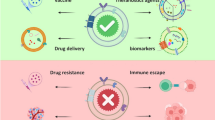
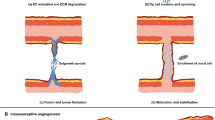
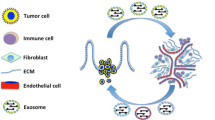
Abstract
Malignant tumours of the digestive system cover a wide range of diseases that affect the health of people to a large extent. Angiogenesis is indispensable in the development, and metastasis of tumours, mainly in two ways: occupation or formation. Vessels can provide nutrients, oxygen, and growth factors for tumours to encourage growth and metastasis, so cancer progression depends on simultaneous angiogenesis. Recently, exosomes have been proven to participate in the angiogenesis of tumours. They influence angiogenesis by binding to tyrosine kinase receptors (VEGFR)-1, VEGFR-2, and VEGFR-3 with different affinities, regulating Yap-VEGF pathway, Akt pathway or other signaling pathway. Additionally, exosomes are potential therapeutic vectors that can deliver many types of cargoes to different cells. In this review, we summarize the roles of exosomes in the angiogenesis of digestive system tumours and highlight the clinical application prospects, directly used as targers or delivery vehicles, in antiangiogenic therapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Background
The digestive system is one of the most important systems in our body, and related tumours frequently occur [1]. Digestive system malignant tumours can be classified into oesophageal, gastric, small intestinal, colon, rectal, appendiceal, anal, hepatic and pancreatic cancer based on the organ from which the abnormal cell proliferation is derived [2]. These tumours cause adverse effects on the physical and mental health of high-risk individuals [3, 4]. According to the report released by the International Agency for Research on Cancer, for both sexes combined, four digestive system cancer types are in the top 10 cancer types for worldwide incidence (23.4% of total cases) and five are in the top 10 for worldwide mortality (35.6% of total cases) [5]. Due to the lack of typical early symptoms, early detection is often difficult and patients often miss the opportunity for optimal treatment [6, 7]. Although endoscopy, cholangiopancreatography and needle aspiration techniques have improved, the invasiveness, uncertainty of operation and high cost restrict large-scale screening [8, 9]. At present, serum carcinoembryonic antigen (CEA), Carbohydrate antigen 19–9 (CA19-9), and Carbohydrate antigen 125 (CA125) are normally used in clinical diagnosis, but both their sensitivity and specificity are low [30], and synaptotagmin 7 (SYT7) [31], have also been reported.
The contents of exosomes are complex and include microRNAs, circular RNAs, long noncoding RNAs (lncRNAs), DNA fragments, proteins, lipids and metabolites (Fig. 1) [32, 33]. These cargoes determine the function of exosomes to a great degree [34, 35]. Exosomes modulate the tissue microenvironment by mediating autocrine, juxtacrine and paracrine interactions, and they also participate in the suppression of antitumour immune responses, cancer angiogenesis, and tumour progression [36]. Exosome uptake by target cells is processed by lipid raft-mediated endocytosis, receptor-mediated endocytosis, phagocytosis, micropinocytosis and membrane fusion [37]. However, the detailed mechanisms remain unclear [32].
Exosomes need to be isolated from biological fluids for various studies [38,117]. In addition to TEX, cancer-associated fibroblast (CAF)-derived exosomes (CAF-exos) can also transmit microRNAs (miRNAs) to CRC. Recently, miR-135b-5p, which is transported by CAF-exos, was reported to downregulate thioredoxin-interacting protein (TXNIP) to promote angiogenesis [ Data sharing is not applicable to this article as no datasets were generated or analysed during the current study. Antiangiogenic therapy Adenosine Angiopoietin-1 Angiopoietin-2 Angiopoietin-like 7 Annexin A1 ADP ribosylation factor 6 B‐cell translocation gene 2 Carbohydrate antigen 125 Carbohydrate antigen 19–9 Cancer-associated fibroblast Carcinoembryonic antigen Claudin-11 C-Type Lectin Domain Family 3 Member B Central nervous system Colorectal cancer Disheveled binding antagonist of beta-catenin 2 Dickkopf-3 Dipeptidyl peptidase IV Endothelial cell Extracellular matrix Erythroblast transformation specific (ETS) related gene Oesophageal squamous cell carcinoma Endosomal sorting complex needed for transport Early-sorting endosome Fibroblast growth factor Forkhead Box M1 D Forkhead Box O Gastric cancer Growth/differentiation factor 15 Golgi phosphoprotein 3 Glucose-regulated protein 78 Hepatocellular carcinoma Hepatocyte growth factor Hexokinase 2 Hepatic stellate cells Human umbilical vein endothelial cells Intussusceptive angiogenesis Intraluminal vesicle Inositol- requiring enzyme 1 alpha Kinesin family member 14 Krüppel-like factor 12 Krüppel-like factor 2 Krüppel-like factor 4 Krev interaction trapped protein 1 Long noncoding RNAs Lysyl oxidase-like 1–4 Late-sorting endosome Histone-lysine N-methyltransferase-3 Matrix metalloproteinase Multivesicular bodies N-ethyl- maleimide-sensitive factor Protease-activated receptor-1 Poly (A) binding protein cytoplasmic 1 Pancreatic cancer Pancreatic ductal adenocarcinoma Platelet-derived growth factor Pyruvate kinase M2 Phospholipase D2 Placental growth factor Promyelocytic leukaemia Periostin Phosphatase and tensin homologue Sprouting angiogenesis Small extracellular vesicles Soluble N-ethyl- maleimide-sensitive factor-attachment protein receptor complex Small nucleolar RNA host gene 16 Synaptotagmin 7 Tumour-associated macrophages Tumour-derived exosomes Tumour microenvironment Thrombospondin-1 Thioredoxin-interacting protein Vessel co-option Vascular endothelial growth factor A Vascular mimicry Zinc finger MYND-type containing 11 Howlader N NA, Krapcho M, Miller D, Brest A, Yu M, Ruhl J, Tatalovich Z, Mariotto A, Lewis DR, Chen HS, Feuer EJ, Cronin KA (eds). SEER Cancer Statistics Review, 1975–2018, National Cancer Institute. Bethesda, MD, https://seer.cancer.gov/csr/1975_2018/, based on November 2020 SEER data submission, posted to the SEER web site, April 2021. Nagtegaal ID, Odze RD, Klimstra D, Paradis V, Rugge M, Schirmacher P, Washington KM, Carneiro F, Cree IA. Board WHOCoTE: the 2019 WHO classification of tumours of the digestive system. Histopathology. 