Abstract
Purpose
We sought to describe the processes undertaken for the systematic selection and consensus determination of the common data elements for inclusion in a national pediatric critical care database in Canada.
Methods
We conducted a multicentre Delphi consensus study of Canadian pediatric intensive care units (PICUs) participating in the creation of a national database. Participants were PICU health care professionals, allied health professionals, caregivers, and other stakeholders. A dedicated panel group created a baseline survey of data elements based on literature, current PICU databases, and expertise in the field. The survey was then used for a Delphi iterative consensus process over three rounds, conducted from March to June 2021.
Results
Of 86 invited participants, 68 (79%) engaged and agreed to participate as part of an expert panel. Panel participants were sent three rounds of the survey with response rates of 62 (91%), 61 (90%) and 55 (81%), respectively. After three rounds, 72 data elements were included from six domains, mostly reflecting clinical status and complex medical interventions received in the PICU. While race, gender, and home region were included by consensus, variables such as minority status, indigenous status, primary language, and ethnicity were not.
Conclusion
We present the methodological framework used to select data elements by consensus for a national pediatric critical care database, with participation from a diverse stakeholder group of experts and caregivers from all PICUs in Canada. The selected core data elements will provide standardized and synthesized data for research, benchmarking, and quality improvement initiatives of critically ill children.
Résumé
Objectif
Nous avons cherché à décrire les processus entrepris pour la sélection systématique et la détermination consensuelle des éléments de données communs à inclure dans une base de données nationale sur les soins intensifs pédiatriques au Canada.
Méthode
Nous avons mené une étude multicentrique de consensus selon la méthode Delphi sur les unités de soins intensifs pédiatriques (USIP) canadiennes participant à la création d’une base de données nationale. Les personnes participant à l’étude étaient des professionnel·les de la santé de l’USIP, du personnel paramédical, des soignant·es et d’autres intervenant·es. Un groupe de travail spécialisé a créé une enquête de base des éléments de données sur la littérature, les bases de données actuelles portant sur les USIP et l’expertise dans le domaine. L’enquête a ensuite été utilisée pour créer un processus de consensus itératif Delphi sur trois cycles, mené de mars à juin 2021.
Résultats
Sur les 86 personnes invitées à participer, 68 (79 %) se sont engagées et ont accepté de participer à un groupe d’experts. Les membres du panel ont reçu trois rondes du sondage, avec des taux de réponse de 62 (91 %), 61 (90 %) et 55 (81 %), respectivement. Après trois cycles, 72 éléments de données provenant de six domaines ont été inclus, reflétant principalement l’état clinique et les interventions médicales complexes reçues à l’USIP. Alors que la race, le genre et la région d’origine ont été inclus par consensus, des variables telles que le statut de minorité, le statut d’autochtone, la langue principale parlée et l’origine ethnique ne l’ont pas été.
Conclusion
Nous présentons le cadre méthodologique utilisé pour sélectionner des éléments de données consensuels destinés à une base de données nationale sur les soins intensifs pédiatriques, avec la participation d’un groupe diversifié d’expert·es et de soignant·es de toutes les USIP au Canada. Les éléments de données de base sélectionnés fourniront des données normalisées et synthétisées pour la recherche, l’analyse comparative et les initiatives d’amélioration de la qualité pour les enfants gravement malades.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Large-scale data-sharing strategies are a valuable asset for improving quality of care in this technologically evolving era of health care.1 Thoughtful quality databases are cost advantageous and efficient for researchers, stakeholders, and health care systems worldwide.2 Patient databases refer to uniform individual patient data collected in a systematic and comprehensive way to serve a predetermined purpose.3,4 In pediatrics, the limited number of patients with specific conditions often precludes conducting large randomized trials. Pediatric patient registries are particularly important because they facilitate the collection of high-quality data from the limited number of pediatric patients with different conditions.5,6 This is especially true for pediatric subspecialties like pediatric critical care.7 There are currently multiple well-established pediatric intensive care unit (PICU) registries worldwide8,9,10,11,12 These PICU registries have become invaluable for: 1) benchmarking and tracking quality-of-care indicators,13,14 2) informing health policymakers, 3) performing high-quality observational research, 4) health care planning and quantification of resource requirements (e.g., informing the pediatric COVID-19 response), and 5) efficiently evaluating patient care and/or health care interventions. Furthermore, network pediatric critical care database trials have been shown to be larger, more likely to be funded, and have a greater dissemination of findings.15 Given the importance of these large-scale databases, the consensus methods used to create them should be highlighted.
