Abstract
The intestinal epithelium acts as the first line of defence against pathogens present in the lumen of the gastrointestinal tract. The epithelium is composed of a single monolayer that includes a variety of cell types, each of which play roles in nutrient and water absorption, antimicrobial defence, and immunomodulation to maintain a homeostatic gut environment. Tight junction (TJ) complexes between adjacent intestinal epithelial cells are responsible for the structural integrity of the gut barrier and controlling the paracellular translocation of luminal contents. The effectiveness of TJs can be impacted by both genetic and environmental factors including microbiota dysbiosis and dietary components. The increased systemic entry of luminal contents has been associated with the development, progression, and/or relapse of autoimmune diseases such as Crohn’s and rheumatoid arthritis. In particular, the extraintestinal spread of luminal microbes possessing components with structural similarity to those of the human host are thought to be involved in the breakdown of immune tolerance towards host components. Here, the structure and function of the intestinal epithelium are discussed as well as the genetic and environmental factors that influence its permeability. There is emphasis on the role of increased intestinal permeability and how the subsequent translocation of luminal contents could be involved in the development and/or exacerbation of autoimmune diseases. This review reinforces how protecting the integrity of the intestinal epithelium and minimising immunological exposure to luminal components, either directly or indirectly, could be a useful strategy in reducing the prevalence and severity of autoimmune diseases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Table of contents
-
Introduction
-
Structural Components of the Gut Barrier & Intestinal Immunity
-
Cellular Components & Their Associated Functions
-
Tight Junctions as Modulators of Intestinal Integrity and Paracellular Translocation
-
The Gut Barrier: An Immunological Hotspot
-
Factors Influencing Intestinal Barrier Integrity
-
Environmental Factors
-
Genetic Predisposition to an Attenuated Gut Barrier
-
Mechanisms of Bacterial Translocation During Enteric Infection
-
The Gut Barrier’s Role in Autoimmune Disease
-
Inflammatory Bowel Disease
-
Coeliac Disease
-
Type 1 Diabetes
-
Systemic Lupus Erythematosus
-
The Gut-Joint Communication Axis in Rheumatic Disease Development
-
Improving Intestinal Barrier Integrity to Limit Inflammatory Disease Development
-
Conclusion
Introduction
Humans have evolved a highly complex and efficient gastrointestinal (GI) tract that plays a pivotal role in nutrient and water absorption, defence against pathogenic microbes, and overall body homeostasis (Allaire et al. 2018). Central to these abilities is the intestinal epithelium, a single monolayer composed of numerous cell types adhered together by tight junction (TJ) protein complexes, which provides selective paracellular permeability to ions, solutes, and water, while also restricting the systemic access of harmful organisms and substances (König et al. 2016; Mu et al. 2017). Such fine modulation of movement across the epithelium demands structural components that aid this process, such as transmembrane proteins including occludin, junctional adhesion molecules, and claudin protein family members that together maintain epithelial cell basolateral polarity (Shin et al. 2006; Bischoff et al. 2014; König et al. 2016). In addition, intestinal epithelial cells (IECs), otherwise known as enterocytes, are adjoined by TJ complexes that are further tethered to an intracellular cytoskeleton permitting communication across the epithelium and regulating processes including the cell cycle (Brenchley and Douek 2012).
The components of the intestinal epithelium modulate immune responses (IRs) in the gut and are essential in establishing immune tolerance towards endogenous molecules or those present in our diet (König et al. 2016). As the main organ involved in nutrient and water uptake, the intestinal barrier is composed of a mucous layer, epithelial layer, and the underlying army of immune cells that prevents further spread of immunogenic components (König et al. 2016). Among the numerous cell types composing the epithelial layer are goblet cells which secrete glycosylated Mucin-2 (Muc2), a key component of the intestinal mucosal layer (Brenchley and Douek 2012; Allaire et al. 2018). Muc2 provides the mucous layer with unique protective properties by virtue of its gel-forming ability that helps restrict excessive bacterial encroachment of the epithelial layer and the underlying lamina propria (Brenchley and Douek 2012; Fine et al. 2020). The protective properties of Muc2 have been demonstrated, with confirmation of a role in flushing away enteric pathogens such as Citrobacter rodentium and Salmonella typhimurium from the mucosal surface (Johansson et al. 2008; Bergstrom et al. 2010; Zarepour et al. 2013). Mice genetically deficient in Muc2 develop spontaneous colitis reinforcing the role of this layer in limiting pathogenic infection and promoting an anti-inflammatory environment (Allaire et al. 2018).
As the largest mucosal surface in the body, spanning up to seventy-five metres in length, the intestinal mucosa plays a pivotal role in human health by limiting pathogenic colonisation in the GI tract and preventing the interaction of harmful compounds with the fragile epithelial monolayer (Smyth 2017). This ultimately promotes healthy functioning of the intestinal epithelium which is required for the effective orchestration of vital processes in the gut, including locomotion, digestion, absorption, as well as modulation of neuroendocrine and immune pathways (Peterson and Artis 2014; Smyth 2017). The role of IECs in the coordination of immune pathways is largely dependent on their ability to express surface receptors, namely Toll-like receptors (TLRs), at their apical and basolateral membranes which are used to detect the presence of harmful stimuli (Abreu 2010; McClure and Massari 2014; Yu and Gao 2015). In addition, enterocytes can secrete antimicrobial peptides (AMPs) such as REGIII-gamma (γ) that are effective against Gram-positive pathogens (Cash et al. 2006; Vaishnava et al. 2008, 2011). Other AMPs secreted by enterocytes include alpha (α) and beta (β) defensins, c-type lectins, cathelicidin, lysozyme, and intestinal alkaline phosphatases, each of which are important in limiting the colonisation and invasion of enteric pathogens (Dupont et al. 2014).
Additional components of the gut barrier hel** to protect the host from infection include members of the intestinal microbiota. These microbes play important roles in restricting the colonisation of pathogenic species, as well as influencing many other aspects of human health (Allaire et al. 2018). An estimated 100 trillion microbes are thought to colonise the human gut, with the majority of these localised to the colon, where bacterial cells outnumber that of the host by up to two orders of magnitude (Brenchley and Douek 2012; Allaire et al. 2018). In the colon alone, it is estimated that 1,000 bacterial species exist constituting a gene library estimated to be one hundred times larger than that of their human host (Brenchley and Douek 2012). Niches associated with the GI tract have unique pH, oxygen levels, and nutrients which provide suitable conditions for microbial colonisation (Roxas and Viswanathan 2018). The intestinal microbiota of healthy individuals is typically dominated by two main phyla: Firmicutes and Bacteroidetes (Brenchley and Douek 2012). The intestinal epithelium is pivotal in separating this microbial hotspot from systemic circulation and distant organs (Luissint et al. 2016).
Members of the microbiota have a significant influence on the gut barrier function and can directly support its integrity through the production of certain metabolites. The production of short-chain fatty acids (SCFAs) following the metabolic breakdown of ingested dietary fibre by gut bacteria provides a nutritional source for colonic epithelial cells (Louis et al. 2010). Of the Firmicutes and Bacteroidetes present in the gut microbiota, Faecalibacterium prausnitzii and Bacteroides thetaiotaomicron along with Clostridium clusters IV and IXa, are among the highest producers of butyrate, a SCFA that without these bacteria would be inaccessible to the host (Roediger 1980; Kau et al. 2011). Butyrate also has a role in the maintenance of an anti-inflammatory environment in the gut. However, exposure to certain external factors such as orally administered antibiotics can diminish the anti-inflammatory activity of SCFAs through the unintentional removal of producing species, thereby reducing its abundance in the gut (Table 1) (Allaire et al. 2018). Gut-colonising microbes and their products are, therefore, important in promoting gut barrier health, and the disruption of microbial communities is associated with a number of downstream consequences (Allaire et al. 2018).
Individuals may be genetically predisposed to an attenuated intestinal barrier either by the expression of abnormal structural components, for example, those involved in TJ protein complexes that modulate paracellular transport across the epithelium (Mu et al. 2017), or by carrying genetic loci leading to the expression of altered surface receptors. These individuals are at an increased risk from environmental factors, including microbes and food components, interacting with the subepithelial immune system and entering systemic circulation to promote chronic inflammation and tissue damage in multiple extraintestinal sites. Genetic abnormalities associated with increased gut permeability or ‘leakage’, include the human leukocyte antigen (HLA) gene involved in the presentation of foreign antigens to T-cells.
The ingestion of certain environmental components can have an influence on intestinal barrier integrity, for example, those present in our diet, or those used as drug delivery vehicles by pharmaceutical companies which facilitate drug uptake by intentionally disrupting intestinal barrier integrity (Table 1) (Hamid et al. 2009). Organic solvents, including alcohol and its metabolites, are known to impair TJ barriers and emulsifiers present in processed foods can alter the composition of the intestinal microbiota, encouraging bacterial encroachment beyond the mucous layer that may lead to epithelial inflammation and damage (Vojdani 2014; Chassaing et al. 2015).
In addition to dietary factors, the brain–gut axis is also likely to play an underappreciated role in modulating gut barrier integrity and permeability. Stress is thought to be a psychological factor that may attenuate intestinal integrity, reinforcing that while environmental and genetic factors are typically considered to have the greatest influence on gut barrier permeability, psychological factors should also be considered (Konturek et al. 2011; Xu et al. 2015).
Although the gut microbiota is well established in providing the host with protection against foreign pathogens, primarily by resisting intestinal colonisation by enteric pathogens, translocation of these microbes across the gut barrier can occur via various mechanisms and often results in undesirable outcomes for the host (Mainous et al. 1991; Gautreaux et al. 1994; Allaire et al. 2018). Microbial size, the presence of virulence factors, defects in host barrier integrity, along with the active uptake and presentation of luminal antigens by cells of the intestinal epithelium are among the factors that contribute to bacterial translocation (Wolochow et al. 1966; Mainous et al. 1991; Macpherson and Uhr 2004; Spadoni et al. 2015; König et al. 2016). Pathological conditions often arise as a consequence of increased intestinal permeability resulting in systemic entry of microbes and their products to cause infection (Brenchley and Douek 2012; Mu et al. 2017; Fine et al. 2020). Diseases associated with microbial translocation include alcoholism, inflammatory bowel disease (IBD), human immunodeficiency virus infection, Hepatitis B and C virus infection, and pancreatitis (Brenchley and Douek 2012). In extreme cases, septic shock may also arise from increased microbial translocation into the bloodstream, with mortality rates of up to 70% being reported (Matot and Sprung 2001; Brenchley and Douek 2012).
Certain enteric pathogens, including species of Vibrio, Escherichia, and Salmonella, promote paracellular migration across the epithelium by secreting enterotoxins which target TJ complexes and increase gut permeability (Wu et al. 2000; Hopkins et al. 2003; Fedwick et al. 2005). Specifically, enteroaggregative and enterotoxigenic Escherichia coli express heat-labile toxins 1 and 2, heat-stable toxins Sta, STb, and enteroaggregative heat-resistant toxin 1 (EAST1) that manipulate the osmotic state of the intestinal lumen by increasing chloride ion secretion from IECs. This alteration leads to increased water and ion entry across TJs into the intestinal lumen, causing symptoms associated with enteric infections including diarrhoea (Dubreuil 2008; Okhuysen and Dupont 2010). In addition, these enterotoxins provide greater opportunity for other microbes, including commensals, to penetrate underlying tissue (Guttman and Finlay 2009). The disruptive nature of enterotoxins on TJ complexes can also lead to the movement of basolateral proteins to apical sites on epithelial cells where they may be utilised by pathogens or their toxins as alternative receptors facilitating infection (Muza-Moons et al. 2003).
Increased intestinal translocation of immunogenic agents, including microbes and dietary components, induces inflammation and increases the risk of develo** autoimmunity or other non-infectious diseases (Table 2). Dysfunctional regulatory factors such as mucins, antimicrobial molecules, cytokines, and immunoglobulins (Igs) are often responsible for impaired barrier integrity that may lead to, or exacerbate, inflammatory diseases including type-1 diabetes (T1D), multiple sclerosis, autoimmune hepatitis, systemic lupus erythematosus (SLE), IBD, and coeliac disease (CD) (Fasano and Shea-Donohue 2005; Tlaskalová-Hogenová et al. 2011; Fasano 2012; Lin et al. 2015; Khaleghi et al. 2016; Mu et al. 2017). In addition, the translocation of commensal microbes to extraintestinal sites has been reported to promote immune profiles characteristic of certain autoimmune diseases (ADs) (Fine et al. 2020). Extraintestinal ADs have been linked to gut barrier dysfunction, with increased mucosal permeability displayed by individuals suffering from inflammatory rheumatic diseases such as rheumatoid arthritis (RA) (Mielants et al. 1991). In addition, gut-associated inflammatory disorders may increase the risk of develo** extraintestinal ADs. From this, it can be proposed that the intestinal epithelium is a pivotal dictator in the development and progression of extraintestinal ADs, with defects in barrier integrity responsible for the systemic spread of luminal contents that contribute to the pathogenesis of certain inflammatory diseases.
In this review, the structure and function of the intestinal epithelium will be discussed, reinforcing the importance of the relationships that exist between barrier components, the mucosal layer, and the underlying immune system, and how effective communication is essential in maintaining both structural integrity and a homeostatic gut environment. Factors which influence barrier integrity including gut colonising microbes, genetic predisposition and environmental factors will also be explored. Furthermore, the role of elevated intestinal permeability in the pathogenesis of certain ADs will also be reviewed. The current review emphasises the importance of the gut barrier in limiting the systemic entry of luminal contents and highlights how strategies to improve its integrity represent a useful approach in reducing the prevalence and burden of gut-associated and extraintestinal inflammatory diseases.
Structural Components of the Gut Barrier and Intestinal Immunity
Cellular Components and Their Associated Functions
The structural architecture of the small and large intestine (colon) varies (Fig. 1), with cells of the small intestine (SI) epithelium harbouring apical extrusions, called villi, which provide a much larger surface area to aid water and nutrient uptake (Allaire et al. 2018). In contrast, the flat mucosal surface of the colon serves to limit structural damage caused by semi-solid stool passing through; however, small invaginations, known as ‘Crypts of Lieberkühn’, do exist in the colon. These invaginations are also present in the SI; however, in this region, they are localised to the folded, villi structures (Spence et al. 2011; Allaire et al. 2018). Lgr5 + stem cells migrate towards the apex of crypts while maturing and differentiating into specialised cell types in the process (Crosnier et al. 2006; Mabbott et al. 2013). Crypts are also continuously recycled and renewed every four to five days in homeostatic conditions. This ensures that IECs shed at the apex of villi in the SI, or crypts in the colon, are replaced effectively (Crosnier et al. 2006; van der Flier and Clevers 2009).
Schematic diagrams illustrating the epithelial components of the a small intestine and b colon. Note the lack of villi in the colon that limits structural damage caused by passing stool. The presence of villi in the small intestine provides a large surface area to encourage optimal absorption of H2O and nutrients
Enterocytes are among the numerous cell types present in the intestinal monolayer (Fig. 1), accounting for an estimated 90% of crypt or villus cells (Mu et al. 2017). In addition to their roles in nutrient and water uptake, enterocytes also secrete transmembrane mucins and AMPs, such as REGIII-γ, which are effective against Gram-positive bacteria (Cash et al. 2006; Vaishnava et al. 2008, 2011; Pelaseyed et al. 2014). Goblet cells are also important secretors present in the gut epithelium, with roles in maintaining the two mucous layers which lie on top of the epithelium by secretion of a glycosylated, gel-forming mucin, Muc2 (Mu et al. 2017). Other components including IgA, enzymes, proteins such as lactoferrin and those with antimicrobial properties including bile acids and AMPs, are also present in these mucosal layers and function collectively to restrict the interaction of luminal contents with the epithelial layer (Singh et al. 2002; Dupont et al. 2014; Mukherjee and Hooper 2015). The resistin-like molecule β (RELM-β) is an AMP present in the mucosal layer and acts as a pro-inflammatory secretory product of goblet cells with its activity against Pseudomonas aeruginosa and C. rodentium being reported previously in human and murine experimental models, respectively (Artis et al. 2004; Propheter et al. 2017). Goblet cells also have a role in the surveillance of harmful stimuli present in the gut lumen through antigen uptake and transport to underlying components of the subepithelial immune system such as dendritic cells (McDole et al. 2012; Howe et al. 2014; Allaire et al. 2018). A further subtype of goblet cells also exists, known as ‘sentinel’ goblet cells (Birchenough et al. 2016). These cellular security guards express TLRs used to sense and endocytose bacterial products and also promote the downstream activation and release of inflammatory mediators (Birchenough et al. 2016). During this process, additional goblet cells are recruited to carry out mucin granule exocytosis thereby protecting the depths of the intestinal crypts by physically restricting bacterial interactions (Allaire et al. 2018).
Enteroendocrine cells are also present in the intestinal epithelium and secrete a range of hormones that influence the physiological environment of the gut. These cells are separated into eight subsets, including D cells (secrete somatostatin), G cells (secrete gastrin), and enterochromaffin cells (secrete 5-hydroxytryptamine (HT)/serotonin), each of which carries out specialised functions depending on the hormone they secrete (Crosnier et al. 2006; Gribble and Reimann 2016; Johansson and Hansson 2016; Rodríguez-Colman et al. 2017). The secretion of hormones by these cells can be influenced by certain intestinal microbes. For example, production of the neurotransmitter, 5-HT/serotonin, was reportedly upregulated in the presence of spore-forming Clostridia species, while germ-free mice displayed lower 5-HT/serotonin levels in their colon and blood as well as reduced intestinal motility (Kashyap et al. 2013; Yano et al. 2015; Allaire et al. 2018). The production of this neurotransmitter is also influenced by other luminal components, including bile acids and bacterial-derived metabolites, which stimulate tryptophan hydroxylase 1 expression, an enzyme involved in 5-HT production (Reigstad et al. 2015; Yano et al. 2015).
Certain epithelial cell types, such as Paneth and M cells, are unique to the SI and are localised to subepithelial regions dense in immune cells called Peyer’s patches (Allaire et al. 2018). Despite the rapid turnover rates for most cell types present in the intestinal epithelium, Paneth cells have a life span of up to two months and play an important role in the defence against harmful microbes (Mu et al. 2017). Central to their antimicrobial qualities is their ability to secrete large granules harbouring AMPs and enzymes such as α-defensins and lysozymes (Vaishnava et al. 2008; Gong et al. 2010; Coulombe et al. 2016; Bel et al. 2017). M cells also contribute to the high concentrations of AMPs present in the intestinal mucosa and additionally produce IgA (Allaire et al. 2018). In addition, the structural properties of M cells make them suitable for their roles in IRs within the SI as these cells possess a large basolateral pocket permitting intimate contact with components of the subepithelial immune system, including dendritic cells and lymphocytes (Ohno 2016).
Tuft cells are another cell type present in the intestinal epithelium and play an important role in the IR against helminth and protozoan parasites. This is primarily through the secretion of cytokines associated with type_2 IRs (Hendel et al. 2022). The increased secretion of interleukin (IL)-25 by tuft cells following helminth infection has been reported to activate group 2 innate lymphoid cells encouraging subsequent tuft cell proliferation by the secretion of IL-13 (von Moltke et al. 2016). The secretion of IL-25 by tuft cells can also be modulated by the SCFA, succinate, produced by members of the intestinal microbiota, particularly species of the Bacteroidetes phylum who are prolific producers of this metabolite (Connors et al. 2019). Tuft cells express succinate receptor 1 on their surface which detects the presence of this in SCFA in the gut lumen stimulating downstream signalling pathways and leading to a type-2 IR (Nadjsombati et al. 2018).
