Abstract
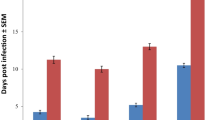
Mice infected with T. evansi cause various clinical manifestations and histopathological changes. The aim of this study was to compare the histopathological lesions of mice infected with T. evansi Bang 87 isolates (high virulence) and Pml 287 isolates (low virulence). A total of 15 susceptible mice (DDY) were divided into three groups (five mice/group): Groups I and II each were infected with 104 T. evansi of high virulence (Bang87) and low virulence (Pml 287), respectively, whereas group III served as a control group. A total of three mice from group I, and one mouse from each group II and III were killed at 4 dpi. A total of two mice from each group II and III were killed at 24 dpi. Two remaining mice from each group were observed until succumb. Mice of group I and group II at 4 dpi showed no gross lesions. However, mice of group I showed very acute animal death at 5 dpi and showed mild to moderate histopathological lesions at 4 dpi, namely non-suppurative encephalitis, non-suppurative pneumonia, hepatitis non-suppurative with intravascular trypanosomiasis, tubular degeneration and necrosis. Group II showed chronic death at 26 dpi with significant gross pathological changes at 24 dpi in spleen (swelling 10 times than normal size) accompanied by severe non-suppurative encephalitis, cholangiohepatitis non-suppurative and bile duct proliferation, diffused splenic necrosis. The result of this study is expected to be used as a basis for improved treatment management in cattle infected with high virulence T. evansi isolates that are need to be handled appropriately to avoid fatal consequences.







Similar content being viewed by others
References
Amin DN, Vodnala SK, Masocha W, Sun B, Kristensson K, Rottenberg ME (2012) Distinct toll-like receptor signals regulate cerebral parasite load and interferon α/β and tumor necrosis factor α-dependent T-cell infiltration in the brains of Trypanosoma brucei-infected mice. J Infect Dis 205:320–332
Antoine-Moussiaux N, Magez S, Desmecht D (2008) Contributions of experimental mouse models to the understanding of African trypanosomiasis. Trends Parasitol 24:411–418
Bal MS, Singla LD, Kumar H, Vasudev A, Gupta K, Juyal PD (2012) Pathological studies on experimental Trypanosoma evansi infection in Swiss albino mice. J Parasit Dis. 36:260–264
Baral TN, De Baetselier P, Brombacher F, Magez S (2007) Control of Trypanosoma evansi Infection Is IgM mediated and does not require a type I inflammatory response. J Infect Dis 195:1513–1520. https://doi.org/10.1086/515577
Berlin D, Loeb E, Baneth G (2009) Disseminated central nervous system disease caused by Trypanosoma evansi in a horse. Vet Parasitol 161:316–319
Biswas D, Choudhury A, Misra K (2001) Histopathology of Trypanosoma (Trypanozoon) evansi infection in bandicoot rat. I. Visceral organs. Exp Parasitol. 99:148–159
Damayanti R, Graydon R, Ladd P (1994) The pathology of experimental Trypanosoma evansi infection in the Indonesian buffalo (Bubalus bubalis). J Comp Pathol 110:237–252
Dargantes AP, Campbell RSF, Copeman DB, Reid SA (2005) Experimental Trypanosoma evansi infection in the goat. II. Pathology. J Comp Pathol. 133:267–276
Darji A, Beschin A, Sileghem M, Heremans H, Brys L, De Baetselier P (1996) In vitro simulation of immunosuppression caused by Trypanosoma brucei: active involvement of gamma interferon and tumor necrosis factor in the pathway of suppression. Infect Immun 64:1937–1943
Daulouede S, Bouteille B, Moynet D, De Baetselier P, Courtois P, Lemesre J, Buguet A, Cespuglio R, Vincendeau P (2001) Human macrophage tumor necrosis factor (TNF)-alpha produc- tion induced by Trypanosoma brucei gambiense and the role of TNF-alpha in parasite control. J Infect Dis 183:988–991
De Menezes VT, Queiroz OA, Gomes MAM, Marques MAP, Jansen AM (2004) Trypanosoma evansi in inbred and Swiss-Webster mice: distinct aspects of pathogenesis. Parasitol Res 94:193–200