To inform and improve our distinct practices, all 17 Canadian PICUs actively endorse and are participating in the creation of a national database; collectively entitled Canadian Pediatric Intensive Care Consortium (CanPICC). Our overall aims are to build on individual PICU data collection strategies to include in a standardized data set, establish a data-sharing platform, and establish a governance model for a nationwide pediatric critical care database in Canada. A fundamental early aim of the national data initiative is to determine the common data elements for this CanPICC database. Design goals include capitalizing on existing clinical data collection, establishing broad ontology and standardizing data elements,16 and creating a dynamic infrastructure for linking data. The objective of this study was to develop national consensus-based common data elements for inclusion in a database of patients admitted to Canadian PICUs.
Methods
Study design
The study outlines the methodological framework for a modified Delphi consensus of variable selection for a national pediatric critical care database. The study had two phases: 1) a process to determine the initial data elements, and 2) a modified Delphi method to develop consensus with three iterations. The Delphi method is recommended in health care as a reliable approach to achieving consensus for a clinical problem.17 The study reporting followed the Checklist for Reporting of Survey Studies (Electronic Supplementary Material [ESM] eAppendix 1).
Phase 1: survey creation and determining initial data elements
The Delphi survey was created using the methodology outlined by Burns et al.18 We employed a modified Delphi consensus approach.17 An initial list of data elements for consideration was generated at a virtual meeting by a focus group (made up of physicians and nurses) separate from the expert panel used in phase 2. The group used a review of the literature, local PICU databases, existing national PICU registries,8,9,10,12,19 and their clinical expertise to generate the domains and the item variables for inclusion in the survey. While the review was not systematic, we searched for all relevant publications related to similar data sets/registries and contacted authors for data elements if these were not available on a website or link. All item sources were reviewed and classified into six data element domains (demographics, PICU admission characteristics, severity of illness scores, PICU therapies, PICU adverse events, and disposition). Item reduction was then performed to remove redundant elements. For data elements made up of multiple items (e.g., PRISM3 scores), all individual items were displayed. Patient and family insights and suggestions were provided by two family representatives (from two centres), along with stakeholder input from the Canadian Institute for Health Information (CIHI) solicited to review the survey draft, prior to dissemination.20 At the end of each domain, there was an open text field for suggestions or comments. The survey was piloted for face and content validity, and readability (n = 3), and then pretested among a small sample of clinicians (n = 3). Internal consistency was excellent with a Cronbach’s alpha of 0.90, but test-retest measure of reliability with Pearson’s r was low (n = 0.5).
The survey also included a selection of potential modules, with open-ended options, that panel members had to rank in terms of importance. Modules were a group of more specific nonmandatory data elements that could be added to the minimal data set for a distinct research project, or as a future expansion of the national database. A sample survey is available in the ESM eAppendix 2.
Phase 2: a modified Delphi method to develop consensus
We then conducted a prospective multicentre iterative survey of health care professionals and experts in pediatric critical care from March to June 2021. The Delphi study is reported according to the Checklist for Reporting Survey Studies, endorsed by the EQUATOR network.21 This initiative was funded by the Canadian Critical Care Trials Group (CCCTG) and the study was approved by the Research Ethics Board of the CHU Sainte-Justine Hospital and the Montreal Children’s Hospital (Montreal, QC, Canada; # MP-2021-3285).
