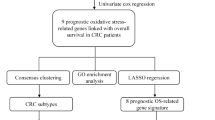
Abstract
Background
Colon cancer (CC) is a prevalent malignant tumor that affects the colon in the gastrointestinal tract. Its aggressive nature, strong invasiveness, and rapid progression make it a significant health concern. In addition, oxidative stress can lead to the production of reactive oxygen species (ROS) that surpass the body's antioxidant defense capacity, causing damage to proteins, lipids, and DNA, potentially promoting tumor development. However, the relationship between CC and oxidative stress requires further investigation.
Methods
We collected gene expression data and clinical data from 473 CC patients from The Cancer Genome Atlas (TCGA) dataset. Additionally, we obtained 433 oxidative stress genes from Genecards (https://www.genecards.org/). Using univariate, multivariate, and LASSO Cox regression analyses, we developed predictive models for oxidative stress-related genes in CC patients. To validate the models, we utilized data from the Gene Expression Omnibus (GEO) database. We assessed the accuracy of the models through various techniques, including the creation of a nomogram, receiver operating characteristic curve (ROC) analysis, and principal component analysis (PCA). The Cytoscape program was utilized to identify hub genes among differentially expressed genes (DEGs) in tumor patients using the TCGA dataset. Subsequently, we conducted survival analysis, clinical relevance analysis, and immune cell relevance analysis for the intersected genes obtained by combining the hub genes with the genes from the predictive models. Moreover, we investigated the mRNA expression and potential functions of these intersected genes using a range of experimental approaches.
Results
In both the TCGA and GSE17538 datasets, patients classified as high-risk had significantly shorter overall survival compared to those in the low-risk group (TCGA: p < 0.001; GSE17538: p = 0.010). As a result, we decided to further investigate the role of SERPINE1. Our survival analysis revealed that patients with high expression of SERPINE1 had a significantly lower probability of survival compared to those with low expression (p < 0.05). Additionally, our clinical correlation analysis showed a significant relationship between SERPINE1 expression and T, N, and M stages, as well as tumor grade. Furthermore, our immune infiltration correlation analysis demonstrated notable differences in multiple immune cells between the high- and low-expression groups of SERPINE1. To validate our findings, we conducted experimental tests and observed that knocking down SERPINE1 in colon cancer cells resulted in significant reductions in cell viability and proliferation. Interestingly, we also noticed an increase in oxidative stress parameters, such as ROS and MDA levels, while the levels of reduced GSH decreased upon SERPINE1 knockdown. These findings suggest that the antineoplastic effect of silencing SERPINE1 may be associated with the induction of oxidative stress.
Conclusion
In conclusion, this study introduces a new approach for the early diagnosis and treatment of CC, and further exploration of SERPINE1 could potentially lead to a significant advancement.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Colon cancer (CC) is a malignant tumor that originates from the mucosal epithelium of the colon. It accounts for over 1 million new cases worldwide each year, posing a serious threat to human health and survival [1]. CC has the second-highest mortality rate and the third-highest prevalence rate among all tumors [2]. While the incidence rate is decreasing among the elderly, it is increasing in younger individuals, causing the overall CC population to become younger [3]. Despite recent advancements in surgery, radiotherapy, targeted therapy, and immunotherapy, the 5-year survival rate for CC remains at 40–60% [4]. Early detection of CC is challenging as early symptoms are often not noticeable, and standard tumor markers have low sensitivity and specificity. Therefore, it is crucial to study diagnostic and prognostic biomarkers related to CC in order to improve the prognosis for individuals with this disease.
Oxidative stress is the physiological and pathological response of cells and tissues to the generation of reactive oxygen radicals (ROS) and reactive nitrogen radicals (RNS) in both internal and external environments [5]. An imbalance of oxidative stress can lead to the oxidation of nucleic acids, proteins, and lipids by ROS, promoting malignancy. Damaged DNA then enters the cytoplasm, triggering an interferon-mediated innate immune response that further stimulates ROS production. This creates a vicious cycle that maintains the inflammatory environment of the tumor [6, 7]. Tumor cells often have higher levels of ROS compared to normal tissues due to abnormal activation of oncogenes, inactivation of tumor suppressor genes, and metabolic reprogramming induced by hypoxia in the tumor microenvironment [8, 9]. Over 20 years ago, Haklar et al. observed a significant increase in ROS levels in CC tumor tissues through chemiluminescence analysis [10]. Other studies have investigated the association between genetic variants in antioxidant protective mechanism genes and the risk of CC [11]. Additionally, chronic inflammation caused by infections can increase ROS production in the colon, leading to oxidative stress in the colonic mucosa and impacting colon carcinogenesis [12]. Additionally, chronic inflammation caused by infections can increase ROS production in the colon, leading to oxidative stress in the colonic mucosa and impacting colon carcinogenesis [13]. However, further exploration is needed to understand the critical biological pathways regulated by oxidative stress and investigate the role of epigenetic factors in the pathogenesis of CC.
Therefore, the identification of prognostic genes and novel signature molecules associated with oxidative stress in colon cancer may provide valuable insights for the development of anti-CC strategies.
2 Materials and methods
2.1 Acquisition of mRNA expression data and clinical data
Clinical information, including general information, stage, and prognosis of CC samples, was obtained from The Cancer Genome Atlas (TCGA) (https://portal.gdc.cancer.gov/). As of October 2022, mRNA transcriptome data and connected medical records of 473 CC patients were obtained from the TCGA database. GSE17538 gene expression profiles were acquired from Gene Expression Omnibus (GEO) (https://www.ncbi.nlm.nih.gov/gds/), which included comprehensive transcriptome data for 238 patients with CC (platform: GPL570). Supplementary Table S1 provides detailed information on the patients.
