Abstract
Introduction
Chimeric antigen receptor T-cell (CAR T) therapies have transformed diffuse large B-cell lymphoma (DLBCL) treatment. It is important to better understand their use in Medicare Fee-for-Service (FFS) patients, who often differ from commercially insured populations in important ways.
Methods
We analyzed Medicare FFS claims data, focusing on the utilization patterns across three CAR T products—lisocabtagene maraleucel (liso-cel), tisagenlecleucel (tisa-cel), and axicabtagene autoleucel (axi-cel)—which are indicated for the treatment of DLBCL. Our investigation covered the period from 2021 through 2022. This analysis spanned a 180-day period prior to CAR T procedure and extended to a 90-day post-CAR T. Utilization of healthcare services, healthcare spending, and comorbidities were assessed in the pre- and post-periods. Clinical trial and PPS-exempt center claims were removed from the analysis. Statistical comparisons between inpatient and outpatient cohorts were made using Wilcoxon’s rank-sum tests for continuous variables and Chi-square tests or Fisher’s exact tests for categorical variables.
Results
Among the total 391 CAR T claims assessed, most of the CAR T therapies were administered in the inpatient setting (79%) compared to outpatient (21%). CAR T therapy in the inpatient setting received an average Medicare cost of US$498,723 ($276,138—$1,066,524), while the average Medicare cost for outpatient CAR T claims was $414,393 ($276,980—$849,878). There was a higher 3-month average post-period cost for those hospitals utilizing CAR T in the outpatient setting than the inpatient setting ($15,794 vs. $10,244). Despite the higher post-period cost, when looking at the CAR T procedure and pre- and post-periods as a single episode, beneficiaries receiving outpatient CAR T had less cost for the total episode of care ($587,908 vs. $529,188). Follow-up inpatient claims were also assessed post-CAR T procedure for 30 days. The rate of post-CAR T inpatient re-admission was significantly lower for beneficiaries receiving the index CAR T in the inpatient setting (21%) compared to outpatient CAR T (59%). Days between index CAR T discharge and IP admission were also significantly shorter for OP CAR T compared to IP CAR T (8.0 vs. 14.1 days, p < 0.0001). Additionally, IP CAR T had a longer ALOS on the admission claim (6.9 vs. 6.2 days).
Conclusion
CAR T therapy for the treatment of LBCL has become more common within the Medicare population, primarily in the inpatient setting. This study helps understand providers’ cost and associated patient care around CAR T administration. The data show that the average cost received by hospitals encompasses the expenses related to both the CAR T drug and the medical services delivered to patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Chimeric antigen receptor T-cell (CAR T) therapies have transformed the treatment of diffuse large B-cell lymphoma (DLBCL), yet understanding of their utilization, costs, and outcomes in the Medicare Fee-for-Service (FFS) patients is limited. |
Medicare FFS patients often have significant differences from commercially insured populations, such as older age and higher comorbidity burdens, highlighting a need for focused analysis on this group. |
The study aimed to analyze CAR T therapy utilization patterns, healthcare costs, and patient outcomes specifically within the Medicare FFS system, hypothesizing that setting of care (inpatient vs. outpatient) significantly impacts these factors. |
What was learned from the study? |
CAR T therapy is predominantly administered in the inpatient setting within the Medicare population, with higher costs incurred for inpatient administration compared to outpatient settings. |
Despite higher immediate post-treatment costs in outpatient settings, the total cost of care during the full episode was lower for outpatient CAR T, suggesting cost-effectiveness over the inpatient approach. |
The study findings indicate a potential shift towards outpatient administration could be economically beneficial and feasible with appropriate patient selection and management, challenging the traditional inpatient-centric model. |
Introduction
Large B-cell lymphoma (LBCL) encompasses several subtypes, with diffuse large B-cell lymphoma (DLBCL) being the most prevalent and well-known subtype. LBCLs, including DLBCL, are a significant subset of non-Hodgkin lymphomas, accounting for a significant percentage of cases globally [1]. As an often fast-growing, aggressive form of cancer, DLBCL requires timely and effective therapeutic interventions to ensure improved patient outcomes and survival rates [2]. Treatment modalities for DLBCL have seen considerable advancements, from conventional chemotherapy regimens to targeted therapies, providing more options to these patients [3].