Study sites and participants
We invited participants from all PICUs across Canada to complete the surveys. Canadian PICU study sites included BC Children’s Hospital and Victoria Children’s Hospital in British Columbia; Stollery Children’s Hospital and Alberta Children’s Hospital in Alberta; Royal University Hospital in Saskatchewan; Children’s Hospital of Winnipeg in Manitoba; The Hospital for Sick Children in Toronto, McMaster Children’s Hospital, Children’s Hospital at London Health Sciences Centre, Children’s Hospital of Eastern Ontario, and Kingston Health Sciences Centre in Ontario; CHU Sainte-Justine, Montreal Children’s Hospital, Centre Mère Enfant Soleil du CHU de Québec-Université Laval, and Centre Hospital Universitaire de Sherbrooke in Quebec; IWK Health Center in Nova Scotia; and Janeway Children’s Health and Rehabilitation Centre in Newfoundland and Labrador. We sought to include a diverse sample of stakeholder participants with relevant expertise in clinical care, quality improvement, or research. Lists of CCCTG members with an interest in pediatric critical care were used to identify at least one contact person in each site, who then provided the study team with contacts of health care experts to be invited. Health care experts included physicians, scientists, research assistants, nurse managers, advanced practice nurse clinicians, hospital administrators, health care administrators, and public health and patient/family representatives. For those not working directly in pediatric critical care, a knowledge of the Canadian PICU landscape and functioning were necessary for inclusion. There were no specific exclusion criteria for participants. Participation and engagement of potentially eligible expert participants was solicited with an online recruitment letter. Consent to participate was implied by answering and returning the survey.
We aimed to obtain a final sample size of 60 PICU health care experts to ensure that we sampled one to three experts from each of the 17 Canadian PICUs, with parents and stakeholder group members making up the remaining participants. Sending invitations to 85 potential participants would allow for a 10–15% nonresponse rate and 10% drop-out rate. This is in kee** with the recommended Delphi methodology suggesting a minimum of 12–35 participants.16,22
Survey process
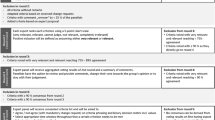
Three rounds of surveys were planned for this process, each one month apart. Participant demographics were collected at the start of the first-round survey. In the first-round, participants selected which data elements would ideally be included in the data set. For the first two rounds of the Delphi process, all questions were evaluated on a five-point nominal scale by selecting the level of importance for inclusion of each data element (“mandatory,” “important,” “optional,” “remove,” “not my area of expertise”). “Consensus” was obtained if 70% or more of eligible responses were within the same response category on the five-point scale.23 If consensus was achieved in the “mandatory” category, these elements were included in the data set. “Disagreement” was defined as 35% or more of responses falling in both of the two extreme ranges of possible options (“mandatory” and “remove”) on the nominal scale. All other combinations of panel answers were considered “partial agreement.” Where consensus was achieved for a data element, that question was removed from subsequent survey rounds, including all of the items that made up that data element. For the second and third round, participants were asked to revisit their consideration of each remaining data element with anonymized results from the prior rounds, shared in the form of proportions of participants selecting each response for each data element. Each of the first two rounds allowed participants to add comments and potential additional data elements. In the third round, to finalize item selection, response options were modified to a four-point scale to “include,” “exclude,” “add to a module,” or “not my area of expertise,” with consensus maintained as a 70% response in the same category. In all rounds, participants were instructed not to account for feasibility of data element collection but rather to select the most important data elements for inclusion in the minimal mandatory data set. This was specified so as not to discourage inclusion of elements related to social determinants of health, including self-reported data elements.
Completion of the survey was anonymous. Study data were collected, managed, and confidentially stored using REDCap electronic data capture tools hosted at the Montreal Children’s Hospital.24,25
Analysis
Results were summarized as counts and proportions. The primary outcome was consensus on the importance of data element inclusion into the database. In the calculation of the proportion of consensus for a data element, if a participant selected “Not my area of expertise” for a given question, they were not included in the denominator calculation for that question, or in the number of participants who answered that question.
