Abstract
Living benthic foraminifera have been widely used as ecological indicators in coastal ecosystems. There is, however, a lack of studies on their response to trace element pollution in tropical estuarine systems. Here we analyze the living assemblages of benthic foraminifera, collected in 2016, in the Cachoeira River Estuary (CRE) in northeastern Brazil, to understand their response to natural and anthropogenic stressors, including trace element pollution. Some species were good bioindicators of specific environmental conditions, such as the agglutinant Paratrochammina clossi which preferred mangrove areas and anoxic conditions. In addition, the calcareous Ammonia tepida and Cribroelphidium excavatum, dominant within the whole system disregarding organic or trace element pollution, seem to resist even in the areas most polluted by trace elements. Interestingly, C. excavatum showed a particular positive relationship with trace element pollution (specifically by Cu and Pb), outnumbering the opportunistic A. tepida in the areas with higher pollution of these metals. However, for other species, it is still difficult to constrain to which parameters they respond (i.e., Haynesina germanica and Elphidium gunteri, which in the present study seem to respond to natural conditions, whereas in the literature they are regarded as indicators of trace element and organic pollution, respectively). Therefore, our findings shed light on the response of benthic foraminiferal species in a highly polluted and highly mixed tropical estuarine system and highlight the need to understand the complexity of these environments when applying foraminiferal biological indexes to avoid imprecise conclusions.
Similar content being viewed by others

Avoid common mistakes on your manuscript.
Introduction
Tropical estuarine ecosystems and their surrounding areas are commonly affected by anthropogenic stressors and activities, such as harbors, industries, fishing, agriculture, and disposal of domestic sewage (often in natura) (Flemer and Champ 2006; Maríns et al. 2007). When the estuary’s capacity for dispersion is exceeded, organic and inorganic contaminants start to accumulate and alter the quality of the water and sediment (e.g., eutrophication process, enhanced toxicity) (Rabalais 2002; Tappin 2002). Among these contaminants, toxic metals (hereinafter referred to as trace elements) have become a global problem and are a major concern in the scientific and public communities. Because they have a high affinity for the fine sediment fraction and do not degrade, they can accumulate or even be biomagnified along the trophic chain (Ip et al. 2004; Gu et al. 2011), posing a threat to marine life (Stankovic et al. 2014).
Among the marine organisms, the benthic community is potentially impacted by the trace-element accumulation in the sediment, since they are directly exposed to the contaminants. Metal-impacted benthic communities are commonly characterized by reduced abundance, lower species diversity, and shifts in community composition from sensitive to tolerant taxa (Clements et al. 1992 and references therein). To investigate the impact on the benthic community, it is therefore important to understand the spatial distribution of the trace elements, their accumulation mechanisms in the sediment (e.g., influence of hydrodynamics, organic matter, and other environmental variables), and how they affect the benthic community distribution.
For this purpose, benthic foraminifera have been proven to represent an excellent low-cost tool, being regarded as good bioindicators of both natural stress and human interference in estuarine areas (Alve 1995). Previous studies based on the modification of their assemblage’s composition and diversity have shown their potential to evaluate the environmental conditions and the ecological quality status (i.e., Alve et al. 2009, 2016; Bouchet et al. 2012, 2013, 2020; Barras et al. 2014; Dimiza et al. 2016; Jorissen et al. 2018). In particular, the sensitivity of these organisms to the combined effects of ocean warming and local impacts (Prazeres et al. 2017; Bergamin et al. 2019), to organic pollutants (Alve 1995; Burone et al. 2006; Martins et al. 2016a), and more specifically to trace element pollution (Frontalini et al. 2009, 2018; Frontalini and Coccioni 2011; Jayaraju et al. 2011; Martins et al. 2013; Mojtahid et al. 2006) has been well demonstrated. However, these methodologies have been used more extensively in temperate environments, as they have been historically limited in tropical areas.
Nonetheless, in recent decades, the number of studies using foraminifera as bioindicators in tropical areas has increased, which a focus on the foraminiferal response to natural (Debenay et al. 2001; Duleba and Debenay 2003; Laut et al. 2016a; Belart et al. 2019) and anthropogenic (Vilela et al. 2004, 2011; Teodoro et al. 2009; Eichler et al. 2015; Laut et al. 2016b, 2021a, b; Belart et al. 2018; Raposo et al. 2018; Pregnolato et al. 2018; Martins et al. 2020) environmental stresses. A few studies addressing the effect of trace element pollution on the benthic foraminiferal community have also been performed (Debenay and Fernandez 2009; Lacuna and Alviro 2014; Martínez-Colón et al. 2009, 2018a; b; Sánchez et al. 2020). However, along the Brazilian coastline, most of these investigations were based on the total assemblage (not distinguishing living from dead organisms) and therefore could not constrain the effect of the trace elements in the living organisms (e.g., Vilela et al. 2004, 2011; Donnici et al. 2012; Damasio et al. 2020), with the exception of the studies by Duleba et al. (2018), Martins et al. (2020), and Castelo et al. (2022) in Southeastern Brazil. This highlights an important gap in such studies along the extensive Brazilian coastline and in tropical regions as a whole.
To accurately understand (and predict) the global impact of trace elements on the living benthic organisms’ communities, it is crucial to comprehend how they affect the different marine environments across the globe. Therefore, in our study, we aim to fill this gap by providing new insights into the living benthic foraminifera as bioindicators of natural and anthropogenic stressors, particularly focusing in the response to trace element pollution. For this, we investigated a tropical mesotidal estuary in the northeastern coast of Brazil, the Cachoeira River Estuary (CRE). The CRE is an estuarine system that receives discharges enriched in organic and inorganic pollutants from agricultural and industrial activities, as well as from an inefficient sewage treatment plant (Souza et al. 2009), and that is regarded to be affected by trace element pollution (Laut et al. 2021c). Given the severe anthropogenic influence on this estuary, we aim to contribute to knowledge about the ecology of benthic foraminifera in coastal areas that are exposed to similar highly urbanized conditions.
Study Area
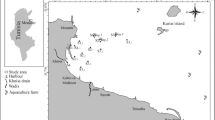
The CRE (14° 45′ to 14° 50′ S and 39° 05′ to 39° 01′ W), located in the city of Ilhéus in the state of Bahia (NE Brazil), comprises an area of approximately 16 km2 and is characterized by a semidiurnal mesotidal regime, with a tidal range of 2 m (Souza et al. 2009; Bahia 2017). It is formed by the convergence of the Cachoeira, Itacanoeira, and Santana rivers (Fig. 1) and represents the largest estuary in the southern part of the state (Almeida et al. 2006). The climate in the region is tropical, warm, and humid, with an annual temperature of 23.3 °C and precipitation exceeding 2000 mm per year (Schiavetti et al. 2005). The average fluvial discharge in the basin is 24.1 m3/s and quickly responds to precipitation, which is higher mainly between November and January (Bahia 2017).
The CRE is a typical tropical estuary dominated by mangroves, which cover an area of approximately 13 km2 of vegetation in shrub and semi-shrub stages and a restinga vegetation strip with trees and undergrowth vegetation over sandy deposits of Quaternary origin (Barbosa and Domingues 1996). The decomposition of Rhizophora mangle and Laguncularia racemosa leaves acts as a natural source of organic matter (OM) in the estuary (Oliveira et al. 2013). The mangroves have been suffering serious damage from landfills, domestic and industrial effluent discharges, and the removal of sand from the river to meet construction demand (Fidelman 2005). The mangrove vegetation removal, in addition to the contaminants and OM contributions from rivers and the continent, turns the CRE into a vulnerable ecosystem (Fidelman 2005). In fact, the estuary has already been identified as a super eutrophic environment (Lucio 2010; Oliveira et al. 2013). This condition is made worse during periods of drought due to lower fluvial discharge (Lucio 2010). In addition, a sewage treatment plant (STP) was installed in the CRE in 2000. This increased the discharge of effluent highly enriched in nutrients and therefore intensified the eutrophication processes in the system (Souza et al. 2009; Silva et al. 2015).
Methods
Sampling
In November 2016, 30 stations were sampled (Fig. 1) for both biotic and abiotic parameters. The stations were distributed along 11 transects. The stations in the middle of the channel were identified with the letter “A” and the stations in the margins with the letters “B” and “C”. Due to navigation difficulties, only one station was sampled in transect 1 (CH01-C) and two stations in transect 2 (CH02-A and CH02-C). The geographic coordinates of the stations are reported in Table S1. According to the tide report (station Port of Ilhéus–Malhado), the higher syzygy tide was 2.1 m, whereas the lower one was 0.1 m during the collection period (DHN 2016).
The definition of the estuary sectors was considered in accordance with a previous study in the CRE (Silva et al. 2015). The estuary mouth was represented by the station CH01-C and the transect CH02. The lower estuary was represented by the transects CH03-CH08 (where CH05 and CH06 represent the connection with the Itacanoeira and Santana rivers, respectively). And the upper estuary was represented by the transects CH09–CH11 (Fig. 1). All abiotic variables of the water and the sediment were treated accordingly to the respective standard methodology, as described by Laut et al. (2021c).
Physical and Chemical Properties of the Water
Physical and chemical parameters of the seawater were obtained at the interface between the water and sediment, using a multiparametric probe (YSI 6600-V2 Xylem Water Solutions, Singapore). The depth ranged from 1.1 to 6.1 m depending on the station. Salinity, temperature, pH, dissolved oxygen (DO), chlorophyll-a (Chl-a), total dissolved solids (TDS), turbidity (NTU +), and the transparency of the water (using a Secchi disk) were measured (detailed measurements for each station are listed in Table S1).
Geochemical Analyses of the Sediment
The sediment samples were collected from the side of a vessel with a small adapted Ekman-type sampler (with ca. 3-L capacity), which had an upper aperture that allowed the separation of the surface of the sediment without major disturbances in the layer. The uppermost part of the sediment was collected for grain-size (ca. 300 mL) and geochemical analyses (50 mL, from the uppermost first cm). The granulometric analysis followed the protocol detailed by Laut et al. (2021c).
For the OM, the sediment samples were dried in an oven at 100 °C. A standard aliquot (80 g) was then treated with hydrogen peroxide (H2O2 10%) to remove the OM, weighted again, and the percentage of OM in the aliquot was calculated. The OM can be interpreted as all organic compounds from living or dead animals or vegetal vestiges, including carbon, hydrogen, oxygen, nitrogen, or other elements. Then, as a final step before the grain-size analysis, the carbonate content was removed by adding hydrochloric acid (HCl 36%) until stabilization of the reaction was achieved and no dissolution of carbonate could be observed (adapted from Suguio 1973). To determine sediment particle size, approximately 1 g of the samples without OM and carbonate content were treated with a sodium hydroxide solution (NaOH) medium dispersant for 24 h and then analyzed using a Particle Size Analyzer (CILAS 1190-3P Instruments, Germany).
Total organic carbon (TOC) and total sulphur (TS) were analyzed with a carbon and sulphur analyzer (LECO SC-632 LECO, Australia) in accordance with the protocols from the American Society for Testing Materials (protocol ASTM D4239, ASTM 2008) and the United States Environmental Protection Agency (protocol NCEA C1282, Schumacher 2002). The C/S ratio was also calculated and used as a proxy of sediment oxygenation.