2020;76:182–8. Thomsen MK, Jørgensen MD, Pedersen L, Erichsen R, Sørensen HT, Mikkelsen EM. Mental disorders, participation, and trajectories in the Danish colorectal cancer programme: a population-based cohort study. Lancet Psychiatry. 2023;10:518–27. Housini M, Dariya B, Ahmed N, Stevens A, Fiadjoe H, Nagaraju GP, Basha R. Colorectal cancer: genetic alterations, novel biomarkers, current therapeutic strategies and clinical trials. Gene. 2024. https://doi.org/10.1016/j.gene.2023.147857. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA-A Cancer J Clin. 2021;71:209–49. Ladabaum U, Dominitz JA, Kahi C, Schoen RE. Strategies for colorectal cancer screening. Gastroenterology. 2020;158:418–32. Correa P. Gastric cancer: overview. Gastroenterol Clin N Am. 2013;42:211. Tang YB, Anandasabapathy S, Richards-Kortum R. Advances in optical gastrointestinal endoscopy: a technical review. Mol Oncol. 2021;15:2580–99. Baiu I, Visser B. Endoscopic retrograde cholangiopancreatography. JAMA. 2018;320:2050. Gu YL, Lan C, Pei H, Yang SN, Liu YF, **ao LL. Applicative value of serum CA19-9, CEA, CA125 and CA242 in diagnosis and prognosis for patients with pancreatic cancer treated by concurrent chemoradiotherapy. Asian Pac J Cancer Prev. 2015;16:6569–73. Wang W, Xu X, Tian B, Wang Y, Du L, Sun T, Shi Y, Zhao X, **g J. The diagnostic value of serum tumor markers CEA, CA19-9, CA125, CA15-3, and TPS in metastatic breast cancer. Clin Chim Acta. 2017;470:51–5. Gao Y, Wang J, Zhou Y, Sheng S, Qian SY, Huo X. Evaluation of serum CEA, CA19-9, CA72-4, CA125 and ferritin as diagnostic markers and factors of clinical parameters for colorectal cancer. Sci Rep. 2018;8:2732. Essola JM, Zhang MJ, Yang HY, Li FZ, **a BZ, Mavoungou JF, Hussain A, Huang YY. Exosome regulation of immune response mechanism: pros and cons in immunotherapy. Bioactive Mater. 2024;32:124–46. Frydrychowicz M, Kolecka-Bednarczyk A, Madejczyk M, Yasar S, Dworacki G. Exosomes—structure, biogenesis and biological role in non-small-cell lung cancer. Scand J Immunol. 2015;81:2–10. McCoy-Simandle K, Hanna SJ, Cox D. Exosomes and nanotubes: control of immune cell communication. Int J Biochem Cell Biol. 2016;71:44–54. Wortzel I, Dror S, Kenific CM, Lyden D. Exosome-mediated metastasis: communication from a distance. Dev Cell. 2019;49:347–60. Tang B, Ma W, Lin Y. Emerging applications of anti-angiogenic nanomaterials in oncotherapy. J Control Release. 2023;364:61–78. Maiborodin I, Mansurova A, Chernyavskiy A, Romanov A, Voitcitctkii V, Kedrova A, Tarkhov A, Chernyshova A, Krasil’nikov S. Cancer angiogenesis and opportunity of influence on tumor by changing vascularization. J Pers Med. 2022. https://doi.org/10.3390/jpm12030327. Ribeiro MF, Zhu H, Millard RW, Fan GC. Exosomes function in pro- and anti-angiogenesis. Curr Angiogenes. 2013;2:54–9. Wang Z, Dabrosin C, Yin X, Fuster MM, Arreola A, Rathmell WK, Generali D, Nagaraju GP, El-Rayes B, Ribatti D, et al. Broad targeting of angiogenesis for cancer prevention and therapy. Semin Cancer Biol. 2015;35(Suppl):S224–43. Yao J, Chen Y, Lin Z. Exosomes: mediators in microenvironment of colorectal cancer. Int J Cancer. 2023. https://doi.org/10.1002/ijc.34471. Kikuchi S, Yoshioka Y, Prieto-Vila M, Ochiya T. Involvement of extracellular vesicles in vascular-related functions in cancer progression and metastasis. Int J Mol Sci. 2019. https://doi.org/10.3390/ijms20102584. van Niel G, D’Angelo G, Raposo G. Shedding light on the cell biology of extracellular vesicles. Nat Rev Mol Cell Biol. 2018;19:213–28. Wu H, Fu M, Liu J, Chong W, Fang Z, Du F, Liu Y, Shang L, Li L. The role and application of small extracellular vesicles in gastric cancer. Mol Cancer. 2021;20:71. Doyle LM, Wang MZ. Overview of extracellular vesicles, their origin, composition, purpose, and methods for exosome isolation and analysis. Cells. 2019. https://doi.org/10.3390/cells8070727. Kalluri R, LeBleu VS. The biology, function, and biomedical applications of exosomes. Science. 2020. https://doi.org/10.1126/science.aau6977. Zhang L, Yu D. Exosomes in cancer development, metastasis, and immunity. Biochim Biophys Acta Rev Cancer. 2019;1871:455–68. Carlton JG, Baum B. Roles of ESCRT-III polymers in cell division across the tree of life. Curr Opin Cell Biol. 2023;85:102274. Pfitzner A-K, Zivkovic H, Bernat-Silvestre C, West M, Peltier T, Humbert F, Odorizzi G, Roux A. Vps60 initiates alternative ESCRT-III filaments. J Cell Biol. 2023. https://doi.org/10.1083/jcb.202206028. Clip**er AK, Naismith TV, Yoo W, Jansen S, Kast DJ, Hanson PI. IST1 regulates select recycling pathways. Traffic. 2023. https://doi.org/10.1111/tra.12921. Liu X, Li R, Chen X, Yao J, Wang Q, Zhang J, Jiang Y, Qu Y. SYT7 is a key player in increasing exosome secretion and promoting angiogenesis in non-small-cell lung cancer. Cancer Lett. 2023;577:216400. Aslan C, Maralbashi S, Salari F, Kahroba H, Sigaroodi F, Kazemi T, Kharaziha P. Tumor-derived exosomes: implication in angiogenesis and antiangiogenesis cancer therapy. J Cell Physiol. 