Abstract
Introduction
Switching disease-modifying therapy (DMT) may be considered for relapsing–remitting multiple sclerosis (RRMS) if a patient’s current therapy is no longer optimal. This was particularly important during the recent COVID-19 pandemic because of considerations around immune deficiency and impaired vaccine response associated with B cell-depleting DMTs. This real-world, single-center study aimed to evaluate change or decline in functional ability and overall disease stability in people with RRMS who were switched from B cell-depleting ocrelizumab (OCRE) to diroximel fumarate (DRF) because of safety concern related to the COVID-19 pandemic.
Methods
Adults with RRMS were included if they had been clinically stable for ≥ 1 year on OCRE. Data collected at baseline and 1 year post switch included relapse rate, magnetic resonance imaging (MRI), blood work for assessment of peripheral immune parameters, the Cognitive Assessment Battery (CAB), optical coherence tomography (OCT), and patient-reported outcomes (PROs).
Results
Participants (N = 25) had a mean (SD) age of 52 (9) years, and a mean (SD) duration of 26 (8) months’ treatment with OCRE before the switch to DRF. Median washout duration since the last OCRE infusion was 7 months (range 4–18 months). No participants relapsed on DRF during follow-up, and all remained persistent on DRF after 1 year. There were no significant changes in peripheral immune parameters, other than an increase in the percentage of CD19+ cells 1 year after switching (p < 0.05). Similarly, there were no significant changes in CAB, OCT, and PROs.
Conclusion
These preliminary findings suggest that transition to DRF from OCRE may be an effective treatment option for people with RRMS who are clinically stable but may need to switch for reasons unrelated to effectiveness. Longer follow-up times on larger samples are needed to confirm these observations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
People with multiple sclerosis may have concerns over immune suppression. |
We assessed outcomes in patients who switched from ocrelizumab to diroximel fumarate. |
No participants relapsed during 1-year follow-up and all remained on treatment |
No significant change in peripheral immune parameters, except increased CD19+ cells. |
No significant changes in cognitive, ocular, or patient-reported outcomes were observed. |
Introduction
There are currently over 20 disease-modifying therapies (DMTs) available for the treatment of relapsing–remitting multiple sclerosis (RRMS) including oral, injectable, and infused medications [1]. The choice of DMT in people with multiple sclerosis (MS) is typically considered on the basis of efficacy and safety, as well as frequency of treatment and route of administration [2]. Escalation, de-escalation, or switching of DMT may be considered if a DMT is no longer optimal for a person with MS [3, 4], and understanding when to make such treatment decisions is of key importance to optimizing the balance of risks and benefits [5]. During the recent global COVID-19 pandemic, other factors related to DMT choice, such as the ability to mount an effective response to vaccination and risk of infection, had to be carefully considered.
In RRMS, B cell-depleting therapies have demonstrated marked reduction in relapses and in disability worsening, and their effect on disease activity as measured by magnetic resonance imaging (MRI) is robust [6]. However, their high efficacy is not without risk. B cell-depleting therapies are reported to be associated with a reduction of protective immunoglobulin synthesis [7], increased rate of infections [8,9,10], and impaired humoral immune response to vaccination [11, 12], albeit T cell response to vaccination may be preserved [13]. Infections are of particular concern as they have been reported to cause pseudo-relapses [14, 15] and, consequently, worsening of neurologic symptoms [16]. Furthermore, there is evidence to suggest that exacerbations associated with systemic infection in people with MS may lead to more sustained neurological damage than non-infection-related exacerbations [17]. Should de-escalation be considered as a result of concerns of immune deficiency, infection risk, impaired vaccine response, immunosenescence or other factors, a DMT from the fumarates class may be an alternative option for disease control. The benefits include a potential reduced risk of infections [8] and improved ability to mount antibody responses to both infections and vaccines compared with B cell-depleting therapies [18, 19]. In addition, a de-escalation strategy may offer the potential for better disease control in some patients compared to DMT discontinuation. In a randomized, controlled, non-inferiority trial, there was an increased risk of new MRI activity in patients older than 55 years with stable disease who discontinued DMT compared to those who remained on treatment [20].
Diroximel fumarate (DRF) is an orally administered fumarate approved in the USA for adults with relapsing forms of MS, and in Europe for adults with RRMS [21, 22]. As of 31 December 2022, approximately 33,989 people had been treated with DRF, representing 35,420 patient-years of exposure. Of these, 1477 people (1718 patient-years) were from clinical trials [23]. Switching treatment to DRF has been reported previously for people with MS who had received glatiramer acetate or interferons as their most recent DMT before initiating DRF in the open-label, phase 3 EVOLVE-MS-1 study (NCT02634307); in these participants, no new safety signals were observed for DRF, and favorable clinical outcomes were reported [24].
