (2017). https://doi.org/10.1016/j.jpowsour.2017.03.101
B. Katryniok, H. Kimura, E. Skrzyńska, J.-S. Girardon, P. Fongarland, M. Capron, R. Ducoulombier, N. Mimura, S. Paul, F. Dumeignil, Selective catalytic oxidation of glycerol: perspectives for high value chemicals. Green Chem. 13(8), 1960–1979 (2011). https://doi.org/10.1039/C1GC15320J
D. Liu, J.-C. Liu, W. Cai, J. Ma, H.B. Yang, H. **ao, J. Li, Y. **ong, Y. Huang, B. Liu, Selective photoelectrochemical oxidation of glycerol to high value-added dihydroxyacetone. Nat Commun 10, 1–8 (2019). https://doi.org/10.1038/s41467-019-09788-5
R. Ciriminna, M. Pagliaro, Oxidation of tartronic acid and dihydroxyacetone to sodium mesoxalate mediated by TEMPO. Tetrahedron Lett, 45(34), 6381–6383 (2004). https://doi.org/10.1016/j.tetlet.2004.07.021
A.A.A. Raman, H.W. Tan, and A. Buthiyappan, Two-Step Purification of Glycerol as a Value Added by Product From the Biodiesel Production Process. Front. Chem. 774–783 (2019). https://doi.org/10.3389/fchem.2019.00774
T. Valliyappan, N. Bakhshi, A.K. Dalai, Pyrolysis of glycerol for the production of hydrogen or syn gas. Bioresour. Technol. 99(10), 4476–4483 (2008). https://doi.org/10.1016/j.biortech.2007.08.069
H. Sulistyo, I. Hapsari, Budhijanto, W.B. Sediawan, S.S. Rahayu, M.M. Azis. Heterogeneous catalytic reaction of glycerol with acetone for solketal production. in MATEC Web of Conferences. (2019)
G. Bagnato, A. Iulianelli, A. Sanna, A. Basile, Glycerol Production and Transformation: A Critical Review with Particular Emphasis on Glycerol Reforming Reaction for Producing Hydrogen in Conventional and Membrane Reactors. Membranes 7, 1–31 (2017). https://doi.org/10.3390/membranes7020017
S.K. Green, J. Lee, H.J. Kim, G.A. Tompsett, W.B. Kim, G.W. Huber, The electrocatalytic hydrogenation of furanic compounds in a continuous electrocatalytic membrane reactor Green Chem. 15(7), 1869–1879 (2013). https://doi.org/10.1039/C3GC00090G
J.J. Roylance, T.W. Kim, K.-S. Chon, Efficient and Selective Electrochemical and Photoelectrochemical Reduction of 5-Hydroxymethylfurfural to 2,5-Bis(hydroxymethyl)furan using Water as the Hydrogen Source. ACS Catal. 6(3), 1840–1847 (2016). https://doi.org/10.1021/acscatal.5b02586
A.E. Roz, P. Fongarland, F. Dumeignil, M. Capron, Glycerol to Glyceraldehyde Oxidation Reaction Over Pt-Based Catalysts Under Base-Free Conditions. Front. Chem. 7, 1–9 (2019). https://doi.org/10.3389/fchem.2019.00156
R.M. Castagna, J.M. Sieben, A.E. Alvarez, M.M.E. Duarte, Electrooxidation of ethanol and glycerol on carbon supported PtCu nanoparticles. Int J Hydrogen Energy 44, 5970–5972 (2019). https://doi.org/10.1016/j.ijhydene.2019.01.090
Y. Liu, W. Yu, D. Raciti, D.H. Gracias, C. Wang*, Electrocatalytic Oxidation of Glycerol on Platinum. J. Phys. Chem. C 123, 426–432 (2019). https://doi.org/10.1002/celc.201900311
B. Habibi, N. Delnavaz, Electrooxidation of glycerol on nickel and nickel alloy (Ni–Cu and Ni–Co) nanoparticles in alkaline media. RSC Adv. 6, 31797–31806 (2016). https://doi.org/10.1039/c5ra26006j
Y. Li, X. Wei, L. Chen, J. Shi, M. He, Nickel-molybdenum nitride nanoplate electrocatalysts for concurrent electrolytic hydrogen and formate productions. Nat Commun 10, 5335–5347 (2019). https://doi.org/10.1038/s41467-019-13375-z