2019;234:16885–903. Li Y, Zhao J, Yu S, Wang Z, He X, Su Y, Guo T, Sheng H, Chen J, Zheng Q, et al. Extracellular vesicles long RNA sequencing reveals abundant mRNA, circRNA, and lncRNA in human blood as potential biomarkers for cancer diagnosis. Clin Chem. 2019;65:798–808. Shen Y, TanTai J. Exosomes secreted by metastatic cancer cells promotes epithelial mesenchymal transition in small cell lung carcinoma: the key role of Src/TGF-β1 axis. Gene. 2024;892:147873. Park AY, Lee JO, Jang Y, Kim Y-J, Lee JM, Kim S-Y, Kim BJ, Yoo KH. Exosomes derived from human dermal fibroblasts protect against UVB-induced skin photoaging. Int J Mol Med. 2023. https://doi.org/10.3892/ijmm.2023.5323. Ludwig N, Whiteside TL. Potential roles of tumor-derived exosomes in angiogenesis. Expert Opin Ther Targets. 2018;22:409–17. Sabatke B, Rossi IV, Sana A, Bonato LB, Ramirez MI. Extracellular vesicles biogenesis and uptake concepts: a comprehensive guide to studying host-pathogen communication. Mol Microbiol. 2023. https://doi.org/10.1111/mmi.15168. Özdemir S, Çomaklı S, Küçükler S, Aksungur N, Altundaş N, Kara S, Korkut E, Aydın Ş, Bağcı B, Çulha MH, Öztürk G. Integrative analysis of serum-derived exosomal lncRNA profiles of alveolar echinococcosis patients. Gene. 2024;892:147884. Chen P, Liu Z, **ao H, Yang X, Li T, Huang W, Zhou H. Effect of tumor exosome-derived Lnc RNA HOTAIR on the growth and metastasis of gastric cancer. Clin Trans Oncol. 2023;25:3447–59. Liao M, Qin M, Liu L, Huang H, Chen N, Du H, Huang D, Wang P, Zhou H, Tong G. Exosomal microRNA profiling revealed enhanced autophagy suppression and anti-tumor effects of a combination of compound phyllanthus urinaria and lenvatinib in hepatocellular carcinoma. Phytomedicine. 2024;122:155091. Yang P, Huang Y, Zhu Y, Wang Q, Guo Y, Li L. Plasma exosomes proteome profiling discovers protein markers associated with the therapeutic effect of Chaihu-Longgu-Muli decoction on temporal lobe epilepsy. J Ethnopharmacol. 2024;318:116928. Chen Y-X, Deng Z-H, Xue G, Qiang D, Juan Y, Chen G-H, Li J-G, Zhao Y-M, Zhang H-T, Zhang G-X, Qian J-X. Exosomes derived from mesenchymal stromal cells exert a therapeutic effect on hypoxia-induced pulmonary hypertension by modulating the YAP1/SPP1 signaling pathway. Biomed Pharmacother. 2023;168:115816. Tavasolian F, Lively S, Pastrello C, Tang M, Lim M, Pacheco A, Qaiyum Z, Yau E, Baskurt Z, Jurisica I, et al. Proteomic and genomic profiling of plasma exosomes from patients with ankylosing spondylitis. Ann Rheum Dis. 2023;82:1429–43. Gardiner C, Di Vizio D, Sahoo S, Thery C, Witwer KW, Wauben M, Hill AF. Techniques used for the isolation and characterization of extracellular vesicles: results of a worldwide survey. J Extracell Vesicles. 2016;5:32945. Altıntaş Ö, Saylan Y. Exploring the versatility of exosomes: a review on isolation, characterization, detection methods, and diverse applications. Anal Chem. 2023;95:16029–48. Théry C, Witwer KW, Aikawa E, Alcaraz MJ, Anderson JD, Andriantsitohaina R, Antoniou A, Arab T, Archer F, Atkin-Smith GK, et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the international society for extracellular vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles. 2018;7:1535750. Witwer KW, Goberdhan DC, O’Driscoll L, Théry C, Welsh JA, Blenkiron C, Buzás EI, Di Vizio D, Erdbrügger U, Falcón-Pérez JM, et al. Updating MISEV: Evolving the minimal requirements for studies of extracellular vesicles. J Extracell Vesicles. 2021;10:e12182. Lv Q, Wang Y, Tian W, Liu Y, Gu M, Jiang X, Cai Y, Huo R, Li Y, Li L, Wang X. Exosomal miR-146a-5p derived from human umbilical cord mesenchymal stem cells can alleviate antiphospholipid antibody-induced trophoblast injury and placental dysfunction by regulating the TRAF6/NF-κB axis. J Nanobiotechnol. 2023;21:419. Dong L, Zieren RC, Horie K, Kim CJ, Mallick E, **g YZ, Feng MX, Kuczler MD, Green J, Amend SR, et al. Comprehensive evaluation of methods for small extracellular vesicles separation from human plasma, urine and cell culture medium. J Extracell Vesicles. 2020. https://doi.org/10.1002/jev2.12044. Liu B, Yang H, Song Y-S, Sorenson CM, Sheibani N. Thrombospondin-1 in vascular development, vascular function, and vascular disease. Semin Cell Dev Biol. 2024;155:32–44. Moshe DL, Baghaie L, Leroy F, Skapinker E, Szewczuk MR. Metamorphic effect of angiogenic switch in tumor development: conundrum of tumor angiogenesis toward progression and metastatic potential. Biomedicines. 2023. https://doi.org/10.3390/biomedicines11082142. Watabe T, Takahashi K, Pietras K, Yoshimatsu Y. Roles of TGF-β signals in tumor microenvironment via regulation of the formation and plasticity of vascular system. Semin Cancer Biol. 2023;92:130–8. Olejarz W, Kubiak-Tomaszewska G, Chrzanowska A, Lorenc T. Exosomes in angiogenesis and anti-angiogenic therapy in cancers. Int J Mol Sci. 2020. https://doi.org/10.3390/ijms21165840. Kumar S, Sharife H, Kreisel T, Bar-Lev L, Grunewald M, Keshet E. Isolation of tumor cells based on their distance from blood vessels. Bio-Protoc. 2020. https://doi.org/10.2176/BioProtoc.3628. Ye Z-W, Yu Z-L, Chen G, Jia J. Extracellular vesicles in tumor angiogenesis and resistance to anti-angiogenic therapy. Cancer Sci. 2023;114:2739–49. Zanotelli MR, Reinhart-King CA. Mechanical forces in tumor angiogenesis. Adv Exp Med Biol. 2018;1092:91–112. Dudley AC, Griffioen AW. The modes of angiogenesis: an updated perspective. Angiogenesis. 2023;26:477–80. LaBelle SA, Poulson AM, Maas SA, Rauff A, Ateshian GA, Weiss JA. Spatial configurations of 3D extracellular matrix collagen density and anisotropy simultaneously guide angiogenesis. PLoS Comput Biol. 2023;19:e1011553. Wang Y, Zeng X, Chen P, Du W, Pei Y, Wang G, Liu B-F. On-chip-angiogenesis based on a high-throughput biomimetic three-dimensional cell spheroid culture system. Analyst. 2023;148:3870–5. Betz C, Lenard A, Belting HG, Affolter M. Cell behaviors and dynamics during angiogenesis. Development. 