Abstract
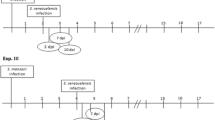
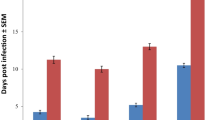
To investigate the response of mice to concomitant infections with Trypanosoma brucei (Tb), Plasmodium berghei (Pb) and Heligmosomoides bakeri (Hb) infections. Each group of 6 mice was either infected with Pb + Tb + Hb, Pb + Tb, Pb + Hb, Tb + Hb, Pb, Tb, Hb or remained as uninfected controls. Hb infected mice each received 200 infective larvae (L3) of Hb orally, Tb infected mice each received 2 × 10−6 organisms through the intraperitoneal route while Pb infected mice received 1 × 10−5 parasitized red blood cells through the intraperitoneal route. PCV, body weights (BW), faecal egg counts (FEC), Tb parasitaemia, Pb parasitaemia, clinical signs and worm burdens (WB) were determined. FEC were highest in Pb + Tb + Hb and least in Hb group and the difference was significant (P < 0.05). WB was significantly higher in mice with concurrent infections. PCV of infected mice was lower than that of uninfected controls and the difference was significant between Pb + Tb + Hb infected and uninfected controls. The difference in weight loss was significant between Pb + Tb + Hb infected and controls. Mortalities occurred in Pb + Tb + Hb, Tb + Hb and Pb + Hb infected mice. Mortalities and low PCV and low BW were indications that concomitant infections were more pathogenic to the mice than single infections, pathogenicity increasing with increasing number of parasite species involved.







Similar content being viewed by others
References
Bell RG, Adams LS, Ogden RW (1984) Trypanosoma musculi with Trichinella spiralis or Heligmosomoides polygyrus: concomittant infections in the mice. Exp Parasitol 58:8–18
Booth M, Bundy DAP, Albonico M, Chwaya HM, Alawi KS, Savioli L (1998) Associations among multiple geohelminth species infections in school children from Pemba Island. Parasitology 116:85–93
Brooker S, Miguel EA, Moulin S, Luoba AI, Bundy DAP, Kremer M (2000) Epidemiology of single and multiple species of helminth infections among school children in Busia District, Kenya. East Afr Med J 77:157–161
Chie**a SN, Wakelin D, Goyal PK (2003) Trypanosome-induced modulation of responses to concurrent helminth infection. Res Vet Sci 74:47–53
Chie**a SN, Musongong GA, Fakae BB, Behnke JM, Ngongeh LA, Wakelin D (2005) The modulatory influence of Trypanosoma brucei on Haemonchus contortus in Nigerian West African Dwarf goats segregated into weak and strong responders to the nematode. Vet Parasitol 128:29–40
Chruz-Chan JV, Quijano-Hernano-Hernandez I, Ramirez-Sierra MJ, Dumonteil E (2010) Dirofilaria immitis and Trypanosoma cruzi natural co-infection in dogs. Vet J 186:399–401
Coleman ER, Ednam EJ, Semprivivo HL (1988) Leishmania mexicana: effect of concomitant malaria on cutaneous leishmaniasis. Development of lesions in a Leishmania-susceptible (BALB/c) strain of mouse. Exp Parasitol 65:269–276
Cox FEG (2001) Concomitant infections, parasites and immune responses. Parasitology 122:S23–S38
Dahl C, Permin A, Christensen JP, Bisgaard M, Muhairwa AP, Peterson KMD, Poulsen JSD, Jensen AL (2002) The effect of concurrent infections with Pasteurella multocida and Ascaridia galli on free range chickens. Vet Microbiol 86:313–324
Dallas AB (1976) Interaction between Trypanosoma brucei and Plasmodium berghei in concurrent infections in mice. J Trop Med Hyg 79:182–188
Darji A, Lucas R, Magez E, Torreel S, Palacois J, Seleghm M, Bajyana Songa E, Hommers R, de Baestcelier P (1992) Mechanisms underlying trypanosome-elicited immunosuppression. Ann Soc Bel Med Trop 72(Suppl. 1):27–38
Druilhe P, Tall A, Sokhna C (2005) Worms can worsen malaria: towards a new means to roll back malaria? Trends Parasitol 21:359–362
Ezeamama EA, McGarvey ST, Hogan J, Lapane LK, Bellinger CD, Acosta LP, Leenstra T, Olveda RM, Kurtis JD, Fiedman FJ (2012) Treatment for Schistosoma japonicum, reduction of intestinal parasite load, and cognitive test score improvements in school-aged children. Plos Negl Trop Dis 6(5):e1634
Ezenwa VO, Etienne RS, Luikart G, Beja-Pereira A, Jolles AE (2010) Hidden consequences of living in a wormy world: nematode-induced immune suppression facilitates tuberculosis invasion in African buffalo. Am Nat 176:613–624
Fakae BB (2001) Primary infection of TO outbred mice with differing intensities of H. polygyrus infective larvae. Int J Agric Sci Biol Sci 1:1–4
Fakae BB, Harrison LTS, Ross CA, Sewell MH (1994) Helignosomoides polygyrus and Trypanosoma congolense. Infections in mice: a laboratory model for concurrent gastro-intestinal nematode and trypanosome infection. Parasitology 108:61–68
