Abstract
Introduction
Vitiligo, a chronic autoimmune skin depigmentation disease with an unpredictable course, has been associated with several comorbid autoimmune and psychological conditions. Our current understanding of vitiligo burden and management in the real world is limited. This real-world analysis presents data on vitiligo epidemiology, comorbidities, and treatment of patients in Israel.
Methods
This retrospective study analyzed data from the Maccabi Health Services database. Prevalent patients with vitiligo in 2021 were matched to patients in the general population on the basis of age group, gender, and socioeconomic status. Patient demographics, vitiligo incidence and prevalence, comorbidities, and treatment patterns are reported. Data are presented as percentages, mean, median, P values, and standard mean differences (SMD).
Results
In this analysis, 11,412 patients with vitiligo were matched to patients from the general population. Incidence and prevalence rates increased over time from 2005 to 2021. Compared to the general population, patients with vitiligo were more likely to have an immune-mediated comorbidity (29.7% vs 18.4% [P < 0.001; SMD 0.27]) or psychological comorbidity (18.7% vs 15.9% [P < 0.001; SMD 0.07]). Comorbidities included atopic dermatitis (patients with vitiligo vs general population 12.5% vs 8.4%), psoriasis (5.8% vs 3.6%), Hashimoto’s thyroiditis (2.9% vs 1.1%), alopecia areata (2.2% vs 0.9%), depression (10.8% vs 9.5%), and sleep disorder/insomnia (5.9% vs 4.4%). Only 74.8% of all patients with vitiligo had ever received treatment, with topical corticosteroids (51.5%) and calcineurin inhibitors (36.5%) most commonly prescribed. At the end of 2021, 83.7% of patients were untreated.
Conclusion
Patients with vitiligo are more likely to have various immune-related and psychological comorbidities, highlighting the significant impact of the condition on well-being. Nearly a quarter of patients had never received treatment, with many receiving only topical treatments, and medication persistence was low. This highlights the lack of adequate treatment in this population and the need for more effective management options.
Similar content being viewed by others

Avoid common mistakes on your manuscript.
Why carry out this study? |
Our current understanding of vitiligo burden and management in the real world is limited. |
This analysis aims to add to our understanding of vitiligo epidemiology, comorbidities, and treatment patterns using data on a large, real-world patient population derived from the Maccabi Health Services database. |
What was learned from the study? |
This study showed that the prevalence of vitiligo was approximately 0.5% in 2021, with nearly a quarter of patients with vitiligo having never received treatment. Moreover, this study also found that patients with vitiligo were more likely than the general population to have various immune-related and psychological comorbidities. |
These findings highlight the systemic burden of disease, the lack of adequate treatment in this population, and the need for more effective management options, such as early diagnosis and appropriate intervention. |
Introduction
Vitiligo is a chronic immune-mediated skin depigmentation disease characterized by chalky-white patches on the skin [1]. Depigmentation is attributed to melanocyte destruction by CD8+ T cells resulting from an interplay of genetic predisposition, environmental triggers, melanocyte stress, and dysregulated innate and adaptive immune responses [2, 3]. Vitiligo can affect anywhere on the body, most commonly the face, hands, feet, and extremities [4]. The disease course of vitiligo is unpredictable, and depigmentation may progress at varying rates [5, 6].
As a systemic disease, vitiligo has been associated with several comorbid conditions, such as thyroid disease and skin, joint, and bowel conditions [7,8,9]. Vitiligo has been reported to have important psychosocial effects which impact many areas of life, including employment and academic performance [10,11,12].
The current standard of care in most parts of the world includes topical corticosteroids (CS) and topical calcineurin inhibitors (CI), along with narrowband ultraviolet B (NB-UVB) light therapy [13, 14]. Other interventions include vitamin D analogues, targeted phototherapy, melanocyte grafting, and full body depigmentation [15]. Recently, topical ruxolitinib (a Janus kinase [JAK]-1 inhibitor) was approved in the USA and Europe for patients with up to 10% body surface area (BSA) affected by vitiligo [16, 17]; there are currently no approved systemic treatments. Despite some emerging evidence, our understanding of the holistic disease burden and management of patients with vitiligo in daily practice remains limited.
