Abstract
Introduction
Anaplastic lymphoma kinase (ALK) tyrosine kinase inhibitors (TKIs) are standard first- and second-line treatment for advanced ALK+ non-small cell lung cancer (NSCLC). We evaluated outcomes in patients with ALK+ NSCLC receiving third-line ALK TKI versus non-ALK-directed therapy.
Methods
Flatiron Health OncoEMR data were extracted for patients with ALK+ NSCLC initiating first-line ALK TKI between January 2015 and March 2022 followed by second-line ALK TKI and third-line ALK TKI (group A) or non-TKI therapy (group B). Time-to-treatment discontinuation (TTD) and overall survival (OS) were analyzed using multivariate modelling.
Results
Among patients receiving third-line ALK TKI (A, n = 85) or non-TKI therapy (B, n = 43), most received first-line crizotinib (A/B: 64%/60%) and second-line alectinib (36%/30%), ceritinib (24%/19%), or lorlatinib (15%/30%). Common third-line treatments were lorlatinib/alectinib (41%/33%) in A and immunotherapy, chemotherapy, or chemotherapy + immunotherapy (30%/28%/21%) in B. Group A versus B had longer TTD of first-line treatment (hazard ratio [HR] 0.62, 95% confidence interval [CI] 0.41–0.93; p = 0.020) and second-line treatment (HR 0.50, 95% CI 0.33–0.75; p < 0.001) and longer OS from start of first-line treatment (HR 0.32, 95% CI 0.19–0.54; p < 0.001) and second-line treatment (HR 0.40, 95% CI 0.24–0.66; p < 0.001). For third-line treatment, median TTD (A/B) was 6.2/2.4 months (HR 0.61, 95% CI 0.37–1.00; p = 0.049) and OS was 17.6/6.5 months (HR 0.57, 95% CI 0.33–0.98; p = 0.042).
Conclusions
Patients receiving third-line non-ALK-directed therapy had suboptimal outcomes on prior TKIs. Patients with longer duration of prior ALK TKI treatment appeared to benefit from third-line ALK TKIs.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Optimal treatment sequencing beyond two lines of anaplastic lymphoma kinase (ALK) tyrosine kinase inhibitor (TKI) therapy in ALK+ non-small cell lung cancer (NSCLC) has not been established. |
Real-world data can inform clinical decision-making and help identify unmet needs in patients with ALK+ NSCLC. |
This study of electronic health record data from the Flatiron Health OncoEMR database explored real-world treatment patterns and outcomes of time-to-treatment discontinuation (TTD) and overall survival (OS) in patients with ALK+ NSCLC who received two lines of ALK TKI therapy followed by third-line treatment with another ALK TKI or a non-ALK-directed therapy. |
What was learned from the study? |
Among 128 patients with ALK+ NSCLC who received first- and second-line therapy with an ALK TKI followed by third-line ALK TKI (group A, n = 85) or non-ALK therapy (group B, n = 43), A versus B had longer TTD of first- and second-line treatment and OS from start of first- and second-line treatment. |
For third-line treatment, median TTD (A/B) was 6.2/2.4 months and OS was 17.6/6.5 months. |
Patients receiving third-line non-ALK-directed therapy had suboptimal outcomes on prior TKIs, while patients with longer prior ALK TKI duration appeared to benefit from third-line ALK TKIs. |
Digital features
This article is published with digital features, including a graphical abstract, to facilitate understanding of the article. To view digital features for this article, go to https://doi.org/10.6084/m9.figshare.25796914.
Introduction
Anaplastic lymphoma kinase gene (ALK) rearrangements occur in 3–8% of patients with NSCLC [1,2,3]. Most patients with ALK+ NSCLC present with locally advanced or metastatic disease at the time of diagnosis [4], which may reflect the aggressiveness of these tumors. Tyrosine kinase inhibitors (TKIs) targeting anaplastic lymphoma kinase (ALK; including crizotinib, alectinib, brigatinib, ceritinib, and lorlatinib) are the standard of care for first- and second-line treatment of advanced ALK+ NSCLC [5]. However, over time most patients develop resistance to an ALK TKI, owing to the development of on-target alterations in ALK (e.g., ALK mutations or gene amplification) [6, 7], ALK-independent resistance mechanisms (e.g., activation of bypass signaling pathways) [6, 7], and progression in the central nervous system (CNS) due to inadequate CNS penetration [8,9,10].
