Abstract
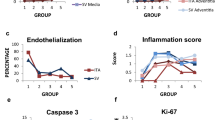
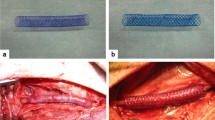
Although autologous vein grafting is essential, the high vein failure rate and specific clinical interventions are not clear, so a potential treatment is critically needed; thus, complex analyses of the relationship between pathobiological and physiological processes in preclinical are essential. The interposition of the femoral vein was performed in a canine model. Maximized expansion and velocity were measured at 8 weeks post-implantation, and a relative decrease was observed at 12 weeks. However, NI formation and NI/Media ratio significantly increased time dependently, and differences between the mechanical properties were observed. Additionally, RhoA-mediated TNF-α induced by rapid structural changes and high shear stress was confirmed. After adaptation to the arterial environment, vascular remodeling occurred by SMC proliferation and differentiation, apoptosis and autophagy were induced through YAP activity without vasodilation and RhoA activity. Our results show that understanding pathobiological processes in which time-dependent physiological changes contribute to vein failure can lead to a potential strategy.
Graphical abstract
The implanted vein graft within the arterial environment undergoes pathobiological processes through RhoA and YAP activity, leading to pathophysiological changes.






Similar content being viewed by others
Abbreviations
- NI:
-
Neo-intimal
- PAOD:
-
Peripheral arterial occlusive disease
- CAOD:
-
Coronary artery obstructive disease
- VSMC:
-
Vascular smooth muscle cell
- PCNA:
-
Proliferation cell nuclear antigen
- IL-1β :
-
Interleukin-1β
- RhoA:
-
Ras homolog gene family, member A TNF-α tumor necrosis factor- α
- SMC:
-
Smooth muscle cell
- YAP:
-
Yes-associated protein
- CFD:
-
Computational fluid dynamics
- WSS:
-
Wall shear stress
References
Weintraub, W. S. E. A. (2012). Comparative effectiveness of revascularization strategies. New England Journal of Medicine, 366, 1467–1476. https://doi.org/10.1056/NEJMoa1110717
Deb, S. E. A. (2013). Coronary artery bypass graft surgery versus percutaneous interventions in coronary revascularization: a systematic review. JAMA, 310, 2086–2095.
S.T. Tranbaugh RF, Swistel DG, et al. (2017) Coronary Artery Bypass Graft Surgery Using the Radial Artery, Right Internal Thoracic Artery, or Saphenous Vein as the Second Conduit. The Annals of Thoracic Surgery 104 553–559. https://doi.org/10.1016/j.athoracsur.2016.11.017.
Schachner, T., Laufer, G., & Bonatti, J. (2006). In vivo (animal) models of vein graft disease. European J Cardio-Thoracic Surg, 30(3), 451–463.
C.H. Wang X, Lin PH, Lumsden AB, Yao Q, Chen C. (2006) Mouse models of neointimal hyperplasia: techniques and applications. Medical Science Monitor (12) 177–185.
S.A.R. Majid Jadidi, Mahmoud Habibnezhad, Eric Anttila, Alexey Kamenskiy (2021) Mechanical, structural, and physiologic differences in human elastic and muscular arteries of different ages: Comparison of the descending thoracic aorta to the superficial femoral artery, Acta Biomaterialia 119 268–283. https://doi.org/10.1016/j.actbio.2020.10.035.
Schachner, G. L. T., & Bonatti, J. (2006). In vivo (animal) models of vein graft disease. Vascular Endovascular Surgical, 30, 451–463. https://doi.org/10.1016/j.ejcts.2006.06.015
He, J., Bao, Q., Yan, M., Liang, J., Zhu, Y., Wang, C., & Ai, D. (2018). The role of Hippo/yes-associated protein signalling in vascular remodelling associated with cardiovascular disease. British Journal of Pharmacology, 175(8), 1354–1361. https://doi.org/10.1111/bph.13806
Boopathy, G. T., & Hong, W. (2019). Role of hippo pathway-YAP/TAZ signaling in angiogenesis. Frontiers in Cell and Developmental Biology, 7, 49.
Q. Yang, D. Lei, S. Huang, Y. Yang, C. Jiang, H. Shi, W. Chen, Q. Zhao, Z. You, X. Ye (2020) A novel biodegradable external stent regulates vein graft remodeling via the Hippo-YAP and mTOR signaling pathways. Biomaterials 258 120254.
Uemura, M., Nagasawa, A., & Terai, K. (2016). Yap/Taz transcriptional activity in endothelial cells promotes intramembranous ossification via the BMP pathway. Scientific reports, 6(1), 1–12.
Choi, H.-J., Kim, N.-E., Kim, B. M., Seo, M., & Heo, J. H. (2018). TNF-α-induced YAP/TAZ activity mediates leukocyte-endothelial adhesion by regulating VCAM1 expression in endothelial cells. International Journal of Molecular Sciences, 19(11), 3428.
Yamaguchi, H., & Taouk, G. M. (2020). A Potential role of YAP/TAZ in the interplay between metastasis and metabolic alterations. Frontiers in Oncology, 10, 928.