This real-world analysis aims to add to our understanding of vitiligo epidemiology, comorbidities, and treatment patterns using the Maccabi Health Services (MHS) database.
Methods
Study Design
A retrospective, cohort study assessed vitiligo incidence, and a cross-sectional study was used to describe patient characteristics and management of vitiligo. This analysis used the MHS electronic database, a large Israeli health maintenance organization accounting for 25% of the population (2.4 million people), with data available since 1993 and less than 1% of patients lost per year.
Study Population
To be included as a vitiligo case, patients must have fulfilled ≥ 1 of the following criteria:
-
≥ 1 diagnosis code from a dermatologist
-
≥ 1 diagnosis code from a hospital plus ≥ 1 diagnosis code from any physician
-
≥ 2 diagnosis codes from any physician
-
≥ 1 diagnosis code from any physician plus purchase of a topical CS within 6 months before or 12 months after the first vitiligo diagnosis
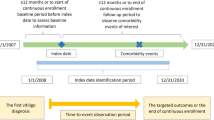
The International Classification of Diseases, Ninth Revision (ICD-9) diagnosis codes used were 709.01 (vitiligo) and 374.53 (hypopigmentation of the eyelid). Included patients were first diagnosed between January 1, 2005 and December 31, 2021, and were MHS members ≥ 12 months before and after the first diagnosis.
Patients who met the vitiligo case definition were defined as incident patients with vitiligo. Those who were also MHS members enrolled by the last day of 2021 were defined as prevalent patients with vitiligo and matched to patients without vitiligo from the general population (GP) on the basis of age, sex, and socioeconomic status (SES).
Demographics, Comorbidities, and Vitiligo-Related Variables
Baseline demographics for patients with vitiligo and the matched GP included age (on December 31, 2021), sex, SES, smoking status, the Charlson Comorbidity Index (CCI) [18], body mass index (BMI), and vitiligo among relatives reported for patients who had ≥ 1 MHS-member relative (sibling, parent, and/or child). The two groups were categorized according to various comorbidities (Supplementary Table S1). Patients with vitiligo were further examined according to age at diagnosis, disease duration, diagnosing physician/setting, and disease severity.
As a result of a lack of clinical variables assessing disease severity within the MHS database, severity was categorized on the basis of treatment received: mild vitiligo was defined by ≥ 1 purchase of a topical CS or CI and moderate to severe disease by ≥ 1 purchase of a systemic CS and/or ≥ 1 purchase of a systemic immunosuppressant (IS), and/or ≥ 1 course of phototherapy or photochemotherapy. Phototherapy was defined as NB-UVB therapy and photochemotherapy was defined as psoralen and UVA (PUVA) therapy. Disease severity was based on categorization used for atopic dermatitis, a disease with similar treatment options [19].
Being treated for vitiligo was defined by ≥ 1 purchase/record of any of the following treatments: topical CS, topical, CI, systemic CS, systemic IS, phototherapy, photochemotherapy, vitamin D analogues, laser therapy, and skin graft. Treatment sequence was defined according to treatments used alone or as combinations. To distinguish between different lines of therapy, a new line was defined if purchased/recorded ≥ 60 days after the previous line.
Statistical Analyses
Annual incidence rates of vitiligo (overall and by subgroup) were assessed from 2005 to 2021. Prevalence rates (overall and by subgroup) were analyzed as of December 31, 2021. Incidence and prevalence were reported as rates per 100,000 patients.
Demographics, comorbidities, and vitiligo-related variables were presented as total numbers, mean ± standard deviation (SD) and median (interquartile range [IQR]) for continuous variables, and percentages for categorical variables. Comorbidities were analyzed individually and grouped by type (i.e., immune-mediated, psychological, and additional). Vitiligo-related treatments were evaluated for prevalent patients (overall and by subgroup) and by presence of comorbidities. Comparison was assessed by standardized mean differences (SMD) and Wilcoxon rank sum test, Kruskal–Wallis rank sum test, or Pearson’s chi-square test, where applicable.