Tight Junctions as Modulators of Intestinal Integrity and Paracellular Translocation
The paracellular transport of important molecules across the intestinal epithelium is regulated by transmembrane and intracellular proteins that interlink to form TJs. Over forty TJ proteins have been identified to date, each of which facilitate cellular adhesion and the maintenance of epithelial cell polarity promoting gut barrier integrity (Yamazaki et al. 2008; König et al. 2016). TJs play an important role in preventing the downward diffusion of apical surface receptors to the basolateral membrane thereby maintaining cellular polarity and ensuring IECs can detect external stimuli in the gut lumen and stimulate the appropriate downstream response (König et al. 2016). TJ complexes are primarily composed of transmembrane proteins including occludins, junctional adhesion molecules, and claudin family members that function collectively to form pores with selective permeability to ions and molecules (Brenchley and Douek 2012; König et al. 2016).
TJs localised at the apex of the lateral membrane of IECs are often referred to as apical junction complexes and are composed of the apical-most TJs, adhesion junctions and desmosomes, each of which are involved in the separation of apical and basolateral membrane domains and maintaining gut barrier integrity (Turner and Turner 2010; Quiros and Nusrat 2014; Roxas and Viswanathan 2018). Components of apical junction complexes retain an anchored position in the cell membrane by establishing interconnective networks between adjacent cells and associating with intracellular adaptor proteins linked to the intracellular cytoskeleton (Zihni et al. 2016). Despite the importance of TJs in cell–cell adhesion, it is the more basal desmosome structures that are primarily responsible for the mechanical strength of the intestinal epithelium by acting as “spot-welds” that clutch cells together (Roxas and Viswanathan 2018). Nonetheless, TJs play a pivotal role in limiting the paracellular translocation of enteric pathogens into systemic circulation and their subsequent spread to extraintestinal sites (Fine et al. 2020).
In contrast to larger molecules that are actively transcytosed by enterocytes to permit their access beyond the intestinal epithelium, the translocation of smaller molecules occurs via several different paracellular routes (Fine et al. 2020). The high-capacity pore pathway is permeable to molecules up to 10 angstroms (Å) and is primarily controlled by claudin proteins. The paracellular translocation of macromolecules up to 125 Å is carried out by a low-capacity leak pathway regulated by zonula occludens (ZO), occludins, and tricellulins (Odenwald and Turner 2017; France and Turner 2017). A third pathway also exists at intestinal sites subject to erosion and ulcerative damage permitting the passage of microbes and their products across the gut barrier as an outcome of its impaired integrity (Odenwald and Turner 2017; France and Turner 2017).
Endocytosis, migration, and recycling are among the dynamic processes involved in TJ assembly and disassembly, each of which are modulated by enzymes of various signalling pathways including protein kinase C, mitogen-activated protein kinases, Rho GTPases and myosin light chain kinases (Ulluwishewa et al. 2011; König et al. 2016). The plasticity of TJs is controlled by a range of external molecules, primarily proteins, that can be either of endogenous or exogenous origin. Certain pathogenic virulence factors, such as those secreted by enteric pathogenic E. coli, alter the cytoskeletal network of IECs to stimulate TJ disassembly and increased gut permeability (Mu et al. 2017; Roxas and Viswanathan 2018; Pakbin et al. 2021). The peri junctional actomyosin ring localised to the periphery of IECs is an example component of the intracellular cytoskeleton that is modulated by the presence of certain virulence factors promoting its contraction. This is a result of the phosphorylation of myosin light chain that occurs in the peri junctional actomyosin ring to encourage TJ disassembly (Cunningham and Turner 2012; Quiros and Nusrat 2014; Roxas and Viswanathan 2018). The function of TJs and expression of their associated components is also influenced by secretory products from other cell types in the gut barrier, for example, S-nitrosoglutathione released by enteric glial cells stimulates TJ protein expression and reduces intestinal permeability (Costantini et al. 2012).
The Gut Barrier: An Immunological Hotspot
Enterocytes are important mediators of the IRs generated in the GI tract. These cells are involved in a complex interconnected network with the microbiota and underlying immune cells that as a collective act as a major site of defence against enteric threats (Allaire et al. 2018). Furthermore, these epithelial components express various surface receptors, including TLRs to sense endogenous danger signals, or pathogen-associated molecular patterns (PAMPs) to detect microbes. The subsequent release of mucins and AMPs as well as the release of chemokines and cytokines recruits immune cells enabling pathogen concentrations to be controlled at a distance from the epithelial layer where systemic access is most likely to be achieved (Johansson et al. 2008; Ermund et al. 2013; Allaire et al. 2018). Despite being present at the apical surface the majority of IEC-associated TLRs are localised to the basolateral membrane. Examples include TLR2 and TLR9, which once activated promote downstream expression of cytokines and chemokines to elicit an IR (Abreu 2010; McClure and Massari 2014; Yu and Gao 2015; Allaire et al. 2018). In contrast, stimulation of apical TLR9 by luminal contents induces a net inhibitory effect, reinforcing the unique ability of IECs to distinguish between signals despite using the same receptors at different spatiotemporal positions (Lee et al. 2006). This specificity is central to the ability of IECs to distinguish between harmless structures associated with microbiota residents and those of enteric pathogens, thereby limiting the occurrence of exaggerated IRs in the gut (Allaire et al. 2018).
While microbes and their products can breach the intestinal epithelium during normal cellular processes including luminal antigen sampling, dead cell extrusion, or programmed cell death, their systemic spread is restricted by specialised immune cells residing within the underlying lamina propria (Smith et al. 2005; Roxas and Viswanathan 2018). Macrophages are among the abundant cell types present in the subepithelial immune system and are distinguished from other immune cell types by the absence of certain receptors, including LPS co-receptor (CD14), Fc receptors (CD89, CD16, CD32, and CD64) or IL-2 and IL-3 receptors (Lee et al. 1985; Smythies et al. 2005). The lack of these receptors ensures GI macrophages are unable to respond to ligands that would typically activate other immune cell types thereby preventing excessive IRs (Brenchley and Douek 2012). However, the expression of other surface receptors, including TLR2, 4, 5 and 9, enables GI macrophages to carry out antigenic clearance from the lamina propria via phagocytic uptake, while also maintaining an anti-inflammatory gut environment (Smith et al. 2001; Smythies et al. 2005; Brenchley and Douek 2012).
Like macrophages, M cells and CD103+ dendritic cells are involved in luminal antigen sampling to monitor the presence of harmful stimuli. These cells may also provide an avenue for the systemic entry of commensal and pathogenic bacteria ultimately facilitating their dissemination throughout the human host (Niess et al. 2005; Chieppa et al. 2006; Farache et al. 2013; Doran et al. 2013; Ribet and Cossart 2015). However, antigen sampling of luminal microbes is necessary for immunological development, bacterial clearance and the production of IgA, the latter of which is important in restricting microbial adhesion to the mucosal layer and coating commensal species to limit their interaction with immune system (Johansen et al. 1999; Macpherson and Uhr 2004; Niess et al. 2005; Woof and Russell 2011; Mazzini et al. 2014; Bunker and Bendelac 2018). The production of IgA can also be influenced by the presence of certain commensals including Enterobacter cloacae. This bacterium can be taken up by M cells and transported to mesenteric lymph nodes where an IgA response is induced (Macpherson and Uhr 2004). The newly produced dimeric or oligomeric forms of IgA then enter the intestinal lumen via transcytosis and play an important role in mucosal defence against enteric pathogens (Brenchley and Douek 2012).
The release of inflammatory mediators during infection or as a consequence of abnormal immune signalling pathways, increases the risk of microbial translocation. Interferon-gamma (IFN-γ) is released from both activated T-cells and natural killer cells and attenuates gut barrier integrity by promoting the endocytic uptake of TJ components such as claudin (Prasad et al. 2005; Chiba et al. 2006). Other inflammatory mediators including tumour necrosis factor (TNF) and IL-13 also have a negative impact on gut barrier integrity by decreasing the expression of claudin proteins (Prasad et al. 2005; Chiba et al. 2006). While this may facilitate the entry of immune cells to fight infectious agents, it also provides an avenue for luminal contents including commensal microbes, pathogens, and dietary components, to bypass the intestinal epithelium and enter systemic circulation. In addition, the presence of the pro-inflammatory mediators, TNF and IL-1, also impair gut barrier integrity by activation of cell death pathways, such as apoptosis, thereby promoting elevated intestinal permeability (Ruemmele et al. 2002). IECs counteract TNF by the production of various factors including the ubiquitin-editing enzyme, A20, required for the termination of certain immune signalling pathways activated in response to surface detection of TNF or PAMPs (Brenchley and Douek 2012). The importance of this protein in immune regulation is reinforced by the multiorgan inflammation reported in A20 deficient mice and the association of mutations in this enzyme with IBD (Brenchley and Douek 2012; Ma and Malynn 2012).
The immunological conditions of the intestinal lumen are heavily influenced by members of the gut microbiota. This has been demonstrated through the ability of intestinal microbes to promote the repair and proliferation of gut barrier components following their innate recognition (Kitajima et al. 2001; Rakoff-Nahoum et al. 2004; Johansson et al. 2010). Mice deficient in TLRs display severe tissue damage and mucosal ulcers, suggesting that microbial recognition at the surface of IECs is of great importance in maintaining gut barrier health (Kitajima et al. 2001; Rakoff-Nahoum et al. 2004; Johansson et al. 2010). The mucous layer can also be restored by commensal microbial products, including LPS and peptidoglycan, which have been reported to elevate mucous secretion through interaction with certain TLRs (Petersson et al. 2011; Johansson et al. 2015).
Numerous IRs are regulated by the microbiota. One example is the induction of mucosal IgA secretion from plasma cells by Acaligenes, a commensal bacterium naturally residing within gut localised dendritic cells and involved in promoting mucosal defence (Obata et al. 2010; Shibata et al. 2018). The production of AMPs from IECs is also modulated by intestinal microbes. B. thetaiotaomicron has been shown to induce the release of Ang4 from mouse Paneth cells demonstrating that antimicrobial activity in the intestinal lumen is dependent on the presence of certain commensals (Hooper et al. 2003; Mu et al. 2017). Conversely, products of IECs can also directly influence the composition of the microbiota. Intestinal alkaline phosphatase, active in both anchored and secreted forms, promotes the growth of LPS-suppressing bacteria such as Bifidobacterium, and limits the number of LPS-producing E. coli (Eliakim et al. 1991; Nakano et al. 2009; Kaliannan et al. 2015). This reinforces how communication between the host and the microbiota occurs in a bidirectional manor.
Factors Influencing Intestinal Barrier Integrity
Environmental Factors
Residents of the Intestinal Microbiota
The maintenance of IEC physiology is heavily dependent on the metabolites produced by bacterial members of the gut microbiota (Allaire et al. 2018). It is estimated up to 10% of all IEC gene transcriptions are modulated by commensal species in the gut lumen, many of which are involved in controlling immunological, metabolic, and cellular proliferative pathways (Sommer et al. 2015). The importance of commensal bacteria in the gut has been demonstrated previously, for example, germ-free mice displayed an underdeveloped mucous layer as well as reduced mucin production and IEC proliferation, each of which are required for gut barrier health (Kim and Ho 2010). In addition, the recognition of commensal structures by IEC TLRs had a positive influence on paracellular permeability by promoting the normal distribution of TJ proteins required for intestinal integrity (Rakoff-Nahoum et al. 2004; Cario et al. 2007).
Butyrate is a well-established SCFA metabolite produced primarily by F. prausnitzii, B. thetaiotaomicron and Clostridium clusters IV and IXa following the fermentation of dietary fibre (Roediger 1980; Kau et al. 2011). This metabolite acts as a nutrient source for colonocytes. Membrane transport proteins are essential in its uptake, and it is then metabolised via β-oxidation and the tricarboxylic acid cycle (Borthakur et al. 2006; Gupta et al. 2006). The uptake of butyrate by colonocytes helps provide optimal colonising conditions for members of the intestinal microbiota which are predominantly obligate anaerobes. This is achieved via the increased oxygen uptake by colonocytes which then promotes epithelial hypoxia thereby supporting the anaerobic lifestyle of many important bacteria present in the intestinal mucosa (Itoh and Freter 1989; Byndloss et al. 2017; Litvak et al. 2018). This SCFA also has an influence on the immunological conditions in the gut. In the murine mucosa, butyrate has been reported to induce the differentiation of regulatory T-cells (Tregs) to maintain intestinal homeostasis (Atarashi et al. 2011; Furusawa et al. 2013). Given the importance of butyrate in supporting commensal colonisation and promoting an anti-inflammatory gut environment, it is not surprising that reduced concentrations have consequences for the host. Butyrate concentrations can be lowered by the unintentional removal of butyrate-producing species occurring after the use of orally administered antibiotics. As an outcome, colonocytes shift to anaerobic metabolism characterised by reduced oxygen consumption thereby facilitating the growth of facultative anaerobes such as Enterobactericeae, which exploit the loss of epithelial hypoxia through aerobic respiration (Byndloss et al. 2017; Litvak et al. 2018). Consequently, the numbers of commensal obligate anaerobes, including Clostridia and Bacteroidia, are reduced due to elevated oxygen concentrations in the intestinal mucosa, which increases the risk of enteric infection (Winter et al. 2013). Nonetheless, restoration of butyrate-producing species to help treat enteric infections can be achieved through various strategies including dietary interventions (Ramakrishna et al. 2000; Bach Knudsen et al. 2018) and bacteriotherapy using faecal microbiota transplantation (Zhu et al. 2021). Although the latter of these can be associated with certain adverse complications (Baxter and Colville 2016), this approach has been successful in treating infections caused by Clostridium difficile (Kumar and Fischer 2020).
Enteric Infection
The intestinal lumen faces continuous exposure to enteric pathogens whose aim is to access the circulatory system to facilitate their systemic spread and proliferation. To counteract the impact of infectious agents, IECs have evolved strategies to inhibit spread, for example, physical expulsion from the monolayer into the faecal stream (Allaire et al. 2018). Antimicrobial responses to enteric pathogens are also modulated by commensal gut colonising bacteria. Bacteroides thuringiensis is effective in the removal of nosocomial C. difficile by the production of a narrow-spectrum bacteriocin that disrupts the target bacterial cell membrane (Rea et al. 2010, 2011; Lessa et al. 2015; Mathur et al. 2017). IRs to enteric pathogens can also occur within the intracellular environment. These are dependent on inflammasome activation following the detection of PAMPs which induces physiological changes in the cell including sloughing, cytokine secretion or coordinated cell death, each of which limit bacterial spread (Lamkanfi and Dixit 2017; Allaire et al. 2018). The inflammatory response to Salmonella infection by IECs involves rearrangement of the actin cytoskeleton to encapsulate infected cells undergoing pyroptosis, preventing bacterial dissemination across the intestinal monolayer (Rosenblatt et al. 2001; Rauch et al. 2017). In addition, caspase activation, which occurs during these intracellular responses, mediates the formation of ring-shaped pores at the cell membrane through Gasdermin D processing. This leads to destabilisation of ionic gradients and subsequent water influx and membrane rupture (Ding et al. 2016; Aglietti et al. 2016; DiPeso et al. 2017).
Despite inflammatory IRs being effective in combatting pathogenic invasion on most occasions, certain enteric pathogens have evolved mechanisms that enable them to utilise the inflammatory environments established in response to their presence. C. rodentium (colon) and Salmonella elevate O2 and nitrate concentrations above the epithelium causing a shift in IEC metabolism, similar to the impact caused by a reduction in butyrate-producing microbes described previously (Rivera-Chávez et al. 2016; Lopez et al. 2016). In addition, crypt hyperplasia is commonly observed during C. rodentium infection resulting in an increased turnover of IECs and leading to a greater number of immature enterocytes becoming localised to the apical site of crypts. These under developed IECs ferment glucose to lactate rather than consuming oxygen which would normally aid commensal colonisation (Gill et al. 2012; Fan et al. 2015; Rivera-Chávez et al. 2017).
The increased production of inflammatory mediators in response to enteric pathogens has an impact on intestinal barrier function. Paneth cells undergo degranulation, luminal extrusion and apoptosis following exposure to IFN-γ (Allaire et al. 2018) and, given that these cells are major contributors to mucosal defence primarily through their ability to secrete lysozymes and defensins, their declining numbers encourages the expansion of harmful microbes including species of Escherichia and Shigella (Farin et al. 2014). The induction of TNF-α also impairs barrier integrity by inducing cytoskeletal alterations in IECs. This results in endocytic uptake of TJ components and provides infectious agents with an opportunity to enter systemic circulation (Roxas and Viswanathan 2018). Although inflammatory mediators increase intestinal permeability facilitating the infiltration of immune cells, their elevated abundance can lead to the erosive loss of important gut barrier components (Fig. 2) (Roxas and Viswanathan 2018). The regulated release of these mediators is therefore essential in maintaining gut barrier integrity.
Enteric pathogens contribute to structural damage of the intestinal epithelium, either directly via the production of toxins or indirectly by inducing pro-inflammatory IRs in the gut. Such damage promotes the shedding of IECs, particularly during intracellular infection to prevent bacterial dissemination across the monolayer
Diet
Dietary intake has a significant role in sha** the microbial communities residing within our gut and is therefore an important environmental factor which dictates intestinal barrier integrity. The change from a diet consisting primarily of maternal milk during infancy to one which involves solid, nutrient-dense foods, is essential in promoting a stable gut microbiota (Bäckhed et al. 2015). In the western world, however, solid food diets consist largely of processed and genetically modified foods, that despite hel** meet the demands of a growing population, often possess ingredients that may have consequences for human health. Genetically modified crop strains are designed to resist herbicides and pesticides, and processed foods harbour additional components (i.e. food additives) used to extend their shelf life and limit microbial contamination (Smyth 2017). Many of the food additives included in processed foods have a direct impact on the composition of microbial communities present in the gut (Table 1). The emulsifying agent, carboxymethylcellulose, has been reported to alter the intestinal microbiota and promote chronic inflammation, and polysorbate 80 has been found to have an erosive impact on the intestinal mucosal layer (Chassaing et al. 2015; Naimi et al. 2021). The mucosal layer can also be attenuated as a result of reduced dietary fibre intake which encourages the growth of mucus-degrading species such as Bacteroides caccae and Akkermansia muciniphila (Desai et al. 2016). Despite the mucin degrading properties of A. muciniphila, it has been reported that supplementation with this bacterium increases the number of mucin secreting goblet cells in mice. The production of SCFAs following mucin degradation is likely to act as an energy source for IECs to aid further mucin secretion. This could provide an explanation for findings from previous studies which confirmed the capacity of A. muciniphila to restore reduced mucosal thickness and decreased AMP production seen in mice fed high-fat diets (Everard et al. 2013, 2014; Shin et al. 2014).
The western diet is composed primarily of foods high in saturated fat, glucose, salt, and synthetic additives, each of which can have a negative impact on gut barrier integrity (Lam et al. 2012; Kleinewietfeld et al. 2013; Lerner and Matthias 2015a). Excessive fat intake has previously been demonstrated in mice to increase intestinal permeability, confirmed by high levels of circulating gut-derived LPS and reduced TJ protein expression (Cani et al. 2007, 2008). High-fat intake can also disrupt gut microbial communities by reducing the numbers of commensal Lactobacillus and elevating the numbers of Oscillibacter, a bacterial genus associated with reduced expression of the TJ component, ZO-1 (Lam et al. 2012; Mu et al. 2017).