The trace elements were extracted by an acid digestion in double distilled nitric acid (2 M HNO3), using a ratio of 50 mg of dried sample to 0.5 mL of acid for 4 h and heating at 100 °C. After cooling, the volume was adjusted to 5 mL with ultrapure water (resistivity > 18 MΩ cm) and analyzed with an inductively coupled plasma-mass spectrometer (ICP-MS, model ELAN DRC II, Perking Elmer-Sciex, Norwalk, CT, USA). The analysis followed the certified protocol DORM-4 by the National Research Council Canada (NRC) (Willie et al. 2012). Performing the extraction with the 2 M HNO3, as well as not using any complexing agents, allowed only a partial extraction of the elements in the sediment. Therefore, this method provides the releasable fraction (defined here as the fraction of free metals in the sediment, i.e., the bioavailable fraction), which is more effective to risk assessment for trace element pollution than the total fraction (Takáč et al. 2009). The analyzed chemical elements were aluminum (Al), arsenic (As), cadmium (Cd), chromium (Cr), cesium (Cs), copper (Cu), iron (Fe), mercury (Hg), magnesium (Mg), manganese (Mn), nickel (Ni), lead (Pb), strontium (Sr), and zinc (Zn).
To identify possible anthropic contributions, we calculated some pollution indicators, such as the Contamination Factor and the Modified Degree of Contamination Index (CF and mCd, respectively, Spagnoli et al. 2021) and the Pollution Load Index (PLI, Tomlinson et al. 1980). The CF is an index developed to evaluate the contamination of a given toxic substance in a basin based on the background concentrations, and the mCd represents the sum of all CFs of the elements analyzed for such a basin (Spagnoli et al. 2021). The PLI represents the pollution level in three possible classes, which are PLI = 0: unpolluted pristine conditions; PLI = 1: baseline levels of pollutants; and PLI > 1: progressive deterioration of the environmental quality by pollution (Tomlinson et al. 1980). The CF, mCd, and PLI were calculated for each station according to the following equations:
where Celement is the concentration of the element in a given station, Cbackground is the background concentration of the area, and \(CFn\) are all the CFs of the elements within a specific station. As the background concentrations were not yet investigated in the CRE, we considered previously published papers in the Bahia state from estuary settings (i.e., Barros et al. 2008; Fostier et al. 2016; Friedmann Angeli et al. 2019; Almeida et al. 2020). It is important to highlight that we are aware that using the regional background concentrations could result in a biased calculation if the estuaries considered are dissimilar to the CRE. One could argue that another option would be to consider the average global concentrations of trace elements in shale or granite. However, this would likewise add bias to the calculation. Therefore, we decided to keep the regional background concentrations. The background concentrations are reported in Table S2.
Foraminiferal Analysis
Sediment samples were collected in three replicates by independent deployments of the sediment grab at each station. A total of 50 mL of sediment from the first centimeter was taken from each replicate and stained with rose-Bengal (2 g/L) to identify the living specimens. The sediment was wet sieved (500–63 µm) and dried at 60 °C for 48 h. The sorting of foraminifera from the sediments was performed under a stereoscope microscope. A micro-splitter was used in the case of too many specimens in one sample. In contrast, when there were too few individuals, the minimum number of 100 specimens per replicate was targeted (Fatela and Taborda 2002). When the average of the replicates did not show the minimum number of specimens, we discarded the respective station from the statistical analyses. The foraminiferal density FD (individuals/50 mL) in each sample was calculated based on the average number of specimens counted in the three replicates and the number of times the samples were split (if that was the case).
We calculated the species richness (S = number of species) and the Fischer α index in PAST software (Hammer et al. 2001) and the diversity of the Shannon Wiener diversity index (H’) by the formula H’ = ∑pilnpi, where pi is the proportion of the species in the samples. The taxonomic identification of the species largely followed Brönnimann (1979), Boltovskoy et al. (1980), Poag (1981), Loeblich and Tappan (1987), Walton and Sloan (1990), Yassini and Jones (1995), Martins and Gomes (2004), Laut et al. (2014, 2017), and Raposo et al. (2016). All the species names were revised on the online platform World Register of Marine Species-WoRMS (Hayward et al. 2021).
Multivariate Analysis
An additive logarithmic transformation log (1 + X) was used prior to statistical analysis to remove the effects of orders of magnitude differences between variables (i.e., biotic and abiotic) and to normalize the data (Brakstad 1992; Manly 1997). A principal component analysis (PCA) was carried out to reduce the data matrices composed of several variables to a small number of components representing the main modes of variation. The PCA was performed on selected environmental parameters (i.e., salinity, pH, DO, PLI, mCd, mud, sand, TOC, and C/S) as primary variables. The PCA also allowed us to plot supplementary variables, namely diversity indices and relative abundances of benthic foraminiferal species (> 2% in at least one sample) (Frontalini et al. 2009). In this way, the PCA was performed only using the primary variables, whereas the supplementary ones were solely projected without affecting the analysis and used to understand their changes against the principal components. Statistical analyses were performed using Statistica 8.0 (Weiß 2007).
Multiple regression analyses were performed to detect the significant environmental variables that affected the species distribution. For this, we built a model for estimating species distribution change based on the selected environmental variables that are the same as in the PCA. To see which environmental variables are significant in their distribution, we examined the coefficients table, which shows the estimate of regression beta coefficients and the associated t-statistic and p-values. The species which showed a significant p-value are shown in Table 1 and the models for all species are reported in the Online Resource 1. The multiple regression analyses were performed using the software R 4.1.1 (R Core Team 2021).
To create a better representation of the empirical data (i.e., environmental variables, community metrics, and dominant species), interpolation maps were created (Azpurua and Ramos 2010) within the software ArcMap 10.5®. Several methods and settings were tested, and the Inverse Distance Weighting (IDW) method resulted as the most reliable one to represent the sediment and biotic variable distribution in the CRE, and the Spline method to represent the water-related variables. We defined the interpolation settings as the following: cell size = 3, power = 2, number of points = 12, and the color scheme was represented by the color ramp as spectrum full light, with 30 classes and a classification method of equal intervals. The metric coordinates were in accordance with the datum WGS84 (UTM 24S). The coverage of our sampling includes nearby sites (maximum distance of 1.8 km among transects) located in strategic regions to include the different environmental gradients (seawater and river input, connection to other estuaries, connection to mangroves, connection to the sewage treatment plant). Therefore, this coverage is appropriate for the interpolations that show the distribution of the sedimentary and water-related environments within the CRE. All the data used for the interpolations is shared in Table S1 (environmental variables) and Table S3 (biotic variables).
Results
Water and Sediment Characterization
Abiotic data has been previously described and discussed by Laut et al. (2021c). The environmental assessment of the physical and (geo)chemical properties of the water and sediment is presented in Figs. 2, 3, 4, and 5 (see Table S1 for detailed results).
The estuary mouth showed a salinity of ca. 37 and a pH of around 8.6. These prevailing marine conditions extended until the middle of the estuary (CH08 transect). Then salinity and pH gradually decreased, reaching 1.40 and 7.41, respectively, in the upper estuary (Fig. 2). The temperature varied from 27.3 to 29.8 °C. The stations closest to the ocean had the lowest temperatures, whereas the inland regions had the highest ones. The DO showed a maximum of 6.60 mg/L at the mouth and a minimum of 0.00 mg/L in the region close to the Itacanoeira River. The Chl-a values varied from a minimum of 0.90 μg/L at the mouth to 15–45 μg/L between the lower and upper estuary sectors. The transparency ranged between 0.5 and 1.6 m, with the lowest value in the station beside the STP (CH10-C) in the upper estuary. The TDS showed a minimum of 1.77 g/L in the upper estuary and a maximum of 36.9 g/L at the mouth. The NTU + varied between 0.90 and 61.7, with the highest values in the lower part of the estuary.
The CRE sediments were mainly represented by sand and some silty sands with OM ranging from 0.6% (CH03-A) to 53% (CH10-C) (Fig. 3 and Table S1). The TOC and TS showed minimum values of 0.01% and 0.05%, respectively, in the mouth (CH03-C), and a maximum in the central region of the estuary (TOC of 4.67% at CH09-B and TS of 1.21% at CH06-B). The C/S ratio was higher in the area between the lower and upper estuary (Fig. 3).
Trace element concentrations are shown in Figs. 4 and 5 and are listed in detail in Table S1. Higher concentrations of these elements were mainly found in the middle estuary, where the values reached 29.0 μg/g (As), 43.0 μg/g (Cr), 114 μg/g (Cu), 497 μg/g (Mn), 37.6 μg/g (Ni), 877 μg/g (Pb), and 390 μg/g (Zn). Cadmium was commonly found in low concentrations throughout the estuary (up to 0.57 μg/g). The concentrations of Hg were high throughout the estuary (up to 0.32 μg/g). The highest values of Cs (2.57 μg/g) and Sr (475 μg/g) were found in the mouth region (Table S1).
Maximum values of CF of Cu, Mg, Mn, Ni, and Pb were found in the middle estuary, and maximum values of Cd, Hg, Mn, and Ni were found in the upper estuary. The PLI ranged between 0.1 and 7.2, in which CH03-A (close to the mouth) presented the lowest PLI and CH08-B (at the beginning of the upper estuary) the highest PLI (Table S1).
Foraminiferal Analysis
Forty-nine living benthic foraminiferal taxa were identified throughout the CRE, represented by 30 calcareous species and 19 agglutinated ones (Table S3). The upper CRE included four stations with low (< 100 ind/50 mL) (CH09-B, CH09-C, CH10-B, CH11-B) or barren FD (CH11-A and CH11-C). The only station with low FD in the lower part of the estuary was CH04-C (Fig. 6). The highest FD (3,500 ind/50 mL) was found in CH08-A. The species list and all the community metrics are reported in Table S3. The H’ diversity was highest (1.55) at station CH01-C at the mouth of the CRE. This site also had the highest species richness (22 species) (Fig. 6). The lowest value of diversity was in the upper estuary, at station CH10-C (0.49). The calcareous species were dominant in most of the estuary and were replaced by agglutinated species in the innermost area (Fig. 6).
The CRE was dominated by Ammonia tepida, which presented high relative abundances of up to 89.1%. Three other species were identified in most of the stations with significant relative abundances: Ammonia parkinsoniana (up to 30.8%), Cribroelphidium excavatum (up to 40.4%), and Paratrochammina clossi (up to 100%). Haynesina germanica was also found in several stations, but with lower relative abundances (up to 10%).
Multivariate Analyses
The PCA showed that ~62.1% of data variance can be explained by the first two principal components (factors). In particular, the eigenvalues of component 1 (horizontally, 40.8 of inertia) and component 2 (vertically, 21.3 of inertia) are 3.7 and 1.9, respectively. Sand, mud, TOC, PLI, mCd, and C/S are the predominant variables in the first component, whereas the major contributors to the second component are pH, salinity, and DO (Fig. 7). On the basis of the distribution of these environmental variables in the PCA plane, the first component can be tentatively interpreted as a grain-size gradient, and the second component as the marine influence gradient (higher marine influence is defined as the higher influence of pH, salinity, and DO). Supplementary variables, namely the relative abundance of species and the diversity indices, were plotted over the PCA plane to reveal their relationships in relation to the first two components. In particular, diversity indices are positively related to the second component (i.e., the degree of marine influence), so the highest values of S and Fischer α indexes appear to be mostly found in the areas the highest marine influence of the estuary. Some of the species appear to be influenced by the grain-size gradient, such as C. excavatum, which shows relative abundances positively related with an increased percentage of fine sediments, which are enriched in TOC and pollutants. On the other hand, B. striatula exhibits the opposite pattern (Fig. 7). Additionally, some species show a preference for areas with less marine influence, such as P. clossi, A. parkinsoniana, Miliammina fusca, and Ammotium morenoi being placed towards negative values of the second component.