Unlike traditional treatments, CAR T therapy offers a novel immunotherapeutic approach that involves genetically modifying a patient's own T-cells to specifically target and eliminate cancer cells [4, 5]. This method has not only provided a new avenue for patients who have relapsed or are refractory to initial therapies but has also demonstrated superiority over standard-of-care (SOC) treatments in the second-line (2L) treatment setting [6, 7]. CAR T therapies, including lisocabtagene maraleucel (liso-cel), tisagenlecleucel (tisa-cel), and axicabtagene ciloleucel (axi-cel), have shown promising clinical outcomes [8]. These therapies have often led to durable remissions [9,10,11], offering hope from improved outcomes to patients.
In the administration of CAR T therapies, the process of Medicare payment is central to understanding the overall healthcare landscape. Following the provision of the CAR T, a claim is submitted by the healthcare provider or system to a regional Medicare Administrative Contractor (MAC). The claim submission, which must occur within one year of the service date, is a critical element in the healthcare reimbursement system [12, 13]. The MAC plays a key role in processing these claims and issuing a Remittance Advice (RA), which accompanies the electronic payment to the provider, and provides payment details as well as any necessary adjustments [12]. This mechanism determines the direct CMS reimbursement associated with CAR T therapies and is also an indicator of the scrutiny these treatments have within government programs, such as Medicare.
The method of reimbursement for CAR T is based on the place of service designation of inpatient (IP) or outpatient (OP). When an OP case is admitted into the same hospital within 72 h, the CAR T infusion will be designated by CMS as IP and paid based on the claim's DRG (diagnosis-related grou**). DRG payment rates are set by CMS' Inpatient Prospective Payment System. In IP claims, the CAR T product is not reimbursed individually but rather it is part of a single payment for the entire IP stay. When a case is solely OP (without any IP admission within 72 h), the CAR T product is paid under CMS' Outpatient Prospective Payment System and is set to Average Sales Price (ASP) plus 6%. However, payments in both the IP and OP systems are adjusted by hospital-specific variables like geography and hospital teaching status. The distinction of a CAR T claim being IP, or OP has financial implications for the hospital provider.
While the effectiveness of CAR T therapies has been established by a growing evidence base of clinical trials and real-world studies, a gap remains in understanding their application and reimbursement for Medicare recipients. Medicare patients represent an elderly population with a higher prevalence of comorbidities compared to commercially insured patients [14, 15]. Recent registry data have added to the evidence suggesting that CAR T-cell therapy is feasible for the elderly, while not compromising on the efficacy shown in clinical trials [16, 17]. Therefore, understanding the utilization patterns and cost implications associated with CAR T therapies is important for informed clinical decision-making.
This study aims to explore Medicare Fee-for-Service (FFS) claims data, analyzing CAR T therapy utilization, costs, and outcomes in the DLBCL treatment setting.
Methods
Data Source and Study Design
This study utilized data from the Centers for Medicare & Medicaid Services (CMS) 100% Research Identifiable Files (RIF) database. The dataset included administrative claims from Medicare FFS Parts A and B for the period spanning from 2021 to 2022. A retrospective, non-interventional cohort study design was employed to investigate the utilization patterns of three major CAR T products available for treatment of DLBCL: lisocabtagene maraleucel (liso-cel), tisagenlecleucel (tisa-cel), and axicabtagene autoleucel (axi-cel). [The list of applicable ICD-10-PCS and Healthcare Common Procedure Coding System (HCPCS) codes used to identify the CAR T products is found in Supplemental Material.] The index date was defined as the date of CAR T therapy infusion. Patients were categorized into either the IP or OP cohorts based on the setting of their index CAR T administration/infusion. The analysis focused on resource use and Medicare reimbursement amounts for both cohorts during the study period.