Desquesnes M, Holzmuller P, Lai D, Dargantes A, Lun ZR, Jittaplapong S (2013) Trypanosoma evansi and surra: a review and perspectives on origin, history, distribution, taxonomy, morphology, hosts, and pathogenic effects. Biomed Res Int 2013:194176. https://doi.org/10.1155/2013/194176
Drury RA, Wallington EA (1980) Carleton’s histological techniques, 5th edn. Oxford University Press, New York
Eckersall PD, Gow JW, McComb C, Bradley B, Rodgers J, Murray M, Kennedy PGE (2001) Cytokines and the acute phase response in post-treatment reactive encephalopathy of Trypanosoma brucei brucei infected mice. Parasitol Int 50:15–26
Enwezor FNC, Sackey KB (2005) Camel trypanosomosis: a review. Vet Arch [Internet]. 75:439–452. http://hrcak.srce.hr/index.php?show=clanak&id_clanak_jezik=50720&lang=en
Garba U, Ak Sackey, Lawal A, Esievo K, Bisalla M, Sambo J (2017) Gross and histopathological alterations in experimental Trypanosoma Evansi infection in donkeys and the effect of isometamidium chloride treatment. J Vet Sci Anim Husb 5:1–10
Gardiner P, Mahmoud M (1990) Salivariantry- trypanosomes causing disease in livestock outside Sub-Saharan Africa. In: Baker JR (ed) Parasitic protozoa, vol 3. Academic Press, New York, pp 1–68
Ghaffar MA, El-Melegy MA, Afifi AF, El-Aswad BE, Elkady N, Atia A (2016) The histopathological effects of Trypanosoma evansi on experimentally infected mice. Menoufia Med J 29:868–873
Haines DM, Sudarto MW, Tabe LH (1990) Immunohistochemical demonstration of Trypanosoma evansi in tissues of experimentally infected rats and a naturally infected water buffalo. J Parasitol 76:162–167
Kitani H, Black SJ, Nakamura Y, Naessens J, Murphy NB, Yokomizo Y, Gibson J, Iraqi F (2002) Recombinant tumor necrosis factor alpha does not inhibit the growth of African trypanosomes in axenic cultures. Infect Immun 70:2210–2214
Londsdale-Eccles JDGD (2002) Trypanosome hydrolases and the blood-brain barrier. Trends Parasitol 18:17–19
Luckins AG (1988) Trypanosoma evansi in Asia. Parasitol Today 4:137–142
Magez S, Radwanska M, Beschin A, Sekikawa K, De Baetselier P (1999) Tumor necrosis factor alpha is a key mediator in the regulation of experimental Trypanosoma brucei infections. Infect Immun 67:3128–3132
Magez S, Radwanska M, Drennan M, Fick L, Baral TN, Allie N, Jacobs M, Nedospasov S, Brombacher F, Ryffel B, Baetselier PD (2007) Tumor necrosis factor (TNF) receptor–1 (TNFp55) signal transduction and macrophage-derived soluble TNF are crucial for nitric oxide-mediated Trypanosoma congolense parasite killing. J Infect Dis 196:954–962. https://doi.org/10.1086/520815
Masocha W, Robertson B, Rottenberg ME, Mhlanga J, Sorokin L, Kristensson K (2004) Cerebral vessel laminins and IFN-γ define Trypanosoma brucei brucei penetration of the blood-brain barrier. J Clin Invest. 114:689–694
Mekata H, Konnai S, Mingala CN, Abes N, Gutierrez C, Dargantes A, Witola W, Inoue N, Onuma M, Murata S, Ohashi K (2013) Isolation, cloning, and pathologic analysis of Trypanosoma evansi field isolates. Parasitol Res 112:1513–1521
Mertens KA, Taylor B (1999) Immune response of cattle infected with African trypanosomes. Mem Inst Oswaldo Cruz 94:239–244
Morrison W, Murray M, Sayer P, Preston J (1981) The pathogenesis of experimentally induced Trypanosoma brucei infection in dog. II. Changes in the lymphoid organs. Am J Pathol 102:182–194
Morrison W, Murray M, Whitelaw D, Sayer P (1983) Pathology of infection with Trypanosoma brucei: disease syndromes in dogs and cattle resulting from severe tissue damage. Contrib Microbiol Immunol 7:103–109
Naessens J, Kitani H, Momotani E, Sekikawa K, Nthale JM, Iraqi F (2004) Susceptibility of TNF-α-deficient mice to Trypanosoma congolense is not due to a defective antibody response. Acta Trop 92:193–203
Ngeranwa J, Gathumbi P, Mutiga E, Agumbah G (1993) Pathogenesis of Trypanosoma (brucei) evansi in small east African goats. Res Vet Sci 54:283–289
Queiroz A, Cabello P, Jansen A (2000) Biological and biochemical characterization of isolate of Trypanosoma evansi from Pantanal of Matogrosso–Brazil. Vet Parasitol 92:107–118