The multiple regression analyses revealed the significant environmental variables in the species distributions (Table 1), which were DO, C/S, Sand, mCd, and PLI. Only four species had their distributions significantly affected by those variables, and the mode in which they were affected (either positively or negatively) was particular to each species. For instance, the dominant species A. tepida was positively related to DO and negatively related to sand and mCd, whereas P. clossi was negatively related to DO and not related to any other environmental variable. Although the species Bolivina doniezi is not present in high abundance in the estuary, it is found in many stations, and it shows significant association with sand and PLI and a negative association with mCd. The most striking result, however, was with the species C. excavatum, which showed a positive and very strong relationship with mCd and C/S.
Discussion
Environmental Characterization of the Estuary
The CRE system shows great environmental variability due to marine influence in the mesotidal regime, the fluvial input by many freshwater discharges, and the OM from several sources (e.g., mangrove vegetation natural deposition, STP discharges, households with poor sanitation).
On the basis of our data, it is possible to observe a higher salinity, lower pH, lower temperature, and higher TDS values in the lower estuary that are related to the enhanced marine influence (Fig. 2). The fluvial influence starts to be more evident around the transects CH07 and CH08, delimiting the upper estuary region, as documented in previous studies in the CRE (Souza et al. 2009; Silva et al. 2015; Laut et al. 2021c). Although this well-defined boundary could be identified based on water parameters, the sedimentary system is much more dynamic and complex.
Relatively higher values of OM, TOC, and TS are associated with the upper estuary. The increased content of organic material and higher Chl-a in the water match well with previous studies that classified the area as a eutrophic or hypertrophic region with higher input of nutrients and higher Chl-a concentrations (Silva et al. 2015). Despite this, high values of OM, TOC, and TS are also found in the lower estuary, mainly in connection with the Itacanoeira and Santana rivers (Fig. 3 and Table S1). Higher OM can be attributed to the contribution of the mangroves (Oliveira et al. 2013), but extreme values above 40% identify highly impacted areas characterized by diffuse sources (poor household sanitation around station CH06–B) and/or point sources (the STP next to the stations CH09–C and CH10–C) of OM input (Laut et al. 2021c). The values of OM in the CRE are comparatively higher than those of other estuarine systems associated with mangroves along the coast of Brazil, such as the Potengi estuary, São João estuary, Paraíba do Sul Delta, Surui Estuary, and Itacorubí estuary, which revealed an OM range between 0.3 and 14.3% (Laut et al. 2016b).
The TOC values found in the CRE are comparable to the values found in highly industrialized regions with significant organic input from commercial ports, domestic sewage, and/or runoff from agricultural fields (e.g., Jobos Bay in Puerto Rico, TOC: 3.7–10.3%, Martínez-Colón et al. 2021; and Guanabara Bay in Brazil, TOC: 2.1–5.5%, Martins et al. 2020). However, the anthropogenic influence alone cannot be related to higher values of TOC everywhere. For instance, the Arade estuary in Portugal (0.62–1.81%; Laut et al. 2014) and the Walton backwater in England (0.07%–1.97%; Aston and Hewitt 1977), located in temperate regions, are affected by high anthropogenic activity but show lower TOC values. Considering that TOC is a quantitative and not a qualitative (i.e., terrestrial or marine) estimation of the total organic carbon, the organic matter found in the CRE could also be refractory, originating from the mangroves. Therefore, it is likely that the high levels of TOC in the CRE come from a combination of both natural (mangroves) and anthropogenic (STP, urban area discharge) sources.
The CRE also shows similar TS values to those found in other impacted coastal ecosystems along the Brazilian coast (Laut et al. 2016b and references there included). The C/S ratio indicates the oxygenation conditions of the sedimentary environment. As proposed by Duleba et al. (2018), values of C/S > 5 indicate oxygenated bottom water and mostly oxic sediment; C/S between 5 and 1.5 defines sedimentary deposits that undergo periods of anoxia, whereas C/S < 1.5 suggests background water and anoxic sediment. Using this classification, six stations would be classified as oxic (CH01–C, CH08–A, CH10–A, CH10–B, CH11–A, and CH11–B), four as anoxic (CH02–A, CH03–A, CH03–C, and CH06–A), and the others as having experienced periods of anoxia. This trend correlates well with the low value of DO observed throughout most of the system and highlights the great spatial heterogeneity of the sedimentary environment. Overall, the fact that the CRE shows high OM and TOC as in other tropical polluted areas (e.g., Martins et al. 2020; Martínez-Colón et al. 2021) suggests environmental degradation and the effects of STP discharges and OM of terrestrial origin in the ecosystem.
Trace Elements in the Sediment
The PLI, which gives an overall assessment of sediment trace element pollution, indicates progressive deterioration of environmental quality in the CRE. In fact, the PLI values shown in the present study are higher than in other polluted areas worldwide (Martins et al. 2015a, c; Damak et al. 2019; Francescangeli et al. 2020). The trace elements that are mainly responsible for this high PLI are the As, Cu, Hg, Ni, and Zn, concentrations of which exceed the US Environmental Protection Agency (US-EPA) Effects Range Low (ER-L), and Pb, with concentrations exceeding the Effects Range Median (ER-M) (Table S1). The ER-L indicates the concentration below which toxic effects are scarcely observed or predicted, while the ER-M indicates the concentration above which effects are generally or always observed (Long et al. 1995). Therefore, the Pb in the CRE already reaches toxic concentrations.
The proximity of the estuary to several agricultural fields of cocoa cultivation (mainly located upstream along the Cachoeira river; Cassano et al. 2009), which are associated with the use of chemical fertilizers, is likely an important source of trace elements in the CRE (Chepote et al. 2012). In the case of Zn, this metal that is present in many fertilizers (e.g., in the form of zinc sulfate) could also be enhanced by local fires in the agricultural areas (Chiba et al. 2011). In addition, Cu and Pb are also associated with agricultural activity, such as the use of several pesticides with arsenates and metal–organic compounds that could reach the estuary through percolation or runoff processes (Tiller 1989). Zn, Cu, and Pb also originate from urban activities (Dalto et al. 2006).
Aside from cocoa cultivation and other agricultural activities (such as cattle breeding), industrial activities in Itabuna and Ilhéus, as well as their high urbanization, have been identified as sources of contamination in aquatic macrophytes along the CRE (Klumpp et al. 2002). In fact, the different metals were associated with specific sources such as agricultural (Cu), industrial (Cr), and urban (Al, Cu) sources (Klumpp et al. 2002). High urbanization has also been related to pollution by Hg in coastal environments (Ferraro et al. 2006, 2009; Mirlean et al. 2009) and could be related to high values around the CRE as well. Therefore, there is a combination of several different pollution sources in the hydrographic basin of the Cachoeira River: an industrial hub including paint and dye factories, many agricultural fields with extensive use of pesticides and fertilizers, and untreated and diffuse discharge of urban sewage (Manzini et al. 2010).
Besides the anthropogenic influence, another possible explanation for the high number of elements exceeding the US-EPA thresholds as well as the high PLI values could be the influence of the rainy season (conditions prevailing during sampling). As previously shown as a relevant factor in other studies (i.e., in the Maracaípe River estuary, Northeastern Brazil, Coimbra et al. 2015; in the Pearl River estuary, southern China, Ip et al. 2004; and in a New Caledonian lagoon, Dalto et al. 2006), the rainfall conditions can enhance the mobilization of the metals in the sediments (Coimbra et al. 2015). In fact, Laut et al. (2021c) suggested an influence of sediment oxygenation, which can also increase with rainfall conditions and in regions under marine influence (Duleba et al. 2018). These observations therefore highlight the importance of more seasonal studies in the area.
Foraminiferal Assemblages’ Metrics and Estuarine Conditions
The living assemblage of the CRE was dominated by A. tepida but also represented by typical estuarine species from the South Atlantic, such as the calcareous A. parkinsoniana, Bolivina inflata, Bolivina striatula, B. doniezi, C. excavatum, Cribroelphidium poeyanum, and the agglutinated species Ammoastuta inepta, Ammoastuta salsa, Ammotium cassis, Ammotium morenoi (previously referred as Ammotium salsum), Arenoparrella mexicana, Haplophragmoides wilberti, M. fusca, and P. clossi (Bonetti and Eichler 1997; Barbosa and Suguio 1999; Debenay and Guillou 2002; Duleba and Debenay 2003; Disaró 2006; Burone and Pires-Vanin 2006; Souza et al. 2010, Teodoro et al. 2010; Donnici et al. 2012; Laut et al. 2011, 2012, 2016b; Martins et al. 2016b).
The heterogeneous distribution of the foraminiferal assemblages is associated with the high spatial variability in the ecosystem of the CRE. Higher values of FD, S, and diversity (Fisher’s α and H’) indices are associated with the areas of higher marine influence (PCA, Fig. 7), as also observed in other estuarine ecosystems worldwide (e.g., the coast of Vendée, France, Armynot du Châtelet et al. 2004; the Arade Estuary, Portugal, Laut et al. 2014; and the Guadiana Estuary, Camacho et al. 2015; Laut et al. 2016a). The lower FD throughout the estuary, with the dominance of very few species, is an indication of a system under environmental stress. Our results suggest that the stress is mainly triggered by anthropogenic sources. Both the community metrics and PCA reveal that the majority of the species are negatively related to the pollutants (i.e., PLI and mCd) and, in part, positively related to coarser grain size. Given the high affinity of trace elements for finer sediments (Ip et al. 2004; Gu et al. 2011), they could be acting as a trap for these elements, explaining the negative relation of the species distribution to the mud fraction. The complex combination of influencing factors such as marine influence, pollutant concentrations, and grain-size gradients highlights the importance of using a multivariate approach to identify the main parameters impacting the foraminiferal distribution.
A limited number of specimens (low FD) and a domination of the assemblage by agglutinated species are found in the uppermost part of the CRE (CH10 and CH11 transects) and this implies that the stronger fluvial influence makes the environmental conditions unfavorable for most of the foraminifera species. A similar trend was observed by Laut et al. (2021c) in the Almada Estuary, adjacent to the CRE, showing a clear relation between higher foraminiferal diversity and the marine influence. Foraminiferal diversity is also reduced in the anoxic sites connected to the northwards Itacanoeira estuary (CH05-A, CH05-B, CH05-C). These sites correspond to a densely urbanized area and show high percentages of OM in the sediment, which could trigger high microbial activity and lead to very low DO. As a result, this region is dominated by the opportunistic species (A. tepida) and the anoxic-resistant species (P. clossi) (Table S3).