2.2 Identification of differentially expressed oxidative stress genes (DEOSGs)
A total of 433 oxidative stress protein domains with correlation scores ≥ 10 were collected from GeneCards (https://www.genecards.org) (Supplementary Table S2). The FPKM values were used to standardize the expression data of the TCGA dataset for comparison, and gene expression related to oxidative stress was retrieved [14]. The extracted genes were then filtered using the 'limma' package based on a false discovery rate (FDR) < 0.05 and |log2 fold change (FC)|≥ 1.5, resulting in the identification of DEOSGs in CC tissues [2.18 Determination of ROS production SW480 and CACO-2 cells were seeded in 6-well plates and incubated for 24 h. Following transfection, the cells were incubated at 37 °C for approximately 30 min with serum-free media containing DCFH-DA (Beyotime, China). Flow cytometry was used to analyze the plates and obtain the outcomes of the treated cells, which were then preserved. MDA and GSH levels were measured using the MDA Assay Kit (Beyotime; S0131; China) and GSH Assay Kit (Nan**g Jiancheng Bioengineering Institute; A006-2-1; China) according to the provided directions. Gene expression in CC and para-tumor tissues was compared using univariate ANOVA, while categorical variables were compared using Pearson chi-square tests. Overall survival between the two patient groups was assessed using stratified log-rank testing and Kaplan–Meier analysis. The independent prognostic efficacy of the risk model was evaluated using univariate and multivariate Cox regression models. R program (v4.2.1) and the Perl language (version 5.30.0) were used for all statistical analyses. Statistical significance was defined as P < 0.05.2.19 MDA and GSH assay
2.20 Statistical analysis
3 Results
3.1 Identification of DEOSGs
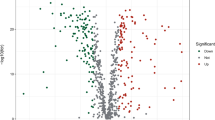
The study design is illustrated in Supplementary Figure S1. Using the TCGA database, we compared the expression of 433 genes related to oxidative stress in 473 CC tissues. We identified 36 down-regulated genes and 29 up-regulated genes, which had a false discovery rate (FDR) < 0.05 and a |log2 fold change (FC)|≥ 1.5. Comprehensive data on the differentially expressed oxidative stress-related genes (DEOSGs) can be found in Supplementary Table S3. The top 50 genes with the greatest increase and decrease are shown in Supplementary Figure S2A. Additionally, all DEOSGs are depicted in the volcano plot (Supplementary Figure S2B).
3.2 Screening of prognostic oxidative stress-related genes in CC
Through a univariate Cox regression analysis in the TCGA database, we have identified 23 prognostic oxidative stress-related genes for CC (P < 0.05). Genes with a hazard ratio (HR) less than 1 are considered low-risk genes, while genes with HR greater than 1 are considered high-risk genes. The 95% confidence intervals for the HR values are shown in brackets in supplementary Figure S3A. The waterfall plot illustrates variations in the distribution of somatic mutations in the 23 DEOSGs (supplementary Figure S3B), and the co-occur plot indicates the co-occurrence between these 23 DEOSGs (supplementary Figure S3C).
3.3 Construction and evaluation of a prognostic model for CC based on oxidative stress-related genes
Through LASSO regression analysis, we identified 16 genes (NGF, IL13, RPS6KA5, TERT, CDKN2A, SERPINE1, CD36, PPARGC1A, ACADL, ACOX1, POMC, BDNF, MSRA, DDIT3, GSTM1, CPT2) based on the best λ value to construct a prognostic model (Supplementary Figure S4A–B). The risk score is calculated using the following formula: risk score = (0.0708 * NGF exp.) + (−2.4907 * IL13 exp.) + (−0.1846 * RPS6KA5 exp.) + (0.7210 * TERT exp.) + (0.1351 * CDKN2A exp.) + (0.1102 * SERPINE1 exp.) + (0.1850 * CD36 exp.) + (−0.3145 * PPARGC1A exp.) + (0.3644 * ACADL exp.) + (−0.0843 * ACOX1 exp.) + (0.1684 * POMC exp.) + (0.5691 * BDNF exp.) + (−0.2211 * MSRA exp.) + (0.2156 * DDIT3 exp.) + (−0.1499 * GSTM1 exp.) + (−0.2575 * CPT2 exp.). We divided the 473 individuals with colorectal cancer into low- and high-risk groups by using the median score obtained from the risk score method. Subsequently, the patients with different risks were further divided into two groups based on PCA for oxidative stress genes and model genes (Supplementary Figure S4C–D). Analysis of the TCGA database revealed a significant difference in overall survival between the low- and high-risk groups (P < 0.001) (Supplementary Figure S4E). To validate the model, we used the GSE17538 database as an external validation group. The analysis showed a statistically significant difference in overall survival between the low- and high-risk groups (P = 0.010) (Supplementary Figure S4F). Furthermore, in the TCGA cohort, the high-risk group exhibited significantly poorer PFS compared to the low-risk group (P < 0.001) (Supplementary Figure S4G).