Glucose is consumed excessively in many developed countries and while it is essential for cellular respiration, it possesses absorption-enhancing properties that elevates intestinal permeability (Lerner and Matthias 2015a). This is largely due to altered TJ protein distribution promoting the paracellular leakage of luminal contents (Lerner and Matthias 2015a). Glucose mediated increase in intestinal permeability has also been reported to precede the development of the inflammatory disorder, metabolic endotoxaemia, and results in lipid accumulation that contributes to obesity (Do et al. 2018). Diets high in salt levels may also have a negative impact on gut barrier health by promoting a shift towards an inflammatory gut environment dominated by T helper 17 (Th17) cells which increases the risk of develo** IBD (Lerner and Matthias 2015a). The cytokine, IL-17, produced by Th17 cells is known to exacerbate disease symptoms in chemically induced animal models of colitis (Aguiar et al. 2018). In addition, high-salt diets have led to histologically detectable intestinal inflammation in mice models, as confirmed by goblet cell depletion, ulceration, erosion, and destruction of the mucosal layer (Aguiar et al. 2018).
Other common additives utilised by the food industry include microbial transglutaminase (mTG) and, although useful for modifying the functionality of dietary protein, has been found to increase intestinal permeability and suppress pathways involved in protection against enteric pathogens (Santos and Torné 2009; Hu et al. 2011; Kieliszek and Misiewicz 2014; Lerner and Benzvi 2021). The fact that TJ protein components, including claudin and occludin, may act as potential substrates for mTG, in addition to the emulsifying properties of this enzyme, provides a possible explanation for its ability to elevate intestinal permeability (Lerner et al. 2017; Lerner and Matthias 2020). Organic solvents such as ethanol and methanol also impair gut barrier integrity by disrupting the structure of TJs (Yu et al. 2013; Vojdani 2014). It is therefore not surprising that, in contrast to healthy controls, individuals who consume abnormally high amount of alcohol display elevated levels of gut-derived LPS in their plasma (Fukui et al. 1991; Schäfer et al. 2002).
Food chain contaminants, such as persistent organic pollutants (POPs) are present in certain fast-food packaging, pesticides/insecticides, and non-stick coating on cookware (Chiu et al. 2020) and represent another environmental factor with gut barrier-impairing properties. The food chain is estimated to account for over 90% of exposure to certain POPs, such as polychlorinated biphenyls (PCB), detectable in dairy products, fish, and animal fat (Roveda et al. 2006). The ability of these chemicals to increase gut permeability in animal models by disrupting the gut microbial community composition has been reported previously (Choi et al. 2010; Rude et al. 2019). The expression of the TJ protein components can also be altered as a consequence of PCB exposure, with PCB 126 found to reduce the expression of ZO-1 (Chi et al. 2019). Other sources of food chain contamination include marine toxins which are concentrated by shellfish and subsequently consumed by humans. The phytoplanktonic toxin, okadaic acid, is responsible for many cases of human shellfish poisoning. This toxin impairs gut barrier properties by altering TJ protein expression resulting in diarrhoea (Valdiglesias et al. 2013; Dietrich et al. 2019). These examples highlight how the intestinal epithelium is continuously exposed to harmful chemicals present in the diet which can attenuate its efficacy in limiting the systemic entry of luminal components.
Drug Therapy
Numerous drugs and their associated components can also directly attenuate the function of the intestinal barrier and lead to an increased risk of luminal contents entering the systemic circulation. Surfactants such as Cremophor EL and Gelucire 44/14 are often utilised by pharmaceutical industries to purposely disrupt the intestinal barrier and facilitate the uptake of orally administered drugs (Hamid et al. 2009). In addition to this, chemotherapeutics, such as cyclophosphamide, have been demonstrated to increase intestinal permeability and permit the translocation of certain luminal bacteria. For example, bacterial species, including Lactobacillus johnsonii, L. murinus and Enterococcus hirae, were cultured from secondary lymphoid organs following cyclophosphamide administration in mice. However in this instance, the translocation of these bacteria was associated with facilitating tumour control by stimulating pathogenic Th17 cell responses (Viaud et al. 2013).
Proton pump inhibitors (PPI) have also been demonstrated to impair barrier function by inhibiting hydrogen/potassium adenosine triphosphatases, particularly those localised to the gut that play roles in the maintenance of potassium homeostasis (Mullin et al. 2009). This inhibition is likely to influence electrolyte balance in the mucosal layer and may diminish the efficacy of immunological components that are sensitive to pH alterations (Keszthelyi et al. 2010). Changes in pH from PPI exposure has also been reported to increase intestinal permeability, for example, PPI exposure in mice promoted a rise in extracellular pH leading to increased activation and expression of the TJ regulator, myosin light chain kinase (Nighot et al. 2022). The increase in extracellular pH may also lead to impaired intestinal barrier integrity by disrupting microbial communities in the gut that are important regulators of overall gut homeostasis. This was demonstrated previously by Takashima and colleagues who revealed a decrease in the abundance of gut colonising Clostridiales in mice following PPI administration (Takashima et al. 2020; Guo et al. 2020).
Despite reducing inflammation, non-steroidal anti-inflammatory drugs (NSAIDs) can disrupt oxidative phosphorylation and decrease the intracellular concentrations of adenosine triphosphate (ATP) (Somasundaram et al. 1997, 2000). Given that the regulation of the intracellular cytoskeleton is dependent on the presence of ATP to stimulate the contraction of actin-myosin complexes, a reduction in ATP concentration caused by NSAIDs was found to impair TJs and reduce cellular polarity (König et al. 2016). In addition, NSAID-induced enteropathies can involve excessive translocation of luminal contents such as bacteria and their products, bile acids and pepsin, promoting immune activation and ulceration of the intestinal epithelium (Reuter et al. 1997; Fortun and Hawkey 2005; Scarpignato 2008). The impact of NSAIDs on intestinal permeability is reinforced by the estimated 60–80% of individuals using these drugs who display a disrupted gut barrier.
Although antibiotics are useful in treating bacterial infections, they can often be associated with the removal of useful GI tract microbial colonisers and contribute to the development of certain diseases (Ramirez et al. 2020). In addition to the indirect consequences of long-term oral antibiotic consumption on intestinal barrier health, particularly through reducing the numbers of SCFA-producing species, these therapeutics have also been reported to directly impair the gut barrier by reducing the expression of TJ proteins and negatively impacting their morphology. Administration of an antibiotic cocktail including ampicillin, metronidazole, and vancomycin, was found to disrupt ZO morphology while decreasing the expression of occludin and claudin-1 TJ proteins in mice (Feng et al. 2019). The decreased abundance of SCFA-producing bacteria in the gut caused by antibiotic administration has also been associated with enhancing pathogenic colonisation, including by the fungal species Candida albicans known to modulate the permeability and integrity of the gut barrier during infection (Allert et al. 2018; Basmaciyan et al. 2019; Guinan et al. 2019).
Genetic Predisposition to an Attenuated Gut Barrier
Genetic abnormalities leading to the expression of dysfunctional or structurally abnormal barrier components can increase the likelihood of interactions between luminal components and the subepithelial immune system. Individuals who are genetically predisposed to a dysfunctional gut barrier are therefore more susceptible to develo** inflammatory gut disorders (König et al. 2016). This is evident in IBDs such as Crohn’s whereby patients have been reported to express receptor variants to IL-23, a cytokine primarily expressed by macrophages and found in elevated abundance in the intestinal mucosa of Crohn’s patients (Duerr et al. 2006; Kobayashi et al. 2008; Silverberg et al. 2009). This inability to maintain immunological homeostasis in the gut encourages the entry of luminal antigens into underlying tissues where excessive inflammatory responses are induced further (König et al. 2016).
Other gut-associated inflammatory conditions linked to genetic predisposition include CD, a chronic enteropathy that arises following T-cell mediated IRs to gluten present in dietary grains such as wheat, barley, and rye (Smyth 2017). Specifically, it is the gliadin component of gluten that is responsible for stimulating the immune-mediated destruction of the intestinal barrier (Smyth 2017). Variants of the HLA gene responsible for the presentation of foreign antigens to T-cells are among the genetic factors thought to play a role in the aetiology of CD. This is reinforced by the presence of the HLA-DQ2 haplotype in over 90% of coeliac patients, while 10% are estimated to express the HLA-DQ8 variant (Lammers et al. 2008). The interaction of gliadin and its immunomodulatory fragments with chemokine CXC receptor 3 on IECs induces zonulin release and downstream disassembly of TJs which has been reported previously among coeliac patients (Lammers et al. 2008; Smyth 2017). In addition, newly established HLA-gliadin complexes localised to the surface of antigen presenting cells, activate T-cells to promote the release of anti-gliadin and anti-tissue transglutaminase (tTG) antibodies by B cells and plasma cells (Smyth 2017). The interaction of surface localised HLA-gliadin complexes with fellow T-cells also leads to the recruitment of immune system components including natural killer cells which have a destructive impact on the gut barrier through cytokine release (Smyth 2017). This inflammatory environment impacts barrier integrity and provides an avenue for the entry of luminal contents into systemic circulation contributing to the development of gut-associated and extraintestinal inflammatory disorders.
Mechanisms of Bacterial Translocation During Enteric Infection
Various factors including drugs, epigenetic changes, radiation, alcohol, hyperglycaemia, and autonomic dysfunction can create opportunities for microbial translocation by disrupting intestinal barrier integrity and permeability (Fine et al. 2020). Microbial translocation may also occur through natural mechanisms involving passive uptake (Payne et al. 1960). This can occur during physiological processes such as luminal antigen sampling by immune cells and M cells to detect the presence of foreign compounds and stimulate an inflammatory response if necessary (Mainous et al. 1991). Enteric pathogens such as Salmonella and Shigella flexneri exploit sampling by M cells to facilitate their spread. Salmonella typhimurium induces the destruction of M cells following uptake thereby attenuating gut barrier integrity (Fine et al. 2020), and S. flexneri utilises a similar mechanism; however, this bacterium re-enters nearby enterocytes through their basolateral membrane aiding bacterial translocation by promoting cellular death (Ribet and Cossart 2015). Bacterial mediated cytoskeletal changes also occur in IECs during Shigella infection. This feature is largely dependent on the production of SepA which limits the expression of host proteins required for actin remodelling, thereby assisting bacterial spread across adjacent IECs (Roxas and Viswanathan 2018).
Certain enteric pathogens possess mechanisms that enable their direct adherence to IECS while others permit the secretion of effector proteins or enterotoxins via membrane localised secretion systems that typically target TJs thereby increasing intestinal permeability by disrupting the intracellular cytoskeleton of IECs (König et al. 2016). Enteropathogenic E. coli (EPEC) is a prevalent cause of diarrhoea in infants, with clinical manifestations of infection characterised by loss of enterocyte microvilli (Lapointe et al. 2009). This bacterium encodes a type-3 secretion system that directly secretes effector proteins, including Tir, into host cells. This effector protein becomes phosphorylated upon entry into the intracellular environment and is subsequently inserted into the apical membrane. There, Tir induces the formation of a raised pedestal structure which acts as a receptor for the bacterial adhesion protein, intimin (Lapointe et al. 2009; Wong et al. 2011; König et al. 2016; Roxas and Viswanathan 2018). Other virulence factors associated with EPEC include heat-labile toxins 1 and 2, heat-stable toxins Sta, STb, and EAST1. These enterotoxins alter luminal osmolarity by inducing chloride ion secretion from IECs which stimulates water entry into the lumen and results in diarrhoea (Dubreuil 2008; Okhuysen and Dupont 2010).
Enteric pathogens have evolved various strategies that directly target TJ protein complexes (König et al. 2016). Many of these barrier-impairing mechanisms are dependent on their ability to secrete the toxins responsible for the intestinal pathologies associated with infection. The secretion of the toxin, CagA, by Helicobacter pylori, attenuates adhesions between adjacent IECs as well as reducing apical-basal cell polarity by altering the IEC cytoskeleton and encouraging TJ disassembly (Bagnoli et al. 2005; Tapia et al. 2017). This strategy also enables the luminal entry of iron that in turn promotes bacterial growth (König et al. 2016). Other pathogenic species that target TJ protein complexes to disrupt gut barrier integrity include Vibrio cholerae and Aeromonas hydrophila which secrete a hemagglutinin/protease and a pore-forming aerolysin, respectively, facilitating the direct cleavage of occludin (Nava et al. 2013).
Despite being important butyrate producers in the gut, certain species of the Gram-positive, spore-forming Clostridia are labelled as the most prolific toxin producers among bacteria (Roxas and Viswanathan 2018). The Clostridium perfringens enterotoxin induces pore formation at the cell membrane and subsequent cell death as a result of an increased influx of calcium and activation of apoptosis pathways (Chakrabarti et al. 2003). Clostridial toxins A (TcdA) and B (TcdB) utilise TJ components as receptors, specifically the nectin-3 component of adhesion junctions, which leads to downstream cytoskeletal disruptions and cell death (Roxas and Viswanathan 2018). Not only does the resulting barrier disruption promote diarrhoea because of increased water concentration in the intestinal lumen, but there is also a greater opportunity for the development of secondary infections due to deeper tissue penetration by other pathogens and toxins (Guttman and Finlay 2009). The movement of basolateral proteins to the cell apex after TJ disruption may lead to a larger pool of luminally exposed cell surface components that act as receptors for other pathogens and their toxins. This is the case during EPEC infection whereby compromised TJs allow the apical migration of basolateral β-integrin which act as an additional receptor for bacterial intimin (Muza-Moons et al. 2003).
Despite exhibiting an intracellular lifestyle within IECs, it was recently discovered that the foodborne pathogen, Listeria monocytogenes, also employs mechanisms to aid its paracellular transport across the intestinal epithelium potentially contributing to systemic infection (Drolia et al. 2018). The Listeria adhesion protein (LAP) is central to this ability, and like many other virulence factors associated with enteric pathogens, disrupts the cytoskeletal architecture of host IECs. This can be as a consequence of the increased expression of inflammatory cytokines, such as TNF-α and IL-6, that occurs following the interaction of LAP with its host receptor, Hsp60 (Drolia et al. 2018). There is also a direct influence of this virulence factor on host IECs through nuclear factor-kappa B (NF-κB) activation leading to the redistribution of TJ components including claudin-1, occludin, and E-cadherin (Drolia et al. 2018). Interestingly, the inhibition of NF-κB and certain cytoskeletal signalling enzymes restrict TJ disassembly and disruption, thereby restricting the paracellular translocation of L. monocytogenes (Drolia et al. 2018). This suggests that components involved in TJ disassembly may represent useful therapeutic targets by limiting the impact of enteric pathogens on intestinal barrier integrity and restricting their systemic spread.
The Gut Barrier’s Role in Autoimmune Disease
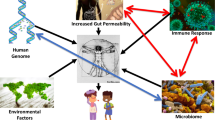
Although the aetiology of ADs remains largely unknown, a combination of environmental, genetic, and microbial factors are thought to be involved (Parks et al. 2014; Smyth 2017). Over eighty ADs exist, with the majority of these typically characterised by the immune system’s inability to distinguish between self-components and those which pose a threat to host health (Smyth 2017). ADs are becoming more prevalent in developed countries of the western world, with environmental factors, specifically dietary components, thought to be major contributing factors (Parks et al. 2014). The intestinal epithelial layer plays an essential role in separating luminal components, including microbes and dietary components, from systemic circulation to limit their interaction with the subepithelial immune system (Luissint et al. 2016). Growing evidence suggests that an attenuated gut barrier and increased translocation of luminal components into systemic circulation potentially contributes to the aetiology and/or progression of gut localised and systemic ADs (Mu et al. 2017; Smyth 2017; Fine et al. 2020).
Inflammatory Bowel Disease
While the aetiology of IBDs remains to be elucidated, there are numerous studies which indicate that genetic abnormalities in barrier components or those involved in immune signalling pathways are likely to be involved in disease pathogenesis. Variations in NOD2 receptors have been found to increase sensitivity to bacterial components and result in overproduction of inflammatory cytokines, such as α-defensin, that can remove beneficial microbes from the intestinal mucosa (Swidsinski et al. 2002; Strober et al. 2014). Furthermore, mutations in genes involved in IL-17 production and TJ protein expression are associated with the development of IBD (Sartor 1997). Disruption of the microbial communities in the gut is frequently reported among IBD patients, and combined with the elevated levels of gut-derived LPS and bacterial DNA also detected, suggests that increased intestinal permeability also contributes to disease pathologies (Targan 2000; Obermeier et al. 2005; Pastor Rojo et al. 2007; Gutiérrez et al. 2009).
Crohn’s disease is suspected to arise from a combination of factors including single-nucleotide polymorphisms leading to the production of structurally abnormal granules from dysfunctional Paneth cells, thereby increasing susceptibility to enteric infection (Yang and Shen 2020). Biopsies from Crohn’s patients with dormant disease have been found to harbour increased numbers of adherent-invasive E. coli at mucosal sites (Deuring et al. 2014). Disruption of the gut microbial communities in Crohn’s patients has been previously reported, however, these disruptions may either exacerbate disease pathologies or be a consequence of the chronic gut inflammation arising from genetic abnormalities that result in dysfunctional immune signalling. This inflammation may account for the low levels of certain commensal Firmicutes, such as F. prausnitzii, reported among Crohn’s patients (Miquel et al. 2013; Liu et al. 2016). Genetic predisposition to IBD development includes the expression of abnormal immune signalling components. IBD patients have been reported to express variants of the IL-23 receptor potentially accounting for excessive inflammation and the immune-mediated removal of commensal species in the gut (Duerr et al. 2006; Silverberg et al. 2009). Exposure to certain food additives may also play a role in the development or exacerbation of Crohn’s through shared structural domains with bacterial components. For example, food additives including emulsifiers, thickeners, and surface-finishing agents harbour domains with structural similarity to mycobacterial lipids known to induce granulomas during tuberculosis infection (Traunmüller 2005).
Coeliac Disease
CD is an immune-mediated disorder impacting the small intestine and is prevalent among IBD patients. CD is characterised by the generation of an excessive inflammatory response to the gliadin component of gluten present in wheat and other grains (König et al. 2016). Dysfunctional TJs have been discovered in coeliac patients and potentially contributes to intestinal leakage providing an access route for gliadin to the underlying immune system in the lamina propria (Schulzke et al. 1998; Pizzuti et al. 2004; Heyman et al. 2012). Other in vitro studies suggest that gliadin itself increases intestinal permeability indirectly by promoting the release of zonulin, a protein that induces TJ disassembly and is present in the submucosa of active coeliac patients at concentrations sixfold greater than healthy controls (Fasano et al. 2000; Clemente et al. 2003; Sander et al. 2005). Due to a shift towards an inflammatory environment, villi of the small bowel epithelium undergo atrophy thereby reducing the ability of enterocytes to absorb nutrients and water (König et al. 2016). The excessive consumption of certain dietary components including emulsifiers, glucose, salt, and saturated fats may potentially increase the risk of develo** CD or exacerbating symptoms in individuals experiencing active disease by disrupting gut barrier integrity (Lerner and Matthias 2015a). Specifically, mTG is included in numerous meat products and possesses emulsifying properties known to increase intestinal permeability as previously described (Kaufmann et al. 2012). Furthermore, mTG stimulates protein cross-linking that may act as novel immunogenic epitopes and induce inflammation (Lerner and Matthias 2015a). Cross-linked proteins are included in bakery products and stimulate an IR in coeliac patients thereby representing a novel role for the influence of food additives, such as mTG, in the development and progression of CD (Lerner and Matthias 2015a).