Logistic regression models computed the adjusted odds ratios (aOR) and 95% confidence intervals (CI) for any immune-mediated comorbidity, any psychological comorbidity, and any type of skin cancer (i.e., melanoma and non-melanoma skin cancer [NMSC]) in prevalent patients with vitiligo versus the matched GP. All models were adjusted for age (as of December 31, 2021), sex, SES, smoking status, CCI, and BMI. Statistical significance was defined as a P value < 0.05 and SMD > 0.1 or ≤ 0.1. Analyses were performed by IBM® SPSS® v.25.0 (IBM, Amrock, NY, USA) and R Foundation for Statistical Computing (Vienna, Austria).
Ethics
The study was approved by the Maccabi Health Services’ Institutional Review Board, reference number MHS-0013-22, who waived the requirement to obtain any informed consent for this secondary analysis of existing data.
Results
Epidemiology
From 2005 to 2021, we identified 12,709 incident patients with vitiligo. Overall incidence of vitiligo increased from 26.3 in 2005 to 36.8 per 100,000 patients in 2021 (Fig. 1). Incidence rates per 100,000 in children and adolescents rose over time from 22.5 and 13.0 in 2005 to 38.1 and 33.0 in 2021, respectively. Adult incidence remained relatively more stable over time (29.8 per 100,000 adults in 2005 and 38.2 per 100,000 in 2021).
Reported prevalence of vitiligo in the MHS population increased from 2005 to 2021 from 26.2 to 445.3 per 100,000. In 2021, the prevalence of vitiligo among children (184.1 per 100,000) was lower than in adolescents (534.8 per 100,000) and adults (511.1 per 100,000); prevalence was similar regardless of sex (Fig. 2).
Demographics and Disease Characteristics
In the 2021 prevalent population, 11,412 patients with vitiligo and 11,412 matched non-vitiligo controls were compared. Mean age in both populations was 42.3 years, with 51.1% being female patients (Table 1). In patients with vitiligo, the mean ± SD age at diagnosis was 34.8 ± 20.5 years, with a mean ± SD disease duration of 7.1 ± 4.8 years. Among patients with vitiligo included in this analysis, 95.5% received their diagnosis from a dermatologist. More patients with vitiligo than the GP had ≥ 1 relative with vitiligo (2.6% vs 1.1% [P < 0.001; SMD 0.11]) and were less likely to have ever smoked (11.0% vs 15.6%).
Among patients with vitiligo in 2021, a total of 4698 (41.2%) were categorized as having mild disease and 3824 (33.5%) were categorized as having moderate to severe disease (Table 2). Severity could not be determined in 2890 cases (25.3%) because no treatment data were available. Patients categorized as having moderate to severe versus mild disease were more likely to be older (mean ± SD 49.9 ± 20.1 vs 39.3 ± 20.5 [P < 0.001; SMD 0.42]), female (54.6% vs 51.0% [P < 0.001; SMD 0.11]), overweight (32.9% vs 27.9%) or obese (21.9% vs 14.0% [P < 0.001; SMD 0.28]), have a disease duration ≥ 4 years (85.5% vs 64.7% [P < 0.001; SMD 0.40]), and be first diagnosed later in life (mean ± SD 40.4 ± 19.6 vs 32.6 ± 20.1 [P < 0.001; SMD 0.32]).