To evaluate the risk signature as an independent predictive variable, we used univariate and multivariate Cox regression models. The results showed that the risk score was an independent predictor of a worse patient prognosis (HR = 3.461, 95% CI 2.552–4.693) based on univariate Cox regression analysis (Supplementary Figure S5A). After adjusting for other confounding variables, the multivariate Cox regression analysis demonstrated that the risk score remained a significant predictive variable for individuals with CC (HR = 2.973, 95% CI 2.124–4.162) (Supplementary Figure S5B). We assessed the sensitivity and specificity of the model using ROC analysis, and found that the areas under the ROC curves for 1, 3, and 5 years were 0.706, 0.730, and 0.781, respectively (Supplementary Figure S5C). Over five years, the prognostic model showed superior predictive accuracy compared to other clinical characteristics in the TCGA cohort (Supplementary Figure S5D).
To determine the association between each clinicopathological characteristic and the risk score, we analyzed age, sex, tumor grade, and TNM stage in the TCGA cohort. The results indicated that the risk score was positively associated with T-stage, except for T1 and T2 stages (Supplementary Figure S5E). Patients with a higher N stage (Supplementary Figure S5F) or M1 stage (Supplementary Figure S5G) of CC were significantly associated with higher risk scores. Regarding tumor grade, the risk score was positively associated with tumor grade, except for stages III and IV (Supplementary Figure S5H). However, there was no significant relationship between the risk score and age or sex in individuals with CC (all P > 0.05).
3.4 Construction and validation of a prognostic nomogram for CC based on oxidative stress-related genes
Based on the findings of the multivariate Cox regression analysis in the TCGA cohort, we constructed a nomogram using independent risk variables including age, sex, tumor grade, TNM stage, and risk scores (Supplementary Figure S6A). To evaluate the nomogram's prediction ability, we plotted clinical ROC curves and calibration curves. The calibration curve (Supplementary Figure S6B) showed a high consistency between the risk predicted by the nomogram and the detected 1-, 3-, and 5-year survival rates. The nomogram also exhibited superior predictive accuracy compared to other clinical variables over a 5-year period, as indicated by the clinical ROC curve (AUC = 0.819) (Supplementary Figure S6C). Moreover, the Nomo risk score showed a significant association with overall survival according to the Univariate Cox regression analysis [HR = 1.309 (1.238–1.383), P < 0.001, Supplementary Figure S6D]. Additionally, the Nomo risk score was identified as an independent risk factor for overall survival in subjects with CC using multivariate Cox regression analysis [HR = 1.167 (1.075–1.266), P < 0.001, Supplementary Figure S6E].
3.5 Differential analysis of immune cells and GSVA analysis
The analysis of immune cell differences revealed significant variations in the expression of B memory cells, plasma cells, T CD4 memory resting cells, NK cells, Macrophages M0, Dendritic cells resting cells, Dendritic cells activated cells, and Eosinophils between the high- and low-risk groups of the TCGA cohort (P < 0.05) (Supplementary Figure S7A). The MCP-counter algorithm also identified significant differences in the expression of NK cells, myeloid dendritic cells, myeloid dendritic cells, and fibroblasts between the low-risk and high-risk groups (Supplementary Figure S7B). Furthermore, the GSVA analysis revealed that the high-risk group of the TCGA cohort had significantly elevated expression levels of the circadian rhythm mammal, basal cell carcinoma, glycosaminoglycan biosynthesis, chondroitin sulfate, and ECM receptor interaction mechanisms (Supplementary Figure S7C).
3.6 Screening and functional enrichment analysis of DEGs in TCGA cohort of CC
Based on the median risk score mentioned above, a differential analysis was conducted on individuals with CC in the TCGA cohort, resulting in the identification of 70 genes. Among these genes, 17 were found to be down-regulated while 53 were up-regulated (Supplementary Table S4). The GO analysis of these 70 DEGs in CC indicated that biological processes (BP) were mainly associated with muscle contraction and intermediate filament-based mechanisms. CC were dominated by collagen-containing extracellular matrix and endoplasmic reticulum lumen. In terms of molecular function (MF), receptor ligand and signaling receptor activator activity were found to be predominant (Supplementary Figure S8A-B). Furthermore, the KEGG pathway analysis revealed that the main signaling pathways involved were ECM-receptor interaction, Human papillomavirus infection, and PI3K-Akt signaling pathway (Supplementary Figure S8C-D).
3.7 Identification of hub genes and prognostic analysis of CDKN2A and SERPINE1
Using the STRING software, it was discovered that out of the 70 DEGs identified in CC, 63 were involved in constructing PPI networks. The resulting PPI network consisted of 62 edges, with an average node degree of 1.97 and an average local clustering coefficient of 0.393. Statistically, the difference in this PPI network was found to be significant (P < 0.05) (Fig. 1A). To identify the top 15 hub genes based on node degree, the Cyto-Hubba plug-in was utilized, resulting in the selection of FN1, CDKN2A, SFRP2, MYH11, SFRP4, CILP, COL9A3, SERPINE1, WIF1, COMP, ACTG2, KRT14, THBS2, CALB2, and KRT17 (Fig. 1B). Furthermore, by intersecting the top 15 hub genes with the genes used to construct the predictive model for CC, CDKN2A and SERPINE1 were also identified (Fig. 1C). The Human Protein Atlas (HPA, https://www.proteinatlas.org/) offers an IHC-based assay for relative protein abundance [23]. In terms of oxidative stress, SERPINE1 exhibited a higher coefficient than CDKN2A (12.33 vs. 11.43). To examine the protein expression of SERPINE1 in CC and normal tissues, HPA data was utilized, which revealed a noticeable accumulation of SERPINE1 in CC tissues (Fig. 1D, E).