Foraminiferal Species and Environmental Conditions
The observed dominance of A. tepida in many different environmental settings, disregarding the oxygen conditions, the grain size, or the marine (or river) influence, highlights why this species is often considered opportunistic with high tolerance to both natural stress and pollutants (Ruiz et al. 2005; Bouchet et al. 2007; Frontalini and Coccioni 2008; Frontalini et al. 2009; Debenay and Fernandez 2009; Souza et al. 2010; Martins et al. 2013, 2014, 2015a, b; Laut et al. 2014, 2016a). Ammonia tepida is constant throughout the system, even in the upper estuary where the dominance of this calcareous form was unexpected due to higher fluvial influence (e.g., Debenay et al. 2003; Laut et al. 2014; Camacho et al. 2015). In addition, our results reveal that the trace elements present in the sediment do not have a decisive impact on the A. tepida distribution (PLI is not significant in the multiple regression analyses, Table 1). The exception was for Cu and Pb (Fig. 4), where the abundance of this species decreases as concentrations increase (Fig. 6). This exception is reflected in the significant negative relationship of A. tepida to mCd in the multiple regression analyses, since the mCd is directly linked to the higher CFCu and CFPb in the stations where A. tepida has a lower abundance.
The reduction in the relative abundances of A. tepida is associated with an increase in the relative abundances of C. excavatum and suggests a better adaptation of C. excavatum to finer sediments enriched in TOC and pollutants (Fig. 7). This is also revealed by the opposite trend observed between A. tepida and C. excavatum in the multiple regression analyses. While A. tepida is negatively associated with mCd, C. excavatum shows a positive and strong association with the contamination index. Indeed, in the areas that C. excavatum is mainly reported (Fig. 6), there are the highest Cu and Pb concentrations and the highest mCd, which also shows a clear relation to C. excavatum in the PCA. Previous studies have related C. excavatum to higher TOC (Aveiro Lagoon, Martins et al. 2015a) and pollution resulting from industrial effluents and heavy metals in estuarine systems (Sharifi et al. 1991; Armynot du Châtelet and Debenay 2010). However, in other studies, salinity was also an important environmental variable for C. excavatum and it was not possible to distinguish between the impact of pollution and the influence of salinity in their distribution (e.g., Debenay and Guillou 2002; Armynot du Châtelet and Debenay 2010). In the present study, however, this species does not show any relation to salinity or other indicators of marine influence. This is clear in the PCA and in the multiple regression analysis (where the only significant environmental variables for this species were C/S, mCd, and PLI). Therefore, our findings reveal that C. excavatum seems to respond more clearly to pollution than to salinity.
Haynesina germanica, in instead, shows a higher affinity for marine settings than pollution and a higher affinity for the lower estuary. In the present study, it is found in lower relative abundances, which is often the case in Brazilian estuaries (Souza et al. 2010; Laut et al. 2016b, 2021c) but very different than in temperate or Mediterranean regions where it is frequently dominant (Armynot du Châtelet et al. 2004; Horton and Murray 2007; Laut et al. 2014). Haynesina germanica was previously regarded as generalist, associated with either intermediary zones between the upper and lower estuary (Debenay and Guillou 2002; Armynot du Châtelet et al. 2004; Laut et al. 2016b) or with higher marine influence (Huelva Coast, Spain, Ruiz et al. 2005). Interestingly, H. germanica has also been regarded as a bioindicator of heavy metal pollution (Bergamin et al. 2003; Romano et al. 2009; Frontalini et al. 2009; Laut et al. 2014), but this is not indicated by our findings. Therefore, the factors controlling the distribution of this generalist species are still difficult to constrain, but it seems that H. germanica prefers relatively colder waters and marine conditions.
Another species positively related to marine influence was E. gunteri (as also shown in Eichler et al. 2003; Martins et al. 2013, 2014, 2015a, b; Laut et al. 2014). This cosmopolitan species (Debenay and Guillou 2002; Armynot du Châtelet et al. 2004; Laut et al. 2014, 2016a, 2021b) is shown in our study to be negatively related to pollutants (Fig. 7). Therefore, our findings reveal E. gunteri as a potential indicator of good water exchange and reduced anthropogenic influence. This is, however, not the usual observation for this species, which has been previously associated with regions impacted by OM pollution (Eichler et al. 2007; Laut et al. 2014; Vilela et al. 2014). In the CRE, E. gunteri is not present in the regions with the highest OM percentages. Therefore, we cannot observe a clear relationship between E. gunteri and OM since it occurs within a large range of OM from 0.6 to 23.7%. With all observations being considered, we believe that this species’ distribution is likely mainly affected by marine influence rather than OM pollution.
As for the agglutinated species Trochammina inflata, also related to a higher marine influence in the PCA, it was actually reported in a great range of salinity values in the CRE (from 2.75 in CH11-B to 37.0 in CH06-C). This reveals a tolerance of T. inflata to great salinity variations (also suggested by Martins et al. 2013, 2014, 2015a; Laut et al. 2014). In the meantime, it is not possible to observe a clear relationship between this species and pollutants in the multivariate analyses, and therefore, T. inflata is likely a better indicator of natural stress than anthropogenic stress.
The dominant agglutinated species in the CRE, P. clossi, is reported in other regions of the Brazilian coast normally associated with mangroves (Disaró 2006; Laut et al. 2012, 2016c, 2021a), but often in lower relative abundances than in the present study. In the PCA, P. clossi is related to lower marine influence and in the opposite relation to DO. This pattern is confirmed in the multiple regression analyses, which revealed a significant negative relationship between P. clossi and DO. This is highlighted by its highest abundance in strongly anoxic conditions in the CRE and suggests a tolerance (or even a preference) of this species to paralic environments.
Another species showing preference for the lower marine influence in the CRE is the dominant A. parkinsoniana, which has been previously related to intermediate estuary zones around the globe (Debenay and Guillou 2002; Debenay et al. 2002; Laut et al. 2016a) and is sensitive to pollution by trace elements (Frontalini and Coccioni 2008; Coccioni et al. 2009). In the present study, however, there is no clear relation between this species and pollution by trace elements (PLI, mCd). It is also not clear if their distribution is affected by sediment grain size. Therefore, the factors controlling this species are still difficult to constrain, but our results suggest that this species prefers low salinity conditions (16.0 in the upper estuary), and their use as an indicator of anthropogenic influence or trace element pollution should be treated with caution.
Finally, we highlight the importance of increasing the knowledge of the ecology of benthic foraminifera species to trace elements and organic pollutants in highly urbanized estuarine areas to enhance their potential as bioindicators. While for some species, it is clear to which environmental conditions they respond, for other species it is still difficult to constrain given the multiple sources of stress from both anthropogenic and natural causes.
Conclusion
This study reveals that the foraminiferal distribution in the Cachoeira River Estuary is mostly driven by grain size, pollutants, and level of marine influence. In addition, increased runoff and input of freshwater due to the humid conditions typical of subtropical climates leads to highly mixed water and a heterogenic sedimentary environment. This dynamic system is associated with the heterogenic distribution of the living assemblages. The agglutinant species Paratrochammina clossi shows a preference for anoxic conditions, whereas Cribroelphidium excavatum thrives in the areas most polluted by trace elements. In addition, the opportunistic calcareous species Ammonia tepida was not particularly impacted by the trace elements in the sediment, with the exception to Cu and Pb (indicated by the modified degree of contamination index). Interestingly, the sister species Ammonia parkinsiona, previously regarded as sensitive to trace element pollution, did not show a clear relationship with trace elements in the present study. Therefore, our findings reveal that while some species are good bioindicators of specific environmental conditions (i.e., trace element or organic pollution, grain size, marine proximity), other species are still difficult to constrain given the complexity of the environmental gradients in which they are found. Therefore, to enhance the potential of these species to be used in further studies on coastal ecosystems under similar conditions, it is essential to increase knowledge of their ecology. This contribution represents the first assessment in South Bahia (NE Brazil) to evaluate living foraminifera communities and their relationships to trace element pollution as well as to physical–chemical and sedimentological parameters.
References
Almeida, A.O., P.A. Coelho, J.T.A. Santos, and N.R. Ferraz. 2006. Crustáceos decápodos estuarinos de Ilhéus, Bahia, Brasil (Decapod estuarine crustaceans from Ilhéus, Bahia, Brazil). Biota Neotropica 6 (2). https://doi.org/10.1590/S1676-06032006000200024.
Almeida, L.C., J.B. da Silva Júnior, I.F. dos Santos, V.S. de Carvalho, A.S. Santos, G.M. Hadlich, and S.L.C. Ferreira. 2020. Assessment of toxicity of metals in river sediments for human supply: Distribution, evaluation of pollution and sources identification. Marine Pollution Bulletin 158: 111423. https://doi.org/10.1016/j.marpolbul.2020.111423.
Alve, E. 1995. Benthic foraminiferal responses to estuarine pollution; a review. Journal of Foraminiferal Research 25: 190–203. https://doi.org/10.2113/gsjfr.25.3.190.
Alve, E., A. Lepland, J. Magnusson, and K. Backer-Owe. 2009. Monitoring strategies for re-establishment of ecological reference conditions: Possibilities and limitations. Marine Pollution Bulletin 59: 297–310. https://doi.org/10.1016/j.marpolbul.2009.08.011.
Alve, E., S. Korsun, J. Schönfeld, N. Dijkstra, E. Golikova, S. Hess, et al. 2016. Foram-AMBI: A sensitivity index based on benthic foraminiferal faunas from North-East Atlantic and Arctic fjords, continental shelves and slopes. Marine Micropaleontology 122: 1–12. https://doi.org/10.1016/j.marmicro.2015.11.001.
Armynot du Châtelet, E., J.-P. Debenay, and R. Soulard. 2004. Foraminiferal proxies for pollution monitoring in moderately polluted harbors. Environmental Pollution 127: 27–40. https://doi.org/10.1016/S0269-7491(03)00256-2.
Armynot du Châtelet, E., and J.-P. Debenay. 2010. The anthropogenic impact on the western French coasts as revealed by foraminifera: A review. Revue De Micropaléontologie 53: 129–137. https://doi.org/10.1016/j.revmic.2009.11.002.
ASTM - American Society for Testing Materials. 2008. Standard test method for sulfur in the analysis sample of coal and coke using high-temperature tube furnace combustion. v. D4239-08. USA: ASTM International. https://doi.org/10.1520/D4239-08.
Aston, S.R., and C.N. Hewitt. 1977. Phosphorus and carbon distributions in a polluted coastal environment. Estuarine and Coastal Marine Science 5 (2): 243–254. https://doi.org/10.1016/0302-3524(77)90020-2.
Azpurua, M.A., and K.D. Ramos. 2010. A comparison of spatial interpolation methods for estimation of average electromagnetic field magnitude. Progress in Electromagnetics Research 14: 135–145. https://doi.org/10.2528/PIERM10083103.
Bahia. 2017. Plano Estratégico para Revitalização da Bacia do Rio Cachoeira (Strategic Plan for the Revitalization of the Cachoeira River Basin). RP1 - Diagnóstico Ambiental 1 t16014 (Environmental Assessment Report). http://cachoeira.participacaopublica.com/ficheiros/RP1_DiagnosticoAmbiental.pdf. Accessed 21 May 2021.
Barbosa, J.S.F., and J.M.L. Domingues. 1996. Mapa geológico do estado da Bahia - Texto explicativo [Geological map of Bahia State - Explanatory text], 382. Governo do estado da Bahia: Universidade Federal da Bahia.
Barbosa, C.F., and K. Suguio. 1999. Biosedimentary facies of a subtropical microtidal estuary – an example from southern Brazil. Journal of Sedimentary Research 69: 576–587. https://doi.org/10.2110/jsr.69.576.