2016;143:2249–60. Weinstein N, Mendoza L, Gitler I, Klapp J. A network model to explore the effect of the micro-environment on endothelial cell behavior during angiogenesis. Front Physiol. 2017;8:960. Kuczynski EA, Vermeulen PB, Pezzella F, Kerbel RS, Reynolds AR. Vessel co-option in cancer. Nat Rev Clin Oncol. 2019;16:469–93. Eelen G, Treps L, Li X, Carmeliet P. Basic and therapeutic aspects of angiogenesis updated. Circ Res. 2020;127:310–29. Angara K, Borin TF, Arbab AS. Vascular mimicry: a novel neovascularization mechanism driving anti-angiogenic therapy (AAT) resistance in glioblastoma. Transl Oncol. 2017;10:650–60. Yao XY, Xue YB, Ma Q, Bai YJ, Jia P, Zhang YM, Lai BC, He ST, Ma Q, Zhang JB, et al. 221S–1a inhibits endothelial proliferation in pathological angiogenesis through ERK/c-Myc signaling. Eur J Pharmacol. 2023. https://doi.org/10.1016/j.ejphar.2023.175805. Wang Y, Hong Y, Mao S, Pan J, Cui Y, Lu J, Wen T, Wang X, Luo Y. Downregulation of miR-124-3p suppresses the development of the deep retinal blood vessels by enhancing the Stat1/Ripk1 pathway in mouse retinal microglia. Exp Eye Res. 2023;233:109551. Huang J, Wang C, Hou Y, Tian Y, Li Y, Zhang H, Zhang L, Li W. Molecular mechanisms of thrombospondin-2 modulates tumor vasculogenic mimicry by PI3K/AKT/mTOR signaling pathway. Biomed Pharmacother. 2023;167:115455. Apte RS, Chen DS, Ferrara N. VEGF in signaling and disease: beyond discovery and development. Cell. 2019;176:1248–64. Sinha K, Parwez S, Mv S, Yadav A, Siddiqi MI, Banerjee D. Machine learning and biological evaluation-based identification of a potential MMP-9 inhibitor, effective against ovarian cancer cells SKOV3. J Biomol Struct Dyn. 2023. https://doi.org/10.1080/07391102.2023.2240416. Chillà A, Anceschi C, Frediani E, Scavone F, Del Rosso T, Pelagio G, Tufaro A, De Palma G, Del Rosso M, Fibbi G, et al. Inhibition of MMPs supports amoeboid angiogenesis hampering VEGF-targeted therapies via MLC and ERK 1/2 signaling. J Transl Med. 2023;21:102. Chi H, Dong Z, Gan Q, Tang X, **ng J, Sheng X, Zhan W. Matrix metalloproteinase 9 modulates immune response along with the formation of extracellular traps in flounder (Paralichthys olivaceus). Fish Shellfish Immunol. 2023;133:108570. Quintero-Fabian S, Arreola R, Becerril-Villanueva E, Torres-Romero JC, Arana-Argaez V, Lara-Riegos J, Ramirez-Camacho MA, Alvarez-Sanchez ME. Role of matrix metalloproteinases in angiogenesis and cancer. Front Oncol. 2019;9:1370. Zhang W, Zhang X, Huang S, Chen J, Ding P, Wang Q, Li L, Lv X, Li L, Zhang P, et al. FOXM1D potentiates PKM2-mediated tumor glycolysis and angiogenesis. Mol Oncol. 2021;15:1466–85. Ge Z, Zhang Q, Lin W, Jiang X, Zhang Y. The role of angiogenic growth factors in the immune microenvironment of glioma. Front Oncol. 2023;13:1254694. Thijssen VL, Paulis YW, Nowak-Sliwinska P, Deumelandt KL, Hosaka K, Soetekouw PM, Cimpean AM, Raica M, Pauwels P, van den Oord JJ, et al. Targeting PDGF-mediated recruitment of pericytes blocks vascular mimicry and tumor growth. J Pathol. 2018;246:447–58. Yu P, Wilhelm K, Dubrac A, Tung JK, Alves TC, Fang JS, **e Y, Zhu J, Chen Z, De Smet F, et al. FGF-dependent metabolic control of vascular development. Nature. 2017;545:224–8. El-Sheikh MM, Aziz MM, Abdelrahman SSM, Mohmad MAEH. The protective effect of crocin against testicular toxicity induced by ionizing radiation via AKT/FOXO pathway. Environ Toxicol. 2023. https://doi.org/10.1002/tox.23932. Akwii RG, Sajib MS, Zahra FT, Mikelis CM. Role of angiopoietin-2 in vascular physiology and pathophysiology. Cells. 2019. https://doi.org/10.3390/cells8050471. Jiang T, Zhu Z, Zhang J, Chen M, Chen S. Role of tumor-derived exosomes in metastasis, drug resistance and diagnosis of clear cell renal cell carcinoma. Front Oncol. 2022;12:1066288. Gholipour E, Kahroba H, Soltani N, Samadi P, Sarvarian P, Vakili-Samiani S, Hosein Pour Feizi AA, Soltani-Zangbar MS, Baghersalimi A, Darbandi B, et al. Paediatric pre-B acute lymphoblastic leukaemia-derived exosomes regulate immune function in human T cells. J Cell Mol Med. 2022;26:4566–76. Bai S, Wei Y, Liu R, Xu R, **ang L, Du J. Role of tumour-derived exosomes in metastasis. Biomed Pharmacother. 2022;147:112657. Sruthi TV, Edatt L, Raji GR, Kunhiraman H, Shankar SS, Shankar V, Ramachandran V, Poyyakkara A, Kumar SVB. Horizontal transfer of miR-23a from hypoxic tumor cell colonies can induce angiogenesis. J Cell Physiol. 2018;233:3498–514. Ludwig N, Yerneni SS, Azambuja JH, Gillespie DG, Menshikova EV, Jackson EK, Whiteside TL. Tumor-derived exosomes promote angiogenesis via adenosine A2B receptor signaling. Angiogenesis. 2020;23:599–610. Wang Y, Cen A, Yang Y, Ye H, Li J, Liu S, Zhao L. miR-181a, delivered by hypoxic PTC-secreted exosomes, inhibits DACT2 by downregulating MLL3, leading to YAP-VEGF-mediated angiogenesis. Mol Ther Nucleic Acids. 2021;24:610–21. Whiteside TL. Tumor-derived exosomes and their role in cancer progression. Adv Clin Chem. 2016;74:103–41. Zhang R, Lian T, Liu J, Du F, Chen Z, Zhang R, Wang Q. Dendritic cell-derived exosomes stimulated by treponema pallidum induce endothelial cell inflammatory response through the TLR4/MyD88/NF-κB signaling pathway. ACS Infect Dis. 2023;9:2299–305. Mao Y, Wang Y, Dong L, Zhang Y, Zhang Y, Wang C, Zhang Q, Yang S, Cao L, Zhang X, et al. Hypoxic exosomes facilitate angiogenesis and metastasis in esophageal squamous cell carcinoma through altering the phenotype and transcriptome of endothelial cells. J Exp Clin Cancer Res. 2019;38:389. Zhang C, Luo Y, Cao J, Wang X, Miao Z, Shao G. Exosomal lncRNA FAM225A accelerates esophageal squamous cell carcinoma progression and angiogenesis via sponging miR-206 to upregulate NETO2 and FOXP1 expression. Cancer Med. 2020;9:8600–11. Zhang Y, Chen C, Liu Z, Guo H, Lu W, Hu W, Lin Z. PABPC1-induced stabilization of IFI27 mRNA promotes angiogenesis and malignant progression in esophageal squamous cell carcinoma through exosomal miRNA-21-5p. J Exp Clin Cancer Res. 2022;41:111. Zhao Z, Yang S, Zhou A, Li X, Fang R, Zhang S, Zhao G, Li P. Small extracellular vesicles in the development, diagnosis, and possible therapeutic application of esophageal squamous cell carcinoma. Front Oncol. 