Construction of PPI networks and identification of hub genes. A The PPI network showed the interactions of the DEGs (interaction score = 0.4). B Visualization of the PPI network and the candidate hub gene according to the EPC ranking. C A Venn diagram shows the number of overlapped genes between the top ten hub genes and the genes involved in the construction of the prognostic model for CC. D–E Immunohistochemical staining of SERPINE1 gene in normal tissue (D) and cancer tissue (E) of CC in HPA database
3.8 Clinical correlation analysis and immune infiltration analysis of SERPINE1
The relationship between SERPINE1 expression and the clinicopathological characteristics of subjects with CC in the TCGA dataset was investigated. The results revealed a significant correlation between SERPINE1 expression and the cancer grade and TNM stages of individuals with CC. SERPINE1 expression was lower in T2 patients compared to T3 or T4 patients (P = 0.00025 and P = 0.0027, respectively) (Fig. 2A). Additionally, SERPINE1 expression was lower in N0 patients compared to N1 or N2 patients (P = 0.023 and P = 0.0058, respectively) (Fig. 2B). Moreover, SERPINE1 expression was lower in M0 patients compared to M1 patients (P = 0.046) (Fig. 2C). In terms of tumor stage, SERPINE1 expression was lower in Stage I patients compared to Stages II, III, or IV patients (P = 0.011, 0.0012, and 0.00062, respectively) (Fig. 2D). However, there was no correlation between SERPINE1 expression and gender or age (P = 0.60 and 0.32, respectively). Survival analysis using the Kaplan–Meier method revealed that patients with reduced SERPINE1 expression had significantly longer overall survival compared to those with elevated expression (Fig. 2E). To examine the connection between SERPINE1 and immune cell infiltration, a correlation study was conducted to assess the relationship between SERPINE1 expression and the abundance of 22 immune cell types. The results showed that B cells naive, macrophages M0, and activated mast cells were significantly more abundant in the high-expression group of SERPINE1, while plasma cells, T cells CD4 memory resting, NK cells resting, dendritic cells resting, and mast cells resting were more abundant in the low-expression group (Fig. 2F).
Clinical correlation analysis and immune infiltration analysis of SERPINE1. A The relationship between SERPINE1 expression and T stage in TCGA cohort. B The relationship between SERPINE1 expression and N stage in TCGA cohort. C The relationship between SERPINE1 expression and M stage in TCGA cohort. D The relationship between SERPINE1 expression and tumor stage in TCGA cohort. E Kaplan–Meier curves for comparison of the overall survival between SERPINE1 low-and high-expression groups in the TCGA database (P < 0.001). F CIBERSORT score of 22 immune cell infiltrations among TCGA samples of SERPINE1 low-expression and high-expression groups
3.9 The expression of SERPINE1 in CC tissues and cell lines
Following the comprehensive online machine learning analysis, RT-qPCR and western blot were conducted to confirm the significantly higher levels of SERPINE1 mRNA and protein in CC tissues compared to para-tumor tissues (Fig. 3A, B). In order to validate these findings, three different CC cell lines (SW480, CACO-2, and HT29) were selected for in vitro experiments, with human intestinal epithelial cells (NCM460) serving as the control group. The results consistently demonstrated overexpression of SERPINE1 in the CC cancer cell lines (Fig. 3C, D). Furthermore, IHC analysis of human tissues provided additional confirmation of the prominent accumulation of SERPINE1 in tumor regions (Fig. 3E, F; Supplementary Figure S9).
The expression of SERPINE1 in CC tissues and cell lines. A SERPINE1 mRNA expression in CC specimens relative to para-tumor tissues as detected by RT-qPCR. B SERPINE1 protein level in 6 CC patients relative to para-tumor tissues as conducted by western blot. C SERPINE1 expression in 3 different CC cell lines (SW480, CACO-2, and HT29) relative to NCM460 was conducted by RT-qPCR. D SERPINE1 expression in 3 different CC cell lines (SW480, CACO-2, and HT29) relative to NCM460 was conducted by western blot. E–F Immunohistochemical analysis of SERPINE1 expression in CC patients. *p < 0.05; **p < 0.01; ***p < 0.001
3.10 Effects of SERPINE1 knockdown on CC cell viability, proliferation, and oxidative stress
Functional interference techniques were employed to examine the impact of SERPINE1 deletion on the behavior of CC cells, aiming to determine the specific role of SERPINE1 in the initiation and development of CC. Figure 4A–C demonstrate the successful transfection in CC cell lines. The results of CCK-8 analysis revealed that suppressing SERPINE1 significantly reduced cell viability in SW480 and CACO-2 cell lines (Fig. 4D, E). Similarly, the data from colony formation analyses indicated that the clone capacity of SW480 and CACO-2 cell lines was diminished after silencing SERPINE1 (Fig. 4F–I). These findings suggest that SERPINE1 plays a crucial role in promoting the progression of CC. Furthermore, it is well-established that oxidative stress is involved in the occurrence and development of cancers. Therefore, this study investigated the levels of ROS, MDA, and GSH in SW480 and CACO-2 cells (Fig. 4J–M), which revealed a significant increase in MDA and ROS levels and a noticeable decrease in GSH levels after transfecting SERPINE1 compared to the si-ctrl group. In summary, the anti-tumor effect of SERPINE1 knockdown on CC may be partly attributed to inducing oxidative stress.