During the COVID-19 pandemic, in the pre-COVID vaccine era, several studies have shown increased risk of severe COVID-19, hospitalization, ICU admission, the need for artificial ventilation, and mortality among people with MS who were on anti-CD20 medications [25, 26]. At the height of the pandemic, many patients raised concern about continuing ocrelizumab (OCRE) in the setting of increased risk of severe COVID-19 infection. For those who were stable for at least 1 year on OCRE and for whom the risk of severe COVID-19 was deemed greater than the risk of MS exacerbation, a shared decision was made during a routine follow-up meeting to de-escalate treatment. DRF was offered as a suitable substitute owing to its short elimination time, if needed during active infection and because no COVID-19-related safety concerns had been raised with regard to this DMT.
The purpose of this study was to evaluate and track changes in functional ability and overall disease stability in people with RRMS who were switched from the B cell-depleting DMT OCRE to DRF by examining clinical outcomes as well as changes found in peripheral immune parameters, cognitive functioning, optical coherence tomography (OCT) testing, MRI, and patient-reported outcomes (PROs).
Methods
Study Design and Participants
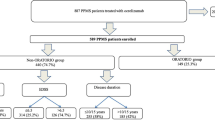
In this real-world, single-center study, all people with RRMS under our care, who switched from treatment with OCRE to DRF, as a result of COVID-19-related concerns were included.
Data were collected at baseline (DRF initiation) and 1 year after the switch. The washout period was calculated as the duration between the last infusion date of OCRE and the first dose of DRF. Although the exact day a patient took the medication based on chart notes alone is difficult to confirm, the closest DRF start dates were noted on the basis of all available documentation. The washout period between treatment switch ranged from 4.2 to 18.4 months (Supplementary Table 1). Those eligible for inclusion were patients treated at South Shore Neurologic Associates Comprehensive MS Care Center, aged ≥ 18 years, had a confirmed diagnosis of RRMS according to McDonald criteria [27], had been clinically stable for ≥ 1 year on OCRE, with an Expanded Disability Status Scale (EDSS) score between 0 and 6.5. Participants were excluded if they had a history of head injury, seizures, or neurological conditions other than MS; a history of drug or alcohol abuse; active psychosis; immunoglobulin (Ig) G < 300 mg/dL; or absolute lymphocyte count (ALC) < 0.5 × 109/L. The exclusion criterion for ALCs was set because fumarates have a recommendation to discontinue therapy if patients have persistent severe lymphopenia (ALC < 0.5 × 109/L).
Data Collection
Data were collected for relapse history; MRI; blood work for assessment of peripheral immune parameters, including concentrations of IgG, IgA, IgM, and IgG subclasses 1–4, and levels of CD4+ , CD8+ , and CD19+ cells; a Cognitive Assessment Battery (CAB) validated for people with MS (NeuroTrax™; NeuroTrax, Medina, NY) assessing memory, processing speed, visuospatial and executive function, among others [28]; OCT using a Heidelberg SPECTRALIS® OCT imaging platform (Heidelberg Engineering Inc., Franklin, MA); and Patient Determined Disease Steps (PDDS), a PRO developed by the North American Research Committee on MS (NARCOMS). PDDS scores range from 0 (normal) to 8 (bedridden). Clinical and PROs are summarized in Table 1.
Brain MRI images were obtained on a 1.5 T, Hitachi Echelon scanner. High-resolution 3D T1-weighted sequences were used to count gadolinium-enhancing lesions (acquisition voxel, 1 × 1 × 1 mm3; field of view, 224 × 224 nm2; inversion time, 666 ms; repetition time, 10.8 ms; echo time, 3.7 ms; flip angle, 10°). Fluid-attenuated inversion recovery (FLAIR) sequences were used for hyperintense lesion count (acquisition voxel, 1 × 1 × 2 mm3; field of view, 256 × 256 mm2; inversion time, 2600 ms; repetition time, 10,179 ms; echo time, 90 ms; flip angle, 90°).
Statistical Analysis
Protocol-defined MS relapse was defined as new or recurrent neurologic symptoms (not associated with fever/infection) lasting ≥ 24 h and accompanied by new neurological findings and change in EDSS score. Paired t tests were used to determine statistically significant differences between data collected at baseline and 1 year post switch (p < 0.05).
Ethics
The study was conducted according to local regulations and in compliance with the principles of the Declaration of Helsinki 1964. All instructions, regulations, and agreements of the protocol, and applicable International Conference on Harmonisation guidelines, were adhered to, and the study protocol was approved.
Results
Participants
Twenty-five people with RRMS who switched from OCRE to DRF met the eligibility criteria and were included in the study. The reason for DMT change for all participants was concern over immunosuppression during the COVID-19 pandemic. Initially 32 patients were identified to switch; however, five were excluded as a result of having < 1 year of being clinically stable on OCRE, and two were excluded for having EDSS scores > 6.5. On average, participants were mean (SD) age of 52 (9) years, and 16/25 (64%) were female. Sixteen percent of patients had a relapse in the year prior to OCRE initiation, but none had active MRI (Table 2). OCRE was started in some people with MS with inactive disease because it is well known that MS-related disease progression may continue independent of relapse and MRI activity and high-efficacy DMTs, such as OCRE, can prevent this silent progression more efficiently than low-efficacy DMTs [29].