Chen, C., **e, J., Rajappa, R., Deng, L., Fredberg, J., & Yang, L. (2015). Interleukin-1β and tumor necrosis factor-α increase stiffness and impair contractile function of articular chondrocytes. Acta Biochimica et biophysica Sinica, 47(2), 121–129.
M.L. Xuelan Zhang, Kun Fang, Jiehua Li, Yuan Peng, Liancun Zheng, Chang Shu (2020) Analysis of the formation mechanism and occurrence possibility of Post-Stenotic Dilatation of the aorta by CFD approach. Computer Methods and Programs in Biomedicine. 194. https://doi.org/10.1016/j.cmpb.2020.105522.
U. Windberger, Bartholovitsch, A., Plasenzotti, R., Korak, K., & Heinze, G. , Whole Blood Viscosity, Plasma Viscosity and Erythrocyte Aggregation in Nine Mammalian Species: Reference Values and Comparison of DataExperimental Physiology 88https://doi.org/10.1113/eph8802496
K.D. Lee J, Lee KJ, Seo IH, Park SH, Jang EH, Park Y, Youn YN, Ryu W (2017) Transfer-molded wrappable microneedle meshes for perivascular drug delivery. Journal of Controlled Release (28;268) 237–246. https://doi.org/10.1016/j.jconrel.2017.10.007.
K.M. Owens GK, Wamhoff BR (2004) Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiological Reviews 84 (3) 767–801. https://doi.org/10.1152/physrev.00041.2003.
H. Zhi, Gong, F. H., Cheng, W. L., Zhu, K., Chen, L., Yao, Y., ... & Li, H. (2018) Tollip Negatively Regulates Vascular Smooth Muscle Cell–Mediated Neointima Formation by Suppressing Akt‐Dependent Signaling. Journal of the American Heart Association 7 (12). https://doi.org/10.1161/JAHA.117.006851.
B.R. Jeremy JY, Johnson JL, Gadsdon P, Vijayan V, Shukla N, Smith FC, Angelini GD. (2004) A bioabsorbable (polyglactin), nonrestrictive, external sheath inhibits porcine saphenous vein graft thickening. The Journal of Thoracic and Cardiovascular Surgery (127(6)) 1766–1772. https://doi.org/10.1016/j.jtcvs.2003.09.054. .
J.T. Murphy GJ, Chamberlain MH, Rizvi SI, Wyatt M, George SJ, Angelini GD, Karsch KR, Oberhoff M, Newby AC. (2007) Short- and long-term effects of cytochalasin D, paclitaxel and rapamycin on wall thickening in experimental porcine vein grafts., Cardiovascular Research (1;73(3)) 607–617. https://doi.org/10.1016/j.cardiores.2006.11.015.
Thomas, A. C. (2012). Animal models for studying vein graft failure and therapeutic interventions. Current Opinion in Pharmacology, 12(2), 121–126. https://doi.org/10.1016/j.coph.2012.01.002
M.K. Chester AH, Yacoub MH. (1998) Expression of vascular adhesion molecules in saphenous vein coronary bypass grafts. Annals of Thoracic Surgery 65(6) 1685–1694. https://doi.org/10.1016/s0003-4975(98)00274-4.
S.M. Alrawi SJ, Raju R, Shirazian D, Acinapura AJ, Cunningham JN (2000) Intercellular and vascular cell adhesion molecule levels in endoscopic and open saphenous vein harvesting for coronary artery bypass surgery., Heart Surgery Forum (3(3)) 241–246.
W.J. Chen SJ, Muller DW (1994) Adenovirus-mediated gene transfer of soluble vascular cell adhesion molecule to porcine interposition vein grafts. Circulation., (89(5)) 1922–1930. https://doi.org/10.1161/01.cir.89.5.1922.
G.S. Wan S, Berry C, Baker AH, Vein graft failure: current clinical practice and potential for gene therapeutics. Gene Therapy 19 (6) (2012) 630–636. https://doi.org/10.1038/gt.2012.29.
J.P. Milani-Nejad N (2014) Small and large animal models in cardiac contraction research: advantages and disadvantages. Pharmacology and Therapeutics 141 (3) 235–249. https://doi.org/10.1016/j.pharmthera.2013.10.007.
J.C. Kohn, Zhou, D. W., Bordeleau, F., Zhou, A. L., Mason, B. N., Mitchell, M. J., ... & Reinhart-King, C. A. (2015) Cooperative effects of matrix stiffness and fluid shear stress on endothelial cell behavior. Biopolymers Journal 108 (3) 471–478. https://doi.org/10.1016/j.bpj.2014.12.023.
Z. Jiang, Wu, L., Miller, B. L., Goldman, D. R., Fernandez, C. M., Abouhamze, Z. S., ... & Berceli, S. A. (2004) A novel vein graft model: adaptation to differential flow environments. American Journal of Physiology - Heart and Circulatory Physiology 286 (1) H240-H245. https://doi.org/10.1152/ajpheart.00760.2003.
M.D. Wakefield TW, Henke PK. , Mechanisms of venous thrombosis and resolution. Arteriosclerosis, Thrombosis, and Vascular Biology 28 (3) (2008) 387–391. https://doi.org/10.1161/ATVBAHA.108.162289.