The effect of SERPINE1 knockdown on CC cell viability, proliferation as well as oxidative stress in vitro. A The efficiency of SERPINE1 knockdown (si- SERPINE1) was assessed by western blot in SW480 and CACO-2 cells. B Quantitative analysis of western blot in SW480 cell. C Quantitative analysis of western blot in CACO-2 cell. D The viability of SW480 cell was assessed by CCK-8 assays. E The viability of CACO-2 cell was assessed by CCK-8 assays. F–G The clone capacity of SW480 cell was evidenced by colony formation assay. H–I The clone capacity of CACO-2 cell was evidenced by colony formation assay. J–K ROS level of SW480 and CACO-2 cells. L MDA level of SW480 and CACO-2 cells. M GSH level of SW480 and CACO-2 cells. *p < 0.05; **p < 0.01; ***p < 0.001
4 Discussion
CC is the most prevalent malignant tumor of the gastrointestinal system, ranking third among all malignant tumors in terms of incidence. The prognosis of CC is poor, and its pathogenesis remains poorly understood. As research on oxidative stress in intestinal diseases advances, there is increasing evidence from experimental and clinical data suggesting that ROS-mediated oxidative stress plays a vital role in CC development [11, 24]. Therefore, it is crucial to explore the function of oxidative stress-related genes in CC. This exploration may help elucidate the pathogenesis of CC, identify specific biomarkers, and discover therapeutic targets for patients with this condition.
The aim of this investigation was to identify prognostic markers for CC based on oxidative stress-related genes and develop a predictive model. Expression data from individuals with CC were obtained from the TCGA database, and a total of 204 differentially expressed oxidative stress genes (DEOSGs) were identified through differential expression analysis. Uni-variate Cox regression analysis using the survival package in the R program was performed to investigate the relationship between the expression of these genes and CC prognosis. This analysis resulted in the identification of 23 oxidative stress genes associated with CC prognosis. Subsequently, multivariate Cox regression analysis was conducted on these 23 genes, leading to the identification of 16 genes significantly associated with CC prognosis. These genes include NGF, IL13, RPS6KA5, TERT, CDKN2A, SERPINE1, CD36, PPARGC1A, ACADL, ACOX1, POMC, BDNF, MSRA, DDIT3, GSTM1, and CPT2. A predictive model for CC was developed using these 16 genes.
Each patient in the TCGA cohort was assigned a risk score using the Lasso regression model. The participants were divided into high- and low-risk groups based on the median value of the scores to assess the predictive value and reliability of the model. The survival study revealed that the prognosis of the high-risk group was significantly worse than that of the low-risk group. To validate the model, we also categorized patients with CC in GSE17538 into high- and low-risk groups based on their risk scores, which yielded the same result. The ROC analysis results showed that the AUC for 1-, 3-, and 5-year predictions were 0.706, 0.730, and 0.781, respectively, indicating excellent prognostic predictive ability of the model for CC. Univariate and multivariate Cox regression analyses identified the risk score as an independent predictive factor. The nomogram also demonstrated similar patterns, with survival, ROC, and univariate and multivariate regression analyses showing superior efficacy of the nomogram compared to other clinicopathological features.
Based on the median risk scores, differential analysis of CC subjects in the TCGA cohort was conducted to identify 70 differentially co-expressed genes with similar expression trends. Further analysis of the top 15 genes with the highest node degrees was performed using PPI networks. These 16 genes overlapped with the 16 genes involved in the model construction, leading to the identification of CDKN2A and SERPINE1. The HPA database revealed higher SERPINE1 protein expression in CC tissues compared to the healthy group [23]. Additionally, we observed that SERPINE1 had a higher oxidative stress coefficient than CDKN2A (12.33 vs. 11.43). Therefore, SERPINE1 was ultimately identified as the hub gene.
SERPINE1, a member of the serine protease inhibitor (serpin) superfamily, is primarily found in the gallbladder, liver, bladder, and placental tissues. It acts as a key suppressor of fibrinogen activator and plays a crucial role in regulating fibrinolysis [25, 26]. Moreover, SERPINE1 is significantly expressed in various tumor tissues and has been linked to cancer progression and metastasis [27]. Kim et al. conducted a study where they directly treated colon cancer cells with purine-based antiplatelet agents, confirming the increased expression of SERPINE1 in colon cancer. They observed that the overexpression of SERPINE1 enhanced cellular mobility in cancer cells, indicating its association with colon cancer metastasis [28]. Additionally, Chen et al. suggested that SERPINE1 may regulate the expression of VEGF and IL-6 through the JAK-STAT3 inflammatory and VEGF signaling pathways, thereby influencing GC cell invasion and migration [29]. Mazzoccoli et al. investigated SERPINE1 expression in 50 CC specimens, para-tumor tissues, and CC cell lines. They found that SERPINE1 expression was elevated in CC and highly proliferative CC cell lines, and it was also associated with tumor aggressiveness and invasiveness [30].
It was discovered that SERPINE1 hinders the tumor healing effects of miR148a-3p in CC, which includes cell growth and invasion [31]. Through univariate Cox regression and clinical correlation analyses, it was determined that high expression of SERPINE1 indicates a poorer prognosis, higher tumor staging, and grading. Wang et al. utilized the CIBERSORT method to evaluate the relationship between differential expression of SERPINE1 and immune cell infiltration. They ultimately identified SERPINE1 as a potential regulator of immune cell infiltration, capable of interacting with eight immune cell types, thereby resha** the tumor microenvironment in colon cancer development and progression [32]. Our analysis of the relationship between SERPINE1 and 22 types of immune cells revealed that SERPINE1 is associated with numerous immune cells, suggesting its involvement in the immune infiltration process during CC development. These findings collectively suggest that SERPINE1 may play a role in CC progression and impact prognosis through its involvement in the regulation of oxidative stress. This study demonstrated that both SERPINE1 mRNA and protein levels were found to be overexpressed in human CC tumor tissues and cells. Functional studies were conducted using si-SERPINE1 transfection, which showed that interference with SERPINE1 significantly reduced the viability and growth of CC cells. Additionally, SERPINE1 inhibition led to a restoration of ROS and MDA accumulation and hindered the GSH content.