The mean (SD) duration of treatment with OCRE before the switch to DRF was 26 (8) months. No relapses were recorded on OCRE. Only one patient had several non-enhancing new T2 lesions on MRI, performed on OCRE, before switching to DRF, 12 months following OCRE start, compared to a previous scan, 2 months prior to OCRE start. This patient had a clinical relapse immediately prior to OCRE initiation. The mean (SD) interval from the last dose of OCRE to initiation of DRF was 8.3 (3.4) months. All patients received OCRE infusions every 6 months. Study follow-up was 1 year for all 25 patients; zero patients were lost to follow-up.
Relapse Rate, MRI, Persistence, and Patient-Reported Outcomes
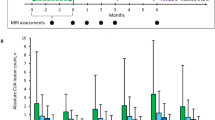
No participants relapsed on DRF during follow-up, and all remained persistent on DRF at 1 year (Table 3). MRI activity, as assessed 6–12 months post DRF start, remained stable, and none of the patients had active MRI after de-escalation to DRF. There was no significant difference observed between scores on any of the PROs assessed at baseline (DRF initiation) and at 1 year following the switch to DRF (n = 17; all p > 0.05) (Fig. 1 and Supplementary Fig. 1).
Median PRO scores at baseline on OCRE and 1 year post switch to DRF. Median and interquartile range is shown. Higher scores indicate worse outcome for PROs, except for PROMIS Social Roles. DRF diroximel fumarate, HADS-A Hospital Anxiety and Depression Scale—Anxiety, HADS-D Hospital Anxiety and Depression Scale—Depression, MFES Modified Falls Efficacy Scale, MSWS-12 12-Item Multiple Sclerosis Walking Scale, OCRE ocrelizumab, PDSS Patient Determined Disease Steps, PRO patient-reported outcome, PROMIS Patient-Reported Outcome Measurement Information System
Cognitive Assessment Battery
There was no significant difference observed between CAB assessments at baseline (DRF initiation) and those carried out 1 year after switching to DRF (n = 17; all p > 0.05), with most CAB parameters representing average cognitive function at both assessment points (Fig. 2).
Mean CAB parameters at baseline on OCRE and 1 year post switch to DRF. Error bars represent SD. Cognitive Assessment Domain Scores: > 115 = above average; 115–100 = average; 99–85 = below average; < 85 = abnormal. CAB Cognitive Assessment Battery, DRF diroximel fumarate, OCRE ocrelizumab, SD standard deviation
Ocular Tomography
OCT assessments showed no significant difference between retinal nerve fiber layer (RNFL) thickness measured at baseline following treatment with OCRE and at 1 year after the switch to DRF (n = 12; all p > 0.05) (Table 4).
Blood Work
Peripheral immune parameters were available for 23 patients before starting DRF, as well as for 15 patients after a year on DRF (Table 5). There were no significant changes in levels of IgG, IgA, IgM, IgG subclasses 1–4, CD4+ cells, CD8+ cells, or CD4+/CD8+ ratio between baseline (DRF initiation) and 1 year post switch (all p > 0.05 for the 15 patients with available measurements at two time points). There was, however, a significant (p < 0.05) increase in the percentage of CD19+ cells 1 year after switching to DRF compared with baseline measurements following treatment with OCRE (Table 5). Notably, 7 out of 15 patients (47%) for whom CD19+ count on DRF was available still had CD19+ count below the lower limit of normal, i.e., < 70 cells/µL. Mean (SD) ALC count was 1327 (343) cells/µL at baseline versus 1480 (704) cells/µL at 1 year post switch.
Adverse Effects
One patient on OCRE reported significant post-infusion fatigue. Five patients on DRF reported flushing and four patients on DRF reported gastrointestinal discomfort.
Discussion
In this study of people with RRMS who switched from OCRE to DRF treatment as a result of concerns around immunosuppression during routine clinical care, all participants remained relapse free and had no MRI activity after 1 year of follow-up. After the switch in treatment, there were no significant changes in peripheral immune parameters apart from the expected rise in CD19+ B cell count, cognitive outcomes, or OCT outcomes (all p > 0.05); PROs also remained stable. Mean PDDS showed a small increase from 3.83 at baseline to 4.23 after 1 year on DRF, but this was not statistically significant.
This study is the first to report outcomes in a cohort of clinically stable people with MS who switched from OCRE to DRF. Although the results should be interpreted with caution because of a limited sample size and duration of follow-up, they are encouraging. The findings from this study support DRF as a potential effective treatment option for people who have been stable on OCRE but may need to switch for reasons unrelated to effectiveness.