Faye D, Osaer S, Goossens B, Van Winghem J, Dorny P, Lejon V, Losson B, Geerts S (2002) Susceptibility of WAD goat and F1 crosses with the susceptible Sahelian breed to experimental Trypansoma congolense infection and interactions with helminth infections and different levels of diet. Vet Parasitol 108:117–136
Kabara H, Fukuma T, Nakazawa S (1990) The effect of Plasmodium berghei infection on mice infected with low-virulent strains of Trypanosoma cruzi or Leishmania donovani. Trop Med 32(1):45–47
Lewinsohn R (1975) Anaemia in mice with concomitant Schistosoma mansoni and Plasmodium berghei yoelii infection. Trans R Soc Trop Med Hyg 69:51–56
MAFF (1977) (Ministry of Agriculture, Fisheries and Food Agricultural Development and Advisory Service). Manual of veterinary parasitological laboratory techniques. Technical bulletin no. 18, HMSO, London, pp 36–37
Mbaya WA, Aliyu MM, Nwosu OC, Egbe-Nwiyi T (2009) The relationship between parasitaemia and anaemia in concurrent Trypanosoma brucei and Haemonchus contortus in red fronted gazelles (Gazella rufrifons). Veterinarski Archiv 79(5):451–460
Mengistu L, Berhanu E, Fekede B (2004) Increased parasitaemia and delayed parasite clearance in Schistosoma mansoni and Plasmodium berghi co-infected mice. Acta Trop 91(2):161–166
Momoh J, Longe AO (2014) In vivo anti-plasmodial and in vitro antioxidant activity of ethanolic leaf extract of Alstonia boonei (ewe ahun) and its effect on some biochemical parameters in swiss albino mice infected with plasmodium berghei nk 65. Eur Sci J 10:68–82
Murua R (1975) Effects of temperature on hatching and development of Nippostrongylus dubius in aerated water. J Helminthol 49:293–296
Mwangi TW, Bethony JM, Brooker S (2006) Malaria and helminth interactions in humans: an epidemiological viewpoint. Ann Trop Med Parasitol 100:551–570
Nacher M (2004) Interactions between worm infections and malaria. Clin Rev Allergy Immunol 26:85–92
Ngongeh LA (2013) A model for the study of gastrointestinal nematode infections. Lambert Academic Publishing, Saarbrücken
Onah DN, Wakelin D (2000) Murine model studies of the practical implication of trypanosome-induced immunosuppression in vaccine-based disease control programmes. Vet Immunol Immunopathol 74:241–294
Permin A, Christensen JP, Bisgaard M (2006) Consequences of concurrent Ascaridia galli and Escherichia coli infections in chickens. Acta Vet Scand 47:43–54
Petney TN, Andrews RH (1998) Multiparasite communities in animals and humans: frequency, structure and pathogenic significance. Int J Parasitol 28:377–393
Philips RS, Wakelin D (1974) Suppression of immunity in mice to the nematode Trichuris muris by concurrent infection with rodent piroplasms. Trans R Soc Trop Med Hyg 68:276
Telfer S, Lambin X, Birtles R, Beldomenico P, Burthe S, Paterson S, Begon M (2010) Species interactions in a parasite community drive infection risk in a wildlife population. Science 330:243–246
Tetsutani K, Ishiwata K, Ishida H, Tu L, Torii M, Hamano S, Himeno K, Hisaeda A (2009) Concurrent infection with Heligmosomoides polygyrus suppresses anti-Plasmodium yoelii protection partially by induction of CD4(+)CD25(+)Foxp3(+) Treg in mice. Eur J Immunol 39:2822–2830
Ward JW, Elsea JR (1997) Animal case and use in drug fate and metabolism. In: Edward RJ, Jean LH (eds) Methods and techniques, 1st edn. Markel Dekker, New York
Zucker JR, Campbell CC (1993) Malaria: principles of prevention and treatment. Infect Dis Clin North Am 7:547–567
Author information
Authors and Affiliations
Contributions
LAN Designed and oversee the smooth running of the study. Performed the infections and recorded and carried out quality control of the data. Also, performed faecal egg counts, read Plasmodium berghei slides (P. berghei parasitaemia), conducted worm counts and preparation and submission of the manuscript. AO Performed T. brucei infections, prepared smears for P. berghei parasitaemia, taking of T. brucei parasitaemia and feed procurement and feeding. MIW Responsible for taking T. brucei parasitaemia, body weight readings of mice and faecal egg counts. SKG He ensured the equipment were functional and ready for use all the time. He participated in collection of specimens and taking the PCV and Plasmodium parasitaemia of the mice.
Corresponding author
Ethics declarations
Conflict of interest
We declare that we do not have any conflict of interest.
Rights and permissions
About this article
Cite this article
Ngongeh, L.A., Onyeabor, A., Wosu, M.I. et al. Response of outbred albino mice to concomitant Heligmosomoides bakeri, Plasmodium berghei and Trypanosoma brucei infections. J Parasit Dis 41, 1105–1113 (2017). https://doi.org/10.1007/s12639-017-0943-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12639-017-0943-1