C.L. Bracey, P.R. Ellis, and G.J. Hutchings, Application of copper-gold alloys in catalysis: Current status and future perspectives. Chem. Soc. Rev. 38 (2009). https://doi.org/10.1039/B817729P
T. Eisaa, H.O. Mohameda, Y.-J. Choi, S.-G. Park, R. Ali, M. Ali, Abdelkareem, Sang-EunOh, and Kyu-JungCha, Nickel nanorods over nickel foam as standalone anode for direct alkaline methanol and ethanol fuel cell. Int J Hydrogen Energy 45(10), 5948–5959 (2020). https://doi.org/10.1016/j.ijhydene.2019.08.071
M.E. Ghaith, G.A. El-Nagar, M.G.A. El-Moghny, H.H. Alalawy, M.E. El-Shakre, M.S. El-Deab, Electrocatalysis by design: Enhanced electro-oxidation of glycerol at NiOx nanoparticle modified 3D porous carbon felts. Int J Hydrogen Energy 45(16), 9658–9668 (2020). https://doi.org/10.1016/j.ijhydene.2020.01.213
N. Hidayati, K. Scott, Electro-oxidation of Ethanol on Carbon Supported PtSn and PtSnNi Catalysts. Bull. Chem. React. Eng. 11(1), 10–20 (2016). https://doi.org/10.9767/bcrec.11.1.399.10-20
R.O. Apaydin, B. Ebin, S. Gurmen. Direct production of nanostructured copper-nickel (Cu-Ni) alloy particles. In 3RD International advances in applied physics and materials science congress. Antalya, Turkey: AIP Publishing. (2013)
X. An, K. Li, and J. Tang, Cu2O/Reduced Graphene Oxide Composites for the Photocatalytic Conversion of CO2. ChemSusChem. 7, 1086–1093 1 (2014). https://doi.org/10.1002/cssc.201301194
J.Y. Zheng, T.-K. Van, A.U. Pawar, C.W. Kim, Y.S. Kang, One-step transformation of Cu to Cu2O in alkaline solution. RSC Adv. 4, 18616–18620 (2014). https://doi.org/10.1039/C4RA01174K
I. Ohno, O. Wakabayashi, S. Haruyama, Anodic Oxidation of Reductants in Electroless Plating. J. Electrochem. Soc. 132, 2323 (1985). https://doi.org/10.1149/1.2113572
J. Zhao, N. Li, G. Cui, J. Zhao, Study on Immersion Tin Process by Electrochemical Methods and Molecular Orbital Theory. J. Electrochem. Soc. 153, 848–853 (2006). https://doi.org/10.1149/1.2358119
M. Morimoto, Y. Takatsuji, R. Yamasaki, H. Hashimoto, I. Nakata, T. Sakakura, T. Haruyama, Electrodeposited Cu-Sn Alloy for Electrochemical CO2 Reduction to CO/HCOO. Electrocatalysis 9, 323–332 (2018). https://doi.org/10.1007/s12678-017-0434-2
H.W. Lee, N.M. Shinde, P.V. Shinde, J.M. Yun, P.K. Song, K.H. Kim, High energy and power density of self-grown CuS@Cu2O core-shell supercapattery positrode. J Solid State Electrochem. 23, 2609–2617 (2019). https://doi.org/10.1007/s10008-019-04351-0
Y. Zhu, T. Liu, L. Li, S. Song, R. Ding1, Nickel-based electrodes as catalysts for hydrogen evolution reaction in alkaline media. Ionics 24, 1121–1127 (2018). https://doi.org/10.1007/s11581-017-2270-z
X. Hao, J. Dong, X. Mu, J. Wei, C. Wang, W. Ke, Influence of Sn and Mo on Corrosion Behavior of Ferrite-pearlite Steel in the Simulated Bottom Plate Environment of Cargo Oil Tank. J Mater Sci Technol. 35, 799–811 (2019). https://doi.org/10.1016/j.jmst.2018.11.012
A.M.B. Honorato, M. Khalid, A.A.d.S. Curvelo, H. Varela, A.S. Shahgaldi, Trimetallic Nanoalloy of NiFeCo Embedded in Phosphidated Nitrogen Doped Carbon Catalyst for Efficient Electro-Oxidation of Kraft Lignin. Polymers 14 (2022). https://doi.org/10.3390/polym14183781