Barras, C., F.J. Jorissen, C. Labrune, B. Andral, and P. Boissery. 2014. Live benthic foraminiferal faunas from the French mediterranean coast: Towards a new biotic index of environmental quality. Ecological Indicators 36: 719–743. https://doi.org/10.1016/j.ecolind.2013.09.028.
Barros, F., V. Hatje, M.B. Figueiredo, W.F. Magalhães, H.S. Dórea, and E.S. Emídio. 2008. The structure of the benthic macrofaunal assemblages and sediments characteristics of the Paraguaçu estuarine system, NE, Brazil. Estuarine, Coastal and Shelf Science 78 (4): 753–762. https://doi.org/10.1016/j.ecss.2008.02.016.
Belart, P., I. Clemente, D. Raposo, R. Habib, E.K. Volino, A. Vilar, M.V.A. Martins, L.F. Fontana, M.L. Lorini, G. Panigai, F. Frontalini, M.S.L. Figueiredo, S.G. Vasconcelos, and L. Laut. 2018. Living and dead Foraminifera as bioindicators in Saquarema Lagoon System, Brazil. Latin American Journal of Aquatic Research 46 (5): 1055–1072. https://doi.org/10.3856/vol46-issue5-fulltext-18.
Belart, P., R. Habib, D. Raposo, J.M. Ballalai, I. Clemente, M.V.A. Martins, F. Frontalini, M.S.L. Figueiredo, M.L. Lorini, and L. Laut. 2019. Seasonal dynamics of benthic foraminiferal biocoenosis in the tropical Saquarema Lagoonal System (Brazil). Estuaries and Coasts 42: 822–841. https://doi.org/10.1007/s12237-018-00514-w.
Bergamin, L., E. Romano, M. Gabellini, A. Ausili, and M.G. Carboni. 2003. Chemical-physical and ecological characterisation in the environmental project of a polluted coastal area: the Bagnoli case study. Mediterranean Marine Science 4: 5–20. https://doi.org/10.12681/mms.225.
Bergamin, L., L. Di Bella, L. Ferraro, V. Frezza, G. Pierfranceschi, and E. Romano. 2019. Benthic foraminifera in a coastal marine area of the eastern Ligurian Sea (Italy): Response to environmental stress. Ecological Indicators 96: 16–31. https://doi.org/10.1016/j.ecolind.2018.08.050.
Boltovskoy, E., G. Giussani, S. Watanabe, and R. Wright. 1980. Atlas of Benthic Shelf Foraminifera of the Southwest Atlantic, 147p. Netherlands: Springer, Netherlands.
Bonetti, C., and B.B. Eichler. 1997. Benthic foraminifera and thecamoebians as indicators of river/sea gradients in the estuarine zone of Itapitangui River – Cananéia/SP, Brazil. Anais Da Academia Brasileira De Ciências 69 (4): 545–563.
Bouchet, V.M.P., J.-P. Debenay, P.-G. Sauriau, J. Radford-Knoery, and P. Soletchnik. 2007. Effects of short-term environmental disturbances on living benthic foraminifera during the Pacific oyster summer mortality in the Marennes-Oléron Bay (France). Marine Environmental Research 64 (3): 358–383. https://doi.org/10.1016/j.marenvres.2007.02.007.
Bouchet, V.M.P., E. Alve, B. Rygg, and R.J. Telford. 2012. Benthic foraminifera provide a promising tool for Ecological Quality assessment of marine waters. Ecological Indicators 23: 66–75. https://doi.org/10.1016/j.ecolind.2012.03.011.
Bouchet, V.M.P., E. Alve, B. Rygg, and R.J. Telford. 2013. Erratum: Benthic foraminifera provide a promising tool for ecological quality assessment of marine waters (Ecological Indicators (2012) 23: 66–75). Ecological Indicators 26: 183. https://doi.org/10.1016/j.ecolind.2012.10.002.
Bouchet, V.M.P., N. Deldicq, N. Baux, J.-C. Dauvin, J.-P. Pezy, L. Seuront, and Y. Méar. 2020. Benthic foraminifera to assess ecological quality statuses: The case of salmon fish farming. Ecological Indicators 117: 106607. https://doi.org/10.1016/j.ecolind.2020.106607.
Brakstad, F. 1992. A comprehensive pollution survey of polychlorinated dibenzo-p-dioxins and dibenzofurans by means of principal component analysis and partial least squares regression. Chemosphere 25: 1611–1629. https://doi.org/10.1016/0045-6535(92)90309-F.
Brönnimann, P. 1979. Recent benthonic foraminifera from Brazil Morphology and ecology Part IV: Trochamminids from the Campos shelf with description of Paratrochammina n. gen. Paläontologische Zeitschrift 53 (1): 5–25. https://doi.org/10.1007/BF02987785.
Burone, L., and A.M.S. Pires-Vanin. 2006. Foraminiferal assemblages in Ubatuba Bay, south-eastern Brazilian coast. Scientia Marina 70: 203–217. https://doi.org/10.3989/scimar.2006.70n2203.
Burone, L., N. Venturini, P. Sprechmann, P. Valente, and P. Muniz. 2006. Foraminiferal responses to polluted sediments in the Montevideo coastal zone, Uruguay. Marine Pollution Bulletin 52: 61–73. https://doi.org/10.1016/j.marpolbul.2005.08.007.
Camacho, S., D. Moura, S. Connor, D. Scott, and T. Boski. 2015. Ecological zonation of benthic foraminifera in the lower Guadiana Estuary (southeastern Portugal). Marine Micropaleontology 114: 1–18. https://doi.org/10.1016/j.marmicro.2014.10.004.
Cassano, C.R., G. Schroth, D. Faria, J.H.C. Delabie, and L. Bede. 2009. Landscape and farm scale management to enhance biodiversity conservation in the cocoa producing region of southern Bahia, Brazil. Biodiversity and Conservation 18: 577–603. https://doi.org/10.1007/s10531-008-9526-x.
Castelo, W.F.L., M.V.A. Martins, M. Martínez-Colón, L.C. Silva, C.M. Menezes, T. Oliveira, S.H.M. Sousa, O. Aguilera, L. Laut, V. Laut, W. Duleba, F. Frontalini, V.M.P. Bouchet, E.A. Châtelet, F. Francescangeli, M.C. Geraldes, A.T. Reis, and S. Bergamashi. 2022. Bioaccumulation of potentially toxic elements in Ammonia tepida (foraminifera) from a polluted coastal area. Journal of South American Earth Sciences 115: 103741. https://doi.org/10.1016/j.jsames.2022.103741.
Chepote, R.E., G.A. Sodré, E.L. Reis, R.G. Pacheco, P.C.L. Morocco, and R.R. Valle. 2012. Recomendações de corretivos e fertilizantes na cultura do cacaueiro no Sul da Bahia. Boletim Técnico No. 203. Ministério da Agricultura, Pecuária e Abastecimento. [Recommendations for repairs and fertilizers in cocoa cultivation in South Bahia. Technical Bulletin No. 203. Ministry of Agriculture, Livestock and Supply]. ISSN 0100–0845. https://www.gov.br/agricultura/pt-br/assuntos/ceplac/publicacoes/boletins-tecnicos-bahia/bt-203.pdf. Accessed 3 September 2021.
Chiba, W.A.C., M.D. Passerini, and J.G. Tundisi. 2011. Metal contamination in benthic macroinvertebrates in a sub-basin in the southeast of Brazil. Brazilian Journal of Biology 71 (2): 391–399. https://doi.org/10.1590/S1519-69842011000300008.
Clements, W.H., S.C. Donald, and J.H. Van Hassel. 1992. Assessment of the impact of heavy metals on benthic communities at the Clinch River (Virginia): Evaluation of an Index of Community Sensitivity. Canadian Journal of Fisheries and Aquatic Sciences 49 (8): 1686–1694. https://doi.org/10.1139/f92-187.
Coccioni, R., F. Frontalini, A. Marsili, and D. Mana. 2009. Benthic foraminifera and trace element distribution: A case study from the heavily polluted lagoon of Venice (Italy). Marine Pollution Bulletin 59: 257–267. https://doi.org/10.1016/j.marpolbul.2009.08.009.
Coimbra, C.D., G. Carvalho, H. Philippini, M.F.M. Silva, and E. Neiva. 2015. Determinação da concentração de metais traço em sedimentos do estuário do Rio Maracaípe - PE/Brasil (Determination of trace elements concentration in sediments from the Maracaípe River estuary - PE/Brazil). Brazilian Journal of Aquatic Science and Technology 19 (2): 58–75. https://doi.org/10.14210/bjast.v19n2.4863.
Dalto, A.G., A. Grémare, A. Dinet, and D. Fichet. 2006. Muddy-bottom meiofauna responses to metal concentrations and organic enrichment in New Caledonia South-West Lagoon. Estuarine, Coastal and Shelf Science 67 (4): 629–644. https://doi.org/10.1016/j.ecss.2006.01.002.
Damak, M., F. Frontalini, B. Elleuch, and M. Kallel. 2019. Benthic foraminiferal assemblages as pollution proxies along the coastal fringe of the Monastir Bay (Tunisia). Journal of African Earth Sciences 150: 379–388. https://doi.org/10.1016/j.jafrearsci.2018.11.013.
Damasio, B.V., C.T. Timoszczuk, B.S.M. Kim, S.H.M. Sousa, M.C. Bícego, E. Siegle, and R.C.L. Figueira. 2020. Impacts of hydrodynamics and pollutants on foraminiferal fauna distribution in the Santos Estuary (SE Brazil). Journal of Sedimentary Environments 5: 61–86. https://doi.org/10.1007/s43217-020-00003-w.
Debenay, J.-P., W. Duleba, C. Bonetti, S.H.M. Sousa, and B.B. Eichler. 2001. Pararotalia cananeiaensis n sp: Indicator of marine influence and water circulation in Brazilian coastal and paralic environments. Journal of Foraminiferal Research 31 (2): 152–163. https://doi.org/10.2113/0310152.
Debenay, J.P., and J.J. Guillou. 2002. Ecological transitional indicated by foraminiferal assemblages in paralic environments. Estuaries 25 (6): 1107–1120. https://doi.org/10.1007/BF02692208.
Debenay, J.P., D. Guiral, and M. Parra. 2002. Ecological factors acting on the microfauna in mangrove swamps. The case of foraminiferal assemblages in French Guiana. Estuarine, Coastal and Shelf Science 55: 509–533. https://doi.org/10.1006/ecss.2001.0906.
Debenay, J.P., P. Carbonel, M.-T. Morzadec-Kerfourn, A. Cazaubon, M. Denèfle, and A.-M. Lézine. 2003. Multi-bioindicator study of a small estuary in Vendée (France). Estuarine, Coastal and Shelf Science 58: 843–860. https://doi.org/10.1016/S0272-7714(03)00189-6.
Debenay, J.-P., and J.-M. Fernandez. 2009. Benthic foraminifera records of complex anthropogenic environmental changes combined with geochemical data in a tropical bay of New Caledonia (SW Pacific). Marine Pollution Bulletin 59 (8–12): 311–322. https://doi.org/10.1016/j.marpolbul.2009.09.014.
DHN - Diretoria de Hidrografia e Navegação (Hydrography and Navigation Directorate). 2016. Tábuas de marés (Tide tables). Marinha do Brasil. https://www.marinha.mil.br/chm/tabuas-de-mare. Accessed 7 October 2016.