Comorbidities
Patients with vitiligo versus GP had increased odds of develo** ≥ 1 immune-mediated comorbidity (aOR [95% CI] 1.85 [1.73, 1.97]; P < 0.001). Indeed, a greater proportion of patients with vitiligo had ≥ 1 immune-mediated comorbidity versus GP (29.7% vs 18.4% [P < 0.001; SMD 0.27]); the most common were atopic dermatitis (12.5% vs 8.4%; SMD 0.13), Hashimoto’s thyroiditis (2.9% vs 1.1%; SMD 0.13), and alopecia areata (2.2% vs 0.9%; SMD 0.11) (all P < 0.001) (Fig. 3). Patients with vitiligo versus GP were more likely to experience anemia (22.5% vs 17.5%; P < 0.001; SMD 0.12) and itch (4.2% vs 2.7%; P < 0.001; SMD 0.08) (Table 3). Importantly, a lower percentage of patients with vitiligo had skin cancer (2.6% vs 3.4%; P < 0.001; SMD 0.05) and reduced odds of develo** skin cancer (aOR [95% CI] 0.67 [0.57, 0.80]; P < 0.001).
Patients with vitiligo versus GP had increased odds of develo** ≥ 1 psychological comorbidity (aOR [95% CI] 1.23 [1.14, 1.32]; P < 0.001). In patients with vitiligo, 18.7% versus 15.9% of GP had ≥ 1 psychological comorbidity (P < 0.001; SMD 0.07); the most common included depression (10.8% vs 9.5%), sleep disorder/insomnia (5.9% vs 4.4%), and anxiety (3.7% vs 3.0%) (all P < 0.01; SMD < 0.1; Fig. 4). Psychological comorbidities were significantly (P < 0.001; SMD > 0.1) more common in adults than in children or adolescents (overall 21.8% vs 4.6% and 7.7%).
A significantly (P < 0.001) greater percentage of patients categorized with moderate to severe versus mild disease had ≥ 1 immune-mediated comorbidity (37.0% vs 29.7% [SMD 0.26]), or any additional comorbidity (36.1% vs 25.6%; SMD 0.22). Patients with moderate to severe versus mild disease were more likely to have any psychological comorbidity (24.8% vs 15.9%), evidenced by increased rates of depression (15.7% vs 8.5%), sleep disorder/insomnia (8.9% vs 4.6%), and anxiety (5.6% vs 2.7%) (Supplementary Table S2).
Treatment Patterns
In the 2021 prevalent vitiligo population, a total of 8537 (74.8%) had ever received any treatment, indicating that 25.2% of patients had never been treated (Table 2). Importantly, at the end of 2021, only 16.3% of patients had ever received any treatment. Mean ± SD time to first treatment was 14.7 ± 29.5 months. Patients with moderate to severe versus mild disease were more likely to have waited longer for initial treatment (mean ± SD 17.6 ± 30.7 vs 12.4 ± 28.3 [P < 0.001; SMD 0.30]), as was the case for adults compared with children and adolescents (Supplementary Table S3).
Among all patients, topical CS and topical CI were most common (51.5% and 36.5%, respectively), followed by systemic CS (27.1%), phototherapy (7.4%), and photochemotherapy (2.2%) (Fig. 5). Female versus male patients (77.0% vs 72.5%; SMD 0.1), and adults versus children and adolescents (77.0% vs 63.2% and 68.5%; SMD 0.20), were more likely to have ever received treatment (all P < 0.05). Adults were more likely than children and adolescents to receive topical CS (54.9% vs 33.9% and 42.0%) and systemic treatments, including phototherapy (8.1% vs 3.6% and 5.1%) (both P < 0.05; SMD 0.13). In contrast, adolescents and children were more likely to receive topical CI (40.6% and 40.2% vs 35.4%; P < 0.05; SMD 0.07). Patients with ≥ 1 immune-mediated disease versus those without (83.0% vs 71.3%; P < 0.001; SMD 0.28), as well as those diagnosed with depression versus without (81.2% vs 76.3%; P < 0.001; SMD 0.12), were more likely to have ever received treatment (Fig. 6).