Patient Population
The study population consisted of adult patients who met the following criteria: a diagnosis of DLBCL, receipt of CAR T therapy following the DLBCL diagnosis, and continuous eligibility in Medicare Parts A and B for 6 months prior to the CAR T administration and 3 months post-administration (or through death). Patients associated with claims linked to clinical trials were excluded, including patients who were managed on expanded access studies for products that did not meet FDA manufacturing targets.
Study Measures
The primary study outcomes were healthcare resource utilization (HRU) and Medicare reimbursement amounts. Baseline characteristics, including demographics, treatment details, and Charlson Comorbidity Index (CCI), were assessed over the 6-month period preceding the index date. HRU was evaluated from CAR T patient claims during the study period and encompassed IP admissions, OP visits, and Medicare reimbursement amounts, total claim charges, and total pharmacy charges. Total reimbursement amounts, encompassing CAR T infusion and post-infusion reimbursements (including IP, emergency room (ER), OP, other medical services, and pharmacy drugs), were assessed over the study period. For the CAR T IP cohort, reimbursement amounts were exclusively assessed among patients who received CAR T in Prospective Payment System (PPS) hospitals due to substantial differences in reimbursement policies between PPS and PPS-exempt (i.e., cancer) hospitals. CCI was calculated for each therapy in the 6 months prior to the CAR T administration. The total CCI score was calculated by summing the scores across the 19 CCI comorbidities, as identified by ICD-10 diagnosis code, prior to their CAR T administration.
Downstream HRU across all sites of care, including IP, Part B Drug service, OP, oncology specialist, general practitioner, home health, skilled nursing facility (SNF), durable medical equipment (DME), and hospice service after patients received their CAR T infusion were included for both patient groups that received CAR T in IP and OP settings.
Statistical Analysis
Comparisons between the CAR T IP and CAR T OP cohorts were made using appropriate statistical tests, including Wilcoxon’s rank-sum tests for continuous variables and Chi-square tests or Fisher’s exact tests for categorical variables. HRU during the study period was expressed as monthly incidence, calculated as the total number of events divided by total follow-up months. Reimbursement amounts were described on a per-patient-per-month (PPPM) basis.
Ethical Approval
This article does not include any research involving human participants or animals that was conducted by any of the authors. All data and findings presented in this paper are derived from secondary sources and data was not identifiable.
Results
Sample Selection
A total of 391 adult patients diagnosed with DLBCL who received CAR T therapy (identification codes in Supplemental Table 1) and met the study eligibility criteria were included in this analysis (see Fig. 1 for summary of selection/attrition). Among these patients, the majority (78% of the sample) received their index administration in the IP setting and were assigned to the CAR T inpatient (CAR T IP) cohort, while the remaining (22%) received their index administration in the OP setting and were allocated to the CAR T outpatient (CAR T OP) cohort.
Baseline Characteristics
Baseline patient demographics are summarized in Table 1. The mean age of patients in the CAR T IP cohort was 71.9 years, with a median age of 72.0 years. In this cohort, 61% of patients were male, and 90% were white. Most patients in the CAR T IP cohort (94%) qualified for Medicare due to their age (65+ years old), and 7% were dual enrollees having both Medicare and Medicaid coverage. Additionally, 8% of patients in this population were eligible for the Low-Income Subsidy (LIS) program, and 81% were enrolled in Medicare Part D, with 10% of those enrolled in Part D qualifying for LIS.
For the CAR T OP cohort, the mean/median age was 71.7/71.0 years, respectively. Both cohorts had a similar percentage of male patients at 62% for OP and 61% for IP. A significant portion (95%) of these patients were eligible for Medicare coverage due to age (65 + years old). No significant difference was found in CCI between IP and OP service.
Comparison of HRU in CAR T IP versus OP Cohorts
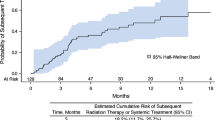
In our analysis, we examined the patterns of healthcare resource utilization (HRU), and associated Medicare spending, for CAR T patients in the IP and OP settings. Notable findings emerged regarding the differences in HRU between these two settings (Figs. 2, 3, 4, 5).