Dimiza, M.D., M.V. Triantaphyllou, O. Koukousioura, and P. Hallock. 2016. The Foram Stress Index: A new tool for environmental assessment of soft-bottom environments using benthic foraminifera. A case study from the Saronikos Gulf, Greece, Eastern Mediterranean. Ecological Indicators 60: 611–621. https://doi.org/10.1016/j.ecolind.2015.07.030.
Disaró, S. 2006. Foraminíferos em ecossistemas de manguezal e marismas salgadas. In Revisões em Zoologia - I: Volume comemorativo dos 30 anos do Curso de Pós-Graduação em Zoologia da Universidade Federal do Paraná, ed. E.L.A. Monteiro-Filho and J.M.R. Aranha, 67–85. Curitiba: Secretaria do Meio Ambiente do Estado do Paraná.
Donnici, S., R. Serandrei-Barbero, M. Bonardi, and M. Sperle. 2012. Benthic foraminifera as proxies of pollution: The case of Guanabara Bay (Brazil). Marine Pollution Bulletin 64: 2015–2028. https://doi.org/10.1016/j.marpolbul.2012.06.024.
Duleba, W., and J.P. Debenay. 2003. Hydrodynamic circulation in the estuaries of Estação Ecológica Juréia-Itatins, Brazil, inferred from foraminifera and thecamoebian assemblages. Journal of Foraminiferal Research 33 (1): 62–93. https://doi.org/10.2113/0330062.
Duleba, W., A.C. Teodoro, J.P. Debenay, M.V.A. Martins, S. Gubitoso, L.A. Pregnolato, L.M. Lerena, S.M. Prada, and J.E. Bevilacqua. 2018. Environmental impact of the largest petroleum terminal in SE Brazil: A multiproxy analysis based on sediment geochemistry and living benthic foraminifera. PLoS ONE 13 (2): e0191446. https://doi.org/10.1371/journal.pone.0191446.
Eichler, P.P.B., B.B. Eichler, L.B. Miranda, E.R.M. Pereira, P.B.P. Kfouri, F.M. Pimenta, A.L. Bérgamo, and C.G. Vilela. 2003. Benthic foraminiferal response to variations in temperature, salinity, dissolved oxygen and organic carbon, in the Guanabara Bay, Rio de Janeiro, Brazil. Anuário Do Instituto De Geociências 26: 36–51.
Eichler, P.P.B., B.B. Eichler, L.B. de Miranda, and A.R. Rodrigues. 2007. Modern foraminiferal facies in a subtropical estuarine channel, Bertioga, Sao Paulo, Brazil. The Journal of Foraminiferal Research 37 (3): 234–247. https://doi.org/10.2113/gsjfr.37.3.234.
Eichler, P.P.B., A.R. Rodrigues, E. da Rocha Mendes, B.B. Pereira, A. Kahn. Eichler, and H. Vital. 2015. Foraminifera as environmental condition indicators in Todos os Santos Bay (Bahia, Brazil). Open Journal of Ecology 5 (7): 326–342. https://doi.org/10.4236/oje.2015.57027.
Fatela, F., and R. Taborda. 2002. Confidence limits of species proportions in microfossil assemblages. Marine Micropaleontology 45: 169–174. https://doi.org/10.1016/S0377-8398(02)00021-X.
Ferraro, L., M. Sprovieri, I. Alberico, F. Lirer, L. Prevedello, and E. Marsella. 2006. Benthic foraminifera and heavy metals distribution: A case study from the Naples Harbour (Tyrrhenian Sea, Southern Italy). Environmental Pollution 142 (2): 274–287. https://doi.org/10.1016/j.envpol.2005.10.026.
Ferraro, L., S. Sammartino, M.L. Feo, P. Rumolo, D. Salvagio Manta, E. Marsella, and M. Sprovieri. 2009. Utility of benthic foraminifera for biomonitoring of contamination in marine sediments: A case study from the Naples Harbour (Southern Italy). Journal of Environmental Monitoring 11: 1226–1235. https://doi.org/10.1039/B819975B.
Fidelman, P.I.J. 2005. Contribuição para a mitigação dos impactos da macrófita aquática Eichhorniacras sipes sobre a zona costeira da região Sul da Bahia [Contribution for Eichhornia crassipes impact migration in the South coast of Bahia coastal zone]. Journal of Integrated Coastal Zone Management 4 (3): 1–5.
Flemer, D.A., and M.A. Champ. 2006. What is the future fate of estuaries given nutrient over enrichment, freshwater diversion and low flows? Marine Pollution Bulletin 52: 247–258. https://doi.org/10.1016/j.marpolbul.2005.11.027.
Fostier, A.H., F.N. Costa, and M.G.A. Korn. 2016. Assessment of mercury contamination based on mercury distribution in sediment, macroalgae, and seagrass in the Todos os Santos bay, Bahia, Brazil. Environmental Science and Pollution Research 23 (19): 19686–19695. https://doi.org/10.1007/s11356-016-7163-6.
Francescangeli, F., M. Quijada, E. Armynot du Châtelet, F. Frontalini, A. Trentesaux, G. Billon, and V.M.P. Bouchet. 2020. Multidisciplinary study to monitor consequences of pollution on intertidal benthic ecosystems (Hauts de France, English Channel, France): Comparison with natural areas. Marine Environmental Research 160: 105034. https://doi.org/10.1016/j.marenvres.2020.105034.
Friedmann Angeli, J.L., B. Rubio, B.S.M. Kim, P.A.L. Ferreira, E. Siegle, and R.C.L. Figueira. 2019. Environmental changes reflected by sedimentary geochemistry for the last one hundred years of a tropical estuary. Journal of Marine Systems 189: 36–49. https://doi.org/10.1016/j.jmarsys.2018.09.004.
Frontalini, F., and R. Coccioni. 2008. Benthic foraminifera for heavy metal pollution monitoring: A case study from the central Adriatic Sea coast of Italy. Estuarine, Coastal and Shelf Science 76: 404–417. https://doi.org/10.1016/j.ecss.2007.07.024.
Frontalini, F., C. Buosi, S. Da Pelo, R. Coccioni, A. Cherchi, and C. Bucci. 2009. Benthic foraminifera as bio-indicators of trace element pollution in the heavily contaminated Santa Gilla lagoon (Cagliari, Italy). Marine Pollution Bulletin 58: 858–877. https://doi.org/10.1016/j.marpolbul.2009.01.015.
Frontalini, F., and R. Coccioni. 2011. Benthic foraminifera as bioindicators of pollution: A review of Italian research over the last three decades. Revue De Micropaléontologie 54: 115–127. https://doi.org/10.1016/j.revmic.2011.03.001.
Frontalini, F., M. Greco, L. Di Bella, F. Lejzerowicz, E. Reo, A. Caruso, C. Cosentino, A. Maccotta, G. Scopellitie, M.P. Nardelli, M.T. Losada, E. Armynot du Châtelet, R. Coccioni, and J. Pawlowski. 2018. Assessing the effect of mercury pollution on cultured benthic foraminifera community using morphological and eDNA metabarcoding approaches. Marine Pollution Bulletin 129 (2): 512–524. https://doi.org/10.1016/j.marpolbul.2017.10.022.
Gu, B., Y. Bian, C.L. Miller, W. Dong, X. Jiang, and L. Liang. 2011. Mercury reduction and complexation by natural organic matter in anoxic environments. PNAS 108 (4): 1479–1483. https://doi.org/10.1073/pnas.1008747108.
Hammer, Ø., D.A.T. Harper, and P.D. Ryan. 2001. PAST: Paleontological Statistics Software Package for Education and Data Analysis. Palaeontologia Electronica 4. http://palaeo-electronica.org/2001_1/past/issue1_01.htm. Accessed 21 May 2021.
Hayward, B.W., T. Cedhagen, M. Kaminski, and O. Gross. 2021. World Foraminifera database. http://www.marinespecies.org/foraminifera. Accessed 21 May 2021.
Horton, B.P., and J.W. Murray. 2007. The roles of elevation and salinity as primary controls on living foraminiferal distributions: Cowpen Marsh, Tees Estuary, UK. Marine Micropaleontology 63: 169–186. https://doi.org/10.1016/j.marmicro.2006.11.006.
Ip, C.C.M., X.D. Li, G. Zhang, J.G. Farmer, O.W.H. Wai, and Y.S. Li. 2004. Over one hundred years of trace metal fluxes in the sediments of the Pearl River Estuary, South China. Environmental Pollution 132: 157–172. https://doi.org/10.1016/j.envpol.2004.03.028.
Jayaraju, N., B.C.S.R. Reddy, K.R. Reddy, and A.N. Reddy. 2011. Impact of iron ore tailing on foraminifera of the Uppateru River Estuary, East Coast of India. Journal of Environmental Protection 2 (3): 213–220. https://doi.org/10.4236/jep.2011.23025.
Jorissen, F., M.P. Nardelli, A. Almogi-Labin, C. Barras, L. Bergamin, E. Bicchi, et al. 2018. Develo** Foram-AMBI for bio-monitoring in the Mediterranean: Species assignments to ecological categories. Marine Micropaleontology 140: 33–45. https://doi.org/10.1016/j.marmicro.2017.12.006.
Klumpp, A., K. Bauer, C. Franz-Gerstein, and M. de Menezes. 2002. Variation of nutrient and metal concentrations in aquatic macrophytes along the Rio Cachoeira in Bahia (Brazil). Environment International 28 (3): 165–171. https://doi.org/10.1016/S0160-4120(02)00026-0.
Lacuna, M.L.D.G., and M.P. Alviro. 2014. Diversity and abundance of benthic foraminifera in nearshore sediments of Iligan City, Northern Mindanao, Philippines. Animal Biology & Animal Husbandry 6(1): 10–26. https://www.proquest.com/scholarly-journals/diversity-abundance-benthic-foraminifera/docview/2018603153/se-2?accountid=14136.
Laut, L.L.M., F.S. Silva, A.G. Figueiredo Jr., and V.M. Laut. 2011. Assembleias de foraminíferos e tecamebas associadas a análises sedimentológicas e microbiológicas no delta do rio Paraíba do Sul, Rio de Janeiro Brasil. [Foraminiferal and thecamoebians assemblages associated with sedimentological and microbiological analyzes in the Paraíba do Sul River Delta, Rio de Janeiro, Brazil.]. Pesquisas em Geociências 38: 251–267. https://doi.org/10.22456/1807-9806.35162.
Laut, L.L.M., F.S. Silva, M.V. Alves Martins, M.A.C. Rodrigues, J.O. Mendonça, I.M.M.M. Clemente, V.M. Laut, and L.G. Mentzigen. 2012. Foraminíferos do Complexo Sepetiba/ Guaratiba [Foraminifera of the Sepetiba / Guaratiba Complex]. In Baía de Sepetiba: Estado da Arte, ed. M.A.C. Rodrigues, S.D. Pereira, and S.B. Santos, 115–150. Rio de Janeiro: Corbã Ltda.
Laut, L.L.M., I.A. Cabral, M.A.C. Rodrigues, F.S. da Silva, M.V.A. Martins, T. Boski, A.I. Gomes, J.M.A. Dias, L.F. Fontana, V.M. Laut, and J.G. Mendonça-Filho. 2014. Compartimentos Ambientais do Estuário do Rio Arade, Sul de Portugal, com Base na Distribuição e Ecologia de Foraminíferos [Environmental Compartments of the Arade River Estuary, Southern Portugal, Based on the Distribution and Ecology of Foraminifera]. Anuário do Instituto de Geociências – UFRJ 37 (2): 60–74. https://doi.org/10.11137/2014_2_60_74.