Treatment patterns a overall and b by sex, and by age group. *P < 0.05; †SMD > 0.1 or < −0.1; ‡Phototherapy refers to NB-UVB; Photochemotherapy refers to PUVA; §Systemic Immunosuppressants includes methotrexate, azathioprine, cyclosporine. CI calcineurin inhibitors, CS corticosteroids, IS immunosuppressant, NV-UVB narrowband ultraviolet B light, PUVA psoralen + ultraviolet A light, SMD standardized mean difference
Treatment Sequencing
Topical CS and topical CI were the most commonly prescribed first-line treatments in the total population (42.4% and 27.8%, respectively) (Fig. 7). Second- and third-line treatments included higher percentages of systemic CS (36.9% vs 32.0%, respectively) and systemic IS (1.2% vs 12.2%), as well as phototherapy (7.8% vs 14.5%). Combination therapy (covering any combination of systemic and/or topical agents) was more commonly prescribed as a first-line treatment and was less common as second- and third-line therapy (13.7%, 4.3%, vs 1.2%, respectively). Similarly, in first-line therapy, more children and adolescents than adults were treated with topical CI (43.6% and 40.6% vs 24.7%) and had less use of systemic treatments (systemic CS 28.2% and 21.4% vs 38.7%; systemic IS 0.6% and 0.3% vs 1.3%) (Supplementary Fig. S1).
Discussion
The findings of this analysis provide a broad view of the epidemiology, disease characteristics, comorbidities, and treatment patterns of patients with vitiligo in Israel. The results demonstrate that the prevalence of vitiligo in 2021 was approximately 0.5%, with some expected differences across age groups. Increasing incidence over time could be due to increasing awareness of the disease and introduction of new treatment modalities, making it easier for healthcare providers to treat vitiligo. Approximately one-third of patients were considered to have moderate to severe disease. To the best of our knowledge, this is one of the first studies in vitiligo to use prescribed treatments as a proxy for disease severity within a payer/provider database.
In this analysis, patients categorized as having moderate to severe vitiligo had longer disease duration, suggesting a potentially progressive disease course. In a study of patients with vitiligo where lesions were compared on the basis of their response to a combination of phototherapy and a topical CI, the results demonstrated that patients with a shorter disease duration had better responses to treatment [20]. Increased time to diagnosis and first treatment in patients with moderate to severe vitiligo suggest that early diagnosis and intervention could be important factors to modulate disease progression.
Regarding the holistic burden of vitiligo, our results show that close to 30% of patients with vitiligo have at least one immune-mediated comorbidity, a percentage that is slightly higher than other literature has reported. In studies from the USA and Belgium, 23% and 15% of patients with vitiligo, respectively, had comorbid autoimmune conditions [8, 21]. Differences in the study population, sample size, data sources, methodology, symptom underreporting, and geographic or temporal variations may account for differences between our study and others. Similar to our study, Ezzedine et al. showed that significantly greater proportions of patients with vitiligo versus controls had atopic dermatitis (12% vs 10%) and alopecia areata (4% vs 2%) [22]. Furthermore, in this study, patients with moderate to severe disease were more likely to have immune-mediated comorbidities, as were female patients, children, and adolescents, indicating a disproportionate burden. As such, the requirements for monitoring and intervention may vary depending on the patient population.
Findings on comorbidities in this analysis included lower odds of skin cancer in patients with vitiligo, as consistent with published literature [23, 24]. One study demonstrated that patients with vitiligo have up to a threefold decreased probability of develo** NMSC [24]. Another comorbidity to note is the proportion of patients reporting itch (4.2%), as it is generally believed that the disease is not linked to physical discomfort. However, one study showed that 48.1% of patients reported experiencing itch prior to the appearance of new vitiligo lesions [25].
Regarding psychological comorbidities, the significant increase in patients with vitiligo versus GP is consistent with other research and highlights the broader impact of disease on patients’ daily lives [26]. Indeed, one study found that patients with vitiligo had a 25% increased risk of recurrent depressive disorder, and a 23% increased risk of anxiety disorder, compared with a control group [26]. The study also found that those with a psychological comorbidity were twice as likely to be absent from work and unemployed [26]. Another study showed higher levels of depression and worse sleep quality in patients with vitiligo versus a control group [27]. Furthermore, the psychological impact of vitiligo can affect quality of life and social integration [28, 29], especially among patients with greater than 5% BSA, darker skin types, or lesions on the face [30]. As a result of these debilitating effects of vitiligo, it is important that providers routinely assess patients’ quality of life along with treatment efficacy.