Type-1 Diabetes
T1D is an immunological disorder typically characterised by an inability to regulate blood glucose levels as a consequence of the self-mediated destruction of insulin-secreting pancreatic β-cells (Atkinson et al. 2014; Smyth 2017). Microbial translocation across the gut epithelium is thought to play a role in T1D development (Costa et al. 2016). Costa and colleagues confirmed the translocation of gut-associated bacteria to pancreatic lymph nodes increased the development of T1D-associated symptoms (Costa et al. 2016). The role for gut-associated bacteria in T1D development was further reinforced through antibiotic treatment that reduced disease symptoms in experimental models and promoted sterile pancreatic lymph nodes (Costa et al. 2016).
Despite T1D children harbouring greater levels of pro-inflammatory mediators, including interferons and interleukins, in their intestine it has been proposed that such immune profiles are a consequence of disease progression rather than the cause of T1D or its exacerbation (Savilahti et al. 1999; Westerholm-Ormio et al. 2003; Odenwald and Turner 2013). However, numerous studies have reported that increased intestinal permeability often precedes the onset of T1D-associated symptoms suggesting that disruption of gut barrier integrity may have an aetiological role in T1D or at least contribute to disease pathogenesis (Meddings et al. 1999; Damci et al. 2003; Secondulfo et al. 2004; Bosi et al. 2006). Mucosal colonising bacteria may also have a role in promoting T1D development. It has been reported that the elevated intestinal permeability detected among T1D patients is dependent on alterations in zonulin production, which is one of many cellular processes modulated by luminal bacteria (El Asmar et al. 2002). The role of zonulin in T1D was further reinforced by Watts and colleagues who demonstrated that T1D-associated symptoms could be alleviated in disease-prone rodent models following administration of a zonulin inhibitor (Watts et al. 2005). From this it can be suggested that the gut microbiota plays a significant role in the aetiology of T1D by influencing intestinal integrity and, therefore, luminal translocation into systemic circulation.
As mentioned previously, dysfunctional TJs present in coeliac patients most likely enables gliadin to traverse the intestinal epithelium and ultimately interact with the subepithelial immune system. The immunological exposure of gliadin may also increase susceptibility to other immune disorders including Hashimoto’s thyroiditis, autoimmune hepatitis as well as T1D (Ventura et al. 1999). Gliadin has been reported to trigger T1D development in non-obese diabetic mice and Bio Breeding Diabetes Prone rat models. The role of increased intestinal permeability in disease progression was reinforced when reduced levels of T1D development were recorded in rodent models fed gluten-free diets (Visser et al. 2003; Smyth 2017). The role of gliadin in the development of T1D was also confirmed by Norris and colleagues who demonstrated that exposure to gluten-containing grains during infancy increased susceptibility to T1D (Norris et al. 2003). This study involved 900 infants considered genetically susceptible to T1D through either the presence of a HLA gene variant or having a first-degree relative with T1D. The authors revealed that gluten exposure between 0 and 3 months and 7+ months of infancy increased the risk of T1D development, whereas exposure at 4–6 months was associated with a reduced risk. These findings suggest that a possible window exists, whereby exposure to gluten outside of this time period elevates the risk of islet autoimmunity and disease development in high-risk individuals (Norris et al. 2003). It is noteworthy that non-coeliac patients also display morphological abnormalities in their intestinal barrier including reduced abundance, delocalisation, and functionality of enterocyte microvilli as well as displacement of TJ components, each of which is likely to contribute to increased intestinal permeability and disease pathogenesis (Secondulfo et al. 2004). This indicates that while CD may increase the risk of T1D, other factors that attenuate the structural integrity of the gut barrier are involved and that this is an immune disorder with multiple aetiological factors.
Systemic Lupus Erythematosus
SLE is a chronic inflammatory AD arising following loss of immunological tolerance to self-antigens thereby promoting chronic inflammation and tissue damage in multiple organs (Tsokos 2011; Tsokos et al. 2016). Like other ADs, genetic predisposition is thought to play a role in the aetiology of SLE (Moser et al. 2009; Ghodke-Puranik and Niewold 2015) and is highlighted by the common occurrence of disease clustering within families. It has been estimated that first-degree relatives of SLE patients are thirteen times more likely to develop this disorder, while 5–10% of patients also have a second family member with SLE (Lawrence et al. 1987; Alarcón-Segovia et al. 2005; Navarra et al. 2011). Genetic predisposition to SLE includes the expression of TLR variants that promote hyperresponsiveness towards luminal microbes and their products. Activation of TLR4 has been found to increase the risk of SLE development, and bacterial components, including lipoteichoic acid (LTA), are also known to induce SLE (Liu et al. 2006, 2015; Mu et al. 2017). Furthermore, the role of luminal microbes in SLE development is reinforced by the fact that patients express the LTA receptor, TLR2, in greater abundance when compared to healthy controls, and the removal of commensal microbes via antibiotic treatment can ameliorate disease symptoms (Liu et al. 2006, 2015). In addition, TLR2 inhibition has been reported to reduce SLE-associated symptoms further indicating that hyperresponsiveness to gut bacteria is likely to contribute to disease pathogenesis (Pawar et al. 2009; Lartigue et al. 2009; Leiss et al. 2013).
Although genetic predisposition is likely to play a significant role in development, there is growing evidence suggesting that microbial translocation across the intestinal barrier may contribute to the pathogenesis of SLE. Spontaneous translocation of the gut commensal, E. gallinarum, across the intestinal epithelium of autoimmune-prone hosts has been reported to promote SLE, particularly due to the ability of this bacterium to migrate to immunological hotspots including the mesenteric lymph nodes, liver, and spleen (Manfredo Vieira et al. 2018). Direct impacts of E. gallinarum on the gut barrier include reducing the expression TJ protein components, claudin 3 and 5, that are involved in the modulation of paracellular permeability (Fine et al. 2020). This impact may provide an explanation for the presence of E. gallinarum DNA detected in biopsy samples obtained from both SLE and autoimmune hepatitis patients (Manfredo Vieira et al. 2018). Furthermore, the migration of E. gallinarum to extraintestinal sites is thought to induce disease progression by stimulating the systemic production of autoantibodies that cross-react with host double stranded DNA (dsDNA), β2-glycoprotein, endogenous retroviral proteins, and RNA, each of which are common diagnostic biomarkers in SLE (Manfredo Vieira et al. 2018; Fine et al. 2020). Interestingly, the levels of circulating antibodies generated towards E. gallinarum RNA correlated with the presence of human RNA autoantibodies, therefore, indicating the potential existence of shared sequences and epitopes (Fine et al. 2020).
The mesenteric lymph nodes, liver, and spleen were also identified as extraintestinal destinations following the systemic entry of Lactobacillus reuteri. The translocation of this gut commensal led to the exacerbation of SLE-associated symptoms in lupus-prone mice (Zegarra-Ruiz et al. 2019). In addition to this the presence of antibodies which cross-react with host dsDNA and antigens of the gut commensal Ruminococcus gnavus in SLE patients further implies that microbial translocation contributes to disease development and/or progression (Azzouz et al. 2019). Furthermore, increased concentrations of LPS in the plasma of SLE patients in comparison to healthy controls suggests that elevated intestinal permeability contributes to this condition (Shi et al. 2014; Mu et al. 2017; Issara-Amphorn et al. 2018).
The ubiquitin homologue, Bacteroides fragilis ubiquitin (BfUbb), secreted by some strains of the gut bacterium Bacteroides fragilis, is another example of a gut-derived component that may enter systemic circulation following a breach of the intestinal barrier and contribute to the pathogenesis of SLE (Patrick et al. 2011). This homologue is thought to be involved in the aetiology and/or progression of SLE, among other ADs, due to its recognition by anti-human ubiquitin antibodies detected in patients suffering from this condition in previous studies (Muller et al. 1988; Stewart et al. 2018). It has been proposed that exposure to this bacterial homologue may contribute to the breakdown in immune tolerance towards the human form of ubiquitin through the presence of shared epitopes (Stewart et al. 2018). The translocation of BfUbb across the gut barrier is likely to provide an avenue for its immunological exposure. The intestinal epithelial layer, therefore, plays an important role in limiting the translocation of bacterial molecular mimics of host components that could promote the development of ADs such as SLE (Fig. 3) (English et al. 2023).
The Gut-Joint Communication Axis in Rheumatic Disease Development
Rheumatoid Arthritis
RA is a physically debilitating autoimmune disorder characterised by excessive systemic inflammation leading to the progressive destruction of joints among other complications (McInnes and Schett 2011; Lerner and Matthias 2015b). Women and elderly individuals are particularly susceptible to develo** RA, with the reasons for this yet to be fully understood (Lerner and Matthias 2015b). Clinical manifestations including persistent synovitis and auto-antibody production are displayed by most RA patients and are likely to be a consequence of certain genetic abnormalities that increase the risk of develo** this disorder (Lerner and Matthias 2015b). The early diagnosis of RA is often challenging due to the widespread symptoms reported by patients, including pain, swelling, stiffness, and functional impairment, many of which are also associated with other inflammatory diseases (Gibofsky 2014).
Environmental factors are also likely to play a role in the aetiology of RA, with smoking a well-established risk factor elevating susceptibility to disease development. It is estimated that 0.5–2% of adults in industrialised countries suffer from RA, with up to fifty new cases per 100,000 people reported annually (Lerner and Matthias 2015b). Increasing evidence suggests that microbial infection may also contribute to RA development, with Porphyromonas gingivalis, Proteus mirabilis, and Mycobacteria among the bacterial species with potential aetiological roles (Li et al. 2013; Pérez-Barbosa et al. 2015). Of these, there is growing interest in oral P. gingivalis, due to its ability to citrullinate proteins and stimulate the production of anticitrullinated protein/peptide antibodies which are specific biomarkers for RA diagnosis (Perricone et al. 2019).
Gluten Sensitivity as an Aetiological Factor of Rheumatoid Arthritis
It has been proposed that similar microbes are potentially involved in the aetiology of both CD and RA, and include Hepatitis C, Epstein-Barr Virus and certain Mycobacteria (Draborg et al. 2013; Zignego et al. 2015; Machado Ribeiro and Goldenberg 2015). Numerous studies have reinforced these microbes as major contributors to AD development and progression, with epigenetics, molecular mimicry, vitamin D and miRNA suspected to play central roles in their capacity to promote these disorders (Singh et al. 2013; Vojdani 2014; Picascia et al. 2015; Bonaventura et al. 2015). Endocrine, dermatological, neurological, and rheumatological ADs are among the numerous extraintestinal pathologies associated with the development of CD (Lauret and Rodrigo 2013). Sjögren’s syndrome, often characterised by dry eyes and mouth, is the most common rheumatic disorder linked with CD with over half of the patients expressing the HLA-DQ2 haplotype considered a genetically predisposing factor for CD (Lerner and Matthias 2015b). Interestingly, many of the ADs associated with CD are also associated with RA including SLE, T1D, Sjögren’s syndrome and multiple sclerosis (Hemminki et al. 2009; Lerner and Matthias 2015b). From this, it can be proposed that suffering from one inflammatory disorder will likely result in a chain of downstream immunological events that predisposes to the development of other ADs over time.
ADs are characterised primarily by the production of autoantibodies following the breakdown of immune tolerance towards self-components (Eggert et al. 2010). Despite most autoantibodies associated with RA and CD being considered as non-specific, due to their ability to recognise multiple components, there have been reports of circulating antibodies which target TJs and neuronal structures as well as organs such as the pancreas, stomach, and liver in those diagnosed with the latter of these two conditions (Shaoul and Lerner 2007). RA patients on the other hand, display high levels of autoantibodies in their sera that target joint components including collagen and cartilage, stress proteins, and nuclear proteins (Steiner 2007). The presence of the IgG targeting auto-antibody, rheumatoid factor (RF), is also frequently reported in RA patient sera. Interestingly, coeliac patients also display heightened production of RF at mucosal sites in their GI tract, specifically the jejunum, implying that the immunogenic response generated subsequent to gluten exposure may contribute towards RF synthesis and downstream RA pathogenesis (Hällgren et al. 1996; Lerner and Matthias 2015b). The relationship between gluten sensitivity and RA development has been further reinforced by two studies that demonstrated a reduction of antibody levels towards dietary antigens, promotion of anti-inflammatory effects, and protection against atherosclerosis in RA patients who adhered to a gluten-free, vegan-based diet (Hafström et al. 2001; Elkan et al. 2008). Taking these findings into account, along with the elevated subclinical gut inflammation and intestinal permeability observed during earlier studies involving patients with rheumatic disease, it can be proposed that gut-joint communication plays an important role in AD development (Mielants et al. 1991; De Vos et al. 1996). It is important to note that intestinal dysbiosis has also been demonstrated to encourage RA pathogenesis further emphasising the ability of gut microbes to influence human health (Asquith et al. 2014).
The transition of an autoimmune environment from the intestinal mucosa to joints is suspected to be dependent on immunological pathways. In addition, the migration of activated T-cells and newly formed epitope complexes from the gut to the joints is also likely to be involved. The citrullination of epithelial neutrophil-activating peptide 78/CXCL5 chemokine by human peptidylarginine deiminase (PAD) present in the gut, has been reported to stimulate the production of monocyte recruiting chemokines and promote inflammation (Yoshida et al. 2014). Similarly, tTG is also a post-translational modification protein involved in generating novel immunogenic epitopes as a result of protein cross-linking. Both PAD and tTG are active in joints and the intestine, with the latter of these modification proteins being over expressed in the synovium of RA patients suggesting a possible role in disease pathogenesis (Lauzier et al. 2012; Lerner and Matthias 2015b). Importantly, post-translational modification by tTG and PAD is likely to occur more frequently in CD and RA patients given that the removal of apoptotic cellular debris is impaired (Cupi et al. 2014; Poon et al. 2014). The diminished removal of cellular components encourages post-translational modification and consequent generation of novel epitopes that stimulate inflammation. These inflammatory responses comprise immune mediators including TNF, IL-6 and IL-17 that are also likely to contribute to the systemic spread of these newly modified proteins in the joints and stimulate immune-mediated tissue damage (Fig. 4) (Lerner and Matthias 2015b). It can therefore be suggested that an attenuated gut barrier, as a result of genetic and environmental factors, is likely to aid the spread of novel proteins/peptides generated by intestinal PAD and tTG thereby promoting disease development, or at least exacerbation of symptoms (Lerner and Matthias 2015b).
Microbial and dietary factors can negatively impact the structural integrity of the gut to facilitate the systemic spread of novel epitopes produced in the intestinal lumen by host and microbial modification enzymes. Newly formed epitopes disseminate via systemic circulation and accumulate in joints to promote rheumatic diseases such as rheumatoid arthritis
Improving Intestinal Barrier Integrity to Limit Inflammatory Disease Development
Given the negative impact of environmental and genetic factors, it is important to be aware of the strategies that exist which aim to improve gut barrier integrity and overall functioning. The central role of TJ protein complexes in regulating intestinal permeability has inspired the identification of novel therapeutics that limit their disassembly. Larazotide acetate is an inhibitor of the protein modulator zonulin, which is associated with TJ disassembly (Fasano et al. 2000), and its use has been reported to reduce disease symptoms in coeliac patients (Leffler et al. 2015). Tofacitinib, an inhibitor of inflammatory enzymes associated with RA, has recently been reported to prevent cytokine-induced gut barrier dysfunction as well as limiting the delocalisation of TJ proteins, such as ZO-1, in IECs following exposure to IFN-γ (Sayoc-Becerra et al. 2020). These findings have led to tofacitinib being considered as a potential therapeutic agent to address the impaired gut barrier characteristic of patients suffering from IBD. This is primarily due to the capacity of this compound to inhibit inflammatory pathways activated by IFN-γ which is more abundant in the inflamed intestinal tissue of IBD patients as when compared to healthy controls (Kaser et al. 2010). Potassium-competitive acid blockers have also been recently highlighted to limit the development of IBDs, such as colitis, which is often associated with the long-term use of PPIs. Although PPIs are used worldwide to treat acid-related disorders, their excessive consumption can lead to disruption of microbial community composition in the gut by aiding the colonisation of pathogenic species and increasing the risk of IBD development. The use of the potassium-competitive acid blocker, tegoprazan, offers not only an alternative for the treatment of gastric acid-related diseases but may also be used to protect against IBD development. Its therapeutic potential in IBD is due to its ability to improve gut barrier health indirectly by increasing the abundance of anti-inflammatory bacteria, such as B. vulgatus, that also limits the epithelial adhesion of pathogenic bacteria (Son et al. 2022). Drugs with similar barrier promoting properties may, therefore, be a useful strategy to improve the integrity of the intestinal epithelium and limit the development of gut-associated and extraintestinal inflammatory diseases (Smyth 2017).
The growing body of research reinforcing the impact of antibiotic consumption on disrupting microbial communities in the gut has encouraged the discovery of novel compounds that can alleviate such alterations. The β-glucan, lentinan, is a bioactive compound produced by the macrofungus, Lentinus edodes, and has been found to have potential in improving the side effects of broad-spectrum antibiotic consumption. In mice lentinan reversed antibiotic mediated dysbiosis in the gut by increasing the abundance of beneficial species that were also likely to be responsible for the elevated concentrations of SCFAs reported (Ji et al. 2022). Antibiotic mediated disruption of gut microbe communities can also be restored through the consumption of probiotic species of the Lactobacillus genus (Gou et al. 2022). For example, following cyclophosphamide administration L. plantarum has been reported to increase the abundance of SCFA-producing species such as Bifidobacterium, and decrease the abundance of pathogenic species such as E. coli, that can often proliferate as a consequence of broad-spectrum antibiotics through the removal of commensal species (Meng et al. 2019). Dietary interventions may provide a useful strategy in limiting the colonisation of enteric pathogens following antibiotic administration and, therefore, protect the intestinal epithelium. The ingestion of fibre from the acacia plant before and after antibiotic exposure was found to reduce E. coli colonisation in animal models (Maeusli et al. 2022). This indicates that certain plant fibres could be administered along with antibiotics to limit the colonisation of enteric pathogens that disrupt gut barrier integrity.
The consumption of dietary materials with gut barrier promoting properties also represents a useful and cost-effective strategy to reduce the risk of inflammatory disease development. Foods high in fermentable dietary fibre, also known as microbiota-associated carbohydrates (MACs), provide an energy for anaerobic bacteria in the gut leading to the production of SCFAs (Bach Knudsen et al. 2018). These metabolites have a positive influence on gut barrier health by acting as a nutrient source for IECs as discussed previously. The importance of dietary fibre in intestinal barrier health has been previously demonstrated, with mice fed on low-fibre diets found to be colonised with a greater abundance of Proteobacteria while also displaying increased intestinal permeability and an under developed mucosal layer (Schroeder et al. 2018). Supplementation with Bifidobacterium or fibre ameliorated the damage caused to the mucosal layer (Schroeder et al. 2018). In addition, reduced concentrations of MACs in the diet has been associated with increasing the effect antibiotic administration has on disrupting microbial community composition in the gut (Ng et al. 2019). A diet low in MACs may also impair the ability of the intestinal microbiota to recover following antibiotic exposure and ultimately increase the likelihood of prolonged damage to the intestinal barrier (Ng et al. 2019; Tanes et al. 2021). The consumption of dietary fibre is, therefore, a useful strategy in improving the robustness of the intestinal microbiota after exposure to broad-spectrum antibiotics and reduce the risk of develo** inflammatory diseases.