Making decisions about when to escalate, de-escalate, or switch DMTs should be based on disease activity. Disease activity quantified by MRI is a strong determinant of treatment switch, particularly in younger people with RRMS [30]. In our study of adults with RRMS, we used OCT as one of the measures to monitor disease progression. OCT is a rapid, noninvasive, high-resolution imaging technique [31] that can be applied to provide objective, examiner-independent measurements that do not rely on EDSS, MRI, or neurologic assessment. OCT measurements have previously demonstrated a thinner RNFL in individuals with MS versus healthy volunteers [32]; moreover, cross-sectional and longitudinal monitoring of RNFL thickness has been shown to predict both physical and cognitive decline in MS [33,34,35]. Our OCT findings of no significant change in RNFL thickness 1 year following the switch to DRF are therefore reassuring, given that previous studies report OCT cutoffs for cross-sectional measurements of the RNFL of ≤ 87 or ≤ 88 μm (depending on OCT device used) [34, 35], and rate of peripapillary RNFL thinning of > 1.5 μm/year [34], as increasing the risk for disease progression in subsequent years. Supporting the outcomes of the quantitative measures, PROs were assessed across several domains, and all remained stable 1 year after switching to DRF. PROs allow for better understanding of people’s interests and needs regarding their treatment and may also encourage patient participation in treatment management decisions [24, 36, 37].
As expected, we observed a significant increase in CD19+ B cells after 1 year of DRF treatment (Table 5), although, notably, about half of available CD19 counts were still below the lower limit of normal after 1 year on DRF. The slow rate of B cell repopulation undoubtedly contributed to maintained stability. Still, it is reassuring that recurrence of disease activity was not observed, despite the clear increase in CD19 count after switching to DRF.
The importance of B cells in MS pathophysiology has been widely published, and previous studies of anti-CD20 therapies have reported a prolonged duration of action beyond their continuous administration period. Depletion data have shown a median time to B cell repletion of 72 weeks (range 27–175) following a final infusion of 600 mg OCRE; lower limit of normal was defined as 80 cells/μl [38]. A phase 2 study of 104 people with RRMS treated with a single dose of rituximab resulted in remission from both clinical and MRI disease activity over 48 weeks [39]. Rituximab maintenance treatment leads to sustained B cell depletion, although early B cell repopulation has been observed in people with MS during the first 2 years of treatment. A recent study reported that early repopulation of B cells (> 5 cells/mm3) in rituximab-treated people occurred at least once in 38.5% of patients (n = 70/182 patients) with MS and was not associated with a risk of relapse or clinical worsening. Interestingly, in some patients it was correlated with increased clinical benefit [40]. In a retrospective observational study of 225 people with MS conducted in Sweden, no statistically significant differences in relapse rate or MRI activity were observed between people with MS who stopped using rituximab and those who remained on regular treatment [41].
In the phase 2 study of OCRE, 133 people with MS were monitored for 48 weeks without treatment, after previously receiving three cycles of therapy [42]. The reduced relapse rate and MRI activity that was reported persisted into the treatment-free period despite CD19+ B cell repopulation. Our data further support a potential prolonged period of effectiveness of anti-CD20 therapies beyond their regular administration period, in addition to the beneficial effects of DRF administration. Some studies have addressed the differential repopulation of B cells in patients with RRMS after treatment with DRF—including increased numbers of circulating B cells with naïve and regulatory capacity [43,44,45] and altered cytokine production [44]. Therefore, an altered repopulation of B cells has been suggested as a potential mechanism for this long-standing efficacy [46]. Additionally, a possible combination mechanism between the two treatments might account for the longer-term efficacy as people transition from anti-CD20 to DRF. OCRE has been found to be safe and effective in treating people with MS [47, 48]. However, it is important to consider the differences between DMTs in terms of longer-term risk of infection [10] and patient response to vaccination—particularly when looking at when to switch treatments. A retrospective analysis of national data collected prospectively and longitudinally in England found a substantial increase in the risk of SARS-CoV-2 infection in people with MS treated with OCRE compared with the general population, despite widespread vaccination, although potential confounders such as age, comorbidities, and neurological disability were not taken into account in this study [49]. Studies have shown that people taking OCRE and other B cell-depleting DMTs have reduced antibody and memory B cell responses to SARS-CoV-2 vaccines [12, 50,51,52,53]. However, these people can mount a vaccine-specific T cell response [50,51,52], and T cells might provide protection from severe disease by limiting viral replication to the upper respiratory tract [51, 54]. The failure to mount a humoral response to SARS-CoV-2 vaccination with anti-CD20 therapies is likely due to the mode of action of this class of DMTs. In contrast, the humoral response to SARS-CoV-2 vaccination appears to be well preserved in people with MS treated with fumarates [19, 53].
The COVID-19 pandemic introduced new uncertainties into clinical decision-making regarding the balance between efficacy and safety of DMTs. Recent epidemiological studies have shown that despite increased risk of breakthrough COVID-19 on high efficacy DMTs, such as OCRE, vaccination may still be effective in preventing severe infection regardless of DMT [55, 56]. Taken together, DMT choice in the post-vaccination COVID-19 era should still be guided first and foremost by MS disease activity; however, the aforementioned signals of increased risk of breakthrough infection on high efficacy DMTs, such as OCRE, call for considering personal risk of infection during therapeutic decision-making, especially if MS is not highly active.