Patients with any immune-mediated comorbidity or depression were more likely to have received any treatment than patients without these comorbidities. These findings suggest that the increased impact of additional physical or psychosocial burden may potentially drive treatment decision-making.
Despite the current lack of formally approved medications for re-pigmentation in Israel, it is encouraging to see that 74.8% of patients had received ≥ 1 treatment; half of patients received topical CS, followed by topical CI and systemic CS. These treatment patterns are consistent with guidelines recommending topical treatments as first- and second-line therapies in vitiligo [4, 14, 31]. Unfortunately, many of these treatments are frequently only effective in a minority of patients and the broad use of topical treatments does not align with the growing understanding of vitiligo as a systemic disease.
Although 74.8% of all patients with vitiligo were treated at some point, our results show that at the end of 2021, more than 80% of patients were untreated, suggesting a lack of medication persistence. This may be due to potential safety concerns with long-term use of therapies, such as steroids, and/or could be an indicator of treatment dissatisfaction by patients and physicians [14, 32,33,34].
Our findings highlight several unmet needs for patients with vitiligo, such as the lack of efficacious and approved treatment options, in particular, systemic therapies. These results also show an important comorbid burden that should be assessed and treated as part of a comprehensive patient management plan. There is a clear need for further research regarding disease course and factors affecting progression in vitiligo.
A strength of this analysis is that it is, to the best of our knowledge, the largest real-world assessment of patients with vitiligo and one of few analyses on vitiligo outside of the US population. The attrition rate of the MHS database is low, allowing for a comprehensive and longitudinal view of the patient’s experience. Limitations of this analysis included disease severity being defined by treatments recorded. While treatment is a reasonable proxy that has been employed in similar diseases, it may underestimate the proportion of patients with moderate to severe vitiligo due to frequent undertreatment [35, 36]. Moreover, defining severity by treatment inherently excludes patients who have not received treatment and relies on such treatment being administered as prescribed. Furthermore, the absence of disease-specific measures, such as BSA, Vitiligo Area Scoring Index, Vitiligo Extent Score, or location of vitiligo lesions impedes potentially more granular conclusions.
Conclusions
This real-world analysis of patients in Israel showed that the prevalence of vitiligo was approximately 0.5% in 2021. The results highlight the systemic burden of disease, showing that patients with vitiligo were significantly more likely to have a range of immune-mediated and psychological comorbidities. The data on treatment patterns highlight the undertreatment and lack of treatment continuity for many patients with vitiligo, suggesting a need for more effective treatment options. Further research is needed to better understand how timely diagnosis and early treatment may impact disease progression and development of comorbidities.
Data Availability
The datasets generated during and/or analyzed during the current study are not publicly available since the data that support the findings of this study originate from Maccabi Healthcare Services and restrictions apply to the availability of these data. However, these data may be accessed through written request to the authors and/or Maccabi Healthcare Services.
References
Spritz RA. The genetics of generalized vitiligo and associated autoimmune diseases. Pigment Cell Res. 2007;20:271–8.
Ralf Paus L, Schallreuter K, Bahadoran P, et al. Vitiligo pathogenesis: autoimmune disease, genetic defect, excessive reactive oxygen species, calcium imbalance, or what else? Exp Dermatol. 2008;17:139–40.
Rodrigues M, Ezzedine K, Hamzavi I, Pandya AG, Harris JE, Vitiligo Working Group. New discoveries in the pathogenesis and classification of vitiligo. J Am Acad Dermatol. 2017;77:1–13.
Speeckaert R, van Geel N. Distribution patterns in generalized vitiligo. J Eur Acad Dermatol Venereol. 2014;28:755–62.