However, it is important to note that identifying the hub gene is only the initial step in screening biomarkers and therapeutic targets for CC. Further experiments are needed to validate the significance of SERPINE1 in CC development and prognosis, as well as to explore its molecular mechanism in regulating oxidative stress. Moreover, our findings underscore the intricate interplay between oxidative stress, SERPINE-1, and immune infiltration in CC. Further research is needed to elucidate the precise mechanisms underlying these relationships and their implications for CC progression and treatment.
5 Conclusion
In this study, we conducted bioinformatics analysis and validation to screen 16 prognostic genes associated with oxidative stress in CC. These genes are NGF, IL13, RPS6KA5, TERT, CDKN2A, SERPINE1, CD36, PPARGC1A, ACADL, ACOX1, POMC, BDNF, MSRA, DDIT3, GSTM1, and CPT2. Using these 16 genes, we developed a prognostic model for CC that showed strong predictive value and reliability in determining patient outcomes. Additionally, our findings suggest that SERPINE1 may contribute to the development of CC through its regulation of oxidative stress. Therefore, SERPINE1 holds promise as a potential therapeutic target and a novel prognostic biomarker for CC.
Data availability
The raw transcriptome data of this study are available in the TCGA database (TCGA cohorts) (https://portal.gdc.cancer.gov/) and the GEO database (GSE17538) (https://www.ncbi.nlm.nih.gov/gds/). Oxidative stress genes were downloaded and collated from Genecards (https://www.genecards.org/).
Abbreviations
- CC:
-
Colon cancer
- ROS:
-
Reactive oxygen radicals
- RNS:
-
Reactive nitrogen radicals
- TCGA:
-
The Cancer Genome Atlas
- GEO:
-
Gene expression omnibus
- FPKM:
-
Fragments per kilobase of exon model per Million mapped fragments
- DEOSGs:
-
Differentially expressed oxidation stress genes
- HR:
-
Hazard ratio
- PFS:
-
Progression-free survival
- ROC:
-
Receiver operating characteristic curve
- AUC:
-
Area under the curve
- MSigDB:
-
Molecular signatures database
- GSVA:
-
Gene set variation analysis
- DEGs:
-
Differentially expressed genes
- PPI:
-
Protein–protein interaction
- EPC:
-
Edge percolated component
- WB:
-
Western blot
- IHC:
-
Immunohistochemistry
- ROS:
-
Reactive oxygen species
- MDA:
-
Malonic dialdehyde
- GSH:
-
Micro reduced glutathione
References
Ahluwalia P, Kolhe R, Gahlay GK. The clinical relevance of gene expression based prognostic signatures in colorectal cancer. Biochim Biophys Acta Rev Cancer. 2021;1875(2):188513.
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–49.
Siegel RL, Fedewa SA, Anderson WF, Miller KD, Ma J, Rosenberg PS, et al. Colorectal cancer incidence patterns in the United States, 1974–2013. J Natl Cancer Inst. 2017. https://doi.org/10.1093/jnci/djw322.
Siegel RL, Miller KD, Goding Sauer A, Fedewa SA, Butterly LF, Anderson JC, et al. Colorectal cancer statistics, 2020. CA Cancer J Clin. 2020;70(3):145–64.
Sies H, Berndt C, Jones DP. Oxidative stress. Annu Rev Biochem. 2017;86:715–48.
Jelic MD, Mandic AD, Maricic SM, Srdjenovic BU. Oxidative stress and its role in cancer. J Cancer Res Ther. 2021;17(1):22–8.
Dossena S, Marino A. Cellular oxidative stress. Antioxidants (Basel). 2021;10(3):399.
Harris IS, DeNicola GM. The complex interplay between antioxidants and ROS in cancer. Trends Cell Biol. 2020;30(6):440–51.
Sies H, Jones DP. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat Rev Mol Cell Biol. 2020;21(7):363–83.
Haklar G, Sayin-Ozveri E, Yüksel M, Aktan AO, Yalçin AS. Different kinds of reactive oxygen and nitrogen species were detected in colon and breast tumors. Cancer Lett. 2001;165(2):219–24.
Perše M. Oxidative stress in the pathogenesis of colorectal cancer: cause or consequence? Biomed Res Int. 2013;2013:725710.
Svrcek M, Borralho Nunes P, Villanacci V, Beaugerie L, Rogler G, De Hertogh G, et al. Clinicopathological and molecular specificities of inflammatory bowel disease-related colorectal neoplastic lesions: the role of inflammation. J Crohns Colitis. 2018;12(12):1486–98.
Tian T, Wang Z, Zhang J. Pathomechanisms of oxidative stress in inflammatory bowel disease and potential antioxidant therapies. Oxid Med Cell Longev. 2017;2017:4535194.
Zhuang Z, Cai H, Lin H, Guan B, Wu Y, Zhang Y, et al. Development and validation of a robust pyroptosis-related signature for predicting prognosis and immune status in patients with colon cancer. J Oncol. 2021;2021:5818512.