Clinical studies supporting the regulatory approvals of ALK TKIs evaluated their efficacy and tolerability in the first- and second-line settings [11,12,13,14,15,16,17,18,19,20]. However, optimal treatment sequencing beyond two lines of ALK TKI therapy has not been established [5]. Real-world data regarding treatment patterns after initial ALK TKI therapy and the clinical outcomes associated with subsequent lines of therapy are needed to inform clinical decision-making and to identify unmet needs in the long-term care of patients with ALK+ NSCLC. We conducted a study of electronic health record (EHR) data from the Flatiron Health OncoEMR database to describe real-world treatment patterns and outcomes of time-to-treatment discontinuation (TTD) and overall survival (OS) in patients with ALK+ NSCLC who received two lines of ALK TKI therapy followed by third-line treatment with either another ALK TKI or a non-ALK-directed therapy.
Methods
Study Design
This was a retrospective cohort study of real-world data obtained from Flatiron Health OncoEMR, a longitudinal EHR database consisting of de-identified patient-level structured and unstructured data curated through technology-enabled chart abstraction from more than 280 cancer clinics in the USA (approx. 800 care sites) [21, 22]. Secondary use of de-identified patient data is exempted from ethics review and informed consent according to the Department of Health and Human Services regulation 45 CFR 46.104(d)(4).
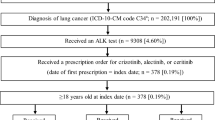
Patients included in this study were adults (age ≥ 18 years) who had a diagnosis of ALK+ NSCLC and initiated first-line therapy with an ALK TKI between January 1, 2015 and March 31, 2022. Eligible patients must have received ALK TKI therapy for first- and second-line treatment of advanced ALK+ NSCLC, followed by third-line therapy with either an ALK TKI (group A) or non-ALK TKI therapy (group B), including chemotherapy, immunotherapy, and others (Fig. 1).
Outcomes and Analyses
We collected data for treatments received for first-, second-, and third-line therapy and baseline demographics, smoking status, Eastern Cooperative Oncology Group (ECOG) performance status, brain metastasis status, time from diagnosis of advanced NSCLC, and duration of follow-up from start of line of therapy for each patient at the start of first-, second-, and third-line therapy. ALK TKIs were categorized as first generation (crizotinib), second-generation (alectinib, brigatinib, or ceritinib), or third-generation (lorlatinib). Key outcomes were TTD and OS from start of first-, second-, and third-line therapy. TTD and OS were analyzed using Kaplan–Meier methods. Hazard ratios (HRs) for comparing TTD and OS between group A and B were calculated using a multivariate Cox proportional hazard model that adjusted for age, sex, ECOG performance status, smoking status, presence of baseline brain metastasis, and time from advanced NSCLC diagnosis to start of each treatment line.
A descriptive subgroup analysis for third-line therapy was conducted that stratified OS and TTD by the time from advanced NSCLC diagnosis to start of the third-line therapy (< 12, 12–24, and ≥ 24 months). p values for this analysis were calculated using a log-rank test.
An inverse probability of treatment weighting (IPTW) method was applied to control for differences in baseline characteristics of age, sex, ECOG performance status, smoking status, presence of baseline brain metastasis, and time from advanced NSCLC diagnosis at the start of each line of therapy.
We conducted a sensitivity analysis of TTD and OS that excluded patients in group B who had received third-line immunotherapy only. For all analyses, a p value of < 0.05 was considered statistically significant. Missing data were not imputed. All data were analyzed using SAS v9.4 (Cary, NC).