Evaluating the Index CAR T Claim
Table 2 summarizes spending associated with the CAR T index event, by cohort. CAR T IPs incurred an average Medicare spend of $498,723, ranging from $276,138 to $1,066,524 (median = $492,898). In contrast, CAR T OP claims exhibited a lower average Medicare spend than CAR T IP (p < 0.001), of $414,393, ranging from $276,980 to $849,878 (median = $421,729). Within the CAR T OP setting, the average Medicare spending was significantly higher for tisa-cel than axi-cel (p = 0.041).
While assessing the index CAR T service by product, the results revealed a significant variation in the average length of stay (ALOS) among the IP CAR T group (p < 0.005); however, a similar pattern was not seen in the OP setting (where claims do not have an analogous “length of stay”).
Claim charges (which are hospital-specific accounting of their services and may not actually represent the payment received) were also significantly higher (p < 0.001) in the IP setting with an average of ~ $2.5 million compared to ~ $1.6 million in the OP setting, as shown in Table 3. The differences in total claim charge between the CAR T products was not statistically significant, and there was remarkable similarity between products within each setting of care.
Evaluating Downstream HRU Post-CAR T
When evaluating HRU after CAR T, we evaluated spending across all sites of care in Medicare (i.e., total cost of care across all of Parts A and B). Table 4 summaries per-patient-per-month (PPPM) spending in both the 6-month pre- and 3-month post-periods.
OP CAR T patients showed significantly higher subsequent total cost of care (TCOC) compared to IP CAR T ($15,794 vs. $10,244, p < 0.0001). For the OP CAR T, 47% of the PPPM post-CAR T spending was from subsequent IP utilization, in contrast to the corresponding 31% of TCOC in the IP setting for IP CAR T. Other sites of care that had higher (non-significant) spend for OP CAR T included: Part B drugs, OP hospital, physician office oncology care, and physician specialists. The IP CAR T cohort had higher PPPM spending (also non-significant) in general practitioner, home health, skilled nursing facility (SNF), durable medical equipment (DME), and hospice.
The significantly higher TCOC rate and IP spending for the OP CAR T cohort was largely due to the rate of subsequent IP admissions (within 30 days) of the index CAR T event. IP hospital admission after CAR T is summarized in Table 5. Among patients receiving IP CAR T, 21% of patients were (re-)admitted to IP hospital within 30-days of discharge from the index CAR T event. OP CAR T had a significantly higher rate of subsequent IP admission (59%, p < 0.0001). Days between index CAR T discharge and IP admission were also significantly shorter for OP CAR T compared to IP CAR T (8.0 vs. 14.1 days, p < 0.0001). Additionally, IP CAR T had a longer ALOS on the admission claim (6.9 vs. 6.2 days), and the average Medicare admission payment was higher (~ $26.9K vs. $22.8K).
Costs of Total CAR T Episode of Care
As shown above, when comparing OP CAR T versus IP CAR T total cost of care (TCOC) for both 3 months pre-period and 6 months post-period (Table 4), the OP CAR T is associated with higher TCOC for both follow-up time periods. However, as shown in Fig. 6 the total spending during the entire episode of CAR T (i.e., the sum of the CAR T claim, 3 months and 6 months average PPPM spending) for beneficiaries receiving OP CAR T incurred lower cumulative costs for the complete episode of care, totaling $529,188, compared to the IP CAR T cohort where the total episode of care incurred costs of $587,908; this discrepancy appears to be due to the higher cost of the CAR T procedure itself in the IP setting.
Total Medicare spend for CAR T episode (i.e. CAR T infusion and 3 months post-CAR T spend). This figure demonstrates that, while post-CAR T spend is higher for CAR T administered in the OP setting, the total episode of care remains significantly lower than for CAR T administered in the IP setting. CAR T chimeric antigen receptor T cell
Discussion
This real-world analysis of Medicare patients with DLBCL CAR T has found distinct patterns in healthcare utilization between IP and OP settings of care. Our study identified 391 patients, predominantly administered CAR T in the IP setting. Notably, this IP group incurred higher Medicare costs for the index CAR T infusion with a mean expense of about $498,723 compared to $414,393 in the OP setting. While there were clear differences in total claim charges between the two settings of care (~ $2.5 million for IP CAR T compared to ~ $1.6 million for OP CAR T), there was also similarity across products in total charges within each setting.