Results
Phase 1
The initial survey produced by the group included 125 items (variables) in the six domains (demographics, n = 22; PICU admission characteristics, n = 26; severity of illness scores, n = 16; PICU therapies, n = 25; PICU adverse events, n = 19; outcomes, n = 16). There were some repeated items with different definitions to allow the Delphi expert group to select the most appropriate definition (e.g., PICU discharge was defined first as the date and time patients were cleared for discharge, and second as the date and time patients physically left the intensive care unit [ICU]). In addition, 17 modules were included for ranking after the Delphi survey. The final survey included 117 data elements divided into six domains: 1) patient demographics, 2) PICU admission, 3) severity of illness, 4) interventions in the PICU, 5) quality assurance, and 6) outcomes.
Phase 2
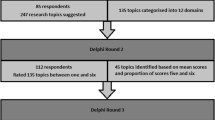
We invited 86 experts to participate in the modified Delphi method phase, 68 (79%) of whom engaged and agreed to participate. There was proportional representation of participants from PICUs across the country, as well as a distributed variety of expertise and lengths of PICU practice (Table 1). Participants were predominantly physicians (63%), recommended by their units for their expertise in acute care data collection, clinical content, research and quality metrics. The 68 expert participants in the panel were sent all three rounds of the survey with response rates of 91% (62/68) in round one, 90% (60/68) in round two, and 81% (55/68) in round three (Figure).
After the first round, 46 of the 117 (39%) data elements were included by consensus, and seven suggested items were added by participants for assessment in the subsequent rounds (Figure). After round two, an additional seven data elements were included by consensus. After limiting the answer options for round three, 24 additional data elements were added to give a total of 77. Of these, data elements that could be derived from other selected items were removed (e.g., Glasgow Coma Scale included in PRISM3 score, night admission/discharge included in time of admission/discharge, readmission), giving a total of 72 distinct included data elements (Table 2). The most highly ranked modules determined to be valuable additions to the minimal database were ventilation (n = 10), sepsis (n = 8), and traumatic brain injury (n = 8).
Discussion
The present modified Delphi consensus process included representatives from all different PICUs across Canada, and identified 72 measurable data elements from six domains, for inclusion in a national PICU database that is currently being operationalized. Active recruitment and engagement of participants across the pediatric critical care community of practice resulted in high response rates from a diverse and heterogenous group of experts, and emphasizes the importance of this work. In contrast to national PICU data sets in other countries created by a small group of individuals, we have shown that development of consensus within a diverse group of experts was possible and resulted in a broad and inclusive group of data elements. Furthermore, we have established a framework to apply this methodology to the creation of other data registries.
The modified Delphi method phase used an initial small focus group of physicians and nurses from various centres to generate a list of variables based on literature, expertise, and other PICU databases. The larger Delphi panel of experts then suggested seven further variables to be added, but none of these were ultimately included in the mandatory data set based on consensus. The small focus group therefore likely appropriately reviewed evidence to select the variables, with their expertise in clinical research, data quality, and data acquisition likely strengthening this selection. The modification of this step in the Delphi process is therefore an efficient and appropriate method of variable preselection prior to consensus generation.
The exclusion of certain data elements in the consensus highlights whether relying solely on consensus methodology is the best approach for selecting a minimal data set. While consensus methodology effectively aggregates the anonymous opinion of the majority, other valuable but less commonly collected or reported data elements may be omitted. Furthermore, there is not a “correct” answer in a Delphi process, but rather a reflection of what the selected participants deem is important. In this case, the majority of selected elements reflected diagnostic categories, advanced medical care delivered, and PICU complications. These choices may reflect the potential of certain interventions to cause significant unintended harm or long-term impacts in patients.
A striking feature of the Delphi results was the lack of consensus regarding inclusion of elements describing social determinants of health. While race, sex, gender, and the first three digits of the postal code (geographic location) were included, elements such as minority status, indigenous status, primary language, family income, education, and ethnicity did not achieve consensus. There may be several reasons for reticence to include these data elements in the data set, including challenges with standardizing data collection, concerns for patient privacy, or lack of understanding of the item’s importance in the child’s health status. Furthermore, physiologic clinical variables may be as easier to standardize and collect than self-reported data of social constructs such as race and ethnicity. Regardless, these data elements remain important determinants of child health inequities in Canada,26 and serve as a foundation for future work in pediatrics in Canada.
