Abstract
In hematologic oncology, acute myeloid leukemia (AML) presents a significant challenge due to its complex genetic landscape and resistance to conventional therapies. Despite advances in treatment, including intensive chemotherapy and hematopoietic stem cell transplantation (HSCT), the prognosis for many patients with AML remains poor. Recently, immunotherapy has emerged as a promising approach to improve outcomes by augmenting existing treatments. Natural killer (NK) cells, a subset of innate lymphoid cells, have garnered attention for their potent cytotoxic capabilities against AML cells. In this review, we discuss the role of NK cells in AML immunosurveillance, their dysregulation in patients with AML, and various therapeutic strategies leveraging NK cells in AML treatment. We explore the challenges and prospects associated with NK cell therapy, including approaches to enhance NK cell function, overcome immune evasion mechanisms, and optimize treatment efficacy. Finally, we emphasize the importance of further research to validate and refine patient-first NK cell-based immunotherapies for AML.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Acute myeloid leukemia (AML) is characterized by the aberrant proliferation of immature myeloid cells, resulting in bone marrow failure and high mortality risk. Despite advancements in conventional therapies, such as chemotherapy and hematopoietic stem cell transplantation (HSCT), AML still exhibits poor long-term survival rates, particularly among high-risk patients. The emergence of immunotherapy has revolutionized cancer treatment by providing innovative methods to target cancer cells while preserving healthy tissues. Natural killer (NK) cells, integral to innate immunity, have garnered significant interest in AML therapy due to their capacity to identify and eradicate malignant cells without prior sensitization. This review explores the intricate relationship between NK cells and AML, analyzing the dysregulation of NK cell function in patients with AML and examining innovative strategies to exploit NK cell-mediated cytotoxicity for therapeutic purposes. The review addresses challenges inherent in NK cell therapy, including AML cell evasion mechanisms and the imperative to optimize NK cell activation and persistence within the hostile tumor microenvironment. In addition, recent advancements in NK cell-based immunotherapies, ranging from adoptive NK cell transfer to chimeric antigen receptor (CAR)-NK cell engineering, are surveyed, with a discussion on their potential implications for AML treatment. Finally, the significance of ongoing research efforts in elucidating the mechanisms underlying NK cell dysfunction in AML is underscored, emphasizing the translation of these insights into effective therapeutic interventions to enhance outcomes for patients with AML.
Acute myeloid leukemia
AML is a hematologic malignancy marked by the accumulation of genetic abnormalities in hematopoietic stem/progenitor cells, impairing differentiation and causing dysregulated proliferation, resulting in autonomous and disordered proliferation of immature blasts (leukemic cells). Intensive chemotherapy is the primary treatment for patients with AML, with some undergoing HSCT based on risk stratification [1].
Recent years have seen the exploration of novel therapeutic options to enhance survival outcomes, including targeted gene and cytokine therapies, along with immunotherapies. Immunotherapy holds promise in leveraging the immune system of the patient to eliminate leukemia cells [2,3,4]. However, achieving better treatment outcomes in AML remains a significant challenge, requiring ongoing efforts.
Natural killer cells
NK cells, a subset of innate lymphoid cells, exhibit robust cytotoxic capabilities, enabling them to directly eliminate virus-infected or cancerous cells without prior activation or exposure to antigens [5, 6]. Unlike T cells, which may cause collateral damage to host tissues, NK cells undergo apoptosis following cytotoxic activity, rendering them potentially advantageous for therapeutic interventions [7].
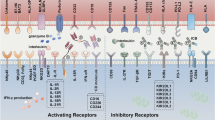
NK cells express two distinct types of receptors on their surface [8]: activating receptors, including natural cytotoxicity receptors (NCRs), killer immunoglobulin-like receptors (KIRs), CD94/NK group 2C (NKG2C), NK group 2D (NKG2D), and DNAX accessory molecule-1 (DNAM-1) [9]; and inhibitory receptors, such as leukocyte immunoglobulin-like receptors (LIRs), inhibitory KIRs, and NK group 2A (NKG2A) [10, 11]. These receptors function through ligand recognition mechanisms.