Results
Patients
Data were extracted for 128 patients with ALK+ NSCLC who received first- and second-line therapy with an ALK TKI followed by third-line ALK TKI (group A, n = 85) or third-line non-ALK-directed treatment (group B, n = 43; Fig. 1). Patient characteristics at the start of each line of therapy are presented in Table 1. Brain metastases were reported in 15% of patients in group A versus 23% in group B at start of first-line therapy but in 33% of patients in each group at start of third-line therapy. Median time from diagnosis of advanced ALK+ NSCLC at start of third-line therapy was 24.5 months (range 4.9–122.2) in group A and 16.5 months (range 4.8–59.1) in group B. Treatments received are summarized by line of treatment in Table 2. The majority had received first-line crizotinib in group A (64%) and group B (60%). The most common second-line ALK TKIs were alectinib (36%) and ceritinib (24%) in group A and alectinib (30%) and lorlatinib (30%) in group B. The most common third-line treatments were lorlatinib (41%) and alectinib (33%) in group A and immunotherapy (30%), chemotherapy (28%), or combined immunotherapy/chemotherapy (21%) in group B.
Outcomes for First- and Second-Line ALK TKI Therapy
The median TTD of first-line ALK TKI therapy was significantly longer in patients who went on to receive third-line ALK TKI therapy (group A, 8.9 months [95% CI 7.2–11.2]) than in patients with third-line non-ALK TKI therapy (group B, 6.6 months [95% CI 4.7–8.4]; HR 0.62 [95% CI 0.41–0.93]; p = 0.020; Fig. S1A). Similarly, the median TTD of second-line ALK TKI therapy was significantly longer in group A (9.2 months [95% CI 5.7–11.3]) than in group B (4.1 months [95% CI 3.0–6.6]; HR 0.50 [95% CI 0.33–0.75]; p < 0.001 Fig. S1B).
Median OS from the start of first-line ALK TKI therapy was significantly longer in patients who later received third-line ALK TKI therapy (group A, 49.6 months [95% CI 37.4–72.1]) versus non-ALK TKI therapy (group B, 21.8 months [95% CI 15.7–33.6]; HR 0.32 [95% CI 0.19–0.54]; p < 0.001; Fig. S2A). The median OS from the start of second-line ALK TKI therapy was also significantly longer in group A (38.5 months [95% CI 23.4–61.1]) than group B (13.6 months [95% CI 10.4–22.1]; HR 0.40 [95% CI 0.24–0.66]; p < 0.001; Fig. S2B).
Outcomes for Third-Line ALK TKI Versus Non-ALK TKI Therapy
Based on Kaplan–Meier estimates and the Cox proportional hazard regression model, median TTD of third-line therapy was 6.2 months (95% CI 3.9–9.4) for patients who received third-line ALK TKI treatment (group A) versus 2.4 months (95% CI 1.4–3.7) for patients treated with third-line non-ALK TKI therapy (group B; HR 0.61 [95% CI 0.37–1.00]; p = 0.049; Fig. 2a, b). Multivariate modelling indicated that poorer ECOG performance status (2–4 vs. 0: HR 1.98 [95% CI 1.06–3.71]; p = 0.030) and shorter time since diagnosis of advanced NSCLC (12–24 months vs. > 24 months: HR 2.42 [95% CI 1.46–4.01]; p < 0.001) were independent predictors of shorter TTD of third-line therapy (Fig. 2b). The numerically longer TTD in group A versus group B was consistent across time-since-diagnosis categories (Table S1). Patients who started third-line therapy ≥ 24 months after diagnosis had median TTD of third-line treatment of 9.4 months (95% CI 5.1–22.8) with ALK TKI therapy versus 3.5 months (95% CI 0.03–not estimable [NE]; p = 0.053) with non-ALK TKI therapy; those who started third-line therapy < 12 months after diagnosis had median TTD of 3.7 months (95% CI 0.95–12.0) versus 1.9 months (95% CI 0.03–5.3; p = 0.080), respectively.