2021;11:732702. Shou Y, Wang X, Chen C, Liang Y, Yang C, **ao Q, Li H, Wang S, Shu J, Tian X, Chen K. Exosomal miR-301a-3p from esophageal squamous cell carcinoma cells promotes angiogenesis by inducing M2 polarization of macrophages via the PTEN/PI3K/AKT signaling pathway. Cancer Cell Int. 2022;22:153. Zhao L, Yu L, Wang X, He J, Zhu X, Zhang R, Yang A. Mechanisms of function and clinical potential of exosomes in esophageal squamous cell carcinoma. Cancer Lett. 2023;553:215993. Shou Y, Wang X, Liang Y, Liu X, Chen K. Exosomes-derived miR-154-5p attenuates esophageal squamous cell carcinoma progression and angiogenesis by targeting kinesin family member 14. Bioengineered. 2022;13:4610–20. Du J, Liang Y, Li J, Zhao JM, Wang ZN, Lin XY. Gastric cancer cell-derived exosomal microRNA-23a promotes angiogenesis by targeting PTEN. Front Oncol. 2020;10:326. **e M, Yu T, **g X, Ma L, Fan Y, Yang F, Ma P, Jiang H, Wu X, Shu Y, Xu T. Exosomal circSHKBP1 promotes gastric cancer progression via regulating the miR-582-3p/HUR/VEGF axis and suppressing HSP90 degradation. Mol Cancer. 2020;19:112. Xue X, Huang J, Yu K, Chen X, He Y, Qi D, Wu Y. YB-1 transferred by gastric cancer exosomes promotes angiogenesis via enhancing the expression of angiogenic factors in vascular endothelial cells. BMC Cancer. 2020;20:996. Chen X, Zhang S, Du K, Zheng N, Liu Y, Chen H, **e G, Ma Y, Zhou Y, Zheng Y, et al. Gastric cancer-secreted exosomal X26nt increases angiogenesis and vascular permeability by targeting VE-cadherin. Cancer Sci. 2021;112:1839–52. Li S, Li J, Zhang H, Zhang Y, Wang X, Yang H, Zhou Z, Hao X, Ying G, Ba Y. Gastric cancer derived exosomes mediate the delivery of circRNA to promote angiogenesis by targeting miR-29a/VEGF axis in endothelial cells. Biochem Biophys Res Commun. 2021;560:37–44. Zhu J, Du S, Zhang J, Huang G, Dong L, Ren E, Liu D. microRNA-10a-5p from gastric cancer cell-derived exosomes enhances viability and migration of human umbilical vein endothelial cells by targeting zinc finger MYND-type containing 11. Bioengineered. 2022;13:496–507. Zhang Z, Sun C, Zheng Y, Gong Y. circFCHO2 promotes gastric cancer progression by activating the JAK1/STAT3 pathway via sponging miR-194-5p. Cell Cycle. 2022;21:2145–64. Iha K, Sato A, Tsai HY, Sonoda H, Watabe S, Yoshimura T, Lin MW, Ito E. Gastric cancer cell-derived exosomal GRP78 enhances angiogenesis upon stimulation of vascular endothelial cells. Curr Issues Mol Biol. 2022;44:6145–57. Qiu S, **e L, Lu C, Gu C, **a Y, Lv J, Xuan Z, Fang L, Yang J, Zhang L, et al. Gastric cancer-derived exosomal miR-519a-3p promotes liver metastasis by inducing intrahepatic M2-like macrophage-mediated angiogenesis. J Exp Clin Cancer Res CR. 2022;41:296. Chen L, Zheng H, Yu X, Liu L, Li H, Zhu H, Zhang Z, Lei P, Shen G. Tumor-secreted GRP78 promotes the establishment of a pre-metastatic niche in the liver microenvironment. Front Immunol. 2020;11:584458. Parri M, Pietrovito L, Grandi A, Campagnoli S, De Camilli E, Bianchini F, Marchio S, Bussolino F, ** B, Sarmientos P, et al. Angiopoietin-like 7, a novel pro-angiogenetic factor over-expressed in cancer. Angiogenesis. 2014;17:881–96. Yamada N, Tsujimura N, Kumazaki M, Shinohara H, Taniguchi K, Nakagawa Y, Naoe T, Akao Y. Colorectal cancer cell-derived microvesicles containing microRNA-1246 promote angiogenesis by activating Smad 1/5/8 signaling elicited by PML down-regulation in endothelial cells. Biochim Biophys Acta. 2014;1839:1256–72. Huang Z, Feng Y. Exosomes derived from hypoxic colorectal cancer cells promote angiogenesis through Wnt4-induced beta-catenin signaling in endothelial cells. Oncol Res. 2017;25:651–61. Yang Y, Zhang L, La X, Li Z, Li H, Guo S. Salvianolic acid A inhibits tumor-associated angiogenesis by blocking GRP78 secretion. Naunyn Schmiedebergs Arch Pharmacol. 2019;392:467–80. Zeng Z, Li Y, Pan Y, Lan X, Song F, Sun J, Zhou K, Liu X, Ren X, Wang F, et al. Cancer-derived exosomal miR-25-3p promotes pre-metastatic niche formation by inducing vascular permeability and angiogenesis. Nat Commun. 2018;9:5395. Hu HY, Yu CH, Zhang HH, Zhang SZ, Yu WY, Yang Y, Chen Q. Exosomal miR-1229 derived from colorectal cancer cells promotes angiogenesis by targeting HIPK2. Int J Biol Macromol. 2019;132:470–7. Yamada N, Nakagawa Y, Tsujimura N, Kumazaki M, Noguchi S, Mori T, Hirata I, Maruo K, Akao Y. Role of intracellular and extracellular microRNA-92a in colorectal cancer. Transl Oncol. 2013;6:482–92. Yamada NO, Heishima K, Akao Y, Senda T. Extracellular vesicles containing MicroRNA-92a-3p facilitate partial endothelial-mesenchymal transition and angiogenesis in endothelial cells. Int J Mol Sci. 2019. https://doi.org/10.3390/ijms20184406. Shang A, Wang X, Gu C, Liu W, Sun J, Zeng B, Chen C, Ji P, Wu J, Quan W, et al. Exosomal miR-183-5p promotes angiogenesis in colorectal cancer by regulation of FOXO1. Aging. 2020;12:8352–71. He Q, Ye A, Ye W, Liao X, Qin G, Xu Y, Yin Y, Luo H, Yi M, **an L, et al. Cancer-secreted exosomal miR-21-5p induces angiogenesis and vascular permeability by targeting KRIT1. Cell Death Dis. 2021;12:576. Bai L, Gao Z, Jiang A, Ren S, Wang B. Circular noncoding RNA circ_0007334 sequestrates miR-577 to derepress KLF12 and accelerate colorectal cancer progression. Anticancer Drugs. 