In addition to their direct cytotoxic effects, NK cells facilitate antibody-dependent cellular cytotoxicity (ADCC) against tumor cells. This process is primarily mediated by the binding of CD16 (FcɤRIIIA) and CD32 (FcɤRIIC) receptors on NK cells to various antibodies. Upon engagement of Fc receptors, NK cell degranulation is triggered, leading to cell death [12]. Furthermore, NK cell activity is subject to modulation by other immune cells, such as activated dendritic cells and macrophages [13].
Several cytokines, such as interleukin (IL)-10 and transforming growth factor (TGF)-β, released from tumor cells can impair NK cell function despite their role in overcoming tumor immune evasion [14, 15]. While the combination of IL-10 and IL-15 has been demonstrated to enhance NK cell cytotoxicity [16], the secretion of IL-10 by regulatory T cells (Tregs) may detrimentally affect NK cell activity [17].
In recent years, NK cells have gained attention due to significant advancements in immunotherapy and its clinical applications [18, 19]. The well-established relationship between tumors and NK cells is evidenced by preclinical studies indicating increased tumor growth rates in the absence of NK cells [23, 24]. Signals through MHC class I (MHC-I) inhibitory mechanisms inhibit the activation signals of NK cells. Downregulation of MHC-I renders tumor cells identifiable and susceptible to NK cell-mediated killing because activation signals override inhibitory signals [25].
Natural killer cells in acute myeloid leukemia
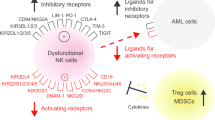
AML blasts are susceptible to attack by T lymphocytes and NK cells due to the expression of MHC class I molecules, leukemia-associated antigens (LAAs), or NK cell activation ligands [26]. However, AML can evade immunity through mechanisms that suppress the anti-leukemic functions of immune cells [27, 28].
Cell numbers
In patients with AML, NK cell numbers are the lowest at diagnosis and upon disease relapse. Conversely, they increase during remission [29, 30]. High reconstitution of NK cells after HSCT is associated with improved 2-year survival rates, reduced CMV reactivation rates, decreased risk of relapse, lower non-relapse mortality rates, and improved progression-free survival [31]. Decreased NK cell numbers on day 60 post-HSCT are associated with an increased risk of relapse [32].
Differentiation
NK cells differentiate from progenitors (CD34dim/CD117−/NKG2A−) into immature CD56+NK cells (CD34−/CD117+/CD56±) and finally to functional CD56bright NK cells (CD34−/CD117±/CD94+). Functional maturation occurs via the expression of NKG2A or KIRs [33]. Patients with a low-maturity profile of NK cells have inferior outcomes, including 3-year overall survival [34], suggesting the importance of NK cell maturation status in predicting long-term outcomes.
Downregulation of activating receptors
The expression of NCRs, such as NKp46, NKp44, and NKp30, is downregulated in NK cells of the majority of patients with AML [35]. In addition, reduced expression of DNAM-1 was observed in NK cells in patients with AML compared to that in controls [36]. Patients with AML with low NCR expression had shorter remission durations, whereas NCR expression was recovered in patients with AML who achieved CR [32], suggesting the importance of activating NK cell receptor expression in AML [37].
Upregulation of inhibitory receptors
The high expression of inhibitory receptors plays a crucial role in AML cell evasion and NK cell immunity [38, 39]. Some studies have reported that recipient leukocyte ligand and donor KIR mismatches lead to reduced relapse rates in patients with AML [40, 41]. Upregulation of CD94/NKG2A is associated with decreased cytotoxicity of NK cells against AML cells [42].
Immune checkpoint molecules
Immune checkpoint molecules, such as PD-1, T cell immunoglobulin, and the ITIM domain (TIGIT) regulate NK cell activity [43]. PD-L1 expression in patients with AML has been reported to modulate recognition by immune cells, correlating with favorable outcomes, such as decreased risk of relapse and extended overall survival [44]. TIGIT, which is expressed in NK cells, binds to CD112, CD113, and CD155 ligands such as DNAM-1. Reduced expression of DNAM-1 in patients with AML may promote binding to TIGIT ligands, sending inhibitory signals, and potentially contributing to tumor immune evasion. Blocking TIGIT enhances NK cell cytotoxicity [45].
In summary, various roles of NK cells in AML progression have been highlighted. Many studies have laid the groundwork for the development of various cell-based therapies targeting AML.