H.J. Saha P, Modarai B, Mattock K, Waltham M, Evans CE, et al. (2011) Leukocytes and the natural history of deep vein thrombosis: current concepts and future directions. Arteriosclerosis, Thrombosis, and Vascular Biology 31 (3) 506–512. https://doi.org/10.1161/ATVBAHA.110.213405.
Q. Wan, Kim, S. J., Yokota, H., & Na, S. (2013) Differential activation and inhibition of RhoA by fluid flow induced shear stress in chondrocytes. Cell Biology International 37 (6) 568–576. https://doi.org/10.1002/cbin.10072.
B. Wojciak-Stothard, & Ridley, A. J. (2003) Shear stress-induced endothelial cell polarization is mediated by Rho and Rac but not Cdc42 or PI 3-kinases.s Journal of Cell Biology 161 (2) 429–439. https://doi.org/10.1083/jcb.200210135.
B.S. Zekun Peng, Yurong Zhang, Miao Wang (2019) Endothelial Response to Pathophysiological Stress, Arteriosclerosis, Thrombosis, and Vascular Biology. (39) 233. https://doi.org/10.1161/ATVBAHA.119.312580.
G.P.v.N.A.a.V.W.M.v (2007) Hinsbergh, Endogenous RhoA Inhibitor Protects Endothelial Barrier, Circulation Research. (101) 7–9. https://doi.org/10.1161/CIRCRESAHA.107.156513.
K.E. Chu Kataoka, Shujiro Inoue, Masao Takemoto, Weihua Ni, Masamichi Koyanagi, Shiro Kitamoto, Makoto Usui, Kozo Kaibuchi, Hiroaki Shimokawa, and Akira Takeshita (2002) Important Role of Rho-kinase in the Pathogenesis of Cardiovascular Inflammation and Remodeling Induced by Long-Term Blockade of Nitric Oxide Synthesis in Rats, Hypertension. (39) 245–250. https://doi.org/10.1161/hy0202.103271.
Chang, Y. J., Huang, H. C., Hsueh, Y. Y., Wang, S. W., Su, F. C., Chang, C. H., Tang, M. J., Li, Y. S., Wang, S. H., Shung, K. K., Chien, S., & Wu, C. C. (2016). Role of Excessive Autophagy Induced by Mechanical Overload in Vein Graft Neointima Formation: Prediction and Prevention. Science and Reports, 26(6), 22147. https://doi.org/10.1038/srep22147.Erratum.In:SciRep.2016Nov14;6:31256
Miyakawa, A. A., Borin, T. F., Campos, L. C. G., Girão-Silva, T., Ribeiro-Silva, J. C., Dallan, L. A. O., & Krieger, J. E. (2019). Activation of Interleukin-1 Beta in Arterialized Vein Grafts and the Influence of the -511C/T IL-1β Gene Polymorphism. J Cardiovasc Dev Dis., 6(2), 20. https://doi.org/10.3390/jcdd6020020
C.a.Z. Huang, Wenwen and Zhu, Yuelin (2019) Drug-eluting stent specifically designed to target vascular smooth muscle cell phenotypic modulation attenuated restenosis through the YAP pathway. American Journal of Physiology - Heart and Circulatory Physiology 317 (3) 541–551. https://doi.org/10.1152/ajpheart.00089.2019.
C.M.H. Paul B. Dieffenbach, Anna Maria F. Coronata, Kyoung Moo Choi, Xaralabos Varelas, Daniel J. Tschumperlin, and Laura E. Fredenburgh (2017) Arterial stiffness induces remodeling phenotypes in pulmonary artery smooth muscle cells via YAP/TAZ-mediated repression of cyclooxygenase-2. American Journal of Physiology 313 (3) 628–647. https://doi.org/10.1152/ajplung.00173.2017.
P. Yuan, Hu, Q., He, X. et al. (2020) Laminar flow inhibits the Hippo/YAP pathway via autophagy and SIRT1-mediated deacetylation against atherosclerosis. Cell Death and Disease 11 141. https://doi.org/10.1038/s41419-020-2343-1.
Acknowledgements
The authors acknowledge the veterinary staff that provided anesthesia support during the procedure.
Funding
This research was supported by a grant of the Korea Health Technology R&D project through 342 the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health 343 & Welfare, Republic of Korea (grant number: HI18C1237), and the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF-2015R1D1A1A01060374).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical Approval
Human studies were not carried out by the authors for this article. All institutional and national guidelines for the care and use of laboratory animals were followed and approved by the appropriate institutional committees.
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported.
Additional information
Communicated by Associate Editor Yihua Bei oversaw the review of this article.
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file2 (MP4 1045 KB)
Rights and permissions
About this article
Cite this article
Jang, E.H., Kim, JH., Ryu, Jy. et al. Time-dependent pathobiological and physiological changes of implanted vein grafts in a canine model. J. of Cardiovasc. Trans. Res. 15, 1108–1118 (2022). https://doi.org/10.1007/s12265-022-10226-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12265-022-10226-z