The trend towards OP administration of CAR T therapy, particularly in aggressive B-cell lymphomas and acute lymphoblastic leukemia, has gained momentum. This shift, supported by a growing understanding of CAR T therapy's safety profile and improved clinical management protocols, highlights its feasibility and safety in the OP setting [18, 19]. The approach may offer reduced health resource utilization and improved use of health system capacity for clinically appropriate patients [20].
Importantly, this study emphasizes the complexity of Medicare reimbursement for CAR T therapy. The IP cohort reports a higher reimbursement, (both in total claim charges, as well as in approved Medicare payments) which may reflect Medicare's payment guidelines. While each product showed usage in both IP and OP settings of care, our claims demonstrated that all three CAR T products had more use in the IP setting (axi-cel 173 vs. 26, liso-cel 87 vs 26, and tisa-cel vs. 47 vs. 32, IP vs. OP, respectively). In addition, while the claim counts are fairly low for OP CAR T (84 for OP compared to 307 IP), there was a notably higher rate of subsequent hospital admission (within 30 days) for patients receiving their index CAR T infusion as OPs (59% vs. 21%, p < 0.0001). Overall, while there was some variance in the component costs from the total episode of care (from 6 months before index, and through 3 months after the index CAR T), the total costs for the IP CAR T episode were $587,908 compared to $529,188 for OP. Taken together, these findings underscore the complexity of integrating such advances into healthcare systems, especially as the treatment landscape continues to evolve [21,22,23].
This study provided an analysis of the claims associated with DLBCL CAR T therapies between the IP and OP settings of care, which could be helpful in sha** future strategies and guidelines for this innovative treatment. While detailed, there are certain limitations common to retrospective claims studies such as this, including the reliance on a financial reporting system such as the Medicare Fee-for-Service program, which does not provide accompanying clinical detail. Also, Medicare claims lack the depth and level of control available in prospective clinical trials, and are more reflective of the real-world in which clinicians practice [24]. In sum, claims are not collected for research purposes, but rather for financial and reimbursement purposes.
In addition, the use of Medicare data limits the generalizability of the findings to other populations. Medicare patients, typically an older population with distinct comorbidities (often including renal disorders), which may differ from younger populations or those with different healthcare coverage. Clinical trials undertaken for regulatory approval often include participants who may not represent the broader real-world population of patients with DLBCL who receive CAR T therapy outside the trial setting [25, 26]. Additionally, cost analyses in this context may not account for all indirect costs or long-term financial impacts of CAR T therapy, and may also be limited to the payer or PBMs perspective, rather than the broader societal perspective.
Since the FDA’s approval of the first CAR T product, there have been many discussions focused on manufacturing costs, market dynamics, and patient access to innovative therapies. For example, adjustments in Medicare payments has impacted hospital IP reimbursements for CAR T therapies [27]. These changes are part of a broader discussion regarding cost compared to traditional treatments, which is confounded by the changing treatment paradigm as CAR T moves into earlier lines of therapy [28]. These findings provide an update to other recent similar Medicare claims analyses, especially with respect to patterns of index CAR T total episode costs, subsequent resource use and also for the HRU associated with CAR T administered in the different settings of care (IP vs. OP), although there were meaningful differences between the products included in the earlier analysis (only tisa-cel and axi-cel were available then), as well as the time period for their analysis (for instance, prior to establishment of the CAR T DRG and/or reimbursement) that make it more of an apples-to-oranges comparison [19].
In conclusion, this discussion provides a real-world analysis of the state of CAR T cell therapies in treating DLBCL in the Medicare population, reinforcing several recent reports addressing the IP versus OP setting of care, and subsequent re-admissions. While it is important to consider limitations common to similar retrospective claims analyses when interpreting these findings, it should also be noted that many of the recent OP programs have been based on significant institutional experience and learning surrounding the safety profile of CAR T therapies. Future research should aim to include more diverse patient populations and to consider prospective studies to validate and expand upon these findings, while also recognizing the changing treatment paradigm, and by including a societal perspective.