Reed S, Pihl D, Grabstein K (1989) Immune deficiency chronic Trypanosoma cruzi infection recombinant IL-1 restores Th function for antibody production. J Immunol. 142:2067–2071
Rodrigues A, Fighera RA, Souza TM, Schild AL, Barros CSL (2009) Neuropathology of naturally occurring Trypanosoma evansi infection of horses. Vet Pathol 46:251–258
Salim B, Bakheit MA, Kamau J, Nakamura I, Sugimoto C (2011) Molecular epidemiology of camel trypanosomiasis based on ITS1 rDNA and RoTat 1.2 VSG gene in the Sudan. Parasit Vectors [Internet]. 4:31. http://www.parasitesandvectors.com/content/4/1/31
Sawitri DH (2016) Virulence study of Trypanosoma evansi isolates from Indonesia and identification of molecular marker based on microsatellite DNA and Cytokine Profile Analyses in Mice (Mus musculus).Thesis. Jakarta. University of Indonesia
Sawitri D, Wardhana A (2019) Biological characteristic of Trypanosoma evansi isolate From Outbrake of Sumba Island and its implication after repeated passaging in mice. J Vet. 20:148–157
Sawitri D, Wardhana A, Wibowo H (2017) Cytokines profile of mice infected by high and low virulences of Indonesian T. evansi isolates. J Ilmu Ternak dan Vet. 22:151–164
Sharma DK, Chauhan PPS, Saxena VK, Agrawal RD (2000) Haematological changes in experimental trypanosomiasis in Barbari goats. Small Rumin Res 38:145–149
Singh V, Singla LD (2013) Trypanosomosis (Surra) in livestock. In: Katoch R, Godara R, Yadav A (eds) Veterinary parasitology in Indian perspective, pp 277–302. Satish Serial Publishing House, Delhi
Singla L, Juyal P, Sandhu B (2001) Clinico-pathological response in Trypanosoma evansi infected and immuno-suppressed buffalo-calves. In: 18th Int Conf WAAVP, WAAVP, Stresa, 26–30 August 2001.
Sivajothi S, Rayulu VC, Sujatha K, Reddy BS (2015) Experimental Trypanosoma evansi infection in albino mice—a histopathological study. Malays J Vet Res. 6:73–80
Subekti D, Sawitri D, Suhardono Wardhana A (2013) Pola Parasitemia dan Kematian Mencit yang Diinfeksi Trypanosoma evansi Isolat Indonesia. J Ilmu Ternak dan Vet. 18:274–290
Sulaiman FA, Adeyemi O (2010) Changes in haematological indices and protein concentrations in Trypanosoma brucei infected rats treated with homidium chloride and diminazene aceturate. EXCLI J. 9:39–45
Taylor K (1998) Immune responses of cattle to African try- panosomes: Protective or pathogenic?”. Int J Parasitol 28:219–240
Uche U, Jones T (1992) Pathology of trypanosomosis associated lipid peroxidation and experimental Trypanosoma evansi infection in raises glutathione concentrations in infected animals. Rabbits. J Comp Pathol 106:299–300
Wolkmer P, Da Silva A, Traesel C, Paim F, Cargnelutti J, Pagnoncelli M, Picada M, Monteiro S, Anjoslopes S (2009) Lipids peroxidation associated with anemia in rats experimentally infected with Trypanosoma evansi. Vet Parasitol 165:41–46
Acknowledgement
The authors gratefully thank to Prof. Dr. dr Mohamad Sadikin DSc and Dr. drsHeri Wibowo MBiomed from University of Indonesia for the support during the study. This study was funded, in part, by Indonesian Agency for Agricultural Research and Development, Ministry of Agriculture in National Budget.
Author information
Authors and Affiliations
Contributions
DHS conducted the research and analyzed the parasitology data while RD analyzed the histopathological findings. DHS and RD are equally contributed in preparing the manuscript writing. Both authors had read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest in this study.
Ethical approval
The research work was conducted at the Indonesian Research Centre for Veterinary Science, Bogor, Indonesia. This study was approved by the ethics Committee of the Faculty of Medicine, University of Indonesia number 124/H2.F1/ETIK/2013.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sawitri, D.H., Damayanti, R. Comparative pathology of mice infected with high and low virulence of Indonesian Trypanosoma evansi isolates. J Parasit Dis 45, 502–511 (2021). https://doi.org/10.1007/s12639-020-01328-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12639-020-01328-z