Natural killer cell therapy for acute myeloid leukemia
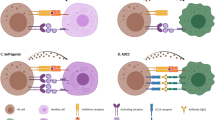
NK cell therapy is an immunotherapeutic option. In patients with AML, the function of NK cells is suppressed, allowing AML cells to evade immune cells. Numerous clinical trials of various NK cell-based immunotherapies are currently underway [2, 4]. An overview of NK cell therapy is illustrated in Fig. 1, and ongoing clinical trials are summarized in Table 1.
Natural killer cell therapy for acute myeloid leukemia. In patients with AML, downregulation of HLA-1 expression in AML cells and a decrease in the expression of activating receptors, such as NCR and DNAM-1, have been observed. In addition, NK cell function is suppressed by inhibitory receptors. CAR-NK cells targeting CD33 or CD123, as well as CIML-NK cells utilizing cytokines, have been developed. Furthermore, antibodies targeting ADCC activity and inhibitory receptors have been developed. DNAM-1, DNAX accessory molecule-1; NCR: natural cytotoxicity receptor; TIGIT, T cell immunoglobulin and ITIM domain; iKIR, inhibitory killer cell immunoglobulin-like receptor; NKG2A, NK group 2A; NKG2DL, NK group 2D ligand; HLA, human leukocyte antigen
Cell source
Autologous NK cells
Peripheral blood NK cells can be expanded ex vivo and reintroduced into the body as autologous NK cells [46]. Autologous NK cell therapy uses patient blood to minimize the risk of graft-versus-host disease (GVHD).
Injected cells can proliferate in vivo; however, in AML, significant therapeutic outcomes are often hindered by the inhibitory effects of human leukocyte antigen (HLA) ligands. In addition, autologous NK cells obtained from heavily pretreated patients may show reduced cytotoxic activity [47].
Allogeneic NK cells
Allogeneic NK cells were obtained from healthy HLA-matched or haploidentical donors, expanded ex vivo, and administered after removing T cells to prevent GVHD [48]. Donor-isolated NK cells show graft-versus-leukemia (GVL) effects against AML cells due to a KIR-KIR ligand (KIR-KIRL) mismatch [49]. Allogeneic NK cell therapy induced complete remission in five of 19 patients with AML with a poor prognosis. The remission rates were significantly higher when KIR ligand-mismatched donors were used [50]. Similar to autologous NK cells, challenges include timely ex vivo expansion, suboptimal activation to demonstrate clinical efficacy, and a lack of in vivo persistence [51].
Cytokines play an important role in NK cell function and have been used to overcome these challenges. IL-2 activates NK cell proliferation, and systemic administration after NK cell infusion may enhance the therapeutic efficacy [52].
The use of IL-15, which is less toxic upon systemic administration than IL-2, has also been investigated [53]. The IL-15 super-agonist complex, ALT-803, was identified as a safe agent in a Phase I trial targeting elderly patients with AML who relapsed after HSCT. Furthermore, NK cells expressing IL-15 or IL-12 can mitigate toxicity from systemic cytokine administration [54, 55].
Umbilical cord blood-derived NK cells
Umbilical cord blood (UCB) provides a readily available off-the-shelf allogeneic NK cell source. UCB-derived NK cells express CD3-CD56+ and are classified as undifferentiated CD56bright or mature CD56dim NK cells [56].
UCB-derived NK cell therapy, either as a maintenance therapy after chemotherapy or in combination with HSCT, has shown promising results [57]. Challenges include improving persistence and develo** strategies to maintain donor NK cell function in vivo [58, 59].
Cell line-derived NK cells
Over the past 20 years, eight cloned NK cell lines, namely, NK-92, YTS, and NKL have been established. Commonly used NK cell lines exhibit differences in phenotype and function [60]. NK-92 is the only cell line that has undergone clinical trials to evaluate safety and efficacy.
NK-92 cells can easily proliferate in vivo but lack many conventional NK cell markers and require irradiation before infusion to prevent uncontrolled proliferation. They demonstrate cytotoxicity against leukemia cells both in vitro and in vivo [61]. Although clinical trials have demonstrated the safety and tolerability of NK-92 cells, their efficacy has not met expectations [62]. NK-92 cells for refractory/relapsed AML did not show antitumor responses after infusion [63, 64]. We revealed that high expression of CD112 is associated with poor prognosis in patients with AML, and the introduction of DNAM-1 into NK-92 cells enhances their anti-AML effect [65].