Other dietary components including amino acids, fatty acids, minerals such as zinc and iron, and vitamins A and D also have the capacity to promote intestinal integrity (Suzuki 2020; Fan et al. 2022). The amino acids, glutamine, and arginine, have been reported to promote gut barrier health by protecting the intestinal mucosa and supporting immune function (Zhu et al. 2003; Funda et al. 2014).
ADs such as SLE are suspected to have a microbial aetiology that may amplify the risk of disease development in genetically susceptible individuals, particularly those with a first-degree relative previously diagnosed with SLE (Navarra et al. 2011). The spontaneous translocation of E. gallinarum to mesenteric lymph nodes, liver, and spleen in AD-prone rodent models induced the production of autoantibodies indicative of SLE, including those which recognise host dsDNA (Manfredo Vieira et al. 2018). While the mechanisms involved in the migration of commensal Enterococcus and their subsequent persistence in extraintestinal environments remain poorly understood, the ability of certain species, for example E. faecalis, to reside within macrophages suggests that these bacteria potentially exploit immune cells to migrate to distant organs (Gentry-Weeks et al. 1999). This indicates that alterations in intestinal epithelium integrity caused by genetic or environmental factors contributes to the translocation of luminal microbes that can become immunogenic when delocalised from their GI niche (Fine et al. 2020). Future research should continue to explore the impact of commensal translocation on AD development/progression to facilitate the development of therapeutics that perhaps target the microbiota to indirectly inhibit chronic inflammation in the gut and beyond.
The intestinal epithelium prevents the translocation of luminal components and maintains a homeostatic environment in the gut making this barrier extremely important in human health. Breakdown in intestinal barrier integrity or immune signalling can result in the translocation of luminal components across the intestinal epithelium which then interact with the underlying immune cells of the lamina propria and induce subsequent inflammation and tissue damage. This translocation provides luminal microbes, and their associated products, with an opportunity to migrate to distant organs and cause IRs that encourage the development of AD or exacerbate disease pathologies. It is, therefore, important that future research continues to elucidate the mechanisms that influence gut barrier integrity (Smyth 2017). This will benefit our understanding of the aetiologies involved in ADs and permit the development of therapeutic strategies that aim to improve intestinal barrier integrity to help tackle the burden caused by inflammatory diseases.
Data Availability
No data was used for the research described in this article.
Abbreviations
- AD:
-
Autoimmune disease
- AMP:
-
Antimicrobial peptide
- ATP:
-
Adenosine triphosphate
- Å:
-
Angstroms
- BfUbb:
-
Bacteroides fragilis Ubiquitin
- CD:
-
Coeliac disease
- dsDNA:
-
Double stranded DNA
- EAST1:
-
Enteroaggregative heat resistant toxin 1
- EPEC:
-
Enteropathogenic E. coli
- GI:
-
Gastrointestinal
- HLA:
-
Human leukocyte antigen
- HT:
-
Hydroxytryptamine
- IBD:
-
Inflammatory bowel disease
- IEC:
-
Intestinal epithelial cell
- IFN:
-
Interferon
- IG:
-
Immunoglobulin
- IL:
-
Interleukin
- IR:
-
Immune response
- LAP:
-
Listeria adhesion protein
- LPS:
-
Lipopolysaccharide
- LTA:
-
Lipoteichoic acid
- MAC:
-
Microbiota-associated carbohydrates
- mTG:
-
Microbial transglutaminase
- Muc2:
-
Mucin-2
- NF-κB:
-
Nuclear factor-kappa B
- NSAIDs:
-
Non-steroidal anti-inflammatory drugs
- PAD:
-
Peptidylarginine deiminase
- PAMP:
-
Pathogen-associated molecular pattern
- PCBs:
-
Polychlorinated biphenyls
- POPs:
-
Persistent organic pollutant
- PPI:
-
Proton pump inhibitor
- RA:
-
Rheumatoid arthritis
- RF:
-
Rheumatoid factor
- SCFAs:
-
Short-chain fatty acids
- SI:
-
Small intestine
- SLE:
-
Systemic lupus erythematosus
- Th17:
-
T helper 17
- TJ:
-
Tight junction
- TLR:
-
Toll-like receptor
- TNF:
-
Tumour necrosis factor
- tTG:
-
Tissue transglutaminase
- T1D:
-
Type-1 diabetes
- ZO:
-
Zonula occludens
References
Abreu MT (2010) Toll-like receptor signalling in the intestinal epithelium: how bacterial recognition shapes intestinal function. Nat Rev Immunol 10:131–144. https://doi.org/10.1038/nri2707
Aglietti RA, Estevez A, Gupta A et al (2016) GsdmD p30 elicited by caspase-11 during pyroptosis forms pores in membranes. Proc Natl Acad Sci U S A 113:7858–7863. https://doi.org/10.1073/pnas.1607769113
Aguiar SLF, Miranda MCG, Guimarães MAF et al (2018) High-salt diet induces IL-17-dependent gut inflammation and exacerbates colitis in mice. Front Immunol 8:1969. https://doi.org/10.3389/fimmu.2017.01969
Alarcón-Segovia D, Alarcón-Riquelme ME, Cardiel MH et al (2005) Familial aggregation of systemic lupus erythematosus, rheumatoid arthritis, and other autoimmune diseases in 1,177 lupus patients from the GLADEL cohort. Arthritis Rheum 52:1138–1147. https://doi.org/10.1002/art.20999
Allaire JM, Crowley SM, Law HT et al (2018) The intestinal epithelium: central coordinator of mucosal immunity. Trends Immunol 39:677–696. https://doi.org/10.1016/j.it.2018.04.002
Allert S, Förster TM, Svensson C-M et al (2018) Candida albicans-induced epithelial damage mediates translocation through intestinal barriers. mBiol 9:e00915-18. https://doi.org/10.1128/mBio.00915-18
Artis D, Wang ML, Keilbaugh SA et al (2004) RELMbeta/FIZZ2 is a goblet cell-specific immune-effector molecule in the gastrointestinal tract. Proc Natl Acad Sci U S A 101:13596–13600. https://doi.org/10.1073/pnas.0404034101
Asquith M, Elewaut D, Lin P, Rosenbaum JT (2014) The role of the gut and microbes in the pathogenesis of spondyloarthritis. Best Pract Res Clin Rheumatol 28:687–702. https://doi.org/10.1016/j.berh.2014.10.018
Atarashi K, Tanoue T, Shima T et al (2011) Induction of colonic regulatory T cells by indigenous Clostridium species. Science 331:337–341. https://doi.org/10.1126/science.1198469
Atkinson MA, Eisenbarth GS, Michels AW (2014) Type 1 diabetes. Lancet 383:69–82. https://doi.org/10.1016/S0140-6736(13)60591-7
Azzouz D, Omarbekova A, Heguy A et al (2019) Lupus nephritis is linked to disease-activity associated expansions and immunity to a gut commensal. Ann Rheum Dis 78:947–956. https://doi.org/10.1136/annrheumdis-2018-214856
Bach Knudsen KE, Lærke HN, Hedemann MS et al (2018) Impact of diet-modulated butyrate production on intestinal barrier function and inflammation. Nutrients 10:1499. https://doi.org/10.3390/nu10101499
Bäckhed F, Roswall J, Peng Y et al (2015) Dynamics and stabilization of the human gut microbiome during the first year of life. Cell Host Microbe 17:690–703. https://doi.org/10.1016/j.chom.2015.04.004
Bagnoli F, Buti L, Tompkins L et al (2005) Helicobacter pylori CagA induces a transition from polarized to invasive phenotypes in MDCK cells. Proc Natl Acad Sci U S A 102:16339–16344. https://doi.org/10.1073/pnas.0502598102
Basmaciyan L, Bon F, Paradis T et al (2019) Candida albicans interactions with the host: crossing the intestinal epithelial barrier. Tissue Barriers 7:1612661. https://doi.org/10.1080/21688370.2019.1612661
Baxter M, Colville A (2016) Adverse events in faecal microbiota transplant: a review of the literature. J Hosp Infect 92:117–127. https://doi.org/10.1016/j.jhin.2015.10.024
Bel S, Pendse M, Wang Y et al (2017) Paneth cells secrete lysozyme via secretory autophagy during bacterial infection of the intestine. Science 357:1047–1052. https://doi.org/10.1126/science.aal4677
Bergstrom KSB, Kissoon-Singh V, Gibson DL et al (2010) Muc2 protects against lethal infectious colitis by disassociating pathogenic and commensal bacteria from the colonic mucosa. PLoS Pathog 6:e1000902. https://doi.org/10.1371/journal.ppat.1000902
Birchenough GMH, Nyström EEL, Johansson MEV, Hansson GC (2016) A sentinel goblet cell guards the colonic crypt by triggering Nlrp6-dependent Muc2 secretion. Science 352:1535–1542. https://doi.org/10.1126/science.aaf7419
Bischoff SC, Barbara G, Buurman W et al (2014) Intestinal permeability—a new target for disease prevention and therapy. BMC Gastroenterol 14:189. https://doi.org/10.1186/s12876-014-0189-7
Bonaventura P, Benedetti G, Albarède F, Miossec P (2015) Zinc and its role in immunity and inflammation. Autoimmun Rev 14:277–285. https://doi.org/10.1016/j.autrev.2014.11.008
Borthakur A, Gill RK, Hodges K et al (2006) Enteropathogenic Escherichia coli inhibits butyrate uptake in Caco-2 cells by altering the apical membrane MCT1 level. Am J Physiol Gastrointest Liver Physiol 290:G30-35. https://doi.org/10.1152/ajpgi.00302.2005
Bosi E, Molteni L, Radaelli MG et al (2006) Increased intestinal permeability precedes clinical onset of type 1 diabetes. Diabetologia 49:2824–2827. https://doi.org/10.1007/s00125-006-0465-3
Brenchley JM, Douek DC (2012) Microbial translocation across the GI tract. Annu Rev Immunol 30:149–173. https://doi.org/10.1146/annurev-immunol-020711-075001
Bunker JJ, Bendelac A (2018) IgA responses to microbiota. Immunity 49:211–224. https://doi.org/10.1016/j.immuni.2018.08.011
Byndloss MX, Olsan EE, Rivera-Chávez F et al (2017) Microbiota-activated PPAR-γ signaling inhibits dysbiotic Enterobacteriaceae expansion. Science 357:570–575. https://doi.org/10.1126/science.aam9949
Cani PD, Amar J, Iglesias MA et al (2007) Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes 56:1761–1772. https://doi.org/10.2337/db06-1491
Cani PD, Bibiloni R, Knauf C et al (2008) Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes 57:1470–1481. https://doi.org/10.2337/db07-1403
Cario E, Gerken G, Podolsky DK (2007) Toll-like receptor 2 controls mucosal inflammation by regulating epithelial barrier function. Gastroenterology 132:1359–1374. https://doi.org/10.1053/j.gastro.2007.02.056
Cash HL, Whitham CV, Behrendt CL, Hooper LV (2006) Symbiotic bacteria direct expression of an intestinal bactericidal lectin. Science 313:1126–1130. https://doi.org/10.1126/science.1127119
Chakrabarti G, Zhou X, McClane BA (2003) Death pathways activated in CaCo-2 cells by Clostridium perfringens enterotoxin. Infect Immun 71:4260–4270. https://doi.org/10.1128/IAI.71.8.4260-4270.2003
Chassaing B, Koren O, Goodrich JK et al (2015) Dietary emulsifiers impact the mouse gut microbiota promoting colitis and metabolic syndrome. Nature 519:92–96. https://doi.org/10.1038/nature14232
Chi Y, Lin Y, Lu Y et al (2019) Gut microbiota dysbiosis correlates with a low-dose PCB126-induced dyslipidemia and non-alcoholic fatty liver disease. Sci Total Environ 653:274–282. https://doi.org/10.1016/j.scitotenv.2018.10.387
Chiba H, Kojima T, Osanai M, Sawada N (2006) The significance of interferon-gamma-triggered internalization of tight-junction proteins in inflammatory bowel disease. Sci STKE 316:pe1. https://doi.org/10.1126/stke.3162006pe1
Chieppa M, Rescigno M, Huang AYC, Germain RN (2006) Dynamic imaging of dendritic cell extension into the small bowel lumen in response to epithelial cell TLR engagement. J Exp Med 203:2841–2852. https://doi.org/10.1084/jem.20061884
Chiu K, Warner G, Nowak RA et al (2020) The impact of environmental chemicals on the gut microbiome. Toxicol Sci 176:253–284. https://doi.org/10.1093/toxsci/kfaa065
Choi YJ, Seelbach MJ, Pu H et al (2010) Polychlorinated biphenyls disrupt intestinal integrity via NADPH oxidase-induced alterations of tight junction protein expression. Environ Health Perspect 118:976–981. https://doi.org/10.1289/ehp.0901751
Clemente MG, De Virgiliis S, Kang JS et al (2003) Early effects of gliadin on enterocyte intracellular signalling involved in intestinal barrier function. Gut 52:218–223. https://doi.org/10.1136/gut.52.2.218
Cohen L, Sekler I, Hershfinkel M (2014) The zinc sensing receptor, ZnR/GPR39, controls proliferation and differentiation of colonocytes and thereby tight junction formation in the colon. Cell Death Dis 5:e1307. https://doi.org/10.1038/cddis.2014.262
Connors J, Dawe N, Van Limbergen J (2019) The role of succinate in the regulation of intestinal inflammation. Nutrients 11:25. https://doi.org/10.3390/nu11010025
Costa FRC, Françozo MCS, de Oliveira GG et al (2016) Gut microbiota translocation to the pancreatic lymph nodes triggers NOD2 activation and contributes to T1D onset. J Exp Med 213:1223–1239. https://doi.org/10.1084/jem.20150744
Costantini TW, Krzyzaniak M, Cheadle GA et al (2012) Targeting α-7 nicotinic acetylcholine receptor in the enteric nervous system: a cholinergic agonist prevents gut barrier failure after severe burn injury. Am J Pathol 181:478–486. https://doi.org/10.1016/j.ajpath.2012.04.005
Coulombe G, Langlois A, De Palma G et al (2016) SHP-2 phosphatase prevents colonic inflammation by controlling secretory cell differentiation and maintaining host-microbiota homeostasis. J Cell Physiol 231:2529–2540. https://doi.org/10.1002/jcp.25407
Crosnier C, Stamataki D, Lewis J (2006) Organizing cell renewal in the intestine: stem cells, signals and combinatorial control. Nat Rev Genet 7:349–359. https://doi.org/10.1038/nrg1840
Cunningham KE, Turner JR (2012) Myosin light chain kinase: pulling the strings of epithelial tight junction function. Ann N Y Acad Sci 1258:34–42. https://doi.org/10.1111/j.1749-6632.2012.06526.x
Cupi ML, Sarra M, De Nitto D et al (2014) Defective expression of scavenger receptors in celiac disease mucosa. PLoS ONE 9:e100980. https://doi.org/10.1371/journal.pone.0100980
Damci T, Nuhoglu I, Devranoglu G et al (2003) Increased intestinal permeability as a cause of fluctuating postprandial blood glucose levels in Type 1 diabetic patients. Eur J Clin Invest 33:397–401. https://doi.org/10.1046/j.1365-2362.2003.01161.x
De Vos M, Mielants H, Cuvelier C et al (1996) Long-term evolution of gut inflammation in patients with spondyloarthropathy. Gastroenterology 110:1696–1703. https://doi.org/10.1053/gast.1996.v110.pm8964393
Desai MS, Seekatz AM, Koropatkin NM et al (2016) A dietary fiber-deprived gut microbiota degrades the colonic mucus barrier and enhances pathogen susceptibility. Cell 167:1339-1353.e21. https://doi.org/10.1016/j.cell.2016.10.043
Deuring JJ, Fuhler GM, Konstantinov SR et al (2014) Genomic ATG16L1 risk allele-restricted Paneth cell ER stress in quiescent Crohn’s disease. Gut 63:1081–1091. https://doi.org/10.1136/gutjnl-2012-303527
Dietrich J, Grass I, Günzel D et al (2019) The marine biotoxin okadaic acid affects intestinal tight junction proteins in human intestinal cells. Toxicol In Vitro 58:150–160. https://doi.org/10.1016/j.tiv.2019.03.033
Ding J, Wang K, Liu W et al (2016) Pore-forming activity and structural autoinhibition of the gasdermin family. Nature 535:111–116. https://doi.org/10.1038/nature18590
DiPeso L, Ji DX, Vance RE, Price JV (2017) Cell death and cell lysis are separable events during pyroptosis. Cell Death Discov 3:17070. https://doi.org/10.1038/cddiscovery.2017.70
Do MH, Lee E, Oh M-J et al (2018) High-glucose or -fructose diet cause changes of the gut microbiota and metabolic disorders in mice without body weight change. Nutrients 10:761. https://doi.org/10.3390/nu10060761
Doran KS, Banerjee A, Disson O, Lecuit M (2013) Concepts and mechanisms: crossing host barriers. Cold Spring Harb Perspect Med 3:a010090. https://doi.org/10.1101/cshperspect.a010090
Draborg AH, Duus K, Houen G (2013) Epstein–Barr virus in systemic autoimmune diseases. Clin Dev Immunol 2013:535738. https://doi.org/10.1155/2013/535738
Drolia R, Tenguria S, Durkes AC et al (2018) Listeria adhesion protein induces intestinal epithelial barrier dysfunction for bacterial translocation. Cell Host Microbe 23:470-484.e7. https://doi.org/10.1016/j.chom.2018.03.004
Dubreuil JD (2008) Escherichia coli STb toxin and colibacillosis: knowing is half the battle. FEMS Microbiol Lett 278:137–145. https://doi.org/10.1111/j.1574-6968.2007.00967.x
Duerr RH, Taylor KD, Brant SR et al (2006) A genome-wide association study identifies IL23R as an inflammatory bowel disease gene. Science 314:1461–1463. https://doi.org/10.1126/science.1135245
Dupont A, Heinbockel L, Brandenburg K, Hornef MW (2014) Antimicrobial peptides and the enteric mucus layer act in concert to protect the intestinal mucosa. Gut Microbes 5:761–765. https://doi.org/10.4161/19490976.2014.972238
Eggert M, Zettl UK, Neeck G (2010) Autoantibodies in autoimmune diseases. Curr Pharm Des 16:1634–1643. https://doi.org/10.2174/138161210791164144
El Asmar R, Panigrahi P, Bamford P et al (2002) Host-dependent zonulin secretion causes the impairment of the small intestine barrier function after bacterial exposure. Gastroenterology 123:1607–1615. https://doi.org/10.1053/gast.2002.36578
Eliakim R, Mahmood A, Alpers DH (1991) Rat intestinal alkaline phosphatase secretion into lumen and serum is coordinately regulated. Biochim Biophys Acta 1091:1–8. https://doi.org/10.1016/0167-4889(91)90213-h
Elkan A-C, Sjöberg B, Kolsrud B et al (2008) Gluten-free vegan diet induces decreased LDL and oxidized LDL levels and raised atheroprotective natural antibodies against phosphorylcholine in patients with rheumatoid arthritis: a randomized study. Arthritis Res Ther 10:R34. https://doi.org/10.1186/ar2388