Another explanation of the apparent stability of this cohort despite de-escalation may be related to age. Indeed, transitioning from a highly efficacious DMT (i.e., OCRE) to a less potent one (i.e., DRF) could entail a reduced level of risk, especially within an older population akin to the study sample. It has been suggested by a meta-analysis of randomized, blinded, MS clinical trials that age has a defining effect on the therapeutic efficacy of immunomodulatory DMTs, so that the differential efficacy of DMTs in inhibiting MS disability is lost with ageing [57]. It was reported that differences between higher-efficacy and moderate-efficacy treatments are typically only demonstrated during earlier stages of MS; therefore, the predicted benefit of receiving immunomodulatory DMTs after the age of 53 years may be limited when considered alongside the exposure to treatment-associated risks [57]. This highlights that higher-efficacy treatments may have more potential benefit in younger people with MS, which should be taken into consideration when initiating and switching therapies. Furthermore, clinical trials evaluating anti-CD20 therapy in progressive MS primarily found more benefit in younger, less disabled people with more inflammatory disease activity. One study reported that 40% of older people with progressive MS still progressed on anti-CD20 therapy (60% remained stable or improved) after 2 years on OCRE, impacting the overall benefit/risk ratio of this treatment [58].
Limitations of this study include those inherent to real-world studies of people with MS in routine clinical care, such as confounding factors, the absence of a comparison arm, and outcomes not being available for all participants. The examined population, being a limited sample, at least partly comprising patients with modest disease activity, impacts the generalizability of these findings. The brief follow-up duration further limits assessing the net value of switching to DRF compared to OCRE’s prolonged effects. This time constraint limits the comprehensive understanding of the long-term implications of the treatment switch. In addition to the limited follow-up time of the study, the sample size of the study was modest since the study was performed proactively during the COVID-19 pandemic, before much of the data became available indicating increased risks of infections and complications on B cell therapies. In addition, longer follow-up time may be required in order to detect significant changes on RNFL thickness.
Conclusion
This analysis characterizes outcomes in people with RRMS who switched from OCRE to DRF in routine care as a result of concerns over immunosuppression during the global COVID-19 pandemic, with a median washout duration of 7 months (range 4–18 months) since the last OCRE infusion. After 1 year of DRF treatment, all patients remained relapse free as well as free from MRI activity. No participants discontinued DRF after 1 year of treatment. PROs, cognition, and OCT outcomes remained stable and there were no significant changes in peripheral immune parameters, aside from increased CD19 counts. These findings suggest that transition to DRF from OCRE because of safety considerations is a potential reasonable treatment strategy for people with RRMS who are stable on OCRE. On the basis of the limited data presented herein, the switch might be feasible primarily for patients exhibiting modest disease activity and of relatively advanced age. Further exploration is warranted prospectively in a larger patient population with longer follow-up.
Data Availability
Request for the data supporting this manuscript should be submitted to https://vivli.org/.
References
National Multiple Sclerosis Society. Disease-modifying therapies for MS 2022. https://www.nationalmssociety.org/managingms/treating-ms/disease-modifying-therapies. Accessed 9 Dec 2022
Burtchell J, Clemmons D, Clemmons J, et al. A targeted literature search and phenomenological review of perspectives of people with multiple sclerosis and healthcare professionals of the immunology of disease-modifying therapies. Neurol Ther. 2022;11(3):955–79.
Cohan SL, Moses H, Calkwood J, et al. Clinical outcomes in patients with relapsing-remitting multiple sclerosis who switch from natalizumab to delayed-release dimethyl fumarate: a multicenter retrospective observational study (STRATEGY). Mult Scler Relat Disord. 2018;22:27–34.
Kresa-Reahl K, Repovic P, Robertson D, Okwuokenye M, Meltzer L, Mendoza JP. Effectiveness of delayed-release dimethyl fumarate on clinical and patient-reported outcomes in patients with relapsing multiple sclerosis switching from glatiramer acetate: RESPOND, a prospective observational study. Clin Ther. 2018;40(12):2077–87.
Rae-Grant A, Day GS, Marrie RA, et al. Practice guideline recommendations summary: disease-modifying therapies for adults with multiple sclerosis: report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology. Neurology. 2018;90(17):777–88.
Sellebjerg F, Blinkenberg M, Sorensen PS. Anti-CD20 monoclonal antibodies for relapsing and progressive multiple sclerosis. CNS Drugs. 2020;34(3):269–80.
Fernández-Velasco JI, Kuhle J, Monreal E, et al. Effect of ocrelizumab in blood leukocytes of patients with primary progressive MS. Neurol Neuroimmunol Neuroinflamm. 2021;8(2):e940.
Nicholas JA, Gudesblatt M, Garabedian M, et al. Dimethyl fumarate is associated with lower rates of infection and lower infection-related healthcare costs when compared with ocrelizumab. Mult Scler Relat Disord. 2022;63: 103921.
Luna G, Al** P, Burman J, et al. Infection risks among patients with multiple sclerosis treated with fingolimod, natalizumab, rituximab, and injectable therapies. JAMA Neurol. 2020;77(2):184–91.
Peters J, Longbrake EE. Infection risk in a real-world cohort of patients treated with long-term B-cell depletion for autoimmune neurologic disease. Mult Scler Relat Disord. 2022;68:104400.