T. Susi, T. Pichler, P. Ayala, X-ray photoelectron spectroscopy of graphitic carbon nanomaterials doped with heteroatoms. Beilstein J Nanotechnol. 6, 177–192 (2015). https://doi.org/10.3762/bjnano.6.17
D.Z. Jeffery, G.A. Camara, The formation of carbon dioxide during glycerol electrooxidation in alkaline media: First spectroscopic evidences. Electrochem Commun 12, 1129–1132 (2010). https://doi.org/10.1016/j.elecom.2010.06.001
A. Zalineeva, A. Serov, M. Padilla, U. Martinez, K. Artyushkova, S. Baranton, C. Coutanceau, P.B. Atanassov, Self-Supported PdxBi Catalysts for the Electrooxidation of Glycerol in Alkaline Media. J. Am. Chem. Soc. 136, 3937–3945 (2014). https://doi.org/10.1021/ja412429f
H. Gao, S. Liao, Z. Liang, H. Liang, F. Luo, Anodic oxidation of ethanol on core-shell structured Ru@PtPd/C catalyst in alkaline media. J Power Sources 196(15), 6138–6143 (2011). https://doi.org/10.1016/j.jpowsour.2011.03.031
Y.H. Ahmad, A.T. Mohamed, K.M. Youssef, S. Kundu, K.A. Mkhoyan, S.Y. Al-Qaradawi, Rational synthesis of ternary PtIrNi nanocrystals with enhanced poisoning tolerance for electrochemical ethanol oxidation. Electrochem. commun. 101, 61–67 (2019). https://doi.org/10.1016/j.elecom.2019.03.001
H. Wang, X. Kou, J. Zhang, J. Li, Large scale synthesis and characterization of Ni nanoparticles by solution reaction method. Bull. Mater. Sci. 31, 97–100 (2008). https://doi.org/10.1007/S12034-008-0017-1
J. Sun, J. Yu, Q. Ma, F. Meng, X. Wei, Y. Sun, N. Tsubaki, Freezing copper as a noble metal–like catalyst for preliminary hydrogenation. Sci. Adv. 4, 1–10 (2018). https://doi.org/10.1126/sciadv.aau3275
A.R. Naghash, T.H. Etsell, S. Xu, XRD and XPS Study of Cu-Ni Interactions on Reduced Copper-Nickel-Aluminum Oxide Solid Solution Catalysts. Chem. Mater. 18, 2480–2488 (2006). https://doi.org/10.1021/cm051910o
J. Zhou, L. Yuan, J. Wang, L. Song, Y. You, R. Zhou, J. Zhang, and J. Xu, Combinational modulations of NiSe2 nanodendrites by phase engineering and iron-do** towards efficient oxygen evolution reaction. J. Mater. Chem. A 8, 8113–8120 (2020). https://doi.org/10.1039/D0TA00860E
O. Fayyaz, A.B. Radwan, M.H. Sliem, A.M. Abdullah, A. Hasan, R.A. Shakoor*, Investigating the Properties of Electrodeposited of Ni-P-ZrC Nanocomposite Coatings. ACS Omega, 6, 33310–33324 (2021). https://doi.org/10.1021/acsomega.1c03117
M. Alsabet, M. Grden, G. Jerkiewicz, Electrochemical Growth of Surface Oxides on Nickel. Part 3: Formation of β-NiOOH in Relation to the Polarization Potential, Polarization Time, and Temperature. Electrocatalysis 6, 60–71 (2015). https://doi.org/10.1007/s12678-011-0067-9
F. Caballero-Briones, J.M. Artes, I. Dı´ez-Pe´rez, P. Gorostiza, and F. Sanz, Direct Observation of the Valence Band Edge by in Situ ECSTM-ECTS in p-Type Cu2O Layers Prepared by Copper Anodization. J.Phys.Chem 113, 1028–1036 (2009). https://doi.org/10.1021/jp805915a
R. Ortiz, O.P. Márquez, J. Márquez, C. Gutiérrez, Necessity of Oxygenated Surface Species for the Electrooxidation of Methanol on Iridium. J. Phys. Chem. B. 100(20), 8389–8396 (1996). https://doi.org/10.1021/jp953185i