2022;33:e409–22. Zheng X, Liu J, Li X, Tian R, Shang K, Dong X, Cao B. Angiogenesis is promoted by exosomal DPP4 derived from 5-fluorouracil-resistant colon cancer cells. Cancer Lett. 2021;497:190–201. Zheng X, Ma N, Wang X, Hu J, Ma X, Wang J, Cao B. Exosomes derived from 5-fluorouracil-resistant colon cancer cells are enriched in GDF15 and can promote angiogenesis. J Cancer. 2020;11:7116–26. Chen C, Liu Y, Liu L, Si C, Xu Y, Wu X, Wang C, Sun Z, Kang Q. Exosomal circTUBGCP4 promotes vascular endothelial cell tip** and colorectal cancer metastasis by activating Akt signaling pathway. J Exp Clin Cancer Res. 2023;42:46. Yin H, Yu S, **e Y, Dai X, Dong M, Sheng C, Hu J. Cancer-associated fibroblasts-derived exosomes upregulate microRNA-135b-5p to promote colorectal cancer cell growth and angiogenesis by inhibiting thioredoxin-interacting protein. Cell Signal. 2021;84:110029. Shi Y, Zhu H, Jiang H, Yue H, Yuan F, Wang F. Cancer-associated fibroblasts-derived exosomes from chemoresistant patients regulate cisplatin resistance and angiogenesis by delivering VEGFA in colorectal cancer. Anticancer Drugs. 2023;34:422–30. Zeng W, Liu Y, Li WT, Li Y, Zhu JF. CircFNDC3B sequestrates miR-937-5p to derepress TIMP3 and inhibit colorectal cancer progression. Mol Oncol. 2020;14:2960–84. Danac JMC, Uy AGG, Garcia RL. Exosomal microRNAs in colorectal cancer: overcoming barriers of the metastatic cascade (Review). Int J Mol Med. 2021. https://doi.org/10.3892/ijmm.2021.4945. Hosseini M, Baghaei K, Hajivalili M, Zali MR, Ebtekar M, Amani D. The anti-tumor effects of CT-26 derived exosomes enriched by MicroRNA-34a on murine model of colorectal cancer. Life Sci. 2022;290:120234. Conigliaro A, Costa V, Lo Dico A, Saieva L, Buccheri S, Dieli F, Manno M, Raccosta S, Mancone C, Tripodi M, et al. CD90+ liver cancer cells modulate endothelial cell phenotype through the release of exosomes containing H19 lncRNA. Mol Cancer. 2015;14:155. Fu X, Liu M, Qu S, Ma J, Zhang Y, Shi T, Wen H, Yang Y, Wang S, Wang J, et al. Exosomal microRNA-32-5p induces multidrug resistance in hepatocellular carcinoma via the PI3K/Akt pathway. J Exp Clin Cancer Res. 2018;37:52. Lin XJ, Fang JH, Yang XJ, Zhang C, Yuan Y, Zheng L, Zhuang SM. Hepatocellular carcinoma cell-secreted exosomal microRNA-210 promotes angiogenesis in vitro and in vivo. Mol Ther Nucleic Acids. 2018;11:243–52. Matsuura Y, Wada H, Eguchi H, Gotoh K, Kobayashi S, Kinoshita M, Kubo M, Hayashi K, Iwagami Y, Yamada D, et al. Exosomal miR-155 derived from hepatocellular carcinoma cells under hypoxia promotes angiogenesis in endothelial cells. Dig Dis Sci. 2019;64:792–802. Zhou Y, Ren H, Dai B, Li J, Shang L, Huang J, Shi X. Hepatocellular carcinoma-derived exosomal miRNA-21 contributes to tumor progression by converting hepatocyte stellate cells to cancer-associated fibroblasts. J Exp Clin Cancer Res. 2018;37:324. Chen Q, Wang X, Zheng Y, Ye T, Liu J, Wang J-Q, Zhang W-F, Wu F-L, Bo H, Shao H, et al. Cancer-associated fibroblasts contribute to the immunosuppressive landscape and influence the efficacy of the combination therapy of PD-1 inhibitors and antiangiogenic agents in hepatocellular carcinoma. Cancer. 2023;129:3405–16. Wu Q, Zhou L, Lv D, Zhu X, Tang H. Exosome-mediated communication in the tumor microenvironment contributes to hepatocellular carcinoma development and progression. J Hematol Oncol. 2019;12:53. Li R, Wang Y, Zhang X, Feng M, Ma J, Li J, Yang X, Fang F, **a Q, Zhang Z, et al. Exosome-mediated secretion of LOXL4 promotes hepatocellular carcinoma cell invasion and metastasis. Mol Cancer. 2019;18:18. Huang XY, Huang ZL, Huang J, Xu B, Huang XY, Xu YH, Zhou J, Tang ZY. Exosomal circRNA-100338 promotes hepatocellular carcinoma metastasis via enhancing invasiveness and angiogenesis. J Exp Clin Cancer Res. 2020;39:20. **e JY, Wei JX, Lv LH, Han QF, Yang WB, Li GL, Wang PX, Wu SB, Duan JX, Zhuo WF, et al. Angiopoietin-2 induces angiogenesis via exosomes in human hepatocellular carcinoma. Cell Commun Signal. 2020;18:46. You LN, Tai QW, Xu L, Hao Y, Guo WJ, Zhang Q, Tong Q, Zhang H, Huang WK. Exosomal LINC00161 promotes angiogenesis and metastasis via regulating miR-590-3p/ROCK axis in hepatocellular carcinoma. Cancer Gene Ther. 2021;28:719–36. Yukawa H, Suzuki K, Aoki K, Arimoto T, Yasui T, Kaji N, Ishikawa T, Ochiya T, Baba Y. Imaging of angiogenesis of human umbilical vein endothelial cells by uptake of exosomes secreted from hepatocellular carcinoma cells. Sci Rep. 2018;8:6765. Wang S, Xu M, Li X, Su X, **ao X, Keating A, Zhao RC. Exosomes released by hepatocarcinoma cells endow adipocytes with tumor-promoting properties. J Hematol Oncol. 2018;11:82. Chen W, Huang L, Liang J, Ye Y, He S, Niu J. Hepatocellular carcinoma cells-derived exosomal microRNA-378b enhances hepatocellular carcinoma angiogenesis. Life Sci. 2021;273:119184. Wang Q, Wang G, Niu L, Zhao S, Li J, Zhang Z, Jiang H, Zhang Q, Wang H, Sun P, et al. Exosomal MiR-1290 promotes angiogenesis of hepatocellular carcinoma via targeting SMEK1. J Oncol. 2021;2021:6617700. Hu K, Li NF, Li JR, Chen ZG, Wang JH, Sheng LQ. Exosome circCMTM3 promotes angiogenesis and tumorigenesis of hepatocellular carcinoma through miR-3619-5p/SOX9. Hepatol Res. 2021;51:1139–52. Li S, Qi Y, Huang Y, Guo Y, Huang T, Jia L. Exosome-derived SNHG16 sponging miR-4500 activates HUVEC angiogenesis by targeting GALNT1 via PI3K/Akt/mTOR pathway in hepatocellular carcinoma. J Physiol Biochem. 2021;77:667–82. Huang XY, Zhang JT, Li F, Li TT, Shi XJ, Huang J, Huang XY, Zhou J, Tang ZY, Huang ZL. Exosomal proteomics identifies RAB13 as a potential regulator of metastasis for HCC. Hepatol Commun. 2023;7:e0006. Dai W, Wang Y, Yang T, Wang J, Wu W, Gu J. Downregulation of exosomal CLEC3B in hepatocellular carcinoma promotes metastasis and angiogenesis via AMPK and VEGF signals. Cell Commun Signal. 