English J, Patrick S, Stewart LD (2023) The potential role of molecular mimicry by the anaerobic microbiota in the aetiology of autoimmune disease. Anaerobe. https://doi.org/10.1016/j.anaerobe.2023.102721
Ermund A, Schütte A, Johansson MEV et al (2013) Studies of mucus in mouse stomach, small intestine, and colon. I. Gastrointestinal mucus layers have different properties depending on location as well as over the Peyer’s patches. Am J Physiol Gastrointest Liver Physiol 305:G341-347. https://doi.org/10.1152/ajpgi.00046.2013
Everard A, Belzer C, Geurts L et al (2013) Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc Natl Acad Sci U S A 110:9066–9071. https://doi.org/10.1073/pnas.1219451110
Everard A, Geurts L, Caesar R et al (2014) Intestinal epithelial MyD88 is a sensor switching host metabolism towards obesity according to nutritional status. Nat Commun 5:5648. https://doi.org/10.1038/ncomms6648
Fan Y-Y, Davidson LA, Callaway ES et al (2015) A bioassay to measure energy metabolism in mouse colonic crypts, organoids, and sorted stem cells. Am J Physiol Gastrointest Liver Physiol 309:G1-9. https://doi.org/10.1152/ajpgi.00052.2015
Fan J, Yang Y, Ma C et al (2022) The effects and cell barrier mechanism of main dietary nutrients on intestinal barrier. Curr Opin Food Sci 48:100942. https://doi.org/10.1016/j.cofs.2022.100942
Farache J, Koren I, Milo I et al (2013) Luminal bacteria recruit CD103+ dendritic cells into the intestinal epithelium to sample bacterial antigens for presentation. Immunity 38:581–595. https://doi.org/10.1016/j.immuni.2013.01.009
Farin HF, Karthaus WR, Kujala P et al (2014) Paneth cell extrusion and release of antimicrobial products is directly controlled by immune cell-derived IFN-γ. J Exp Med 211:1393–1405. https://doi.org/10.1084/jem.20130753
Fasano A (2012) Zonulin, regulation of tight junctions, and autoimmune diseases. Ann N Y Acad Sci 1258:25–33. https://doi.org/10.1111/j.1749-6632.2012.06538.x
Fasano A, Shea-Donohue T (2005) Mechanisms of disease: the role of intestinal barrier function in the pathogenesis of gastrointestinal autoimmune diseases. Nat Clin Pract Gastroenterol Hepatol 2:416–422. https://doi.org/10.1038/ncpgasthep0259
Fasano A, Not T, Wang W et al (2000) Zonulin, a newly discovered modulator of intestinal permeability, and its expression in coeliac disease. Lancet 355:1518–1519. https://doi.org/10.1016/S0140-6736(00)02169-3
Fedwick JP, Lapointe TK, Meddings JB et al (2005) Helicobacter pylori activates myosin light-chain kinase to disrupt claudin-4 and claudin-5 and increase epithelial permeability. Infect Immun 73:7844–7852. https://doi.org/10.1128/IAI.73.12.7844-7852.2005
Feng Y, Huang Y, Wang Y et al (2019) Antibiotics induced intestinal tight junction barrier dysfunction is associated with microbiota dysbiosis, activated NLRP3 inflammasome and autophagy. PLoS ONE 14:e0218384. https://doi.org/10.1371/journal.pone.0218384
Ferretti G, Bacchetti T, Masciangelo S, Saturni L (2012) Celiac disease, inflammation and oxidative damage: a nutrigenetic approach. Nutrients 4:243–257. https://doi.org/10.3390/nu4040243
Fine RL, Manfredo Vieira S, Gilmore MS, Kriegel MA (2020) Mechanisms and consequences of gut commensal translocation in chronic diseases. Gut Microbes 11:217–230. https://doi.org/10.1080/19490976.2019.1629236
Fortun PJ, Hawkey CJ (2005) Nonsteroidal antiinflammatory drugs and the small intestine. Curr Opin Gastroenterol 21:169–175. https://doi.org/10.1097/01.mog.0000153314.51198.58
France MM, Turner JR (2017) The mucosal barrier at a glance. J Cell Sci 130:307–314. https://doi.org/10.1242/jcs.193482
Fukui H, Brauner B, Bode JC, Bode C (1991) Plasma endotoxin concentrations in patients with alcoholic and non-alcoholic liver disease: reevaluation with an improved chromogenic assay. J Hepatol 12:162–169. https://doi.org/10.1016/0168-8278(91)90933-3
Funda DP, Fundova P, Hansen AK, Buschard K (2014) Prevention or early cure of type 1 diabetes by intranasal administration of gliadin in NOD mice. PLOS ONE 9:e94530. https://doi.org/10.1371/journal.pone.0094530
Furusawa Y, Obata Y, Fukuda S et al (2013) Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature 504:446–450. https://doi.org/10.1038/nature12721
Gautreaux MD, Deitch EA, Berg RD (1994) Bacterial translocation from the gastrointestinal tract to various segments of the mesenteric lymph node complex. Infect Immun 62:2132–2134. https://doi.org/10.1128/iai.62.5.2132-2134.1994
Gentry-Weeks CR, Karkhoff-Schweizer R, Pikis A et al (1999) Survival of Enterococcus faecalis in mouse peritoneal macrophages. Infect Immun 67:2160–2165. https://doi.org/10.1128/IAI.67.5.2160-2165.1999
Ghodke-Puranik Y, Niewold TB (2015) Immunogenetics of systemic lupus erythematosus: a comprehensive review. J Autoimmun 64:125–136. https://doi.org/10.1016/j.jaut.2015.08.004
Gibofsky A (2014) Epidemiology, pathophysiology, and diagnosis of rheumatoid arthritis: a synopsis. Am J Manag Care 20:S128-135
Gill N, Ferreira RBR, Antunes LCM et al (2012) Neutrophil elastase alters the murine gut microbiota resulting in enhanced Salmonella colonization. PLoS ONE 7:e49646. https://doi.org/10.1371/journal.pone.0049646
Gong J, Xu J, Zhu W et al (2010) Epithelial-specific blockade of MyD88-dependent pathway causes spontaneous small intestinal inflammation. Clin Immunol 136:245–256. https://doi.org/10.1016/j.clim.2010.04.001
Gou H-Z, Zhang Y-L, Ren L-F et al (2022) How do intestinal probiotics restore the intestinal barrier? Front Microbiol 13:929346. https://doi.org/10.3389/fmicb.2022.929346
Gribble FM, Reimann F (2016) Enteroendocrine cells: chemosensors in the intestinal epithelium. Annu Rev Physiol 78:277–299. https://doi.org/10.1146/annurev-physiol-021115-105439
Guinan J, Wang S, Hazbun TR et al (2019) Antibiotic-induced decreases in the levels of microbial-derived short-chain fatty acids correlate with increased gastrointestinal colonization of Candida albicans. Sci Rep 9:8872. https://doi.org/10.1038/s41598-019-45467-7
Guo P, Zhang K, Ma X, He P (2020) Clostridium species as probiotics: potentials and challenges. J Anim Sci Biotechnol 11:24. https://doi.org/10.1186/s40104-019-0402-1
Gupta N, Martin PM, Prasad PD, Ganapathy V (2006) SLC5A8 (SMCT1)-mediated transport of butyrate forms the basis for the tumor suppressive function of the transporter. Life Sci 78:2419–2425. https://doi.org/10.1016/j.lfs.2005.10.028
Gutiérrez A, Francés R, Amorós A et al (2009) Cytokine association with bacterial DNA in serum of patients with inflammatory bowel disease. Inflamm Bowel Dis 15:508–514. https://doi.org/10.1002/ibd.20806
Guttman JA, Finlay BB (2009) Tight junctions as targets of infectious agents. Biochim Biophys Acta 1788:832–841. https://doi.org/10.1016/j.bbamem.2008.10.028
Hafström I, Ringertz B, Spångberg A et al (2001) A vegan diet free of gluten improves the signs and symptoms of rheumatoid arthritis: the effects on arthritis correlate with a reduction in antibodies to food antigens. Rheumatology (oxford) 40:1175–1179. https://doi.org/10.1093/rheumatology/40.10.1175
Hällgren J, Knutson F, Lavö B, Hällgren R (1996) Increased mucosal synthesis of rheumatoid factor (RF) in coeliac disease. Clin Exp Immunol 103:94–98. https://doi.org/10.1046/j.1365-2249.1996.903599.x
Hamid KA, Katsumi H, Sakane T, Yamamoto A (2009) The effects of common solubilizing agents on the intestinal membrane barrier functions and membrane toxicity in rats. Int J Pharm 379:100–108. https://doi.org/10.1016/j.ijpharm.2009.06.018
Hemminki K, Li X, Sundquist J, Sundquist K (2009) Familial associations of rheumatoid arthritis with autoimmune diseases and related conditions. Arthritis Rheum 60:661–668. https://doi.org/10.1002/art.24328
Hendel SK, Kellermann L, Hausmann A et al (2022) Tuft cells and their role in intestinal diseases. Front Immunol. https://doi.org/10.3389/fimmu.2022.822867
Heyman M, Abed J, Lebreton C, Cerf-Bensussan N (2012) Intestinal permeability in coeliac disease: insight into mechanisms and relevance to pathogenesis. Gut 61:1355–1364. https://doi.org/10.1136/gutjnl-2011-300327
Hooper LV, Stappenbeck TS, Hong CV, Gordon JI (2003) Angiogenins: a new class of microbicidal proteins involved in innate immunity. Nat Immunol 4:269–273. https://doi.org/10.1038/ni888
Hopkins AM, Walsh SV, Verkade P et al (2003) Constitutive activation of Rho proteins by CNF-1 influences tight junction structure and epithelial barrier function. J Cell Sci 116:725–742. https://doi.org/10.1242/jcs.00300
Howe SE, Lickteig DJ, Plunkett KN et al (2014) The uptake of soluble and particulate antigens by epithelial cells in the mouse small intestine. PLoS ONE 9:e86656. https://doi.org/10.1371/journal.pone.0086656
Hu X, Ren J, Zhao M et al (2011) Emulsifying properties of the transglutaminase-treated crosslinked product between peanut protein and fish (Decapterus maruadsi) protein hydrolysates. J Sci Food Agric 91:578–585. https://doi.org/10.1002/jsfa.4229
Issara-Amphorn J, Surawut S, Worasilchai N et al (2018) The synergy of endotoxin and (1→3)-β-d-glucan, from gut translocation, worsens sepsis severity in a lupus model of Fc gamma receptor IIb-deficient mice. J Innate Immun 10:189–201. https://doi.org/10.1159/000486321
Itoh K, Freter R (1989) Control of Escherichia coli populations by a combination of indigenous Clostridia and Lactobacilli in gnotobiotic mice and continuous-flow cultures. Infect Immun 57:559–565. https://doi.org/10.1128/iai.57.2.559-565.1989
Ji X, Su L, Zhang P et al (2022) Lentinan improves intestinal inflammation and gut dysbiosis in antibiotics-induced mice. Sci Rep 12:19609. https://doi.org/10.1038/s41598-022-23469-2
Johansen FE, Pekna M, Norderhaug IN et al (1999) Absence of epithelial immunoglobulin A transport, with increased mucosal leakiness, in polymeric immunoglobulin receptor/secretory component-deficient mice. J Exp Med 190:915–922. https://doi.org/10.1084/jem.190.7.915
Johansson MEV, Hansson GC (2016) Immunological aspects of intestinal mucus and mucins. Nat Rev Immunol 16:639–649. https://doi.org/10.1038/nri.2016.88
Johansson MEV, Phillipson M, Petersson J et al (2008) The inner of the two Muc2 mucin-dependent mucus layers in colon is devoid of bacteria. Proc Natl Acad Sci U S A 105:15064–15069. https://doi.org/10.1073/pnas.0803124105
Johansson MEV, Gustafsson JK, Sjöberg KE et al (2010) Bacteria penetrate the inner mucus layer before inflammation in the dextran sulfate colitis model. PLoS ONE 5:e12238. https://doi.org/10.1371/journal.pone.0012238
Johansson MEV, Jakobsson HE, Holmén-Larsson J et al (2015) Normalization of host intestinal mucus layers requires long-term microbial colonization. Cell Host Microbe 18:582–592. https://doi.org/10.1016/j.chom.2015.10.007
Kaliannan K, Wang B, Li X-Y et al (2015) A host-microbiome interaction mediates the opposing effects of omega-6 and omega-3 fatty acids on metabolic endotoxemia. Sci Rep 5:11276. https://doi.org/10.1038/srep11276
Kaser A, Zeissig S, Blumberg RS (2010) Inflammatory bowel disease. Annu Rev Immunol 28:573–621. https://doi.org/10.1146/annurev-immunol-030409-101225
Kashyap PC, Marcobal A, Ursell LK et al (2013) Complex interactions among diet, gastrointestinal transit, and gut microbiota in humanized mice. Gastroenterology 144:967–977. https://doi.org/10.1053/j.gastro.2013.01.047
Kau AL, Ahern PP, Griffin NW et al (2011) Human nutrition, the gut microbiome and the immune system. Nature 474:327–336. https://doi.org/10.1038/nature10213
Kaufmann A, Köppel R, Widmer M (2012) Determination of microbial transglutaminase in meat and meat products. Food Addit Contam Part A Chem Anal Control Expo Risk Assess 29:1364–1373. https://doi.org/10.1080/19440049.2012.691557
Keszthelyi D, Jansen SV, Schouten GA et al (2010) Proton pump inhibitor use is associated with an increased risk for microscopic colitis: a case-control study. Aliment Pharmacol Ther 32:1124–1128. https://doi.org/10.1111/j.1365-2036.2010.04453.x
Khaleghi S, Ju JM, Lamba A, Murray JA (2016) The potential utility of tight junction regulation in celiac disease: focus on larazotide acetate. Therap Adv Gastroenterol 9:37–49. https://doi.org/10.1177/1756283X15616576
Kieliszek M, Misiewicz A (2014) Microbial transglutaminase and its application in the food industry. A Review. Folia Microbiol (Praha) 59:241–250. https://doi.org/10.1007/s12223-013-0287-x
Kim YS, Ho SB (2010) Intestinal goblet cells and mucins in health and disease: recent insights and progress. Curr Gastroenterol Rep 12:319–330. https://doi.org/10.1007/s11894-010-0131-2
Kitajima S, Morimoto M, Sagara E et al (2001) Dextran sodium sulfate-induced colitis in germ-free IQI/Jic mice. Exp Anim 50:387–395. https://doi.org/10.1538/expanim.50.387
Kleinewietfeld M, Manzel A, Titze J et al (2013) Sodium chloride drives autoimmune disease by the induction of pathogenic TH17 cells. Nature 496:518–522. https://doi.org/10.1038/nature11868
Kobayashi T, Okamoto S, Hisamatsu T et al (2008) IL23 differentially regulates the Th1/Th17 balance in ulcerative colitis and Crohn’s disease. Gut 57:1682–1689. https://doi.org/10.1136/gut.2007.135053
Köhler H, Sakaguchi T, Hurley BP et al (2007) Salmonella enterica serovar Typhimurium regulates intercellular junction proteins and facilitates transepithelial neutrophil and bacterial passage. Am J Physiol Gastrointest Liver Physiol 293:G178–187. https://doi.org/10.1152/ajpgi.00535.2006
Kommineni S, Bretl DJ, Lam V et al (2015) Bacteriocin production augments niche competition by enterococci in the mammalian gastrointestinal tract. Nature 526:719–722. https://doi.org/10.1038/nature15524
Kong J, Zhang Z, Musch MW et al (2008) Novel role of the vitamin D receptor in maintaining the integrity of the intestinal mucosal barrier. Am J Physiol Gastrointest Liver Physiol 294:G208-216. https://doi.org/10.1152/ajpgi.00398.2007
König J, Wells J, Cani PD et al (2016) Human intestinal barrier function in health and disease. Clin Transl Gastroenterol 7:e196. https://doi.org/10.1038/ctg.2016.54
Konturek PC, Brzozowski T, Konturek SJ (2011) Stress and the gut: pathophysiology, clinical consequences, diagnostic approach and treatment options. J Physiol Pharmacol 62:591–599
Kumar V, Fischer M (2020) Expert opinion on fecal microbiota transplantation for the treatment of Clostridioides difficile infection and beyond. Expert Opin Biol Ther 20:73–81. https://doi.org/10.1080/14712598.2020.1689952
Lam YY, Ha CWY, Campbell CR et al (2012) Increased gut permeability and microbiota change associate with mesenteric fat inflammation and metabolic dysfunction in diet-induced obese mice. PLoS ONE 7:e34233. https://doi.org/10.1371/journal.pone.0034233
Lamkanfi M, Dixit VM (2017) In Retrospect: the inflammasome turns 15. Nature 548:534–535. https://doi.org/10.1038/548534a
Lammers KM, Lu R, Brownley J et al (2008) Gliadin induces an increase in intestinal permeability and zonulin release by binding to the chemokine receptor CXCR3. Gastroenterology 135:194-204.e3. https://doi.org/10.1053/j.gastro.2008.03.023
Lapointe TK, O’Connor PM, Buret AG (2009) The role of epithelial malfunction in the pathogenesis of enteropathogenic E. coli-induced diarrhea. Lab Invest 89:964–970. https://doi.org/10.1038/labinvest.2009.69
Lartigue A, Colliou N, Calbo S et al (2009) Critical role of TLR2 and TLR4 in autoantibody production and glomerulonephritis in lpr mutation-induced mouse lupus. J Immunol 183:6207–6216. https://doi.org/10.4049/jimmunol.0803219
Lauret E, Rodrigo L (2013) Celiac disease and autoimmune-associated conditions. Biomed Res Int 2013:127589. https://doi.org/10.1155/2013/127589
Lauzier A, Charbonneau M, Paquette M et al (2012) Transglutaminase 2 cross-linking activity is linked to invadopodia formation and cartilage breakdown in arthritis. Arthritis Res Ther 14:R159. https://doi.org/10.1186/ar3899
Lawrence JS, Martins CL, Drake GL (1987) A family survey of lupus erythematosus. 1. Heritability. J Rheumatol 14:913–921
Lee SH, Starkey PM, Gordon S (1985) Quantitative analysis of total macrophage content in adult mouse tissues. Immunochemical studies with monoclonal antibody F4/80. J Exp Med 161:475–489. https://doi.org/10.1084/jem.161.3.475
Lee J, Mo J-H, Katakura K et al (2006) Maintenance of colonic homeostasis by distinctive apical TLR9 signalling in intestinal epithelial cells. Nat Cell Biol 8:1327–1336. https://doi.org/10.1038/ncb1500
Leffler DA, Kelly CP, Green PHR et al (2015) Larazotide acetate for persistent symptoms of celiac disease despite a gluten-free diet: a randomized controlled trial. Gastroenterology 148:1311-1319.e6. https://doi.org/10.1053/j.gastro.2015.02.008
Leiss H, Niederreiter B, Bandur T et al (2013) Pristane-induced lupus as a model of human lupus arthritis: evolvement of autoantibodies, internal organ and joint inflammation. Lupus 22:778–792. https://doi.org/10.1177/0961203313492869
Lerner A, Benzvi C (2021) Microbial transglutaminase is a very frequently used food additive and is a potential inducer of autoimmune/neurodegenerative diseases. Toxics 9:233. https://doi.org/10.3390/toxics9100233