TTD and OS with third-line therapy in patients who received third-line treatment with an ALK TKI (group A) versus non-ALK TKI therapy (group B). A TTD estimated by Kaplan–Meier methods. B Multivariate Cox proportional hazard model results for TTD. C OS estimated by Kaplan–Meier methods. D Multivariate Cox proportional hazard model results for OS. ALK anaplastic lymphoma kinase, ECOG Eastern Cooperative Oncology Group, OS overall survival, TKI tyrosine kinase inhibitor, TTD time-to-treatment discontinuation. aHazard ratio and p values were calculated using a Cox proportional hazard model that adjusted for age, gender, ECOG performance status, smoking status, presence of baseline brain metastasis, and time from advanced diagnosis at the start of third-line therapy
Median OS after start of third-line therapy was 17.6 months (95% CI 13.0–24.9) for patients treated with third-line ALK TKI therapy (group A) and 6.5 months (95% CI 3.9–8.0) in patients who received third-line non-ALK TKI therapy (group B; HR 0.57 [95% CI 0.33–0.98]; p = 0.042; Fig. 2c). In the multivariate model, poorer ECOG performance status (2–4 vs. 0: HR 2.51 [95% CI 1.26–4.98]; p = 0.009) and shorter time since diagnosis of advanced NSCLC (< 12 vs. > 24 months: HR 1.97 [95% CI 1.07–3.65]; p = 0.031) were independent predictors of shorter OS after start of third-line therapy (Fig. 2d). Median OS was numerically longer with third-line ALK TKI treatment (group A) versus third-line non-ALK TKI treatment (group B) across time-since-diagnosis categories (Table S1). For patients who started third-line therapy ≥ 24 months after diagnosis, median OS was 21.5 months (95% CI 13.0–NE) in group A and 15.6 months (95% CI 2.9–NE; log-rank p = 0.270) in group B; for those who started third-line therapy < 12 months after diagnosis, median OS was 12.0 months (95% CI 2.5–40.6) in group A and 4.1 months (95% CI 0.8–7.6; log-rank p = 0.100) in group B.
After IPTW adjustment, there were no significant between-group differences in baseline characteristics at start of third-line therapy or in category of ALK TKI (first-generation vs. second- or third-generation ALK TKI) received for first- or second-line treatment (Table 3). The IPTW-adjusted median TTD of third-line therapy was 5.8 months (95% CI 2.7–8.6) for patients who received third-line ALK TKI therapy (group A) versus 2.3 months (95% CI 1.4–3.7) with third-line non-ALK TKI therapy (group B; chi-square log-rank p = 0.002; Fig. 3a). Adjusted median OS after start of third-line therapy was 13.0 months (95% CI 9.5–20.5) with ALK TKI therapy (group A) versus 6.5 months (95% CI 2.9–16.6) with non-ALK TKI therapy (group B; chi-square log-rank p = 0.058; Fig. 3b).
Adjusted Kaplan–Meier estimates of A TTD and B OS for third-line therapy after IPTW matching in patients who received third-line treatment with an ALK TKI (group A) versus non-ALK TKI therapy (group B). ALK anaplastic lymphoma kinase, CI confidence interval, df degree of freedom, ECOG Eastern Cooperative Oncology Group, IPTW inverse probability of treatment weighting, TKI tyrosine kinase inhibitor, TTD time-to-treatment discontinuation
In the sensitivity analyses that excluded 13 patients from group B who had received only immunotherapy for third-line treatment, the modified group B included 30 patients who received third-line chemotherapy with or without immunotherapy and/or anti-vascular endothelial growth factor (VEGF) and epidermal growth factor receptor (EGFR) TKI therapy. Median TTD of third-line therapy was 6.2 months (95% CI 3.9–9.4) in group A versus 2.4 months (95% CI 1.4–5.1) in the modified group B (HR 0.64 [95% CI 0.37–1.11]; p = 0.109; Fig. S3A). Median OS from start of third-line therapy was 17.6 months (95% CI 13.0–24.9) in group A versus 6.5 months (95% CI 3.9–7.6) in modified group B (HR 0.56 [95% CI 0.30–1.04]; p = 0.066; Fig. S3B).