NK cells derived from induced pluripotent stem cells (iPSCs) were used as standardized “off-the-shelf” products for all patients. iPSC-derived NK cells have been shown to suppress tumor growth in vivo in leukemia cell lines without exogenous IL-2 or IL-15 [66].
Cytokine-induced memory-like (CIML) NK cell
NK cells activated in response to interleukins, such as IL-12, IL-15, and IL-18, exhibit memory-like features and demonstrate long-term effector functions [67, 68]. No serious adverse events were observed, and clinical trials administering CIML NK cells in combination with chemotherapy to patients relapsing after HSCT are ongoing [69].
Chimeric antigen receptor (CAR)-NK cell
Since the introduction of CAR-T cell therapy, there has been significant interest in enhancing cytotoxic functions through genetic modifications [70, 71].
CAR-T therapy faces challenges, such as cytokine release syndrome, neurological toxicities, off-target effects, and manufacturing timelines.
In contrast, NK cells can avoid toxicity arising from nonspecific recognition of normal cells by binding to human leukocyte antigen (HLA) ligands on leukemia cells. NK cells recognize self and non-self-cells through various activating and inhibitory receptors on their surfaces [72, 73], which are known to not cause GVHD. In addition, they have a short lifespan, favorable cytotoxic profiles, and lower manufacturing costs, making them promising alternatives to T cells [74, 75].
Unmodified NK cell infusions have shown some efficacy in patients with AML, but their short lifespan and modest therapeutic outcomes have shifted the focus to CAR-NK cells, whose efficacy has been evaluated in various preclinical studies and clinical trials.
CAR-NK cells incorporating signaling domains, such as 2B4 or 4-1BB, have demonstrated anti-AML cytotoxic activity both in vitro and in vivo [76]. CAR-NK-92 targeting CD123 is highly expressed in AML cells and is engineered to express human IL-15, which has enhanced in vivo persistence [77]. Other CAR-NK cells targeting CD4 [78], CD276 [79], or NKG2D [80], and attempts to enhance cytotoxic activity by knocking out inhibitory NK cell receptors, such as NKG2A or TIGIT, are underway.
The first CAR-NK cell therapy was reported in 2018, which evaluated the safety and efficacy of third-generation CD33 CAR-NK cells derived from the NK-92 cell line with CD28 and 4-1BB costimulatory domains in three relapsed/refractory (R/R) patients with AML. Although CAR-NK-92 cells can be produced at much lower costs than CAR-T cells, the significant reduction of CAR-NK cell numbers within a week post-infusion due to pre-infusion irradiation and the lack of sustained remission remains a challenge [81].
However, CAR-NK cells have several drawbacks. First, NK cell numbers are limited and their lifespan is short (approximately 1 to 4 weeks). Incorporation of cytokines, such as IL-2 or IL-15, into CAR-NK cells may improve their proliferation and persistence [74]. Various methods, including the administration of cytokines ex vivo or the use of feeder cells, have been used to enhance proliferation [82]. Second, there are no specific optimal targets. Some surface markers of AML cells are shared with hematopoietic stem cells, making it crucial to identify the optimal leukemia-specific markers that can be specifically targeted by CAR-NK cells [83, 84]. In addition, antigen downregulation or loss by AML cells may occur, leading to the development of CAR-NK cells that target the two antigens to overcome this challenge [85]. Although CAR-NK cells typically function only when antigens are expressed on the cell surface, CAR-NK cells that recognize intracellular antigens presented by MHC molecules via TCR have also been developed [86]. Finally, there is a high risk of apoptosis and difficulty in genetic manipulation. It is necessary to optimize methods such as electroporation and the viral vectors used for genetic manipulation [87, 88].
Antibodies
Antibodies targeting tumor-associated antigens
Antibodies targeting tumor-associated antigens play a significant role in inducing ADCC in NK cells. Preclinical studies have investigated Fc-optimized antibodies against various potential antigens, such as IL-1 receptor accessory protein (IL1RAP), CD33, and CD133 [89,90,91]. Furthermore, novel therapies that combine NK cell therapy have shown promising results [92, 93].