2019;17:113. Moh-Moh-Aung A, Fujisawa M, Ito S, Katayama H, Ohara T, Ota Y, Yoshimura T, Matsukawa A. Decreased miR-200b-3p in cancer cells leads to angiogenesis in HCC by enhancing endothelial ERG expression. Sci Rep. 2020;10:10418. Dong SS, Dong DD, Yang ZF, Zhu GQ, Gao DM, Chen J, Zhao Y, Liu BB. Exosomal miR-3682-3p suppresses angiogenesis by targeting ANGPT1 via the RAS-MEK1/2-ERK1/2 pathway in hepatocellular carcinoma. Front Cell Dev Biol. 2021;9:633358. Pessolano E, Belvedere R, Bizzarro V, Franco P, Marco I, Porta A, Tosco A, Parente L, Perretti M, Petrella A. Annexin A1 may induce pancreatic cancer progression as a key player of extracellular vesicles effects as evidenced in the in vitro MIA PaCa-2 model system. Int J Mol Sci. 2018. https://doi.org/10.3390/ijms19123878. Shang D, **e C, Hu J, Tan J, Yuan Y, Liu Z, Yang Z. Pancreatic cancer cell-derived exosomal microRNA-27a promotes angiogenesis of human microvascular endothelial cells in pancreatic cancer via BTG2. J Cell Mol Med. 2020;24:588–604. Fang X, Cai Y, Xu Y, Zhang H. Exosome-mediated lncRNA SNHG11 regulates angiogenesis in pancreatic carcinoma through miR-324-3p/VEGFA axis. Cell Biol Int. 2022;46:106–17. Manuelli V, Pecorari C, Filomeni G, Zito E. Regulation of redox signaling in HIF-1-dependent tumor angiogenesis. FEBS J. 2022;289:5413–25. Guo Z, Wang X, Yang Y, Chen W, Zhang K, Teng B, Huang C, Zhao Q, Qiu Z. Hypoxic tumor-derived exosomal long noncoding RNA UCA1 promotes angiogenesis via miR-96-5p/AMOTL2 in pancreatic cancer. Mol Ther Nucleic Acids. 2020;22:179–95. Chen K, Wang Q, Liu X, Wang F, Yang Y, Tian X. Hypoxic pancreatic cancer derived exosomal miR-30b-5p promotes tumor angiogenesis by inhibiting GJA1 expression. Int J Biol Sci. 2022;18:1220–37. Wu G, Ding X, Quan G, **ong J, Li Q, Li Z, Wang Y. Hypoxia-induced miR-210 promotes endothelial cell permeability and angiogenesis via exosomes in pancreatic ductal adenocarcinoma. Biochem Res Int. 2022;2022:7752277. Wang L, Yang L, Zhuang T, Shi X. Tumor-derived exosomal miR-29b reduces angiogenesis in pancreatic cancer by silencing ROBO1 and SRGAP2. J Immunol Res. 2022;2022:4769385. Yin Z, Ma T, Huang B, Lin L, Zhou Y, Yan J, Zou Y, Chen S. Macrophage-derived exosomal microRNA-501-3p promotes progression of pancreatic ductal adenocarcinoma through the TGFBR3-mediated TGF-beta signaling pathway. J Exp Clin Cancer Res. 2019;38:310. Yang Y, Guo Z, Chen W, Wang X, Cao M, Han X, Zhang K, Teng B, Cao J, Wu W, et al. M2 macrophage-derived exosomes promote angiogenesis and growth of pancreatic ductal adenocarcinoma by targeting E2F2. Mol Ther. 2021;29:1226–38. Borriello R, Cerrito L, Gasbarrini A, Ponziani FR. Pharmacokinetic considerations for angiogenesis inhibitors used to treat hepatocellular carcinoma: an overview. Expert Opin Drug Metab Toxicol. 2023. https://doi.org/10.1080/17425255.2023.2272598. Gadre S, M M, Chakraborty G, Rayrikar A, Paul S, Patra C, Patra M. Development of a highly in vivo efficacious dual antitumor and antiangiogenic organoiridium complex as a potential anti-lung cancer agent. J Med Chem. 2023;66:13481–500. Wang C, Huang M, Lin Y, Zhang Y, Pan J, Jiang C, Cheng M, Li S, He W, Li Z, et al. ENO2-derived phosphoenolpyruvate functions as an endogenous inhibitor of HDAC1 and confers resistance to antiangiogenic therapy. Nat Metab. 2023;5:1765–86. Modest DP, Pant S, Sartore-Bianchi A. Treatment sequencing in metastatic colorectal cancer. Eur J Cancer. 2019;109:70–83. Moawad AW, Szklaruk J, Lall C, Blair KJ, Kaseb AO, Kamath A, Rohren SA, Elsayes KM. Angiogenesis in hepatocellular carcinoma; pathophysiology, targeted therapy, and role of imaging. J Hepatocell Carcinoma. 2020;7:77–89. Annese T, Tamma R, Ruggieri S, Ribatti D. Angiogenesis in pancreatic cancer: pre-clinical and clinical studies. Cancers. 2019. https://doi.org/10.3390/cancers11030381. Rajabi M, Mousa SA. The role of angiogenesis in cancer treatment. Biomedicines. 2017. https://doi.org/10.3390/biomedicines5020034. Zarrin B, Zarifi F, Vaseghi G, Javanmard SH. Acquired tumor resistance to antiangiogenic therapy: mechanisms at a glance. J Res Med Sci. 2017;22:117. Hameed S, Bhattarai P, Dai Z. Nanotherapeutic approaches targeting angiogenesis and immune dysfunction in tumor microenvironment. Sci China Life Sci. 2018;61:380–91. Teleanu RI, Chircov C, Grumezescu AM, Teleanu DM. Tumor angiogenesis and anti-angiogenic strategies for cancer treatment. J Clin Med. 2019. https://doi.org/10.3390/jcm9010084. Mukherjee S, Patra CR. Therapeutic application of anti-angiogenic nanomaterials in cancers. Nanoscale. 2016;8:12444–70. Franchi M, Piperigkou Z, Karamanos KA, Franchi L, Masola V. Extracellular matrix-mediated breast cancer cells morphological alterations, invasiveness, and microvesicles/exosomes release. Cells. 2020. https://doi.org/10.3390/cells9092031. Eguchi T, Sogawa C, Okusha Y, Uchibe K, Iinuma R, Ono K, Nakano K, Murakami J, Itoh M, Arai K, et al. Organoids with cancer stem cell-like properties secrete exosomes and HSP90 in a 3D nanoenvironment. PLoS ONE. 2018;13:e0191109. Thippabhotla S, Zhong C, He M. 3D cell culture stimulates the secretion of in vivo like extracellular vesicles. Sci Rep. 2019;9:13012. Bordanaba-Florit G, Madarieta I, Olalde B, Falcon-Perez JM, Royo F. 3D cell cultures as prospective models to study extracellular vesicles in cancer. Cancers. 2021. https://doi.org/10.3390/cancers13020307. Lai X, Wang M, McElyea SD, Sherman S, House M, Korc M. A microRNA signature in circulating exosomes is superior to exosomal glypican-1 levels for diagnosing pancreatic cancer. Cancer Lett. 2017;393:86–93. Melo SA, Luecke LB, Kahlert C, Fernandez AF, Gammon ST, Kaye J, LeBleu VS, Mittendorf EA, Weitz J, Rahbari N, et al. Glypican-1 identifies cancer exosomes and detects early pancreatic cancer. Nature. 