Abstract
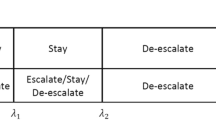
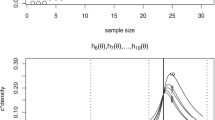
Traditionally, dose-finding process for oncology compound is carried out in phase I dose escalation study and is driven by safety in order to find maximum tolerated dose (MTD). However, with the recent paradigm shift from cytotoxic drugs to new generation of targeted therapies and immuno-oncology therapies, it may be difficult or unnecessary to identify the MTD because of the possible non-monotonic dose–response curves, and efficacy data should be incorporated into the dose-finding process. In this article, we have proposed efficacy-driven dose-finding designs with a safety-driven warm-up phase. Both local investigation and adaptive randomization using the framework of double-sided isotonic regression are investigated. Simulation studies are used to compare the proposed design to the original double-sided isotonic design. The results show that a safety-driven warm-up phase at the beginning can significantly improve the performance of double-sided isotonic regression, and both local investigation and adaptive randomization have good operating characteristics for finding the best dose/dose range under different tested scenarios.
Similar content being viewed by others
References
Ananthakrishnan R, Green S, Li D, LaValley M (2019) 2D (2 dimensional) TEQR design for determining the optimal dose for safety and efficacy. Contemp Clin Trials Commun 16:100461
Babb J, Rogatko A, Zacks S (1998) Cancer phase I clinical trials: efficient dose escalation with overdose control. Stat Med 17(10):1103–1120
FDA (2011) Guidance for industry: clinical considerations for therapeutic cancer vaccine
Ji Y, Liu P, Li Y, Nebiyou Bekele B (2010) A modified toxicity probability interval method for dose-finding trials. Clin Trials 7(6):653–663
Korn EL, Midthune D, Chen TT, Rubinstein LV, Christian MC, Simon RM (1994) A comparison of two phase I trial designs. Stat Med 13(18):1799–1806
Li DH, Whitmore JB, Guo W, Ji Y (2017) Toxicity and efficacy probability interval design for phase I adoptive cell therapy dose-finding clinical trials. Clin Cancer Res 23(1):13–20
Lin Y, Shih WJ (2001) Statistical properties of the traditional algorithm-based designs for phase I cancer clinical trials. Biostatistics 2(2):203–215
Liu S, Yuan Y (2015) Bayesian optimal interval designs for phase I clinical trials. J R Stat Soc 64(3):507–523
Mandrekar SJ, Qing R, Sargent DJ (2010) Model-based phase I designs incorporating toxicity and efficacy for single and dual agent drug combinations: methods and challenges. Stat Med 29(10):1077–1083
Neuenschwander B, Branson M, Gsponer T (2008) Critical aspects of the bayesian approach to phase I cancer trials. Stat Med 27(13):2420–2439
Neuenschwander B, Capkun-Niggli G, Branson M, Spiegelhalter DJ (2010) Summarizing historical information on controls in clinical trials. Clin Trials 7(1):5–18
O’Quigley J, Pepe M, Fisher L (1990) Continual reassessment method: a practical design for phase 1 clinical trials in cancer. Biometrics 33–48
Ratain MJ, Doi T, De Jonge MJ, LoRusso P, Dunbar M, Chiney M, Motwani M, Glasgow J, Petrich AM, Rasco DW et al (2019) Phase 1, first-in-human study of trail receptor agonist fusion protein abbv-621
Schmidli H, Gsteiger S, Roychoudhury S, O’Hagan A, Spiegelhalter D, Neuenschwander B (2014) Robust meta-analytic-predictive priors in clinical trials with historical control information. Biometrics 70(4):1023–1032
Storer BE (1989) Design and analysis of phase I clinical trials. Biometrics 45(3):925–937
Thall PF, Cook JD, Estey EH (2006) Adaptive dose selection using efficacy-toxicity trade-offs: illustrations and practical considerations. J Biopharm Stat 16(5):623–638
Turner T, Wollan P (1997) Locating a maximum using isotonic regression. Comput Stat Data Anal 25(3):305–320
Woolley PV, Schein PS (1979) Clinical pharmacology and phase I trial design. Methods Cancer Res 17:177–198
Zang Y, Lee JJ, Yuan Y (2014) Adaptive designs for identifying optimal biological dose for molecularly targeted agents. Clin Trials 11(3):319–327
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liu, Q., Geng, J., Fleischer, F. et al. Efficacy-Driven Dose Finding with Toxicity Control in Phase I Oncology Studies. Stat Biosci 14, 413–431 (2022). https://doi.org/10.1007/s12561-021-09327-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12561-021-09327-1