Lerner A, Matthias T (2015a) Possible association between celiac disease and bacterial transglutaminase in food processing: a hypothesis. Nutr Rev 73:544–552. https://doi.org/10.1093/nutrit/nuv011
Lerner A, Matthias T (2015b) Rheumatoid arthritis-celiac disease relationship: joints get that gut feeling. Autoimmun Rev 14:1038–1047. https://doi.org/10.1016/j.autrev.2015.07.007
Lerner A, Matthias T (2020) Processed food additive microbial transglutaminase and its cross-linked gliadin complexes are potential public health concerns in celiac disease. Int J Mol Sci 21:1127. https://doi.org/10.3390/ijms21031127
Lerner A, Aminov R, Matthias T (2017) Transglutaminases in dysbiosis as potential environmental drivers of autoimmunity. Front Microbiol 8:66. https://doi.org/10.3389/fmicb.2017.00066
Lessa FC, Mu Y, Bamberg WM et al (2015) Burden of Clostridium difficile infection in the United States. N Engl J Med 372:825–834. https://doi.org/10.1056/NEJMoa1408913
Li S, Yu Y, Yue Y et al (2013) Microbial infection and rheumatoid arthritis. J Clin Cell Immunol 4:174. https://doi.org/10.4172/2155-9899.1000174
Lin R, Zhou L, Zhang J, Wang B (2015) Abnormal intestinal permeability and microbiota in patients with autoimmune hepatitis. Int J Clin Exp Pathol 8:5153–5160
Litvak Y, Byndloss MX, Bäumler AJ (2018) Colonocyte metabolism shapes the gut microbiota. Science 362:eaat9076. https://doi.org/10.1126/science.aat9076
Liu B, Yang Y, Dai J et al (2006) TLR4 up-regulation at protein or gene level is pathogenic for lupus-like autoimmune disease. J Immunol 177:6880–6888. https://doi.org/10.4049/jimmunol.177.10.6880
Liu Y, Liao J, Zhao M et al (2015) Increased expression of TLR2 in CD4(+) T cells from SLE patients enhances immune reactivity and promotes IL-17 expression through histone modifications. Eur J Immunol 45:2683–2693. https://doi.org/10.1002/eji.201445219
Liu T-C, Gurram B, Baldridge MT et al (2016) Paneth cell defects in Crohn’s disease patients promote dysbiosis. JCI Insight 1:e86907. https://doi.org/10.1172/jci.insight.86907
Lopez CA, Miller BM, Rivera-Chávez F et al (2016) Virulence factors enhance Citrobacter rodentium expansion through aerobic respiration. Science 353:1249–1253. https://doi.org/10.1126/science.aag3042
Louis P, Young P, Holtrop G, Flint HJ (2010) Diversity of human colonic butyrate-producing bacteria revealed by analysis of the butyryl-CoA:acetate CoA-transferase gene. Environ Microbiol 12:304–314. https://doi.org/10.1111/j.1462-2920.2009.02066.x
Luissint A-C, Parkos CA, Nusrat A (2016) Inflammation and the intestinal barrier: leukocyte-epithelial cell interactions, cell junction remodeling, and mucosal repair. Gastroenterology 151:616–632. https://doi.org/10.1053/j.gastro.2016.07.008
Ma A, Malynn BA (2012) A20: linking a complex regulator of ubiquitylation to immunity and human disease. Nat Rev Immunol 12:774–785. https://doi.org/10.1038/nri3313
Mabbott NA, Donaldson DS, Ohno H et al (2013) Microfold (M) cells: important immunosurveillance posts in the intestinal epithelium. Mucosal Immunol 6:666–677. https://doi.org/10.1038/mi.2013.30
Machado Ribeiro F, Goldenberg T (2015) Mycobacteria and autoimmunity. Lupus 24:374–381. https://doi.org/10.1177/0961203314559634
Macpherson AJ, Uhr T (2004) Induction of protective IgA by intestinal dendritic cells carrying commensal bacteria. Science 303:1662–1665. https://doi.org/10.1126/science.1091334
Maeusli M, Skandalis N, Lee B et al (2022) Acacia fiber protects the gut from extended-spectrum beta-lactamase (ESBL)-producing Escherichia coli colonization enabled by antibiotics. mSphere 7:e0007122. https://doi.org/10.1128/msphere.00071-22
Mainous MR, Tso P, Berg RD, Deitch EA (1991) Studies of the route, magnitude, and time course of bacterial translocation in a model of systemic inflammation. Arch Surg 126:33–37. https://doi.org/10.1001/archsurg.1991.01410250037005
Manfredo Vieira S, Hiltensperger M, Kumar V et al (2018) Translocation of a gut pathobiont drives autoimmunity in mice and humans. Science 359:1156–1161. https://doi.org/10.1126/science.aar7201
Mathur H, Fallico V, O’Connor PM et al (2017) Insights into the mode of action of the sactibiotic thuricin CD. Front Microbiol 8:696. https://doi.org/10.3389/fmicb.2017.00696
Matot I, Sprung CL (2001) Definition of sepsis. Intensive Care Med 27(Suppl 1):S3-9. https://doi.org/10.1007/pl00003795
Mazzini E, Massimiliano L, Penna G, Rescigno M (2014) Oral tolerance can be established via gap junction transfer of fed antigens from CX3CR1+ macrophages to CD103+ dendritic cells. Immunity 40:248–261. https://doi.org/10.1016/j.immuni.2013.12.012
McClure R, Massari P (2014) TLR-dependent human mucosal epithelial cell responses to microbial pathogens. Front Immunol 5:386. https://doi.org/10.3389/fimmu.2014.00386
McDole JR, Wheeler LW, McDonald KG et al (2012) Goblet cells deliver luminal antigen to CD103+ dendritic cells in the small intestine. Nature 483:345–349. https://doi.org/10.1038/nature10863
McInnes IB, Schett G (2011) The pathogenesis of rheumatoid arthritis. N Engl J Med 365:2205–2219. https://doi.org/10.1056/NEJMra1004965
Meddings JB, Jarand J, Urbanski SJ et al (1999) Increased gastrointestinal permeability is an early lesion in the spontaneously diabetic BB rat. Am J Physiol Gastrointest Liver Physiol 276:G951–G957. https://doi.org/10.1152/ajpgi.1999.276.4.G951
Meng Y, Wang J, Wang Z et al (2019) Lactobacillus plantarum KLDS1.0318 ameliorates impaired intestinal immunity and metabolic disorders in cyclophosphamide-treated mice. Front Microbiol 10:731. https://doi.org/10.3389/fmicb.2019.00731
Mielants H, De Vos M, Goemaere S et al (1991) Intestinal mucosal permeability in inflammatory rheumatic diseases. II. Role of disease. J Rheumatol 18:394–400
Miquel S, Martín R, Rossi O et al (2013) Faecalibacterium prausnitzii and human intestinal health. Curr Opin Microbiol 16:255–261. https://doi.org/10.1016/j.mib.2013.06.003
Moser KL, Kelly JA, Lessard CJ, Harley JB (2009) Recent insights into the genetic basis of systemic lupus erythematosus. Genes Immun 10:373–379. https://doi.org/10.1038/gene.2009.39
Mu Q, Kirby J, Reilly CM, Luo XM (2017) Leaky gut as a danger signal for autoimmune diseases. Front Immunol 8:598. https://doi.org/10.3389/fimmu.2017.00598
Mukherjee S, Hooper LV (2015) Antimicrobial defense of the intestine. Immunity 42:28–39. https://doi.org/10.1016/j.immuni.2014.12.028
Muller S, Briand JP, Van Regenmortel MH (1988) Presence of antibodies to ubiquitin during the autoimmune response associated with systemic lupus erythematosus. Proc Natl Acad Sci U S A 85:8176–8180. https://doi.org/10.1073/pnas.85.21.8176
Mullin JM, Gabello M, Murray LJ et al (2009) Proton pump inhibitors: actions and reactions. Drug Discov Today 14:647–660. https://doi.org/10.1016/j.drudis.2009.03.014
Muza-Moons MM, Koutsouris A, Hecht G (2003) Disruption of cell polarity by enteropathogenic Escherichia coli enables basolateral membrane proteins to migrate apically and to potentiate physiological consequences. Infect Immun 71:7069–7078. https://doi.org/10.1128/IAI.71.12.7069-7078.2003
Nadjsombati MS, McGinty JW, Lyons-Cohen MR et al (2018) Detection of succinate by intestinal tuft cells triggers a type 2 innate immune circuit. Immunity 49:33-41.e7. https://doi.org/10.1016/j.immuni.2018.06.016
Naimi S, Viennois E, Gewirtz AT, Chassaing B (2021) Direct impact of commonly used dietary emulsifiers on human gut microbiota. Microbiome 9:66. https://doi.org/10.1186/s40168-020-00996-6
Nakano T, Inoue I, Alpers DH et al (2009) Role of lysophosphatidylcholine in brush-border intestinal alkaline phosphatase release and restoration. Am J Physiol Gastrointest Liver Physiol 297:G207-214. https://doi.org/10.1152/ajpgi.90590.2008
Nava P, Kamekura R, Nusrat A (2013) Cleavage of transmembrane junction proteins and their role in regulating epithelial homeostasis. Tissue Barriers 1:e24783. https://doi.org/10.4161/tisb.24783
Navarra SV, Ishimori MI, Uy EA et al (2011) Studies of Filipino patients with systemic lupus erythematosus: autoantibody profile of first-degree relatives. Lupus 20:537–543. https://doi.org/10.1177/0961203310385164
Ng KM, Aranda-Díaz A, Tropini C et al (2019) Recovery of the gut microbiota after antibiotics depends on host diet, community context, and environmental reservoirs. Cell Host Microbe 26:650-665.e4. https://doi.org/10.1016/j.chom.2019.10.011
Niess JH, Brand S, Gu X et al (2005) CX3CR1-mediated dendritic cell access to the intestinal lumen and bacterial clearance. Science 307:254–258. https://doi.org/10.1126/science.1102901
Nighot M, Liao P-L, Morris N et al (2022) Long term use of proton pump inhibitor disrupts intestinal tight junction barrier and exaggerates experimental colitis. J Crohns Colitis. https://doi.org/10.1093/ecco-jcc/jjac168
Norris JM, Barriga K, Klingensmith G et al (2003) Timing of initial cereal exposure in infancy and risk of islet autoimmunity. JAMA 290:1713–1720. https://doi.org/10.1001/jama.290.13.1713
Obata T, Goto Y, Kunisawa J et al (2010) Indigenous opportunistic bacteria inhabit mammalian gut-associated lymphoid tissues and share a mucosal antibody-mediated symbiosis. Proc Natl Acad Sci U S A 107:7419–7424. https://doi.org/10.1073/pnas.1001061107
Obermeier F, Dunger N, Strauch UG et al (2005) CpG motifs of bacterial DNA essentially contribute to the perpetuation of chronic intestinal inflammation. Gastroenterology 129:913–927. https://doi.org/10.1053/j.gastro.2005.06.061
Odenwald MA, Turner JR (2013) Intestinal permeability defects: is it time to treat? Clin Gastroenterol Hepatol 11:1075–1083. https://doi.org/10.1016/j.cgh.2013.07.001
Odenwald MA, Turner JR (2017) The intestinal epithelial barrier: a therapeutic target? Nat Rev Gastroenterol Hepatol 14:9–21. https://doi.org/10.1038/nrgastro.2016.169
Ohno H (2016) Intestinal M cells. J Biochem 159:151–160. https://doi.org/10.1093/jb/mvv121
Okhuysen PC, Dupont HL (2010) Enteroaggregative Escherichia coli (EAEC): a cause of acute and persistent diarrhea of worldwide importance. J Infect Dis 202:503–505. https://doi.org/10.1086/654895
Pakbin B, Brück WM, Rossen JWA (2021) Virulence factors of enteric pathogenic Escherichia coli: a review. Int J Mol Sci 22:9922. https://doi.org/10.3390/ijms22189922
Park J-S, Choi JW, Jhun J et al (2018) Lactobacillus acidophilus improves intestinal inflammation in an acute colitis mouse model by regulation of Th17 and treg cell balance and fibrosis development. J Med Food 21:215–224. https://doi.org/10.1089/jmf.2017.3990
Parks CG, Miller FW, Pollard KM et al (2014) Expert panel workshop consensus statement on the role of the environment in the development of autoimmune disease. Int J Mol Sci 15:14269–14297. https://doi.org/10.3390/ijms150814269
Pastor Rojo O, López San Román A, Albéniz Arbizu E et al (2007) Serum lipopolysaccharide-binding protein in endotoxemic patients with inflammatory bowel disease. Inflamm Bowel Dis 13:269–277. https://doi.org/10.1002/ibd.20019
Patrick S (2022) A tale of two habitats: Bacteroides fragilis, a lethal pathogen and resident in the human gastrointestinal microbiome. Microbiology (Reading). https://doi.org/10.1099/mic.0.001156
Patrick S, Jobling KL, O’Connor D et al (2011) A unique homologue of the eukaryotic protein-modifier ubiquitin present in the bacterium Bacteroides fragilis, a predominant resident of the human gastrointestinal tract. Microbiology (Reading) 157:3071–3078. https://doi.org/10.1099/mic.0.049940-0
Pawar RD, Castrezana-Lopez L, Allam R et al (2009) Bacterial lipopeptide triggers massive albuminuria in murine lupus nephritis by activating Toll-like receptor 2 at the glomerular filtration barrier. Immunology 128:e206-221. https://doi.org/10.1111/j.1365-2567.2008.02948.x
Payne JM, Sansom BF, Garner RJ et al (1960) Uptake of small resin particles (1–5 microns diameter) by the alimentary canal of the calf. Nature 188:586–587. https://doi.org/10.1038/188586a0
Pelaseyed T, Bergström JH, Gustafsson JK et al (2014) The mucus and mucins of the goblet cells and enterocytes provide the first defense line of the gastrointestinal tract and interact with the immune system. Immunol Rev 260:8–20. https://doi.org/10.1111/imr.12182
Pérez-Barbosa L, Esquivel-Valerio JA, Arana-Guajardo AC et al (2015) Increased detection of latent tuberculosis by tuberculin skin test and booster phenomenon in early rheumatoid arthritis patients. Rheumatol Int 35:1555–1559. https://doi.org/10.1007/s00296-015-3246-9
Perricone C, Ceccarelli F, Saccucci M et al (2019) Porphyromonas gingivalis and rheumatoid arthritis. Curr Opin Rheumatol 31:517–524. https://doi.org/10.1097/BOR.0000000000000638
Peterson LW, Artis D (2014) Intestinal epithelial cells: regulators of barrier function and immune homeostasis. Nat Rev Immunol 14:141–153. https://doi.org/10.1038/nri3608
Petersson J, Schreiber O, Hansson GC et al (2011) Importance and regulation of the colonic mucus barrier in a mouse model of colitis. Am J Physiol Gastrointest Liver Physiol 300:G327-333. https://doi.org/10.1152/ajpgi.00422.2010
Picascia A, Grimaldi V, Pignalosa O et al (2015) Epigenetic control of autoimmune diseases: from bench to bedside. Clin Immunol 157:1–15. https://doi.org/10.1016/j.clim.2014.12.013
Pizzuti D, Bortolami M, Mazzon E et al (2004) Transcriptional downregulation of tight junction protein ZO-1 in active coeliac disease is reversed after a gluten-free diet. Dig Liver Dis 36:337–341. https://doi.org/10.1016/j.dld.2004.01.013
Poon IKH, Lucas CD, Rossi AG, Ravichandran KS (2014) Apoptotic cell clearance: basic biology and therapeutic potential. Nat Rev Immunol 14:166–180. https://doi.org/10.1038/nri3607
Prasad S, Mingrino R, Kaukinen K et al (2005) Inflammatory processes have differential effects on claudins 2, 3 and 4 in colonic epithelial cells. Lab Invest 85:1139–1162. https://doi.org/10.1038/labinvest.3700316
Propheter DC, Chara AL, Harris TA et al (2017) Resistin-like molecule β is a bactericidal protein that promotes spatial segregation of the microbiota and the colonic epithelium. Proc Natl Acad Sci U S A 114:11027–11033. https://doi.org/10.1073/pnas.1711395114
Quiros M, Nusrat A (2014) RhoGTPases, actomyosin signaling and regulation of the epithelial Apical Junctional Complex. Semin Cell Dev Biol 36:194–203. https://doi.org/10.1016/j.semcdb.2014.09.003
Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F et al (2004) Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell 118:229–241. https://doi.org/10.1016/j.cell.2004.07.002
Ramakrishna BS, Venkataraman S, Srinivasan P et al (2000) Amylase-resistant starch plus oral rehydration solution for cholera. N Engl J Med 342:308–313. https://doi.org/10.1056/NEJM200002033420502
Ramirez J, Guarner F, Bustos Fernandez L et al (2020) Antibiotics as major disruptors of gut microbiota. Front Cell Infect Microbiol 10:572912
Rauch I, Deets KA, Ji DX et al (2017) NAIP-NLRC4 inflammasomes coordinate intestinal epithelial cell expulsion with eicosanoid and IL-18 release via activation of caspase-1 and -8. Immunity 46:649–659. https://doi.org/10.1016/j.immuni.2017.03.016
Rea MC, Sit CS, Clayton E et al (2010) Thuricin CD, a posttranslationally modified bacteriocin with a narrow spectrum of activity against Clostridium difficile. Proc Natl Acad Sci U S A 107:9352–9357. https://doi.org/10.1073/pnas.0913554107
Rea MC, Dobson A, O’Sullivan O et al (2011) Effect of broad- and narrow-spectrum antimicrobials on Clostridium difficile and microbial diversity in a model of the distal colon. Proc Natl Acad Sci U S A 108(Suppl 1):4639–4644. https://doi.org/10.1073/pnas.1001224107
Reigstad CS, Salmonson CE, Rainey JF et al (2015) Gut microbes promote colonic serotonin production through an effect of short-chain fatty acids on enterochromaffin cells. FASEB J 29:1395–1403. https://doi.org/10.1096/fj.14-259598
Reuter BK, Davies NM, Wallace JL (1997) Nonsteroidal anti-inflammatory drug enteropathy in rats: role of permeability, bacteria, and enterohepatic circulation. Gastroenterology 112:109–117. https://doi.org/10.1016/s0016-5085(97)70225-7
Ribet D, Cossart P (2015) How bacterial pathogens colonize their hosts and invade deeper tissues. Microbes Infect 17:173–183. https://doi.org/10.1016/j.micinf.2015.01.004
Rivera-Chávez F, Zhang LF, Faber F et al (2016) Depletion of butyrate-producing Clostridia from the gut microbiota drives an aerobic luminal expansion of Salmonella. Cell Host Microbe 19:443–454. https://doi.org/10.1016/j.chom.2016.03.004
Rivera-Chávez F, Lopez CA, Bäumler AJ (2017) Oxygen as a driver of gut dysbiosis. Free Radic Biol Med 105:93–101. https://doi.org/10.1016/j.freeradbiomed.2016.09.022
Rodríguez-Colman MJ, Schewe M, Meerlo M et al (2017) Interplay between metabolic identities in the intestinal crypt supports stem cell function. Nature 543:424–427. https://doi.org/10.1038/nature21673
Roediger WE (1980) Role of anaerobic bacteria in the metabolic welfare of the colonic mucosa in man. Gut 21:793–798. https://doi.org/10.1136/gut.21.9.793
Rosenblatt J, Raff MC, Cramer LP (2001) An epithelial cell destined for apoptosis signals its neighbors to extrude it by an actin- and myosin-dependent mechanism. Curr Biol 11:1847–1857. https://doi.org/10.1016/s0960-9822(01)00587-5