Among the 29 patients who received a chemotherapy-based regimen for third-line treatment, 24 patients received a pemetrexed-based regimen and five received platinum-doublet–based chemotherapy (e.g., carboplatin/paclitaxel or albumin-bound paclitaxel). In the 24 patients who received third-line pemetrexed-based chemotherapy, the median TTD was 2.8 months (95% CI 1.4–5.4) and median OS was 6.5 months (95% CI 3.9–16.6). For the 23 patients who received immunotherapy with or without chemotherapy for third-line treatment, the median TTD was 3.5 months (95% CI 1.4–5.3) and median OS was 4.9 months (95% CI 2.6–8.0). Among the 13 patients who received only immunotherapy for third-line treatment, the median TTD of third-line therapy was 3.5 months (95% CI 0.03–5.8) and median OS from start of third-line therapy was 4.0 months (95% CI 0.5–NE).
Discussion
In this study of real-world data from medical records in the Flatiron Health OncoEMR database, we evaluated outcomes in patients with ALK+ NSCLC who received two lines of ALK TKI treatment followed by third-line treatment with either an ALK TKI or a non-ALK-directed therapy. We observed that the group of patients with third-line non-ALK-directed therapy (group B) had poorer TTD and OS on first- and second-line ALK TKI treatment than patients in the third-line ALK TKI group (group A). Given that TTD has been shown to be correlated with PFS in patients with ALK+ NSCLC receiving ALK TKIs [23], the shorter TTD for first- and second-line therapy indicates that patients in group B experienced rapid disease progression on prior ALK TKIs. Available data for baseline characteristics were mostly similar between groups. The rate of brain metastases was slightly higher in the third-line non-ALK TKI group at start of first-line treatment (23% vs. 15%) but was the same (33%) in both groups at start of third-line treatment. However, the median time from advanced diagnosis to the start of third-line therapy was approximately 8 months shorter in the group switched to non-ALK TKI therapy for third-line treatment (24.5 months in group A vs. 16.5 months in group B). The shorter time since diagnosis and the shorter TTD of first- and second-line therapy in group B suggest that these patients were switched to non-ALK TKI therapy for third-line treatment because their disease was not well controlled by prior ALK TKIs and that these patients were considered less likely to benefit from third-line ALK TKI treatment.
After IPTW adjustment for baseline characteristics, patients who received third-line ALK TKIs versus non-ALK TKI therapy had longer median TTD of third-line therapy (5.8 vs. 2.3 months; p = 0.002) and median OS from start of third-line therapy (13.0 vs. 6.5 months; p = 0.058). These trends were maintained in the sensitivity analysis that excluded patients who received only immunotherapy from the non-ALK-directed therapy group, indicating that the differences were not attributable to the poor response rates previously reported with immunotherapy in patients with ALK+ NSCLC [24, 25].
The clinical benefit of receiving additional lines of ALK TKI therapy after progression on an ALK TKI is supported by results of a retrospective single-center study, which reported that median OS from start of first-line treatment was 59 months for patients who received multiple lines of ALK TKI treatment (n = 71) versus 41 months for patients who received a single ALK TKI only (n = 73; p = 0.002) [26]. Among patients who were deceased, median OS was 41 months for those who received chemotherapy and TKI therapy (n = 48) and 16 months for patients who received only TKI therapy (n = 26; p < 0.001). Notably, 25–27% of patients who failed crizotinib or a second-generation ALK TKI did not receive any subsequent anticancer treatment, mainly due to rapid clinical deterioration associated with disease progression [26]. The authors concluded that closer disease monitoring and broader molecular profiling may help to lower attrition between treatment lines and potentially improve long-term outcomes [26].
Importantly, sequential lines of treatment for patients with ALK+ NSCLC may be informed by rebiopsy or liquid biopsy at disease progression, because activity against specific ALK resistance mutations and involvement of other oncogenic pathways (e.g., MET and EGFR activation) should be considered when selecting subsequent ALK TKIs or other treatments [7, 27, 28]. Biomarker data for ALK resistance mutations and other oncogenic pathways were not available for the current analyses but are important to consider for future studies of treatment sequencing in this population.