Despite the significant increase in research on understanding outcomes after PICU, no scales associated with global or cerebral functional status were included. In addition, despite data to suggest the importance of transport on PICU patient outcomes,27 none of the transport data elements were included. Given that these pre-ICU admission data are not a part of the ICU electronic medical record, participants may not have included these due to perceived feasibility challenges related to collection and accuracy. Lastly, although patient weight is an included data element, there are no included measures of ideal body weight, body mass index, or height. Given the importance that obesity can have on outcomes of critical illness and long-term health, this exclusion will remain an important variable for future consideration. Data elements that did not achieve the vote of all members (e.g., “master ID” or “primary diagnosis”) highlight the heterogeneity, selective expertise, and knowledge bases of the panel members; each with their own use for the database. In this case, a hybrid approach for common data element inclusion, with oversight of a larger steering committee for example, may be necessary to avoid the omission of key data elements in addition to all those voted on by panel members.
Our study had some limitations. First, a low test-retest value (0.5) suggests a low reliability with equal true and error-related variance. It is unclear whether retesting would produce the same results given the possibility of measurement error.28 Second, despite the diversity of participants and their areas of expertise, the participant numbers were likely over represented by physicians, and limited in allied health professionals such as social workers and nutritionists. An increased presence of stakeholders, patients, and members specialized in equity, diversity, and inclusion may have balanced the perspectives of the group with regard to social determinants of health items. To overcome this limitation, we consulted the CIHI and the Public Health Agency of Canada to discuss adding important items for exploring social determinants of health, such as ethnicity. Improved understanding of the social determinants of health is essential to illuminate health care inequalities that exist for children in Canada, and to inform policy changes to improve health care access and quality for marginalized children. Ensuring that routinely collected data elements reflect the needs of a diverse range of stakeholders will amplify the sustainability of data collaboration. The aim to have a national, population-level data coverage, engaging public health and policy-makers has been emphasized as a means to strengthen our processes. Last, family perspectives were limited in the study as only one patient-partner was involved in this Delphi process.
Ongoing work includes clear ontogeny, definitions, and timing of data collection for the selected data elements, as some of these are not well established in children. In conjunction with pediatric members of the CCCTG, who conducted multiple randomized trials in children, we are harmonizing definitions for included data elements based on multiple recent trials29,30 to standardize data element inclusion. A parallel group has created a governance structure for how national data would be validated, accessed, and used for research and quality improvement. In terms of infrastructure, national partners in data management and adult critical care are creating an infrastructure for an open-source platform at each hospital. To overcome barriers preventing record-level data from leaving the provinces, data storage and analysis would be conducted behind a firewall at each individual site, and only aggregate data would exit the sites.
The core data elements selected in this Delphi study will be instrumental in assessing the determinants, clinical outcomes, and quality of care, of critically ill children in Canada. By pooling granular, standardized, anonymous data that goes beyond what is submitted by hospitals using diagnostic codes, we will have an unprecedented ability to ask targeted questions and learn from and improve our practices through quality improvement initiatives and research. Ensuring that variable coding can be harmonized with other data sources and is standardized to align with pre-existing data standards and international collaborations16 will ease linkage, sharing, and comparability across sites and registries. Overall, this will lead to greater insight in the effectiveness, safety, and quality of critical care.
Conclusion
We present the consensus methodology used to derive the data elements for a national pediatric critical care database, with participation from a diverse group of experts, caregivers, and stakeholders representing every pediatric critical care program in the country. The above-outlined process provides a framework for both stakeholders and clinicians that can be applied to similar initiatives to ensure consensual data element selection for a sustainable, feasible creation of a national database. The selected core data elements will provide standardized and synthesized data for research, benchmarking, and quality improvement initiatives in critically ill children.
References
Nelson EC, Dixon-Woods M, Batalden PB, et al. Patient focused registries can improve health, care, and science. BMJ 2016; 354: i3319. https://doi.org/10.1136/bmj.i3319
Murdoch TB, Detsky AS. The inevitable application of big data to health care. JAMA 2013; 309: 1351–2. https://doi.org/10.1001/jama.2013.393
Brooke EM. The current and future use of registers in health information systems, 1974. Available from URL: https://apps.who.int/iris/handle/10665/36936 (accessed November 2022).