Bar-Or A, Calkwood JC, Chognot C, et al. Effect of ocrelizumab on vaccine responses in patients with multiple sclerosis: the VELOCE study. Neurology. 2020;95(14):e1999–2008.
Achiron A, Mandel M, Dreyer-Alster S, et al. Humoral immune response to COVID-19 mRNA vaccine in patients with multiple sclerosis treated with high-efficacy disease-modifying therapies. Ther Adv Neurol Disord. 2021;14:17562864211012836.
Tortorella C, Aiello A, Gasperini C, et al. Humoral- and T-cell-specific immune responses to SARS-CoV-2 mRNA vaccination in patients with MS using different disease-modifying therapies. Neurology. 2022;98(5):e541–54.
Rodríguez de Antonio LA, García Castañón I, Aguilar-Amat Prior MJ, Puertas I, González Suárez I, Oreja Guevara C. Non-inflammatory causes of emergency consultation in patients with multiple sclerosis. Neurologia (Engl Ed). 2021;36(6):403–11.
Mills EA, Mirza A, Mao-Draayer Y. Emerging approaches for validating and managing multiple sclerosis relapse. Front Neurol. 2017;8:116.
Conway SE, Healy BC, Zurawski J, et al. COVID-19 severity is associated with worsened neurological outcomes in multiple sclerosis and related disorders. Mult Scler Relat Disord. 2022;63: 103946.
Buljevac D, Flach HZ, Hop WC, et al. Prospective study on the relationship between infections and multiple sclerosis exacerbations. Brain. 2002;125(Pt 5):952–60.
Satyanarayan S, Safi N, Sorets T, et al. Differential antibody response to COVID-19 vaccines across immunomodulatory therapies for multiple sclerosis. Mult Scler Relat Disord. 2022;62: 103737.
Jakubecz C, Zhang XS, Woodson S, Serra A, Abboud H. The humoral response to SARS-COV-2 vaccines in MS patients: a case series exploring the impact of DMT, lymphocyte count, immunoglobulins, and vaccine type. Mult Scler Relat Disord. 2022;61:103785.
Corboy JR, Fox RJ, Kister I, et al. Risk of new disease activity in patients with multiple sclerosis who continue or discontinue disease-modifying therapies (DISCOMS): a multicentre, randomised, single-blind, phase 4, non-inferiority trial. Lancet Neurol. 2023;22(7):568–77.
Biogen. Vumerity [Summary of Product Characteristics]. Netherlands2023. p. 2–3.
Biogen. Vumerity [Prescribing Information]. Cambridge, MA2023.
Biogen. Data on file.
Wray S, Then Bergh F, Wundes A, et al. Efficacy and safety outcomes with diroximel fumarate after switching from prior therapies or continuing on DRF: results from the phase 3 EVOLVE-MS-1 study. Adv Ther. 2022;39(4):1810–31.
Sormani MP, De Rossi N, Schiavetti I, et al. Disease-modifying therapies and coronavirus disease 2019 severity in multiple sclerosis. Ann Neurol. 2021;89(4):780–9.
Simpson-Yap S, De Brouwer E, Kalincik T, et al. Associations of disease-modifying therapies with COVID-19 severity in multiple sclerosis. Neurology. 2021;97(19):e1870–85.
Polman CH, Reingold SC, Banwell B, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol. 2011;69(2):292–302.
Golan D, Wilken J, Doniger GM, et al. Validity of a multi-domain computerized cognitive assessment battery for patients with multiple sclerosis. Mult Scler Relat Disord. 2019;30:154–62.
Kappos L, Wolinsky JS, Giovannoni G, et al. Contribution of relapse-independent progression vs relapse-associated worsening to overall confirmed disability accumulation in typical relapsing multiple sclerosis in a pooled analysis of 2 randomized clinical trials. JAMA Neurol. 2020;77(9):1132–40.
Teter B, Agashivala N, Kavak K, Chouhfeh L, Hashmonay R, Weinstock-Guttman B. Characteristics influencing therapy switch behavior after suboptimal response to first-line treatment in patients with multiple sclerosis. Mult Scler. 2014;20(7):830–6.
Galetta KM, Calabresi PA, Frohman EM, Balcer LJ. Optical coherence tomography (OCT): imaging the visual pathway as a model for neurodegeneration. Neurotherapeutics. 2011;8(1):117–32.
Pearson T, Chen Y, Dhillon B, Chandran S, van Hemert J, MacGillivray T. Multi-modal retinal scanning to measure retinal thickness and peripheral blood vessels in multiple sclerosis. Sci Rep. 2022;12(1):20472.
Bsteh G, Hegen H, Teuchner B, et al. Peripapillary retinal nerve fibre layer as measured by optical coherence tomography is a prognostic biomarker not only for physical but also for cognitive disability progression in multiple sclerosis. Mult Scler. 2019;25(2):196–203.
Swinnen S, De Wit D, Van Cleemput L, Cassiman C, Dubois B. Optical coherence tomography as a prognostic tool for disability progression in MS: a systematic review. J Neurol. 2023;270(2):1178–86.