This study has several limitations. The analysis included data from patients starting treatment as early as January 2015, at which time crizotinib was the only ALK TKI approved for first-line treatment; therefore, more than 60% of patients had received first-line crizotinib. Alectinib, brigatinib, and lorlatinib were approved by the US Food and Drug Administration for first-line treatment of advanced ALK+ NSCLC in November 2017, May 2020, and March 2021, respectively [5]. Outcomes would likely differ for populations with greater proportions of patients receiving first-line treatment with alectinib, brigatinib, or lorlatinib. Median OS from start of first-line TKI treatment was shorter in this analysis (21.8–49.6 months) than observed with crizotinib (57.4 months) in the phase 3 ALEX trial [29]. Unlike the current study, the ALEX trial included patients who did not have disease progression on a first- or second-line TKI within the first few years of diagnosis [29]. Patients without early disease progression on a first-line ALK TKI have an excellent prognosis. In addition, patients treated in routine clinical practice may have shorter survival and higher rates of treatment toxicity compared with clinical trial participants owing to differences in patient characteristics and clinical practices [30, 31]. The analysis was limited to the US patient population included in the Flatiron Health OncoEMR database, which may not be representative of the broader population of patients with ALK+ NSCLC. Furthermore, the analysis was limited to patients who had proceeded to third-line therapy, excluding patients who did not receive more than two lines of therapy. The Cox regression and IPTW matching methods used considered only the observed variables. Other unobserved patient characteristics may confound outcomes, an inherent limitation of retrospective studies.
Conclusions
Patients with ALK+ NSCLC who received third-line non-ALK-directed therapy appeared to experience rapid disease progression on prior ALK TKIs. Patients who were treated with prior ALK TKIs for longer appeared to benefit from third-line ALK TKI treatment.
Data Availability
Restrictions apply to the availability of some or all data generated or analyzed during this study because they were used under license. The corresponding author will on request detail the restrictions and any conditions under which access to some data may be provided.
References
Gainor JF, Varghese AM, Ou SH, et al. ALK rearrangements are mutually exclusive with mutations in EGFR or KRAS: an analysis of 1683 patients with non-small cell lung cancer. Clin Cancer Res. 2013;19(15):4273–81.
Kris MG, Johnson BE, Berry LD, et al. Using multiplexed assays of oncogenic drivers in lung cancers to select targeted drugs. JAMA. 2014;311(19):1998–2006.
Koivunen JP, Mermel C, Zejnullahu K, et al. EML4-ALK fusion gene and efficacy of an ALK kinase inhibitor in lung cancer. Clin Cancer Res. 2008;14(13):4275–83.
Shaw AT, Yeap BY, Mino-Kenudson M, et al. Clinical features and outcome of patients with non-small-cell lung cancer who harbor EML4-ALK. J Clin Oncol. 2009;27(26):4247–53.
Owen DH, Singh N, Ismaila N, et al. Therapy for stage IV non-small-cell lung cancer with driver alterations: ASCO living guideline, version 2022.2. J Clin Oncol. 2023;41(5):e10–20.
Pan Y, Deng C, Qiu Z, Cao C, Wu F. The resistance mechanisms and treatment strategies for ALK-rearranged non-small cell lung cancer. Front Oncol. 2021;11:713530.
Gainor JF, Dardaei L, Yoda S, et al. Molecular mechanisms of resistance to first- and second-generation ALK inhibitors in ALK-rearranged lung cancer. Cancer Discov. 2016;6(10):1118–33.
Ou SH, Janne PA, Bartlett CH, et al. Clinical benefit of continuing ALK inhibition with crizotinib beyond initial disease progression in patients with advanced ALK-positive NSCLC. Ann Oncol. 2014;25(2):415–22.
Costa DB, Kobayashi S, Pandya SS, et al. CSF concentration of the anaplastic lymphoma kinase inhibitor crizotinib. J Clin Oncol. 2011;29(15):e443–5.
Solomon BJ, Cappuzzo F, Felip E, et al. Intracranial efficacy of crizotinib versus chemotherapy in patients with advanced ALK-positive non-small-cell lung cancer: results from PROFILE 1014. J Clin Oncol. 2016;34(24):2858–65.
Kim DW, Tiseo M, Ahn MJ, et al. Brigatinib in patients with crizotinib-refractory anaplastic lymphoma kinase-positive non-small-cell lung cancer: a randomized, multicenter phase II trial. J Clin Oncol. 2017;35(22):2490–8.