Data Availability
Data sharing is not applicable due to the use of the Chronic Condition Warehouse (CCW) Virtual Research Data Center (VRDC), which is the intellectual property of the CCW.
References
Teras LR, et al. 2016 US lymphoid malignancy statistics by World Health Organization subtypes. CA Cancer J Clin. 2016;66(6):443–59.
Chapuy B, et al. Molecular subtypes of diffuse large B cell lymphoma are associated with distinct pathogenic mechanisms and outcomes. Nat Med. 2018;24(5):679–90.
Chan JY, et al. Evolving therapeutic landscape of diffuse large B-cell lymphoma: challenges and aspirations. Discov Oncol. 2023;14(1):132.
Feins S, et al. An introduction to chimeric antigen receptor (CAR) T-cell immunotherapy for human cancer. Am J Hematol. 2019;94(S1):S3–9.
Roschewski M, Longo DL, Wilson WH. CAR T-cell therapy for large B-cell lymphoma—who, when, and how? N Engl J Med. 2021;386(7):692–6.
Shargian L, et al. Chimeric antigen receptor T-cell therapy is superior to standard of care as second-line therapy for large B-cell lymphoma: a systematic review and meta-analysis. Br J Haematol. 2022;198(5):838–46.
Elsawy M, et al. Patient-reported outcomes in ZUMA-7, a phase 3 study of axicabtagene ciloleucel in second-line large B-cell lymphoma. Blood. 2022;140(21):2248–60.
Hopfinger G, Jäger U, Worel N. CAR-T cell therapy in diffuse large B cell lymphoma: hype and hope. Hemasphere. 2019;3(2): e185.
Johnson PC, et al. Longitudinal patient-reported outcomes in patients receiving chimeric antigen receptor T-cell therapy. Blood Adv. 2023;7(14):3541–50.
Cappell KM, Kochenderfer JN. Long-term outcomes following CAR T cell therapy: what we know so far. Nat Rev Clin Oncol. 2023;20(6):359–71.
Sermer D, et al. Outcomes in patients with DLBCL treated with commercial CAR T cells compared with alternate therapies. Blood Adv. 2020;4(19):4669–78.
CMS. National coverage determination (NCD 110.24): chimeric antigen receptor (CAR) t-cell therapy. CMS; 2019.
ASTCT. ASTCT CAR-T therapy coding and billing guide. ASTCT; 2024.
Lochner KA, Cox CS. Prevalence of multiple chronic conditions among medicare beneficiaries, United States, 2010. Prev Chronic Dis. 2013;10:E61.
Kilgore KM, et al. Medicare patients receiving chimeric antigen receptor T-cell therapy for non-Hodgkin lymphoma: a real-world look at patient characteristics, healthcare utilization and costs. Biol Blood Marrow Transplant. 2020;26(3):S43–4.
Westin JR, et al. Safety and efficacy of axicabtagene ciloleucel versus standard of care in patients 65 years of age or older with relapsed/refractory large B-cell lymphoma. Clin Cancer Res. 2023;29(10):1894–905.
Jacobson CA, et al. Real-world evidence of axicabtagene ciloleucel for the treatment of large B cell lymphoma in the United States. Transpl Cell Ther. 2022;28(9):581.e1-581.e8.
Myers GD, et al. Perspectives on outpatient administration of CAR-T cell therapy in aggressive B-cell lymphoma and acute lymphoblastic leukemia. J Immunother Cancer. 2021;9(4): e002056.
Hansen DK, et al. The impact of outpatient versus inpatient administration of CAR-T therapies on clinical, economic, and humanistic outcomes in patients with hematological cancer: a systematic literature review. Cancers. 2023;15(24):5746.
Oluwole OO, et al. Chimeric antigen receptor T-cell therapy in the outpatient setting: an expert panel opinion from the American Society for Transplantation and Cellular Therapy. Transpl Cell Ther. 2024;30(2):131–42.