Abdel-Malek ZA, Jordan C, Ho T, Upadhyay PR, Fleischer A, Hamzavi I. The enigma and challenges of vitiligo pathophysiology and treatment. Pigment Cell Melanoma Res. 2020;33:778–87.
Ezzedine KEV, Whitton M, van Geel N. Vitiligo. Lancet. 2015;386(9988):74–84. https://doi.org/10.1016/S0140-6736(14)60763-7.
Gill L, Zarbo A, Isedeh P, Jacobsen G, Lim HW, Hamzavi I. Comorbid autoimmune diseases in patients with vitiligo: a cross-sectional study. J Am Acad Dermatol. 2016;74:295–302.
van Geel N, Speeckaert M, Brochez L, Lambert J, Speeckaert R. Clinical profile of generalized vitiligo patients with associated autoimmune/autoinflammatory diseases. J Eur Acad Dermatol Venereol. 2014;28:741–6.
Hadi A, Wang JF, Uppal P, Penn LA, Elbuluk N. Comorbid diseases of vitiligo: a 10-year cross-sectional retrospective study of an urban US population. J Am Acad Dermatol. 2020;82:628–33.
Parsad D, Dogra S, Kanwar AJ. Quality of life in patients with vitiligo. Health Qual Life Outcomes. 2003;1:1–3.
Silverberg JI, Silverberg NB. Quality of life impairment in children and adolescents with vitiligo. Pediatr Dermatol. 2014;31:309–18.
Simons RE, Zevy DL, Jafferany M. Psychodermatology of vitiligo: psychological impact and consequences. Dermatol Ther. 2020;33:e13418.
Mayo MJ, Carey E, Smith HT, et al. Impact of pruritus on quality of life and current treatment patterns in patients with primary biliary cholangitis. Dig Dis Sci. 2023;68(3):995–1005.
Taieb A, Alomar A, Böhm M, et al. Guidelines for the management of vitiligo: the European Dermatology Forum consensus. Br J Dermatol. 2013;168:5–19.
Agarwal K, Podder I, Kassir M, et al. Therapeutic options in vitiligo with special emphasis on immunomodulators: a comprehensive update with review of literature. Dermatol Ther. 2020;33:e13215.
Hegade VS, Bolier R, Oude Elferink RP, Beuers U, Kendrick S, Jones DE. A systematic approach to the management of cholestatic pruritus in primary biliary cirrhosis. Frontline Gastroenterol. 2016;7:158–66.
Hegade VS, Mells G, Beuers U, et al. Patient experience and characteristics of cholestatic pruritus in the UK-PBC research cohort. Hepatology. 2014;60:362A-A363.
Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–83.
Shrestha S, Miao R, Wang L, Chao J, Yuce H, Wei W. Burden of atopic dermatitis in the United States: analysis of healthcare claims data in the Commercial, Medicare, and Medi-Cal databases. Adv Ther. 2017;34:1989–2006.
Yang Q, Zhang G, Su M, et al. Vitiligo skin biomarkers associated with favorable therapeutic response. Front Immunol. 2021;12:613031.
Sheth VM, Guo Y, Qureshi AA. Comorbidities associated with vitiligo: a ten-year retrospective study. Dermatology. 2014;227:311–5.
Ezzedine K, Seneschal J, Da Silva A, et al. Vitiligo patient population and disease burden in France: VIOLIN study results from the CONSTANCES cohort. J Eur Acad Dermatol Venereol. 2023;37(11):2249–58.
Ferguson J, Eleftheriadou V, Nesnas J. Risk of melanoma and non-melanoma skin cancer in people with vitiligo: UK population-based cohort study. J Investig Dermatol. 2023;143(11):2204–10.
Teulings H, Overkamp M, Ceylan E, et al. Decreased risk of melanoma and nonmelanoma skin cancer in patients with vitiligo: a survey among 1307 patients and their partners. Br J Dermatol. 2013;168:162–71.