Camidge DR, Kim HR, Ahn MJ, et al. Brigatinib versus crizotinib in ALK-positive non-small-cell lung cancer. N Engl J Med. 2018;379(21):2027–39.
Shaw AT, Kim DW, Nakagawa K, et al. Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. N Engl J Med. 2013;368(25):2385–94.
Solomon BJ, Mok T, Kim DW, et al. First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N Engl J Med. 2014;371(23):2167–77.
Peters S, Camidge DR, Shaw AT, et al. Alectinib versus crizotinib in untreated ALK-positive non-small-cell lung cancer. N Engl J Med. 2017;377(9):829–38.
Gadgeel SM, Gandhi L, Riely GJ, et al. Safety and activity of alectinib against systemic disease and brain metastases in patients with crizotinib-resistant ALK-rearranged non-small-cell lung cancer (AF-002JG): results from the dose-finding portion of a phase 1/2 study. Lancet Oncol. 2014;15(10):1119–28.
Shaw AT, Kim DW, Mehra R, et al. Ceritinib in ALK-rearranged non-small-cell lung cancer. N Engl J Med. 2014;370(13):1189–97.
Soria JC, Tan DS, Chiari R, et al. First-line ceritinib versus platinum-based chemotherapy in advanced ALK-rearranged non-small-cell lung cancer (ASCEND-4): a randomised, open-label, phase 3 study. Lancet. 2017;389:917–29.
Shaw AT, Bauer TM, de Marinis F, et al. First-line lorlatinib or crizotinib in advanced ALK-positive lung cancer. N Engl J Med. 2020;383(21):2018–29.
Solomon BJ, Besse B, Bauer TM, et al. Lorlatinib in patients with ALK-positive non-small-cell lung cancer: results from a global phase 2 study. Lancet Oncol. 2018;19(12):1654–67.
Ma X, Long L, Moon S, Adamson BJS, Baxi SS. Comparison of population characteristics In real-world clinical oncology databases in the US: Flatiron Health, SEER, and NPCR. medRxiv. 2020:2020.03.16.20037143.
Birnbaum B, Nussbaum N, Seidl-Rathkopf K, et al. Model-assisted cohort selection with bias analysis for generating large-scale cohorts from the EHR for oncology research. ar**v Prepr., 2020. https://arxiv.org/abs/2001.09765. Accessed 30 Jan 2023.
Blumenthal GM, Gong Y, Kehl K, et al. Analysis of time-to-treatment discontinuation of targeted therapy, immunotherapy, and chemotherapy in clinical trials of patients with non-small-cell lung cancer. Ann Oncol. 2019;30(5):830–8.
Garassino MC, Cho BC, Kim JH, et al. Durvalumab as third-line or later treatment for advanced non-small-cell lung cancer (ATLANTIC): an open-label, single-arm, phase 2 study. Lancet Oncol. 2018;19(4):521–36.
Mazieres J, Drilon A, Lusque A, et al. Immune checkpoint inhibitors for patients with advanced lung cancer and oncogenic driver alterations: results from the IMMUNOTARGET registry. Ann Oncol. 2019;30(8):1321–8.
Elsayed M, Bozorgmehr F, Kazdal D, et al. Feasibility and challenges for sequential treatments in ALK-rearranged non-small-cell lung cancer. Front Oncol. 2021;11:670483.
Horn L, Whisenant JG, Wakelee H, et al. Monitoring therapeutic response and resistance: analysis of circulating tumor DNA in patients with ALK+ lung cancer. J Thorac Oncol. 2019;14(11):1901–11.
Kauffmann-Guerrero D, Kahnert K, Huber RM. Treatment sequencing for anaplastic lymphoma kinase-rearranged non-small-cell lung cancer. Drugs. 2021;81(1):87–100.
Mok T, Camidge DR, Gadgeel SM, et al. Updated overall survival and final progression-free survival data for patients with treatment-naive advanced ALK-positive non-small-cell lung cancer in the ALEX study. Ann Oncol. 2020;31(8):1056–64.