Gliklich RE, NA Dreyer NA, Leavy MB. Registries for evaluating patient outcomes: a user's guide, 2014. Available from URL: https://www.ncbi.nlm.nih.gov/books/NBK208643/ (accessed November 2022).
Duffett M, Choong K, Foster J, et al. High-quality randomized controlled trials in pediatric critical care: a survey of barriers and facilitators. Pediatr Crit Care Med 2017; 18: 405–13. https://doi.org/10.1097/pcc.0000000000001144
LaRovere JM, Jeffries HE, Sachdeva RC, et al. Databases for assessing the outcomes of the treatment of patients with congenital and paediatric cardiac disease--the perspective of critical care. Cardiol Young 2008; 18: 130–6. https://doi.org/10.1017/s1047951108002886
Duffett M, Choong K, Hartling L, Menon K, Thabane L, Cook DJ. Randomized controlled trials in pediatric critical care: a sco** review. Crit Care 2013; 17: R256. https://doi.org/10.1186/cc13083
Virtual Pediatric Systems. Homepage. Available from URL: https://www.myvps.org (accessed November 2022).
ANZICS. Australian and New Zealand Pediatric Intensive Care registry (ANZPIC). Available from URL: https://www.anzics.com.au/australian-and-new-zealand-paediatric-intensive-care-registry-anzpicr/ (accessed November 2022).
EpiMed Solutions. About. Available from URL: https://www.epimedsolutions.com/en/team/ (accessed November 2022).
JSICM. JIPAD (ICU patient registry in Japan) since 2014. 2020. Available from URL: https://www.jsicm.org/en/jipad.html (accessed November 2022).
PICANet. About. Available from URL: https://www.picanet.org.uk (accessed November 2022).
Wetzel RC. Pediatric intensive care databases for quality improvement. J Pediatr Intensive Care 2016; 5: 81–8. https://doi.org/10.1055/s-0035-1568146
Lannon CM, Peterson LE. Pediatric collaborative networks for quality improvement and research. Acad Pediatr 2013; 13: S69–74. https://doi.org/10.1016/j.acap.2013.07.004
Choong K, Duffett M, Cook DJ, Randolph AG. The impact of clinical trials conducted by research networks in pediatric critical care. Pediatr Crit Care Med 2016; 17: 837–44. https://doi.org/10.1097/pcc.0000000000000835
Ward SL, Flori HR, Bennett TD, et al. Design and rationale for common data elements for clinical research in pediatric critical care medicine. Pediatr Crit Care Med 2020; 21: e1038–41. https://doi.org/10.1097/pcc.0000000000002455
McMillan SS, King M, Tully MP. How to use the nominal group and Delphi techniques. Int J Clin Pharm 2016; 38: 655–62. https://doi.org/10.1007/s11096-016-0257-x
Burns KEA, Duffett M, Kho ME, et al. A guide for the design and conduct of self-administered surveys of clinicians. CMAJ 2008; 179: 245–52. https://doi.org/10.1503/cmaj.080372
Institut National d’Excellence en Santé et en services sociaux (INESSS). Indicateurs de qualité pour soutenir une organisation de service optimale en soins intensifs au Québec. Quebec City; INESSS: 2018.
Canadian Institute for Health Information. Care in Canadian ICUs, 2016. Available from URL: https://secure.cihi.ca/free_products/ICU_Report_EN.pdf (accessed November 2022).