Martinez-Lapiscina EH, Arnow S, Wilson JA, et al. Retinal thickness measured with optical coherence tomography and risk of disability worsening in multiple sclerosis: a cohort study. Lancet Neurol. 2016;15(6):574–84.
Repovic P, Robertson D, Kresa-Reahl K, et al. Effectiveness of dimethyl fumarate in patients with relapsing multiple sclerosis switching after suboptimal response to glatiramer acetate, including patients with early multiple sclerosis: subgroup analysis of RESPOND. Neurol Ther. 2021;10(1):169–82.
Hassanein M, Kaczmarek O, Sethi A, et al. Association between cognition and social role participation among patients with multiple sclerosis. The Consortium of Multiple Sclerosis Centers Annual Meeting 2021.
Gibiansky E, Petry C, Mercier F, et al. Ocrelizumab in relapsing and primary progressive multiple sclerosis: pharmacokinetic and pharmacodynamic analyses of OPERA I, OPERA II and ORATORIO. Br J Clin Pharmacol. 2021;87(6):2511–20.
Hauser SL, Waubant E, Arnold DL, et al. B-cell depletion with rituximab in relapsing-remitting multiple sclerosis. N Engl J Med. 2008;358(7):676–88.
Dorcet G, Migné H, Biotti D, et al. Early B cells repopulation in multiple sclerosis patients treated with rituximab is not predictive of a risk of relapse or clinical progression. J Neurol. 2022;269(10):5443–53.
Boremalm M, Sundström P, Salzer J. Discontinuation and dose reduction of rituximab in relapsing-remitting multiple sclerosis. J Neurol. 2021;268(6):2161–8.
Baker D, Pryce G, James LK, Marta M, Schmierer K. The ocrelizumab phase II extension trial suggests the potential to improve the risk: benefit balance in multiple sclerosis. Mult Scler Relat Disord. 2020;44:102279.
Traub J, Traffehn S, Ochs J, et al. Dimethyl fumarate impairs differentiated B cells and fosters central nervous system integrity in treatment of multiple sclerosis. Brain Pathol. 2019;29(5):640–57.
Smith MD, Martin KA, Calabresi PA, Bhargava P. Dimethyl fumarate alters B-cell memory and cytokine production in MS patients. Ann Clin Transl Neurol. 2017;4(5):351–5.
Lundy SK, Wu Q, Wang Q, et al. Dimethyl fumarate treatment of relapsing-remitting multiple sclerosis influences B-cell subsets. Neurol Neuroimmunol Neuroinflamm. 2016;3(2):e211.
Bar-Or A, Calabresi PA, Arnold D, et al. Rituximab in relapsing-remitting multiple sclerosis: a 72-week, open-label, phase I trial. Ann Neurol. 2008;63(3):395–400.
Hauser SL, Bar-Or A, Comi G, et al. Ocrelizumab versus interferon β-1a in relapsing multiple sclerosis. N Engl J Med. 2017;376(3):221–34.
Gelfand JM, Cree BAC, Hauser SL. Ocrelizumab and other CD20(+) B-cell-depleting therapies in multiple sclerosis. Neurotherapeutics. 2017;14(4):835–41.
Garjani A, Patel S, Bharkhada D, et al. Impact of mass vaccination on SARS-CoV-2 infections among multiple sclerosis patients taking immunomodulatory disease-modifying therapies in England. Mult Scler Relat Disord. 2022;57: 103458.
Apostolidis SA, Kakara M, Painter MM, et al. Cellular and humoral immune responses following SARS-CoV-2 mRNA vaccination in patients with multiple sclerosis on anti-CD20 therapy. Nat Med. 2021;27(11):1990–2001.
Brill L, Rechtman A, Zveik O, et al. Humoral and T-cell response to SARS-CoV-2 vaccination in patients with multiple scerosis teated wth orelizumab. JAMA Neurol. 2021;78(12):1510–4.
Gadani SP, Reyes-Mantilla M, Jank L, et al. Discordant humoral and T cell immune responses to SARS-CoV-2 vaccination in people with multiple sclerosis on anti-CD20 therapy. EBioMedicine. 2021;73:103636.
Sormani MP, Inglese M, Schiavetti I, et al. Effect of SARS-CoV-2 mRNA vaccination in MS patients treated with disease modifying therapies. EBioMedicine. 2021;72:103581.
Sette A, Crotty S. Adaptive immunity to SARS-CoV-2 and COVID-19. Cell. 2021;184(4):861–80.
Spierer R, Lavi I, Bloch S, Mazar M, Golan D. Risk of breakthrough COVID-19 after vaccination among people with multiple sclerosis on disease-modifying therapies. J Neurol. 2023;270(10):4632–9.
Capuano R, Prosperini L, Altieri M, et al. Symptomatic COVID-19 course and outcomes after three mRNA vaccine doses in multiple sclerosis patients treated with high-efficacy DMTs. Mult Scler. 2023;29(7):856–65.
Weideman AM, Tapia-Maltos MA, Johnson K, Greenwood M, Bielekova B. Meta-analysis of the age-dependent efficacy of multiple sclerosis treatments. Front Neurol. 2017;8:577.