Antibodies targeting NK cell inhibitory receptors
Inhibitory receptors on NK cells play a crucial role in immune evasion by tumor cells, making them excellent targets for cancer immunotherapy. In addition to MHC-I inhibitory leukocyte immunoglobulin-like receptors (LIR), KIR, and CD94/NKG2A receptors, other NK cell inhibitory receptors have been implicated in cellular dysfunction [94]. CD200R is also known to be an inhibitory receptor for NK cells. Overexpression of CD200 suppresses the antitumor activity of NK cells in patients with AML, leading to an increased risk of relapse; however, inhibition of CD200 has been reported to restore NK cell function [95].
Targeting the KIR2DL1/2/3 NK inhibitory receptors with IPH2101 has shown efficacy both in vivo and in clinical trials [96]. However, no significant improvements were observed in median survival or response rates. Combinations of anti-NKG2A, anti-KIR, and anti-LIR-1 antibodies may be necessary to enhance the therapeutic efficacy [97, 98].
Immune checkpoint inhibitors
Like T cells, NK cells express cytotoxic T lymphocyte-associated protein 4 (CTLA4), LAG3, and PD-1, suggesting that immune checkpoint receptors regulate NK cell activity [99]. TIGIT and CD96 (TACTILE) as well as T cell immunoglobulin and mucin domain 3 (TIM-3) have also been identified as inhibitory receptors expressed on NK cells [100].
In clinical trials, monotherapy with anti-PD-1 antibodies for AML did not show significant efficacy [101]. However, ipilimumab treatment after allogeneic HSCT resulted in favorable responses in 22 patients with various hematological malignancies, including 12 patients with AML [102]. TIM-3 is a co-inhibitory receptor that recognizes galectin-9 as its ligand. Blocking TIM-3 has been shown to restore NK cell function [103, 104], and the efficacy of MBG453 (a TIM-3 antibody), alone or in combination with other agents, has been analyzed.
Clinical trials are also being conducted on anti-LAG3 antibodies and anti-TIGIT therapies among others.
Challenges and prospects
Our understanding of the role of NK cells in leukemia and their function in anti-leukemia therapy has improved dramatically. Patients with leukemia often experience NK cell dysfunction or a reduced number of NK cells. NK cell infusion is beneficial in temporarily restoring these functions. Compared with T cell-targeted therapies, which are costly to manufacture and have lower safety profiles, NK cells are gaining attention in immunotherapy because of their lower neurotoxicity and incidence of cytokine release syndrome in CAR-T therapy.
NK cells represent a promising immunotherapy for rapid tumor detection, antitumor effects, and the ability to enhance immune responses. Despite numerous immune evasion mechanisms, the crucial role of NK cells in AML immune surveillance has been demonstrated. From the adoptive transfer of NK cells and cytokines to immunomodulatory agents, antibodies, and gene editing, NK cells have paved the way for better management of AML.
Several clinical trials have investigated the potential of NK cell infusions in combination with conventional therapies. NK cell therapy offers advantages over other cell therapies, including the recognition of tumor cells independent of MHC, the potential to treat a broader population, ease of expansion in vivo, and cost-effective manufacturing of off-the-shelf products tailored to individual patients.
However, several challenges remain in enhancing the potential of NK cells in AML immunotherapy. These include blocking inhibitory receptors, eliminating Treg activity, and neutralizing immunosuppressive cytokines. Providing the essential cytokines and growth factors necessary for NK cell activation, proliferation, and survival is challenging.
The majority of these studies are still in the preclinical stage, highlighting the need for further research and a deeper understanding of the mechanisms of NK cell dysfunction and immunotherapy-based approaches in AML to validate and implement these therapeutic strategies.
Conclusion
Despite advances in chemotherapy, AML remains a challenging hematologic malignancy to cure. NK cells have shown potent anti-AML effects without causing adverse events, such as GVHD, neurotoxicity, or cytokine release syndrome. However, the effector functions of NK cells are inhibited in immunosuppressive microenvironments. Functional impairment of NK cells by the heterogeneous TME poses a significant obstacle to maintaining anti-AML activity. Achieving desirable outcomes with NK cell therapy requires enhancing NK cell cytotoxicity, improving the recognition of target cells, and maintaining cytotoxic functions. Thus, NK cell therapy may be a viable treatment option for AML.
Data availability
Data sharing is not applicable as no datasets were generated or analysed.