Roveda AM, Veronesi L, Zoni R et al (2006) Exposure to polychlorinated biphenyls (PCBs) in food and cancer risk: recent advances. Ig Sanita Pubbl 62:677–696
Roxas JL, Viswanathan VK (2018) Modulation of intestinal paracellular transport by bacterial pathogens. Compr Physiol 8:823–842. https://doi.org/10.1002/cphy.c170034
Rude KM, Pusceddu MM, Keogh CE et al (2019) Developmental exposure to polychlorinated biphenyls (PCBs) in the maternal diet causes host-microbe defects in weanling offspring mice. Environ Pollut 253:708–721. https://doi.org/10.1016/j.envpol.2019.07.066
Ruemmele FM, Beaulieu JF, Dionne S et al (2002) Lipopolysaccharide modulation of normal enterocyte turnover by toll-like receptors is mediated by endogenously produced tumour necrosis factor alpha. Gut 51:842–848. https://doi.org/10.1136/gut.51.6.842
Sander GR, Cummins AG, Henshall T, Powell BC (2005) Rapid disruption of intestinal barrier function by gliadin involves altered expression of apical junctional proteins. FEBS Lett 579:4851–4855. https://doi.org/10.1016/j.febslet.2005.07.066
Santos M, Torné JM (2009) Recent patents on transglutaminase production and applications: a brief review. Recent Pat Biotechnol 3:166–174. https://doi.org/10.2174/187220809789389180
Sartor RB (1997) Pathogenesis and immune mechanisms of chronic inflammatory bowel diseases. Am J Gastroenterol 92:5S-11S
Savilahti E, Ormälä T, Saukkonen T et al (1999) Jejuna of patients with insulin-dependent diabetes mellitus (IDDM) show signs of immune activation. Clin Exp Immunol 116:70–77. https://doi.org/10.1046/j.1365-2249.1999.00860.x
Sayoc-Becerra A, Krishnan M, Fan S et al (2020) The JAK-inhibitor tofacitinib rescues human intestinal epithelial cells and colonoids from cytokine-induced barrier dysfunction. Inflamm Bowel Dis 26:407–422. https://doi.org/10.1093/ibd/izz266
Scarpignato C (2008) NSAID-induced intestinal damage: are luminal bacteria the therapeutic target? Gut 57:145–148. https://doi.org/10.1136/gut.2007.134502
Schäfer C, Parlesak A, Schütt C et al (2002) Concentrations of lipopolysaccharide-binding protein, bactericidal/permeability-increasing protein, soluble CD14 and plasma lipids in relation to endotoxaemia in patients with alcoholic liver disease. Alcohol Alcohol 37:81–86. https://doi.org/10.1093/alcalc/37.1.81
Schroeder BO, Birchenough GMH, Ståhlman M et al (2018) Bifidobacteria or fiber protects against diet-induced microbiota-mediated colonic mucus deterioration. Cell Host Microbe 23:27-40.e7. https://doi.org/10.1016/j.chom.2017.11.004
Schulzke JD, Bentzel CJ, Schulzke I et al (1998) Epithelial tight junction structure in the jejunum of children with acute and treated celiac sprue. Pediatr Res 43:435–441. https://doi.org/10.1203/00006450-199804000-00001
Secondulfo M, Iafusco D, Carratù R et al (2004) Ultrastructural mucosal alterations and increased intestinal permeability in non-celiac, type I diabetic patients. Dig Liver Dis 36:35–45. https://doi.org/10.1016/j.dld.2003.09.016
Shaoul R, Lerner A (2007) Associated autoantibodies in celiac disease. Autoimmun Rev 6:559–565. https://doi.org/10.1016/j.autrev.2007.02.006
Shi L, Zhang Z, Yu AM et al (2014) The SLE transcriptome exhibits evidence of chronic endotoxin exposure and has widespread dysregulation of non-coding and coding RNAs. PLoS ONE 9:e93846. https://doi.org/10.1371/journal.pone.0093846
Shibata N, Kunisawa J, Hosomi K et al (2018) Lymphoid tissue-resident Alcaligenes LPS induces IgA production without excessive inflammatory responses via weak TLR4 agonist activity. Mucosal Immunol 11:693–702. https://doi.org/10.1038/mi.2017.103
Shin K, Fogg VC, Margolis B (2006) Tight junctions and cell polarity. Annu Rev Cell Dev Biol 22:207–235. https://doi.org/10.1146/annurev.cellbio.22.010305.104219
Shin N-R, Lee J-C, Lee H-Y et al (2014) An increase in the Akkermansia spp. population induced by metformin treatment improves glucose homeostasis in diet-induced obese mice. Gut 63:727–735. https://doi.org/10.1136/gutjnl-2012-303839
Silverberg MS, Cho JH, Rioux JD et al (2009) Ulcerative colitis-risk loci on chromosomes 1p36 and 12q15 found by genome-wide association study. Nat Genet 41:216–220. https://doi.org/10.1038/ng.275
Singh PK, Parsek MR, Greenberg EP, Welsh MJ (2002) A component of innate immunity prevents bacterial biofilm development. Nature 417:552–555. https://doi.org/10.1038/417552a
Singh RP, Massachi I, Manickavel S et al (2013) The role of miRNA in inflammation and autoimmunity. Autoimmun Rev 12:1160–1165. https://doi.org/10.1016/j.autrev.2013.07.003
Smith PD, Smythies LE, Mosteller-Barnum M et al (2001) Intestinal macrophages lack CD14 and CD89 and consequently are down-regulated for LPS- and IgA-mediated activities. J Immunol 167:2651–2656. https://doi.org/10.4049/jimmunol.167.5.2651
Smith PD, Ochsenbauer-Jambor C, Smythies LE (2005) Intestinal macrophages: unique effector cells of the innate immune system. Immunol Rev 206:149–159. https://doi.org/10.1111/j.0105-2896.2005.00288.x
Smyth MC (2017) Intestinal permeability and autoimmune diseases. Biosci Horiz Int J Student Res. https://doi.org/10.1093/biohorizons/hzx015
Smythies LE, Sellers M, Clements RH et al (2005) Human intestinal macrophages display profound inflammatory anergy despite avid phagocytic and bacteriocidal activity. J Clin Invest 115:66–75. https://doi.org/10.1172/JCI19229
Somasundaram S, Rafi S, Hayllar J et al (1997) Mitochondrial damage: a possible mechanism of the “topical” phase of NSAID induced injury to the rat intestine. Gut 41:344–353. https://doi.org/10.1136/gut.41.3.344
Somasundaram S, Sigthorsson G, Simpson RJ et al (2000) Uncoupling of intestinal mitochondrial oxidative phosphorylation and inhibition of cyclooxygenase are required for the development of NSAID-enteropathy in the rat. Aliment Pharmacol Ther 14:639–650. https://doi.org/10.1046/j.1365-2036.2000.00723.x
Sommer F, Nookaew I, Sommer N et al (2015) Site-specific programming of the host epithelial transcriptome by the gut microbiota. Genome Biol 16:62. https://doi.org/10.1186/s13059-015-0614-4
Son M, Park IS, Kim S et al (2022) Novel potassium-competitive acid blocker, tegoprazan, protects against colitis by improving gut barrier function. Front Immunol 13:870817. https://doi.org/10.3389/fimmu.2022.870817
Spadoni I, Zagato E, Bertocchi A et al (2015) A gut-vascular barrier controls the systemic dissemination of bacteria. Science 350:830–834. https://doi.org/10.1126/science.aad0135
Spence JR, Lauf R, Shroyer NF (2011) Vertebrate intestinal endoderm development. Dev Dyn 240:501–520. https://doi.org/10.1002/dvdy.22540
Steiner G (2007) Auto-antibodies and autoreactive T-cells in rheumatoid arthritis: pathogenetic players and diagnostic tools. Clin Rev Allergy Immunol 32:23–36. https://doi.org/10.1007/BF02686079
Stewart L, Edgar JDM, Blakely G, Patrick S (2018) Antigenic mimicry of ubiquitin by the gut bacterium Bacteroides fragilis: a potential link with autoimmune disease. Clin Exp Immunol 194:153–165. https://doi.org/10.1111/cei.13195
Strober W, Asano N, Fuss I et al (2014) Cellular and molecular mechanisms underlying NOD2 risk-associated polymorphisms in Crohn’s disease. Immunol Rev 260:249–260. https://doi.org/10.1111/imr.12193
Suzuki T (2020) Regulation of the intestinal barrier by nutrients: the role of tight junctions. Anim Sci J 91:e13357. https://doi.org/10.1111/asj.13357
Swidsinski A, Ladhoff A, Pernthaler A et al (2002) Mucosal flora in inflammatory bowel disease. Gastroenterology 122:44–54. https://doi.org/10.1053/gast.2002.30294
Takashima S, Tanaka F, Kawaguchi Y et al (2020) Proton pump inhibitors enhance intestinal permeability via dysbiosis of gut microbiota under stressed conditions in mice. Neurogastroenterol Motil 32:e13841. https://doi.org/10.1111/nmo.13841
Tanes C, Bittinger K, Gao Y et al (2021) Role of dietary fiber in the recovery of the human gut microbiome and its metabolome. Cell Host Microbe 29:394-407.e5. https://doi.org/10.1016/j.chom.2020.12.012
Tapia R, Kralicek SE, Hecht GA (2017) Modulation of epithelial cell polarity by bacterial pathogens. Ann N Y Acad Sci 1405:16–24. https://doi.org/10.1111/nyas.13388
Targan SR (2000) Biology of inflammation in Crohn’s disease: mechanisms of action of anti-TNF—a therapy. Can J Gastroenterol 14(Suppl C):13C-16C. https://doi.org/10.1155/2000/409396
Tlaskalová-Hogenová H, Stěpánková R, Kozáková H et al (2011) The role of gut microbiota (commensal bacteria) and the mucosal barrier in the pathogenesis of inflammatory and autoimmune diseases and cancer: contribution of germ-free and gnotobiotic animal models of human diseases. Cell Mol Immunol 8:110–120. https://doi.org/10.1038/cmi.2010.67
Traunmüller F (2005) Etiology of Crohn’s disease: Do certain food additives cause intestinal inflammation by molecular mimicry of mycobacterial lipids? Med Hypotheses 65:859–864. https://doi.org/10.1016/j.mehy.2005.05.040
Tsokos GC (2011) Systemic lupus erythematosus. N Engl J Med 365:2110–2121. https://doi.org/10.1056/NEJMra1100359
Tsokos GC, Lo MS, Costa Reis P, Sullivan KE (2016) New insights into the immunopathogenesis of systemic lupus erythematosus. Nat Rev Rheumatol 12:716–730. https://doi.org/10.1038/nrrheum.2016.186
Turner HL, Turner JR (2010) Good fences make good neighbors: gastrointestinal mucosal structure. Gut Microbes 1:22–29. https://doi.org/10.4161/gmic.1.1.11427
Ulluwishewa D, Anderson RC, McNabb WC et al (2011) Regulation of tight junction permeability by intestinal bacteria and dietary components. J Nutr 141:769–776. https://doi.org/10.3945/jn.110.135657
Vaishnava S, Behrendt CL, Ismail AS et al (2008) Paneth cells directly sense gut commensals and maintain homeostasis at the intestinal host–microbial interface. Proc Natl Acad Sci U S A 105:20858–20863. https://doi.org/10.1073/pnas.0808723105
Vaishnava S, Yamamoto M, Severson KM et al (2011) The antibacterial lectin RegIII-γ promotes the spatial segregation of microbiota and host in the intestine. Science 334:255–258. https://doi.org/10.1126/science.1209791
Valdiglesias V, Prego-Faraldo MV, Pásaro E et al (2013) Okadaic acid: more than a diarrheic toxin. Mar Drugs 11:4328–4349. https://doi.org/10.3390/md11114328
van der Flier LG, Clevers H (2009) Stem cells, self-renewal, and differentiation in the intestinal epithelium. Annu Rev Physiol 71:241–260. https://doi.org/10.1146/annurev.physiol.010908.163145
Ventura A, Magazzù G, Greco L (1999) Duration of exposure to gluten and risk for autoimmune disorders in patients with celiac disease. SIGEP Study Group for Autoimmune Disorders in Celiac Disease. Gastroenterology 117:297–303. https://doi.org/10.1053/gast.1999.0029900297
Viaud S, Saccheri F, Mignot G et al (2013) The intestinal microbiota modulates the anticancer immune effects of cyclophosphamide. Science 342:971–976. https://doi.org/10.1126/science.1240537
Visser J, Groen H, Klatter F et al (2003) The diabetes prone BB rat model of IDDM shows duration of breastfeeding to influence Type 1 diabetes development later in life. Diabetologia 46:1711–1713. https://doi.org/10.1007/s00125-003-1239-9
Vojdani A (2014) A potential link between environmental triggers and autoimmunity. Autoimmune Dis 2014:437231. https://doi.org/10.1155/2014/437231
von Moltke J, Ji M, Liang H-E, Locksley RM (2016) Tuft-cell-derived IL-25 regulates an intestinal ILC2-epithelial response circuit. Nature 529:221–225. https://doi.org/10.1038/nature16161
Wang X, Pierre JF, Heneghan AF et al (2015) Glutamine improves innate immunity and prevents bacterial enteroinvasion during parenteral nutrition. J Parenter Enter Nutr 39:688–697. https://doi.org/10.1177/0148607114535265
Watts T, Berti I, Sapone A et al (2005) Role of the intestinal tight junction modulator zonulin in the pathogenesis of type I diabetes in BB diabetic-prone rats. Proc Natl Acad Sci U S A 102:2916–2921. https://doi.org/10.1073/pnas.0500178102
Westerholm-Ormio M, Vaarala O, Pihkala P et al (2003) Immunologic activity in the small intestinal mucosa of pediatric patients with type 1 diabetes. Diabetes 52:2287–2295. https://doi.org/10.2337/diabetes.52.9.2287
Wexler HM (2007) Bacteroides: the good, the bad, and the Nitty-Gritty. Clin Microbiol Rev 20:593–621. https://doi.org/10.1128/CMR.00008-07
Winter SE, Winter MG, Xavier MN et al (2013) Host-derived nitrate boosts growth of E. coli in the inflamed gut. Science 339:708–711. https://doi.org/10.1126/science.1232467
Wolochow H, Hildebrand GJ, Lamanna C (1966) Translocation of microorganisms across the intestinal wall of the rat: effect of microbial size and concentration. J Infect Dis 116:523–528. https://doi.org/10.1093/infdis/116.4.523
Wong ARC, Pearson JS, Bright MD et al (2011) Enteropathogenic and enterohaemorrhagic Escherichia coli: even more subversive elements. Mol Microbiol 80:1420–1438. https://doi.org/10.1111/j.1365-2958.2011.07661.x
Woof JM, Russell MW (2011) Structure and function relationships in IgA. Mucosal Immunol 4:590–597. https://doi.org/10.1038/mi.2011.39
Wu Z, Nybom P, Magnusson KE (2000) Distinct effects of Vibrio cholerae haemagglutinin/protease on the structure and localization of the tight junction-associated proteins occludin and ZO-1. Cell Microbiol 2:11–17. https://doi.org/10.1046/j.1462-5822.2000.00025.x
**ao S, Li Q, Hu K et al (2018) Vitamin A and retinoic acid exhibit protective effects on necrotizing enterocolitis by regulating intestinal flora and enhancing the intestinal epithelial barrier. Arch Med Res 49:1–9. https://doi.org/10.1016/j.arcmed.2018.04.003
Xu Q, Guo X, Liu J et al (2015) Blockage of protease-activated receptor 1 ameliorates heat-stress induced intestinal high permeability and bacterial translocation. Cell Biol Int 39:411–417. https://doi.org/10.1002/cbin.10408
Yamazaki Y, Okawa K, Yano T et al (2008) Optimized proteomic analysis on gels of cell-cell adhering junctional membrane proteins. Biochemistry 47:5378–5386. https://doi.org/10.1021/bi8002567
Yang E, Shen J (2020) The roles and functions of Paneth cells in Crohn’s disease: a critical review. Cell Prolif 54:e12958. https://doi.org/10.1111/cpr.12958
Yano JM, Yu K, Donaldson GP et al (2015) Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell 161:264–276. https://doi.org/10.1016/j.cell.2015.02.047
Yoshida K, Korchynskyi O, Tak PP et al (2014) Citrullination of epithelial neutrophil-activating peptide 78/CXCL5 results in conversion from a non-monocyte-recruiting chemokine to a monocyte-recruiting chemokine. Arthritis Rheumatol 66:2716–2727. https://doi.org/10.1002/art.38750
Yu S, Gao N (2015) Compartmentalizing intestinal epithelial cell toll-like receptors for immune surveillance. Cell Mol Life Sci 72:3343–3353. https://doi.org/10.1007/s00018-015-1931-1
Yu Q, Wang Z, Li P, Yang Q (2013) The effect of various absorption enhancers on tight junction in the human intestinal Caco-2 cell line. Drug Dev Ind Pharm 39:587–592. https://doi.org/10.3109/03639045.2012.692376
Zarepour M, Bhullar K, Montero M et al (2013) The mucin Muc2 limits pathogen burdens and epithelial barrier dysfunction during Salmonella enterica serovar Typhimurium colitis. Infect Immun 81:3672–3683. https://doi.org/10.1128/IAI.00854-13
Zegarra-Ruiz DF, El Beidaq A, Iñiguez AJ et al (2019) A diet-sensitive commensal Lactobacillus strain mediates TLR7-dependent systemic autoimmunity. Cell Host Microbe 25:113-127.e6. https://doi.org/10.1016/j.chom.2018.11.009
Zhu H, Liu Y, **e X et al (2013) Effect of l-arginine on intestinal mucosal immune barrier function in weaned pigs after Escherichia coli LPS challenge. Innate Immun 19:242–252. https://doi.org/10.1177/1753425912456223
Zhu L-B, Zhang Y-C, Huang H-H, Lin J (2021) Prospects for clinical applications of butyrate-producing bacteria. World J Clin Pediatr 10:84–92. https://doi.org/10.5409/wjcp.v10.i5.84
Zignego AL, Gragnani L, Piluso A et al (2015) Virus-driven autoimmunity and lymphoproliferation: the example of HCV infection. Expert Rev Clin Immunol 11:15–31. https://doi.org/10.1586/1744666X.2015.997214
Zihni C, Mills C, Matter K, Balda MS (2016) Tight junctions: from simple barriers to multifunctional molecular gates. Nat Rev Mol Cell Biol 17:564–580. https://doi.org/10.1038/nrm.2016.80
Acknowledgements
Jamie English is in receipt of a Ph.D. studentship from the Department for Economy, Northern Ireland. Figures were prepared using Biorender software (Biorender.com).
Funding
This work received no specific grant from any funding agency.
Author information
Authors and Affiliations
Contributions
All authors contributed to the conceptualisation of the study. Jamie English performed the literature search and assembly of the first written draft of the manuscript. LS and LC contributed to project administration, supervision, and review and editing of the manuscript, and LS secured the funding. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
English, J., Connolly, L. & Stewart, L.D. Increased Intestinal Permeability: An Avenue for the Development of Autoimmune Disease?. Expo Health 16, 575–605 (2024). https://doi.org/10.1007/s12403-023-00578-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12403-023-00578-5