2015;523:177–82. Yang KS, Im H, Hong S, Pergolini I, Del Castillo AF, Wang R, Clardy S, Huang CH, Pille C, Ferrone S, et al. Multiparametric plasma EV profiling facilitates diagnosis of pancreatic malignancy. Sci Transl Med. 2017. https://doi.org/10.1126/scitranslmed.aal3226. Ariston Gabriel AN, Wang F, Jiao Q, Yvette U, Yang X, Al-Ameri SA, Du L, Wang YS, Wang C. The involvement of exosomes in the diagnosis and treatment of pancreatic cancer. Mol Cancer. 2020;19:132. Li X, Li C, Zhang L, Wu M, Cao K, Jiang F, Chen D, Li N, Li W. The significance of exosomes in the development and treatment of hepatocellular carcinoma. Mol Cancer. 2020;19:1. Zhang H, Wang Y, Bai M, Wang J, Zhu K, Liu R, Ge S, Li J, Ning T, Deng T, et al. Exosomes serve as nanoparticles to suppress tumor growth and angiogenesis in gastric cancer by delivering hepatocyte growth factor siRNA. Cancer Sci. 2018;109:629–41. El-Kattawy AM, Algezawy O, Alfaifi MY, Noseer EA, Hawsawi YM, Alzahrani OR, Algarni A, Kahilo KA, El-Magd MA. Therapeutic potential of camel milk exosomes against HepaRG cells with potent apoptotic, anti-inflammatory, and anti-angiogenesis effects for colostrum exosomes. Biomed Pharmacother. 2021;143:112220. Zhao L, Yu Q, Gao C, **ang J, Zheng B, Feng Y, Li R, Zhang W, Hong X, Zhan YY, et al. Studies of the efficacy of low-dose apatinib monotherapy as third-line treatment in patients with metastatic colorectal cancer and apatinib’s novel anticancer effect by inhibiting tumor-derived exosome secretion. Cancers. 2022. https://doi.org/10.3390/cancers14102492. Gao Y, Yin Z, Qi Y, Peng H, Ma W, Wang R, Li W. Golgi phosphoprotein 3 promotes angiogenesis and sorafenib resistance in hepatocellular carcinoma via upregulating exosomal miR-494-3p. Cancer Cell Int. 2022;22:35. Yang Y-H, Wen C-S, Kuo Y-L, Fu S-L, Lin T-Y, Chen C-M, Wu P-K, Chen W-M, Wang J-Y. GuiLu-Er**an glue extract promotes mesenchymal stem cells (MSC)-induced chondrogenesis via exosomes release and delays aging in the MSC senescence process. J Ethnopharmacol. 2023;317:116784. Alam MR, Rahman MM, Li Z. The link between intracellular calcium signaling and exosomal PD-L1 in cancer progression and immunotherapy. Genes Dis. 2024;11:321–34. Kang C, He H, Liu P, Liu Y, Li X, Zhang J, Ran H, Zeng X, Zhao H, Liu J, Qiu S. Role of dendritic cell-derived exosomes in allergic rhinitis (Review). Int J Mol Med. 2023. https://doi.org/10.3892/ijmm.2023.5320. Bunggulawa EJ, Wang W, Yin T, Wang N, Durkan C, Wang Y, Wang G. Recent advancements in the use of exosomes as drug delivery systems. J Nanobiotechnol. 2018;16:81. Ahmadi M, Rezaie J. Tumor cells derived-exosomes as angiogenenic agents: possible therapeutic implications. J Transl Med. 2020;18:249. Wang J, Wang C, Li Y, Li M, Zhu T, Shen Z, Wang H, Lv W, Wang X, Cheng X, **e X. Potential of peptide-engineered exosomes with overexpressed miR-92b-3p in anti-angiogenic therapy of ovarian cancer. Clin Transl Med. 2021;11:e425. Rizk NI, Abulsoud AI, Kamal MM, Kassem DH, Hamdy NM. Exosomal-long non-coding RNAs journey in colorectal cancer: Evil and goodness faces of key players. Life Sci. 2022;292:120325. Katayama Y, Uchino J, Chihara Y, Tamiya N, Kaneko Y, Yamada T, Takayama K. Tumor neovascularization and developments in therapeutics. Cancers. 2019. https://doi.org/10.3390/cancers11030316. Xu R, Greening DW, Zhu H-J, Takahashi N, Simpson RJ. Extracellular vesicle isolation and characterization: toward clinical application. J Clin Investig. 2016;126:1152–62. Qiu S, **e L, Lu C, Gu C, **a Y, Lv J, Xuan Z, Fang L, Yang J, Zhang L, et al. Gastric cancer-derived exosomal miR-519a-3p promotes liver metastasis by inducing intrahepatic M2-like macrophage-mediated angiogenesis. J Exp Clin Cancer Res. 2022;41:296. Chen Z, **e Y, Chen W, Li T, Chen X, Liu B. microRNA-6785-5p-loaded human umbilical cord mesenchymal stem cells-derived exosomes suppress angiogenesis and metastasis in gastric cancer via INHBA. Life Sci. 2021;284:119222. This work was supported by National Natural Science Foundation of China (82203854, 82102702, 82103322), Key Research and Development Program of Shandong Province (No.2021CXGC011104; No.2019JZZY010104; No.2019GSF108146), Academic promotion programme of Shandong First Medical University (2019QL021), and Special Foundation for Taishan Scholars Program of Shandong Province (No.ts20190978), Natural Science Foundation of Shandong Province of China (ZR2020QH180, ZR2021QH141), Youth Innovation Science and Technology Program of Shandong Provincial Universities (2022KJ187), China postdoctoral science foundation (2022M711970), Clinical Medical Science and Technology Innovation Project of **an (202225046). YL and HW had the idea for the article, YL and HW performed the literature search and finished the manuscript; YL, WC and YDS finished the figures and tables; WC, LS and LPL made critical revisions and proofread the manuscript. All authors read and approved the final manuscript. Not applicable. The authors declare that they have no competing interests. Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. Liu, Y., Wu, H., Sang, Y. et al. Research progress of exosomes in the angiogenesis of digestive system tumour.
Discov Onc 15, 33 (2024). https://doi.org/10.1007/s12672-024-00879-4 Received: Accepted: Published: DOI: https://doi.org/10.1007/s12672-024-00879-4Availability of data and materials
Abbreviations
References
Funding
Author information
Authors and Affiliations
Contributions
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Competing interests
Additional information
Publisher's Note
Rights and permissions
About this article
Cite this article
Keywords