Xu J, Zhou H, Cheng Y, **ang G. Identifying potential signatures for atherosclerosis in the context of predictive, preventive, and personalized medicine using integrative bioinformatics approaches and machine-learning strategies. Epma J. 2022;13(3):433–49.
Shiva UMKS, Kuruva MM, Mitnala S, Rupjyoti T, Venkat RG, Botlagunta S, et al. MicroRNA profiling in periampullary carcinoma. Pancreatology. 2014;14(1):36–47.
Yuan K, Zeng R, Deng P, Zhang A, Liu H, Wang N, et al. Identification and verification of immune-related genes prognostic signature based on ssGSEA for adrenocortical carcinoma (ACC). Int J Gen Med. 2022;15:1471–83.
Mayakonda A, Lin DC, Assenov Y, Plass C, Koeffler HP. Maftools: efficient and comprehensive analysis of somatic variants in cancer. Genome Res. 2018;28(11):1747–56.
Bersanelli M, Mosca E, Milanesi L, Bazzani A, Castellani G. Frailness and resilience of gene networks predicted by detection of co-occurring mutations via a stochastic perturbative approach. Sci Rep. 2020;10(1):2643.
Zhang C, He H, Hu X, Liu A, Huang D, Xu Y, et al. Development and validation of a metastasis-associated prognostic signature based on single-cell RNA-seq in clear cell renal cell carcinoma. Aging (Albany NY). 2019;11(22):10183–202.
Chong W, Shang L, Liu J, Fang Z, Du F, Wu H, et al. m(6)A regulator-based methylation modification patterns characterized by distinct tumor microenvironment immune profiles in colon cancer. Theranostics. 2021;11(5):2201–17.
Zhou J, Zhu Y, Ma S, Li Y, Liu K, Xu S, et al. Bioinformatics analysis identifies DYNC1I1 as prognosis marker in male patients with liver hepatocellular carcinoma. PLoS ONE. 2021;16(10):e0258797.
Thul PJ, Lindskog C. The human protein atlas: a spatial map of the human proteome. Protein Sci. 2018;27(1):233–44.
Carini F, Mazzola M, Rappa F, Jurjus A, Geagea AG, Al Kattar S, et al. Colorectal carcinogenesis: role of oxidative stress and antioxidants. Anticancer Res. 2017;37(9):4759–66.
Planus E, Barlovatz-Meimon G, Rogers RA, Bonavaud S, Ingber DE, Wang N. Binding of urokinase to plasminogen activator inhibitor type-1 mediates cell adhesion and spreading. J Cell Sci. 1997;110(Pt 9):1091–8.
Xu Y, Zhang S, Niu H, Ye Y, Hu F, Chen S, et al. STIM1 accelerates cell senescence in a remodeled microenvironment but enhances the epithelial-to-mesenchymal transition in prostate cancer. Sci Rep. 2015;5:11754.
Chiappetta C, Puggioni C, Carletti R, Petrozza V, Della Rocca C, Di Crisfofano C. The nuclear-cytoplasmic trafficking of a chromatin-modifying and remodelling protein (KMT2C), in osteosarcoma. Oncotarget. 2018;9(55):30624–34.
Kim WT, Mun JY, Baek SW, Kim MH, Yang GE, Jeong MS, et al. Secretory SERPINE1 expression is increased by antiplatelet therapy, inducing MMP1 expression and increasing colon cancer metastasis. Int J Mol Sci. 2022;23(17):9596.
Chen S, Li Y, Zhu Y, Fei J, Song L, Sun G, et al. SERPINE1 overexpression promotes malignant progression and poor prognosis of gastric cancer. J Oncol. 2022;2022:2647825.
Mazzoccoli G, Pazienza V, Panza A, Valvano MR, Benegiamo G, Vinciguerra M, et al. ARNTL2 and SERPINE1: potential biomarkers for tumor aggressiveness in colorectal cancer. J Cancer Res Clin Oncol. 2012;138(3):501–11.
Hu B, Chen Z, Wang X, Chen F, Song Z, Cao C. MicroRNA-148a-3p directly targets SERPINE1 to suppress EMT-mediated colon adenocarcinoma progression. Cancer Manag Res. 2021;13:6349–62.
Wang S, Pang L, Liu Z, Meng X. SERPINE1 associated with remodeling of the tumor microenvironment in colon cancer progression: a novel therapeutic target. BMC Cancer. 2021;21(1):767.
Acknowledgements
Not applicable.
Funding
This study was supported by the Science and Technology Projects in Guangzhou (No. 202201020063).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. The development of methodology and writing, review, and revision of the paper were performed by KY, DH, and XM. Acquisition, analysis, and interpretation of data, and statistical analysis were provided by KY and RZ. The experimental validation was participated in by KY, BW, and ZZ. Financial support was provided by RH and CW. All authors commented on previous versions of the manuscript. KY, DH, and XM contributed equally to this work and share first authorship. RH and CW were both the corresponding authors. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
This study involving human participants was in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The Human Investigation Committee (IRB) of The First Affiliated Hospital of **an University approved this study. Informed consents (Consent to Participate and Consent to Publish) were obtained from all participants, if participants are under 18, from a parent and/or legal guardian.
Consent for publication
Informed consents were obtained from all participants, if participants are under 18, from a parent and/or legal guardian. All the authors are aware and given consent on the submission of this article.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
12672_2023_833_MOESM1_ESM.tif
Additional file1 (TIF 9283 KB)—Figure S1: The workflow of the study design (DEOSGs: differentially expressed oxidative stress genes, DEGs: differentially expressed genes).