Laut, L.L.M., I.M.M.M. Clemente, P. Belart, M.V.A. Martins, F. Frontalini, V.M. Laut, A. Gomes, T. Boski, M.L. Lorini, R.R. Fortes, and M.A.C. Rodrigues. 2016a. Multiproxies (benthic foraminifera, ostracods and biopolymers) approach applied to identify the environmental partitioning of the Guadiana River Estuary (Iberian Peninsula). Journal of Sedimentary Environments 1 (2): 184–201. https://doi.org/10.12957/jse.2016.22534.
Laut, L.L.M., M.V.A. Martins, F.S. da Silva, M.A.C. Crapez, L.F. Fontana, S.B.V. Carvalhal-Gomes, and R.C.C.L. Souza. 2016b. Foraminifera, thecamoebians, and bacterial activity in polluted intertropical and subtropical Brazilian estuarine systems. Journal of Coastal Research 32 (1): 56–69. https://doi.org/10.2112/JCOASTRES-D-14-00042.1.
Laut, L.L.M., M.V. Alves Martins, F. Frontalini, P. Belart, V.F. Santos, M.L. Lorini, R.F. Fortes, F.S. Silva, S.S.S. Vieira, and P.W.M. Souza Filho. 2016c. Biotic (foraminifera and thecamoebians) and abiotic parameters as proxies for identification of the environmental heterogeneity in Caeté river estuary, amazon coast, Brazil. Journal of Sedimentary Environments 1 (1): 1–16. https://doi.org/10.12957/jse.2016.21264.
Laut, L., I. Clemente, M.V.A. Martins, F. Frontalini, D. Raposo, P. Belart, R. Habib, R. Fortes, and M.L. Lorini. 2017. Benthic foraminifera and thecamoebians of Godineau River Estuary, Gulf of Paria, Trinidad Island. Anuário do Instituto de Geociências – UFRJ 40 (2): 118–143. https://doi.org/10.11137/2017_2_118_143.
Laut, L., G. da Matta, G. Camara, P. Belart, I. Clemente, J. Ballalai, E. Volino, and E.C.G. Couto. 2021a. Living and dead foraminifera assemblages as environmental indicators in the Almada River Estuary, Ilhéus, Northeastern Brazil. Journal of South American Earth Sciences 105: 102883. https://doi.org/10.1016/j.jsames.2020.102883.
Laut, L., I. Clemente, and W. Louzada. 2021b. The influence of organic matter compounds on foraminiferal and ostracode assemblages: a case study from the Maricá-Guarapina Lagoon System (Rio de Janeiro, Brazil). Micropaleontology 67 (5): 447–458. https://doi.org/10.47894/mpal.67.5.02.
Laut, L., D. Raposo, I. Clemente, F. Veríssimo, E. Pereira, S.C. de Vasconcelos, J. Balallai, and E. Couto. 2021. Indicadores geoquímicos e biodisponibilidade de elementos-traço em sedimentos do estuário do rio Cachoeira, Ilhéus - BA, Brasil. [Geochemical proxies and bioavailability of trace elements in sediments from the Cachoeira River estuary, Ilhéus - BA, Brazil.]. Anuário do Instituto de Geociências – UFRJ 44: 35952. https://doi.org/10.11137/1982-3908_2021_44_35952.
Loeblich, A.R., and H. Tappan. 1987. Foraminiferal Genera and their Classification. New York: Van Nostrand Reinhold Company.
Long, E.R., D.D. Macdonald, S.L. Smith, and F.D. Calder. 1995. Incidence of adverse biological effects within ranges of chemical concentrations in marine and estuarine sediments. Environmental Management 19: 81–97. https://doi.org/10.1007/BF02472006.
Lucio, M.Z.T.P.Q.L. 2010. Biogeoquímica do Rio Cachoeira (Bahia, Brasil). [Biogeochemistry of the Cachoeira River (Bahia, Brazil).] Masters’ thesis. Programa de Pós-Graduação em Sistemas Aquáticos Tropicais da Universidade Estadual de Santa Cruz. 44p. PDF: https://bit.ly/3wwhY0V. Accessed 14 March 2018.
Manly, B.F.J. 1997. Randomization and Monte Carlo Methods in Biology. New York: Chapman and Hall.
Manzini, F.F., K.B. Sá, and L.M.A. Plicas. 2010. Metais pesados: fonte e ação toxicológica [Heavy metals: source and toxicological effects]. Fórum Ambiental da Alta Paulista 6 (12): 800–815. https://doi.org/10.17271/19800827612201026.
Maríns R.V., F.J.P. Filho, and C.A.S. Rocha. 2007. Geoquímica de Fósforo como Indicadora da Qualidade Ambiental e dos Processos Estuarinos do Rio Jaguaribe- Costa Nordeste Oriental Brasileira. [Phosphorus geochemistry as a proxy of environmental estuarine processes at the Jaguaribe River, Northeastern Brazil]. Quimica Nova 30 (5): 1208–1214. https://doi.org/10.1590/S0100-40422007000500029.
Martínez-Colón, M., P. Hallock, and C. Green-Ruíz. 2009. Strategies for using shallow-water foraminifers as bioindicators of potentially toxic elements: A review. Journal of Foraminiferal Research 39 (4): 278–299. https://doi.org/10.2113/gsjfr.39.4.278.
Martínez-Colón, M., P. Hallock, C. Green-Ruíz, and J. Smoak. 2018a. Temporal variability in potentially toxic elements (PTE’s) and benthic Foraminifera in an estuarine environment in Puerto Rico. Micropaleontology 63 (6): 357–381. https://doi.org/10.47894/mpal.63.6.01.
Martínez-Colón, M., P. Hallock, C. Green-Ruíz, and J. Smoak. 2018b. Benthic foraminifera as bioindicators of potentially toxic elements (PTE) pollution: Torrecilla Lagoon, Puerto Rico. Ecological Indicators 89: 516–527. https://doi.org/10.1016/j.ecolind.2017.10.045.
Martínez-Colón, M., H. Alegría, A. Huber, H. Kubra Gul, and P. Kurt-Karakus. 2021. Bioaccumulation and biomagnification of potential toxic elements (PTEs): An Avicennia germinans-Uca rapax trophic transfer story from Jobos Bay, Puerto Rico. Ecological Indicators 121: 107038. https://doi.org/10.1016/j.ecolind.2020.107038.
Martins, M.V.A., and V.C.R.D. Gomes. 2004. Foraminíferos da Margem Continental NW Ibérica: Sistemática, Ecologia e Distribuição. [Foraminifera from the Continental Iberia NW Iberia: Systematics, Ecology and Distribution.]. Aveiro: Aveiro University Press.
Martins, M.V.A., F. Frontalini, K.M. Tramonte, R.C.L. Figueira, P. Miranda, C. Sequeira, S. Fernández-Fernández, J.A. Dias, C. Yamashita, L.M. Laut, F.S. Silva, M.A.C. Rodrigues, C. Bernardes, R. Nagai, S.M. Sousa, M. Mahiques, B. Rubio, A. Bernabeu, D. Rey, and F. Rocha. 2013. Assessment of the health quality of Ria de Aveiro (Portugal): Heavy metals and benthic foraminifera. Marine Pollution Bulletin 70: 18–33. https://doi.org/10.1016/j.marpolbul.2013.02.003.
Martins, M.V.A., F. Frontalini, M.A.C. Rodrigues, J.A. Dias, L.L.M. Laut, F.S. Silva, I.M.M.M. Clemente, R. Reno, J. Moreno, S. Sousa, N. Zaaboub, M. El Bour, and F. Rocha. 2014. Foraminiferal biotopes and their distribution control in Ria de Aveiro (Portugal): A multiproxy approach. Environmental Monitoring and Assessment 186: 8875–8897. https://doi.org/10.1007/s10661-014-4052-7.
Martins, M.V.A., F. Silva, L.M.L. Lazaro, F. Frontalini, I.M. Clemente, P. Miranda, R. Figueira, S.H.M. Sousa, and J.M.A. Dias. 2015a. Response of benthic foraminifera to organic matter quantity and quality and bioavailable concentrations of metals in Aveiro Lagoon (Portugal). PLoS ONE 10 (2): e0118077. https://doi.org/10.1371/journal.pone.0118077.
Martins, M.V.A., L.L.M. Laut, F.S. Silva, P. Miranda, J.G. Mendonça-Filho, S. Fernández-Fernández, S.S. Sousa, M.A.C. Rodrigues, A.R. Rodrigues, C. Yamashita, E.O. Faria, R.R. Oliveira, and R.H. Nagai. 2015b. Associações de foraminíferos em resposta a variações ambientais da Laguna de Aveiro – Portugal. [Associations of foraminifera in response to environmental variations in the Lagoon of Aveiro - Portugal]. Anuário de Geociências – UFRJ 38: 56–69. https://doi.org/10.11137/2015_2_56_69.
Martins, M.V.A., N. Zaaboub, L. Aleya, F. Frontalini, E. Pereira, P. Miranda, M. Mane, F. Rocha, L. Laut, and M. El Bour. 2015c. Environmental quality assessment of Bizerte Lagoon (Tunisia) using living foraminifera assemblages and a multiproxy approach. PLoS ONE 10 (9): e0137250. https://doi.org/10.1371/journal.pone.0137250.
Martins, M.V.A., A.F.S. Pinto, F. Frontalini, M.C.M. Fonseca, D.L. Terroso, L.L.M. Laut, et al. 2016a. Can benthic foraminifera be used as bio-indicators of pollution in areas with a wide range of physicochemical variability? Estuarine, Coastal and Shelf Science 182: 211–225. https://doi.org/10.1016/j.ecss.2016.10.011.
Martins, M.V.A., L.L.M. Laut, F. Frontalini, C. Sequeira, R. Rodrigues, M.C.F. Fonseca, S. Bergamaschi, E. Pereira, F.P. Delavy, A.G. Figueiredo Jr., P. Miranda, D. Terroso, A.L. Pena, V.M. Laut, R. Figueira, and F. Rocha. 2016b. Controlling factors on the abundance, diversity and size of living benthic foraminifera in the NE sector of Guanabara Bay (Brazil). Journal of Sedimentary Environments 1 (4): 393–410. https://doi.org/10.12957/jse.2016.26872.
Martins, M.V.A., J. Hohenegger, M. Martínez-Colón, F. Frontalini, S. Bergamashi, L. Laut, P. Belart, M. Mahiques, E. Pereira, R. Rodrigues, D. Terroso, P. Miranda, M.C. Geraldes, H. Heringer Villena, T. Reis, O.A. Aguilera-Socorro, S.H.M. Sousa, C. Yamashita, and F. Rocha. 2020. Ecological quality status of the NE sector of the Guanabara Bay (Brazil): A case of living benthic foraminiferal resilience. Marine Pollution Bulletin 58: 111449. https://doi.org/10.1016/j.marpolbul.2020.111449.
Mirlean, N., L. Calliari, P. Baisch, E. Loitzenbauer, and E. Shumilin. 2009. Urban activity and mercury contamination in estuarine and marine sediments (Southern Brazil). Environmental Monitoring and Assessment 157: 583–589. https://doi.org/10.1007/s10661-008-0558-1.