Epstein S, Fong KT, De Jager PL, et al. Evaluation of ocrelizumab in older progressive multiple sclerosis patients. Mult Scler Relat Disord. 2021;55:103171.
Acknowledgements
We thank the participants of this study.
Medical Writing, Editorial, and Other Assistance
Medical writing support for the preparation of this manuscript was provided by Karen Spach, PhD, Excel Scientific Solutions (Fairfield, CT, USA) and editorial support for the preparation of this manuscript was provided by Excel Medical Affairs (Glasgow, UK), under the direction of the authors; funding was provided by Biogen.
Funding
Funding for writing and editorial support, as well as the journal’s Rapid Service and Open Access fees, was provided by Biogen.
Author information
Authors and Affiliations
Contributions
Mark Gudesblatt: contributed to the writing the original draft, and critically reviewed and edited this manuscript. Daniel Golan: contributed to the conceptualization and methodology, and critically reviewed and edited this manuscript. Barbara Bumstead: critically reviewed and edited this manuscript. Marijean Buhse: critically reviewed and edited this manuscript. Myassar Zarif: critically reviewed and edited this manuscript. Sarah A. Morrow: critically reviewed and edited this manuscript. Jacqueline A. Nicholas: critically reviewed and edited this manuscript. Laura M. Hancock: critically reviewed and edited this manuscript. Jeffrey Wilken: critically reviewed and edited this manuscript. Nicole Scott: critically reviewed and edited this manuscript. Anne Gocke: contributed to the conceptualization and critically reviewed and edited this manuscript. James B. Lewin: contributed to the writing the original draft, and critically reviewed and edited this manuscript. Jason P. Mendoza: contributed to the writing the original draft, and critically reviewed and edited this manuscript. Olivia Kaczmarek: critically reviewed and edited this manuscript. Joanna Weller: critically reviewed and edited this manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
Mark Gudesblatt: speaker and consulting for Acorda, Amgen, Biogen, EMD Serono, Medtronic, Novartis, Sanofi Genzyme, Saol Therapeutics, and Teva Pharmaceuticals. Daniel Golan: Speaker and consulting for Bristol Myers Squib, Medison, Merck Serono, Novartis, and Roche. Barbara Bumstead: speaker and consulting for Biogen and BMS. Marijean Buhse: speaker and consulting for Biogen and Genzyme. Myassar Zarif: speaker and consulting for Biogen, BMS, EMD Serono, Horizon, Genentech, Novartis, and Sanofi. Sarah A. Morrow: served on advisory boards for Biogen Idec, EMD Serono, Novartis, Roche, and Sanofi Genzyme; received investigator-initiated grant funds from Biogen Idec, Roche, and Sanofi Genzyme; acted as site PI for multi-center trials funded by BMS, EMD Serono, Novartis, Roche, and Sanofi Genzyme; and received funding from CIHR, MSSC, and NMSS. Dr. Morrow’s new affiliation is Department of Clinical Neurosciences, University of Calgary, Hotchkiss Brain Institute, Calgary, AB, Canada. The affiliation listed in the title was Dr. Morrow’s affiliation at the time of the study. Jacqueline A. Nicholas: has received consultancy fees from Bristol Myers Squibb, EMD Serono, Genentech, MyMSTeam, Novartis, Octave Bio and TG Therapeutics; has received research support from Novartis, Genentech, Alexion, Octave Bio, Sanofi Genzyme, University of Buffalo and PCORI; serves on speakers’ bureau for EMD Serono and TG Therapeutics. Laura M. Hancock: research support from BMS, Clinical & Translational Science Institute and Advancing a Healthier Wisconsin Research and Education Program, and NIH; and speaker honoraria from Can Do MS, the. MS Association of America, and the National MS Society of America. Jeffrey Wilken: nothing to disclose. Nicole Scott, Anne Gocke, James B. Lewin, and Jason P. Mendoza: employees of and hold stock/stock options in Biogen. Olivia Kaczmarek: nothing to disclose. Joanna Weller: nothing to disclose.
Ethical Approval
The study was conducted according to local regulations and in compliance with the principles of the Declaration of Helsinki 1964. All instructions, regulations, and agreements of the protocol, and applicable International Conference on Harmonisation guidelines, were adhered to, and the study protocol was approved by the Institutional review board (IRB). Because this was a retrospective cohort study, based on routinely collected clinical data in a coded database, informed consent has been waived by the IRB.
Additional information
Prior Presentation: Some of these data have been previously presented at the Americas Committee for Treatment and Research in Multiple Sclerosis Forum 2022, West Palm Beach, FL, USA, February 24–26, 2022.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Gudesblatt, M., Bumstead, B., Buhse, M. et al. De-escalation of Disease-Modifying Therapy for People with Multiple Sclerosis Due to Safety Considerations: Characterizing 1-Year Outcomes in 25 People Who Switched from Ocrelizumab to Diroximel Fumarate. Adv Ther (2024). https://doi.org/10.1007/s12325-024-02902-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12325-024-02902-0