Sharma A, Minh Duc NT, Lam Thang TL, et al. A consensus-based checklist for reporting of survey studies (CROSS). J Gen Intern Med 2021; 26: 3179–87. https://doi.org/10.1007/s11606-021-06737-1
Boulkedid R, Abdoul H, Loustau M, Sibony O, Alberti C. Using and reporting the Delphi method for selecting healthcare quality indicators: a systematic review. PLoS ONE 2011; 6: e20476. https://doi.org/10.1371/journal.pone.0020476
Diamond IR, Grant RC, Feldman BM, et al. Defining consensus: a systematic review recommends methodologic criteria for reporting of Delphi studies. J Clin Epidemiol 2014; 67: 401–9. https://doi.org/10.1016/j.jclinepi.2013.12.002
Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009; 42: 377–81. https://doi.org/10.1016/j.jbi.2008.08.010
Harris PA, Taylor R, Minor BL, et al. The REDCap consortium: building an international community of software platform partners. J Biomed Inform 2019; 95: 103208. https://doi.org/10.1016/j.jbi.2019.103208
Canadian Institute for Health Information. In pursuit of health equity: defining stratifiers for measuring health inequality. A focus on age, sex, gender, income, education and geographic location, 2018. Available from URL: https://www.cihi.ca/sites/default/files/document/defining-stratifiers-measuring-health-inequalities-2018-en-web.pdf (accessed November 2022).
Tijssen JA, Allen BN, Bray Jenkyn KM, Shariff SZ. Impact of deferring critically ill children away from their designated pediatric critical care unit: a population-based retrospective cohort study. Healthc Policy 2019; 15: 40–52. https://doi.org/10.12927/hcpol.2019.25939
Matheson GJ. We need to talk about reliability: making better use of test–retest studies for study design and interpretation. PeerJ 2019; 7: e6918. https://doi.org/10.7717/peerj.6918
Spinella PC, Tucci M, Fergusson DA, et al. Effect of fresh vs standard-issue red blood cell transfusions on multiple organ dysfunction syndrome in critically ill pediatric patients: a randomized clinical trial. JAMA 2019; 322: 2179–90. https://doi.org/10.1001/jama.2019.17478
Menon K, McNally D, O'Hearn K, et al. A randomized controlled trial of corticosteroids in pediatric septic shock: a pilot feasibility study. Pediatr Crit Care Med 2017; 18: 505–12. https://doi.org/10.1097/pcc.0000000000001121
Author contributions
Nadia Roumeliotis conceptualized and designed the study, conducted the Delphi process, performed the statistical analysis, summarized results, drafted the initial manuscript, and reviewed and revised the manuscript. Joanne Ramil helped design the study, coordinated REDCap, acquired the data, summarized results, and critically reviewed the article. Daniel Garros provided variable insight and elements, and contributed to the study design, analysis plan, interpretation of results, and critical appraisal of intellectual content. Srinivas Murthy conceptualized the study, provided sponsorship, and critically reviewed the manuscript for important intellectual content. Patricia S. Fontela conceptualized and designed the study, coordinated and supervised data collection, provided sponsorship and critically reviewed the manuscript for important intellectual content. Fuad Alnaji, Macha Bourdages, Valerie Brule, Karen Dryden-Palmer, Fiona Muttalib, Jessica Nicoll, and Michael Sauthier contributed to study design, literature review, interpretation of results, manuscript drafting, and reviewing and revising the manuscript. They are listed in alphabetical order. All authors agree to be accountable for all aspects of the work.
Acknowledgments
Special thanks to Eddy Fan, Oleksa Rewa, and Kusum Menon, CCCTG members, for their revision and comments on this manuscript. Thanks to Anna Gunz, for expertise in data elements relating to transport, to Anne-Marie Guerguerian for variables relating to extracorporeal membrane oxygenation. Thanks as well to Murdoch Leeies for expertise in the promotion of equity, diversity and inclusion data elements.
Disclosures
The authors have no disclosures or conflicts of interest to declare related to this work.
Funding statement
This study was funded by a Grant from the Canadian Critical Care Trials Group (CCCTG).
Editorial responsibility
This submission was handled by Dr. Alexis F. Turgeon, Associate Editor, Canadian Journal of Anesthesia/Journal canadien d’anesthésie.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Roumeliotis, N., Ramil, J., Garros, D. et al. Designing a national pediatric critical care database: a Delphi consensus study. Can J Anesth/J Can Anesth 70, 1216–1225 (2023). https://doi.org/10.1007/s12630-023-02480-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12630-023-02480-9