12672_2023_833_MOESM2_ESM.tif
Additional file2 (TIF 12760 KB)—Figure S2: Identification of DEOSGs in CC from the TCGA database. (A) The heatmap showed the expression levels of the 50 genes that show the greatest difference in increase and decrease, respectively. Red represents high expression and green represents low expression. (B) The volcano plot showed 103 up-regulated genes (red) and 101 down-regulated genes (green) in the TCGA cohort (P < 0.05). Black dots mean meaningless (P > 0.05)
12672_2023_833_MOESM3_ESM.tif
Additional file3 (TIF 1826 KB)—Figure S3: Univariate Cox regression analysis of DEOSGs in CC from the TCGA database. (A) Univariate Cox regression analysis for identification prognostic oxidative stress genes in CC (P < 0.05). HR > 1 are high-risk genes, indicated in red, and HR < 1 are low-risk genes, indicated in green. 95% confidence intervals for HR values were in brackets. (B) Waterfall chart of mutation frequency of 23 oxidative stress genes. (C) Multi-gene correlation map was generated using the 23 oxidative stress genes. A positive value represents a positive correlation; otherwise, a negative correlation. The larger correlation value means a better correlation between two genes. P < 0.01 is indicated with *
12672_2023_833_MOESM4_ESM.tif
Additional file4 (TIF 7605 KB)—Figure S4: Construction and evaluation of oxidative stress-related risk signature in the TCGA cohort. (A) Cross-validation of the LASSO regression. (B) LASSO regression of the 16 prognostic oxidative stress genes. (C) PCA plot for oxidative stress (OS) genes. (D) PCA plot for 16 model genes. (E) Kaplan–Meier curves for comparison of the overall survival between low- and high-risk groups in the TCGA database (P < 0.001), (F) and the GSE17538 database (P = 0.010). (G) PFS analysis between low- and high-risk groups in the TCGA database (P < 0.001)
12672_2023_833_MOESM5_ESM.tif
Additional file5 (TIF 11135 KB)—Figure S5: The independent prognostic value and clinicopathological feature of the risk score. (A) Univariate cox regression analysis for the TCGA cohort. (B) Multivariate cox regression analysis for the TCGA cohort. (C) Time ROC curves for forecasting overall survival in TCGA cohort. (D) Clinical ROC curves were used to evaluate the predictive accuracy of the risk score by forecasting overall survival in the TCGA cohort. (E) The relationship between the risk scores and T stage in TCGA cohort. (F) The relationship between the risk scores and N stage in the TCGA cohort. (G) The relationship between the risk scores and M stage in the TCGA cohort. (H) The relationship between the risk scores and tumor stage in the TCGA cohort
12672_2023_833_MOESM6_ESM.tif
Additional file6 (TIF 8596 KB)—Figure S6: Construction and evaluation of oxidative stress-related nomogram in the TCGA cohort. (A) Nomogram of risk score and other clinical features for predicting CC 1-, 3-, and 5-year overall survival in TCGA cohort. (B) The calibration plot of the 1-year, 3-year, and 5-year survival rates of the nomogram in the TCGA cohort. (C) Clinical ROC curves were used to evaluate the predictive accuracy of the nomogram by forecasting overall survival in the TCGA cohort. (D) Univariate cox regression analysis for the nomo risk score in TCGA cohort. (E) Multivariate cox regression analysis for the nomo risk score in TCGA cohort
12672_2023_833_MOESM7_ESM.tif
Additional file7 (TIF 9974 KB)—Figure S7: Differential analysis of immune cells and GSVA analysis. (A) CIBERSORT score of 22 immune cell infiltrations among TCGA samples of high- and low-risk groups. *p < 0.05; **p < 0.01; ***p < 0.001. (B) Comparison of proportions of different kinds of cells estimated by MCP-counter algorithm. *p < 0.05; **p < 0.01; ***p < 0.001. (C) GSVA heatmap of oxidative stress-related genes of high- and low-risk groups. Red represents high risk and the green represents low risk
12672_2023_833_MOESM8_ESM.tif
Additional file8 (TIF 3592 KB)—Figure S8: Functional analysis of immune cells. (A) Bubble graph for GO enrichment in the TCGA cohort. BP: biological process; CC, cell component; MF, molecular function. (B) Circle diagram which enriched in GO analysis. (C) Barplot graph for KEGG pathways in the TCGA cohort. (D) Circle diagram which enriched in KEGG pathways
12672_2023_833_MOESM9_ESM.tif
Additional file9 (TIF 6339 KB)—Figure S9: (A-B) Three other samples of immunohistochemical analysis of SERPINE1 expression in CC patients. *p < 0.05; **p < 0.01; ***p < 0.001.
12672_2023_833_MOESM12_ESM.csv
Additional file12 (CSV 58 KB)—Table S2: 433 oxidative stress protein domains with correlation scores ≥ 10 were extracted from the gene cards.
12672_2023_833_MOESM13_ESM.xlsx
Additional file13 (XLSX 27 KB)—Table S3: The detailed information of DEOSGs in the TCGA cohort was shown in Supplementary Table S3.
12672_2023_833_MOESM14_ESM.xls
Additional file14 (XLS 7 KB)—Table S4: The detailed information of DEGs in TCGA cohort was shown in Supplementary Table S4.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yuan, K., Hu, D., Mo, X. et al. Novel diagnostic biomarkers of oxidative stress, immune- infiltration characteristics and experimental validation of SERPINE1 in colon cancer. Discov Onc 14, 206 (2023). https://doi.org/10.1007/s12672-023-00833-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12672-023-00833-w