Vachiramon V, Onprasert W, Harnchoowong S, Chanprapaph K. Prevalence and clinical characteristics of itch in vitiligo and its clinical significance. BioMed Res Int. 2017;2017:5617838.
Eleftheriadou V, Thompson A, Nesnas J. Vitiligo diagnosis is associated with an increase in new-onset depression and anxiety: a population-based cohort study in the UK. Br J Dermatol. 2022;187:10.
Öztekin A, Öztekin C. Sleep quality and depression in vitiligo patients. Eurasian J Family Med. 2020;9:35–41.
Ongenae K, Van Geel N, De Schepper S, Naeyaert J-M. Effect of vitiligo on self-reported health-related quality of life. Br J Dermatol. 2005;152:1165–72.
Thompson A, Clarke S, Newell RJ, Gawkrodger D, Collaboration AR. Vitiligo linked to stigmatization in British South Asian women: a qualitative study of the experiences of living with vitiligo. Br J Dermatol. 2010;163:481–6.
Bibeau K, Ezzedine K, Harris JE, et al. Mental health and psychosocial quality-of-life burden among patients with vitiligo: findings from the global VALIANT study. JAMA Dermatol. 2023;159(10):1124–8.
Gawkrodger D, Ormerod A, Shaw L, et al. Guideline for the diagnosis and management of vitiligo. Br J Dermatol. 2008;159:1051–76.
Bae JM, Jeong KH, Choi CW, et al. Development of evidence-based consensus on critical issues in the management of patients with vitiligo: a modified Delphi study. Photodermatol Photoimmunol Photomed. 2021;37:3–11.
Narayan V, Uitentuis S, Luiten R, Bekkenk M, Wolkerstorfer A. Patients’ perspective on current treatments and demand for novel treatments in vitiligo. J Eur Acad Dermatol Venereol. 2021;35:744–8.
Hamzavi IH, Bibeau K, Grimes P, et al. Exploring the natural and treatment history of vitiligo: perceptions of patients and healthcare professionals from the global VALIANT study. Br J Dermatol. 2023;189:ljad245.
Ezzedine K, Sheth V, Rodrigues M, et al. Vitiligo is not a cosmetic disease. J Am Acad Dermatol. 2015;73:883–5.
Silverwood RJ, Forbes HJ, Abuabara K, et al. Severe and predominantly active atopic eczema in adulthood and long term risk of cardiovascular disease: population based cohort study. BMJ. 2018; 361:k1786. https://doi.org/10.1136/bmj.k1786.
Medical Writing, Editorial, and Other Assistance
Medical writing services were provided by Natalie Mitchell, MSc, of Fishawack Facilitate Ltd., part of Avalere Health, and funded by AbbVie.
Funding
AbbVie funded the study and the journal’s Rapid Service Fee, as well as participated in interpretation of data, review, and approval of the manuscript. All authors contributed to the development of the manuscript and maintained control over final content. No honoraria or payments were made for authorship.
Author information
Authors and Affiliations
Contributions
All authors (Yuval Ramot, Vered Rosenburg, Limei Zhou and Stephanie Harbers) contributed to study conception, design and data interpretation. Data collection and analysis were performed by Vered Rosenburg and Limei Zhou. All authors commented on all versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
Y Ramot received speaker honoraria, consultancy fees or travel support from Pfizer, AbbVie, Novartis, Janssen, Sanofi, BI, Neopharm, Dexcel Pharma, Taro, and Lilly. V Rosenberg has nothing to declare. L Zhou and S Harbers are employees of AbbVie and may own AbbVie stock and/or options.
Ethical Approval
The study was approved by the Maccabi Health Services’ Institutional Review Board, reference number MHS-0013-22, who waived the requirement to obtain any informed consent for this secondary analysis of existing data.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Ramot, Y., Rosenberg, V., Zhou, L. et al. Epidemiology and Treatment Patterns of Patients with Vitiligo: A Real-World Analysis. Adv Ther 41, 2890–2906 (2024). https://doi.org/10.1007/s12325-024-02875-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-024-02875-0