Templeton AJ, Vera-Badillo FE, Wang L, et al. Translating clinical trials to clinical practice: outcomes of men with metastatic castration resistant prostate cancer treated with docetaxel and prednisone in and out of clinical trials. Ann Oncol. 2013;24(12):2972–7.
Green AK, Curry M, Trivedi N, Bach PB, Mailankody S. Assessment of outcomes associated with the use of newly approved oncology drugs in medicare beneficiaries. JAMA Netw Open. 2021;4(2):e210030.
Medical Writing, Editorial, and Other Assistance
Medical writing support for the development of this manuscript, under the direction of the authors, was provided by Lela Creutz, PhD, and Lauren Gallagher, RPh, PhD, of Peloton Advantage, LLC, an OPEN Health company, and funded by Takeda Development Center Americas, Inc., Cambridge, MA, and complied with the Good Publication Practice (GPP) guidelines (DeTora LM, et al. Ann Intern Med 2022;175:1298–1304).
Authorship
All authors have made substantial contributions to the conception or design of the work or the acquisition, analysis, or interpretation of data or the creation of new software used in the work; drafted the work or revised it critically for important intellectual content; approved the version to be published; and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy of integrity of any part of the work are appropriately investigated and resolved.
Funding
The work was sponsored by Takeda Development Center Americas, Inc. The sponsor has also funded the journal’s Rapid Service and Open Access fees associated with publication of this article.
Author information
Authors and Affiliations
Contributions
Konstantinos Arnaoutakis: Writing—review & editing. Yin Wan: Conceptualization, Formal analysis, Methodology, Project administration, Resources, Validation, Visualization. Jennifer Elliott: Conceptualization, Investigation, Methodology, Writing—review & editing. Matt Young: Conceptualization. Yu Yin: Data curation, Formal analysis, Methodology. Konstantinos Leventakos: Conceptualization, Writing—review & editing. Huamao M. Lin: Conceptualization, Investigation, Methodology, Writing—review & editing. Anastasios Dimou: Methodology, Supervision, Writing—review & editing.
Corresponding author
Ethics declarations
Conflict of Interest
Konstantinos Arnaoutakis: Consulting activities: Gilead, OncLive/MJH Life Sciences; stocks: Athos Therapeutics. Yin Wan: Employment (Takeda). Jennifer Elliott: Employment (Takeda). Matt Young: Employment (Takeda). Yu Yin: Employment (Takeda). Konstantinos Leventakos: Consulting activities: Boehringer Ingelheim, Amgen, AstraZeneca, Targeted Oncology, Takeda, Jazz Pharmaceuticals, Mirati Therapeutics, Janssen, and Regeneron; research support (to institution): AstraZeneca and Mirati Therapeutics; CME activities: OncLive and MJH Life Sciences. Huamao M. Lin: Employment (Takeda). Anastasios Dimou: Advisory board: TP Therapeutics, AnHeart Therapeutics, Guardant Health, and ChromaCode; clinical trial support: Syntrix Pharmaceuticals, Novartis, Sorrento Therapeutics, AnHeart Therapeutics, Merck, and Guardant Health; honoraria: Roche/Genentech.
Ethical Approval
This was a retrospective cohort study of real-world data obtained from Flatiron Health OncoEMR, a longitudinal electronic health record database consisting of de-identified patient-level structured and unstructured data curated through technology-enabled chart abstraction from more than 280 cancer clinics in the USA. Secondary use of de-identified patient data is exempted from ethics review and informed consent according to the Department of Health and Human Services regulation 45 CFR 46.104(d)(4).
Additional information
Prior Presentation: Data from this manuscript were presented in a poster at the Annual Meeting of the North American Conference on Lung Cancer (NACLC), September 23–25, 2022, Chicago, IL, USA.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Arnaoutakis, K., Wan, Y., Elliott, J. et al. Real-World Treatment Patterns and Outcomes Across Three Lines of Therapy in Patients with ALK+ NSCLC. Adv Ther (2024). https://doi.org/10.1007/s12325-024-02899-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12325-024-02899-6