M.A.A. Rahim, H.B. Hassan, R.M.A. Hamid, A systematic study on the effect of OH− and Ni2+ ions on the electro-catalytic oxidation of methanol at Ni-S-1 electrode. J Power Sources 154(1), 59–65 (2006). https://doi.org/10.1016/j.jpowsour.2005.03.198
H.B. Hassan, Z. Abdel Hamid, H. Mona, Synthesis and performance of electroless Ni-P-TiCN composite coatings on Al substrate. Surf. Interface Anal. 46, 512–520 (2014). https://doi.org/10.1002/sia.5530
W. Chaitree, E.E. Kalu, Z. Liang, Y.D. Yeboaha, Effects of bath composition and thermal treatment on the performance of Co-Ni-Mo-P electrocatalyst supported on carbon for the electro- oxidation of ethanol. J. Alloys Compd. 860, 158404 (2021). https://doi.org/10.1016/j.jallcom.2020.158404
L.-S. Yuan, Y.-X. Zheng, M.-L. Jia, S.-J. Zhan, Nanoporous nickel-copper-phosphorus amorphous alloy film for methanol electro-oxidation in alkaline medium. Electrochim. Acta 154, 54–62 (2015). https://doi.org/10.1016/j.electacta.2014.12.055
A. Bard, L. Faulkner, Electrochemical methods Fundamentals and Applications. JOHN WILEY & SONS, INC. (2001)
V.L. Oliveira, C. Morais, K. Servat, T.W. Napporn, G. Tremiliosi-Filho, K.B. Kokoh, Glycerol oxidation on nickel based nanocatalysts in alkaline medium –Identification of the reaction products. J. Electroanal. Chem. 703, 56–62 (2013). https://doi.org/10.1016/j.jelechem.2013.05.021
V. Oliveira, C. Morais, K. Servat, T. Napporn, G. Tremiliosi-Filho, K. Kokoh, Studies of the reaction products resulted from glycerol electrooxidation on Ni-based materials in alkaline medium. Electrochim Acta 117, 255–262 (2014). https://doi.org/10.1016/j.electacta.2013.11.127
M. S.E., Houache, E. Cossar, S. Ntais, E.A. Baranova, Electrochemical modification of nickel surfaces for efficient glycerol electrooxidation. J. Power Sources 375, 310–319 (2018). https://doi.org/10.1016/j.jpowsour.2017.08.089
V.L. Oliveira, C. Morais, K. Servat, T.W. Napporn, P. Olivi, K.B. Kokoh, G. Tremiliosi-Filho, Kinetic Investigations of Glycerol Oxidation Reaction on Ni/C. Electrocatalysis 6, 447–454 (2015). https://doi.org/10.1007/s12678-015-0261-2
Acknowledgments
This work was supported by Office of the Permanent Secretary Ministry of Higher Education, Science Research and Innovation (OPS MHESI), Thailand Science Research and Innovation (TSRI) (Grant No. RGNS64-216).
Funding
Office of the Permanent Secretary Ministry of Higher Education, Science Research and Innovation (OPS MHESI), Thailand Science Research and Innovation (TSRI) (Grant No. RGNS64-216).
Author information
Authors and Affiliations
Contributions
Wasu Chaitree: Conceptualization, Validation, Investigation, Writing - original draft. Joongjai Panpranot: Resources, Supervision, Writing - review & editing. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Ethical Approval
Not applicable.
Conflict of Interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chaitree, W., Panpranot, J. Electrocatalytic Oxidation of Glycerol using Electrolessly Deposited CuNiSnP Electrocatalysts Supported on Carbon in Alkaline Media. Electrocatalysis 14, 840–856 (2023). https://doi.org/10.1007/s12678-023-00840-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12678-023-00840-z