Mojtahid, M., F. Jorissen, J. Durrieu, F. Galgani, H. Howa, F. Redois, et al. 2006. Benthic foraminifera as bio-indicators of drill cutting disposal in tropical east Atlantic outer shelf environments. Marine Micropaleontology 61: 58–75. https://doi.org/10.1016/j.marmicro.2006.05.004.
Oliveira, A.B., A.E. Rizzo, and E.C.G. Couto. 2013. Assessing decomposition rates of Rhizophora mangle and Laguncularia racemosa leaves in a tropical mangrove. Estuaries and Coasts 36: 1354–1362. https://doi.org/10.1007/s12237-013-9627-y.
Poag, C.W. 1981. Ecologic Atlas of Benthic Foraminifera of the Gulf of Mexico. Woods Hole: Hutchinson Ross.
Prazeres, M., T.E. Roberts, and J.M. Pandolfi. 2017. Variation in sensitivity of large benthic foraminifera to the combined effects of ocean warming and local impacts. Scientific Reports 7: 1–11. https://doi.org/10.1038/srep45227.
Pregnolato, L.A., R.A. Viana, C.C. Passos, M.L. Misailidis, and W. Duleba. 2018. Ammonia-Elphidium Index as a proxy for marine pollution assessment, northeast Brazil. Journal of Sedimentary Environments 3 (3): 176–186. https://doi.org/10.12957/jse.2018.38001.
R Core Team. 2021. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. https://www.R-project.org/. Accessed 5 March 2022.
Rabalais, N.N. 2002. Nitrogen in aquatic ecosystems. AMBIO 31 (2): 102–112. https://doi.org/10.1579/0044-7447-31.2.102.
Raposo, D., V. Laut, I. Clemente, V. Martins, F. Frontalini, F. Silva, M.L. Lorini, R. Fortes, and L. Laut. 2016. Recent benthic foraminifera from the Itaipu Lagoon, Rio de Janeiro (southeastern Brazil). Check List 12 (5): 1–14. https://doi.org/10.15560/12.5.1959.
Raposo, D., I. Clemente, M. Figueiredo, A. Vilar, M.L. Lorini, F. Frontalini, V. Martins, P. Belart, L. Fontana, R. Habib, and L. Laut. 2018. Benthic foraminiferal and organic matter compounds as proxies of environmental quality in a tropical coastal lagoon: The Itaipu lagoon (Brazil). Marine Pollution Bulletin 129: 114–125. https://doi.org/10.1016/j.marpolbul.2018.02.018.
Romano, E., L. Bergamin, A. Ausili, G. Pierfranceschi, C. Maggi, G. Sesta, and M. Gabellini. 2009. The impact of the Bagnoli industrial site (Naples, Italy) on sea-bottom environment. Chemical and textural features of sediments and the related response of benthic foraminifera. Marine Pollution Bulletin 59: 245–256. https://doi.org/10.1016/j.marpolbul.2009.09.017.
Ruiz, F., M.L. González-Regalado, J.G. Pendón, M. Abad, M. Olías, and J.M. Munõz. 2005. Correlation between foraminifera and sedimentary environments in recent estuaries of Southwestern Spain: Applications to holocene reconstructions. Quaternary International 140–141: 21–36. https://doi.org/10.1016/j.quaint.2005.05.002.
Sánchez, A., A. Gómez-León, H. Pérez-Tribouillier, N. Rey-Villiers, M.C. Ortiz-Hernández, G. Rodríguez-Figueroa, and E. Shumilin. 2020. Vertical variability of benthic foraminifera and trace elements in a tropical coastal lagoon in the Gulf of California. Marine Pollution Bulletin 158: 111417. https://doi.org/10.1016/j.marpolbul.2020.111417.
Schiavetti, A., A.C. Schilling, and H.T. Oliveira. 2005. Caracterização sócio-ambiental da bacia hidrográfica do Rio Cachoeira, sul da Bahia, Brasil. [Social-environmental characterization of the hydrographic basin of the Cachoeira River, southern Bahia, Brazil.]. In Conceito de bacias hidrográficas: teorias e aplicações, ed. A. Schiavetti and A.F.M. Camargo, 141–161. Ilhéus: Editus press.
Schumacher, B. 2002. Methods for the determination of total organic carbon (TOC) in soils and sediments: ecological risk assessment support center, 1–23. United States Environmental Protection Agency Method NCEA-C-1282.
Sharifi, A.R., I.W. Croudace, and R.L. Austin. 1991. Benthic foraminiferids as pollution indicators in Southampton Water, southern England, UK. Journal of Micropaleontology 10 (1): 109–113. https://doi.org/10.1144/jm.10.1.109.
Silva, M.A.M., M.F.L. Souza, and P.C. Abreu. 2015. Spatial and temporal variation of dissolved inorganic nutrients, and chlorophyll-a in a tropical estuary in northeastern Brazil: Dynamics of nutrient removal. Brazilian Journal of Oceanography 63 (1): 1–15. https://doi.org/10.1590/S1679-87592015064506301.
Souza, M.F.L., G.F. Eça, M.A.M. Silva, F.A.C. Amorim, and I.P. Lôbo. 2009. Distribuição de nutrientes dissolvidos e clorofila-a no Estuário do Rio Cachoeira, Nordeste do Brasil. [Distribution of dissolved nutrients and chlorophyll-a in the Cachoeira Rio Estuary, Northeastern Brazil]. Atlântica 31: 107–121. https://doi.org/10.5088/atl.2009.31.1.107.
Souza, V.M., L.L.M. Laut, F.S. Silva, A.G. Figueiredo, H. Vital, and E. Frazão. 2010. Benthic foraminifera and bacterial activity as a proxy for environmental characterization in Potengi Estuary, Rio Grande do Norte, Brazil. Anuário do Instituto de Geociências - UFRJ 33 (1): 20–34. https://doi.org/10.11137/2010_1_20-34.
Spagnoli, F., R. de Marco, E. Dinelli, E. Frapiccini, F. Frontalini, and P. Giordano. 2021. Sources and metal pollution of sediments from a coastal area of the central western Adriatic Sea (Southern Marche Region, Italy). Applied Sciences 11: 1118. https://doi.org/10.3390/app11031118.
Stankovic, S., P. Kalaba, and A.R. Stankovic. 2014. Biota as toxic metal indicators. Environmental Chemistry Letters 12: 63–84. https://doi.org/10.1007/s10311-013-0430-6.
Suguio, K. 1973. Introdução a sedimentologia. São Paulo: Ed Edgard Blücher.
Takáč, P., T. Szabová, Ľ Kozáková, and M. Benková. 2009. Heavy metals and their bioavailability from soils in the long-term polluted Central Spiš region of SR. Plant, Soil and Environment 55: 167–172. https://doi.org/10.17221/21/2009-PSE.
Tappin, A.D. 2002. An examination of the fluxes of nitrogen and phosphorus in temperate and tropical estuaries: Current estimates and uncertainties. Estuarine, Coastal and Shelf Science 55: 885–901. https://doi.org/10.1006/ecss.2002.1034.
Teodoro, A., W. Duleba, and C. Lamparelli. 2009. Associações de foraminíferos e composição textural da região próxima ao emissário submarino de esgotos domésticos de Cigarras, canal de São Sebastião, SP, Brasil. Pesquisas em Geociências 36 (1): 79–94. https://doi.org/10.22456/1807-9806.17876.
Teodoro, A.C., W. Duleba, S. Gubitoso, S.M. Prada, C.C. Lamparelli, and J.E. Bevilacqua. 2010. Analysis of foraminifera assemblages and sediment geochemical properties to characterize the environment near Araçá and Saco da Capela domestic sewage submarine outfalls of São Sebastião Channel, São Paulo State, Brazil. Marine Pollution Bulletin 60: 536–553. https://doi.org/10.1016/j.marpolbul.2009.11.011.
Tiller, K.G. 1989. Heavy metal in soils and their environmental significance. In Advances in soil science, ed. K.G. Tiller, 113–114. New York: Springer.
Tomlinson, D.L., J.G. Wilson, C.R. Harris, and D.W. Jeffrey. 1980. Problems in the assessment of heavy-metal levels in estuaries and the formation of a pollution index. Helgoländer Meeresunters 33: 566–575. https://doi.org/10.1007/BF02414780.
Vilela, C.G., D.S. Batista, J.A. Batista-Neto, M. Crapez, and J.J. Mcallister. 2004. Benthic foraminifera distribution in high polluted sediments from Niterói Harbor (Guanabara Bay), Rio de Janeiro, Brazil. Anais Da Academia Brasileira De Ciências 76 (1): 161–171. https://doi.org/10.1590/S0001-37652004000100014.
Vilela, C.G., D.S. Batista, J.A.B. Neto, and R.O. Ghiselli. 2011. Benthic foraminifera distribution in a tourist lagoon in Rio de Janeiro, Brazil: A response to anthropogenic impacts. Marine Pollution Bulletin 62 (10): 2055–2074. https://doi.org/10.1016/j.marpolbul.2011.07.023.
Vilela, C.G., B.O. Figueira, M.C. Macedo, and J.A. Baptista Neto. 2014. Late Holocene evolution and increasing pollution in Guanabara Bay Rio De Janeiro, SE Brazil. Marine Pollution Bulletin 79 (1–2): 175–187. https://doi.org/10.1016/j.marpolbul.2013.12.020.
Walton, W.R., and B.J. Sloan. 1990. The genus Ammonia Brunnich, 1772: Its geographic distribution and morphologic variability. Journal of Foraminiferal Research 20 (2): 128–156. https://doi.org/10.2113/gsjfr.20.2.128.
Weiß, C.H. 2007. StatSoft Inc, Tulsa, OK.: STATISTICA 8.0. AStA 91: 339–341. https://doi.org/10.1007/s10182-007-0038-x.
Willie, S., C. Brophy, V. Clancy, J. Lam, R. Sturgeon, and L. Yang. 2012. DORM-4: Fish protein certified reference material for trace metals. National Research Council Canada. https://doi.org/10.4224/crm.2012.dorm-4.
Yassini, I., and B.G. Jones. 1995. Recent Foraminifera and Ostracoda From Estuarine and Shelf Environments on the Southeastern Coast of Australia. Wollongong: University of Wollongong Press.
Acknowledgements
Prof. Dr. Lazaro Laut deeply thanks the National Council for Scientific and Technological Development- CNPq for the research grant (process 302582/2019-3). We warmly thank Dr. Masoud A. Rostami for his collaboration on the multiple regression analysis and the anonymous reviewers for their contributions to the improvement of the manuscript.
Funding
Open Access funding enabled and organized by Projekt DEAL. This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior–Brasil (CAPES)–Finance Code 001, that provided the Master Fellowships of Débora Raposo. The funds for the sample collections in the field were supported by the project entitled “Foraminíferos e Tecamebas dos estuários do litoral oriental do Brasil” from the INOVA/UNIRIO Program of the Universidade Federal do Estado do Rio de Janeiro (UNIRIO) in partnership with the Universidade Estadual de Santa Cruz (UESC).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Patricia Ramey-Balci
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Raposo, D., Frontalini, F., Clemente, I. et al. Benthic Foraminiferal Response to Trace Elements in a Tropical Mesotidal Brazilian Estuary. Estuaries and Coasts 45, 2610–2631 (2022). https://doi.org/10.1007/s12237-022-01095-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12237-022-01095-5