Oluwole OO, et al. An updated cost-effectiveness analysis of axicabtagene ciloleucel in second-line large B-cell lymphoma patients in the United States. J Med Econ. 2024;27(1):77–83.
Cliff ERS, et al. High cost of chimeric antigen receptor T-cells: challenges and solutions. Am Soc Clin Oncol Educ Book. 2023;43: e397912.
Potnis KC, et al. Cost-effectiveness of chimeric antigen receptor T-cell therapy in adults with relapsed or refractory follicular lymphoma. Blood Adv. 2023;7(5):801–10.
Sherman RE, et al. Real-world evidence—what is it and what can it tell us? N Engl J Med. 2016;375(23):2293–7.
Neelapu SS, et al. Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N Engl J Med. 2017;377(26):2531–44.
Locke FL, et al. Long-term safety and activity of axicabtagene ciloleucel in refractory large B-cell lymphoma (ZUMA-1): a single-arm, multicentre, phase 1–2 trial. Lancet Oncol. 2019;20(1):31–42.
Gustafson K et al. CAR-T reimbursement updated in FY 2024 IPPS final rule; 2024.
Choi G, Shin G, Bae S. Price and prejudice? The value of chimeric antigen receptor (CAR) T-cell therapy. Int J Environ Res Public Health. 2022;19(19):12366.
Funding
Financial support including the Rapid Service Fee and Open Access Fee for the study were provided by Kite Pharma, A Gilead Company.
Author information
Authors and Affiliations
Contributions
James Wu: Overall study supervision, Concept and design, manuscript review, drafting the manuscript. Armin Ghobadi: Data interpretation, manuscript review. Richard Maziarz: Data interpretation, manuscript review. Krish Patel: Data interpretation, manuscript review. Hil Hsu: Data interpretation, manuscript review. Zihao Liu: statistical analysis, drafting the manuscript, manuscript review. Caitlin Sheetz: Concept and design, manuscript review. Peter Kardel: statistical analysis, drafting the manuscript, manuscript review. Christine Fu: Data interpretation, manuscript review.
Corresponding author
Ethics declarations
Conflict of Interest
Hill Hsu is employee of Kite Pharma, A Gilead Company and Christine Fu and James Wu are former employees of Kite Pharma, A Gilead Company. Zihao Liu, Peter Kardel, and Caitlin Sheetz are employees of ADVI Health Inc. Richard Maziarz reports serving as consultant for Autolous, Kite/Gilead, and Novartis, research support from Gamida, Allovir, OrcaBio, Kite/Gilead and Novartis, participating in a DSMB for Athersys, Novartis, Century Therapeutics and VorPharma and a patent with Athersys; no activity has conflict with the material of this article. Armin Ghobadi serving as consultant/advisory board for WUGEN, Atara pharmaceuticals, Celgene/BMS, CRISPR Therapeutics, Autolus, ADC Therapeutics, Nurix Therapeutics, serving as consultant and provide research support for Kite, a Gilead Company and Amgen Inc and Genentech. Krish Patel serve as consultant or received research funding from AstraZeneca, Beigene, BMS, Loxo, Gentech/Roche, Kite, Century, CRISPR, Caribou, Curis, Sana, Fate Therapeutics, Xencor, ADC, Janssen, Nurix, Pfizer, Pharmacyclics.
Ethical Approval
This article does not include any research involving human participants or animals that was conducted by any of the authors. All data and findings presented in this paper are derived from secondary sources and data was not identifiable.
Additional information
Prior Presentation: Tandem Meetings. Transplantation & Cellular Therapy Meetings of ASTCT® and CIBMTR®, February 15-19, Orlando FL, USA.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Wu, J., Ghobadi, A., Maziarz, R. et al. Medicare Utilization and Cost Trends for CAR T Cell Therapies Across Settings of Care in the Treatment of Diffuse Large B-Cell Lymphoma. Adv Ther (2024). https://doi.org/10.1007/s12325-024-02917-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12325-024-02917-7