References
Döhner H, Weisdorf DJ, Bloomfield CD. Acute myeloid leukemia. N Engl J Med. 2015;373(12):1136–52.
Kaweme NM, Zhou F. Optimizing NK Cell-Based Immunotherapy In Myeloid Leukemia: Abrogating an immunosuppressive microenvironment. Front Immunol. 2021;12: 683381.
Hussein BA, Hallner A, Wennström L, Brune M, Martner A, Hellstrand K, et al. Impact of NK cell activating receptor gene variants on receptor expression and outcome of immunotherapy in acute myeloid leukemia. Front Immunol. 2021;12: 796072.
Xu J, Niu T. Natural killer cell-based immunotherapy for acute myeloid leukemia. J Hematol Oncol. 2020;13(1):167.
Abel AM, Yang C, Thakar MS, Malarkannan S. Natural killer cells: Development, maturation, and clinical utilization. Front Immunol. 2018;9:1869.
Ghaemdoust F, Keshavarz-Fathi M, Rezaei N. Natural killer cells and cancer therapy, what we know and where we are going. Immunotherapy. 2019;11(14):1231–51.
Ames E, Murphy WJ. Advantages and clinical applications of natural killer cells in cancer immunotherapy. Cancer Immunol Immunother. 2014;63(1):21–8.
Waldhauer I, Steinle A. NK cells and cancer immunosurveillance. Oncogene. 2008;27(45):5932–43.
Chester C, Fritsch K, Kohrt HE. Natural killer cell immunomodulation: Targeting activating, inhibitory, and co-stimulatory receptor signaling for cancer immunotherapy. Front Immunol. 2015;6:601.
Campbell KS, Hasegawa J. Natural killer cell biology: an update and future directions. J Allergy Clin Immunol. 2013;132(3):536–44.
Pegram HJ, Andrews DM, Smyth MJ, Darcy PK, Kershaw MH. Activating and inhibitory receptors of natural killer cells. Immunol Cell Biol. 2011;89(2):216–24.
Wang W, Erbe AK, Hank JA, Morris ZS, Sondel PM. NK cell-mediated antibody-dependent cellular cytotoxicity in cancer immunotherapy. Front Immunol. 2015;6:368.
Walzer T, Dalod M, Robbins SH, Zitvogel L, Vivier E. Natural-killer cells and dendritic cells: “l’union fait la force.” Blood. 2005;106(7):2252–8.
Fauriat C, Long EO, Ljunggren HG, Bryceson YT. Regulation of human NK-cell cytokine and chemokine production by target cell recognition. Blood. 2010;115(11):2167–76.
Lee HM, Kim KS, Kim J. A comparative study of the effects of inhibitory cytokines on human natural killer cells and the mechanistic features of transforming growth factor-beta. Cell Immunol. 2014;290(1):52–61.
Park JY, Lee SH, Yoon SR, Park YJ, Jung H, Kim TD, et al. IL-15-induced IL-10 increases the cytolytic activity of human natural killer cells. Mol Cells. 2011;32(3):265–72.
Littwitz-Salomon E, Malyshkina A, Schimmer S, Dittmer U. The cytotoxic activity of natural killer cells is suppressed by IL-10(+) regulatory t cells during acute retroviral infection. Front Immunol. 2018;9:1947.
Baragaño Raneros A, López-Larrea C, Suárez-Álvarez B. Acute myeloid leukemia and NK cells: Two warriors confront each other. Oncoimmunology. 2019;8(2): e1539617.
Sivori S, Meazza R, Quintarelli C, Carlomagno S, Della Chiesa M, Falco M, et al. NK cell-based immunotherapy for hematological malignancies. J Clin Med. 2019;8(10):1702.
Guerra N, Tan YX, Joncker NT, Choy A, Gallardo F, **ong N, et al. NKG2D-deficient mice are defective in tumor surveillance in models of spontaneous malignancy. Immunity. 2008;28(4):571–80.
Albertsson PA, Basse PH, Hokland M, Goldfarb RH, Nagelkerke JF, Nannmark U, et al. NK cells and the tumour microenvironment: implications for NK-cell function and anti-tumour activity. Trends Immunol. 2003;24(11):603–9.
Sugioka DK, Gonçalves CE, Bicalho MD. KIR repertory in patients with hematopoietic diseases and healthy family members. BMC Hematol. 2016;16:25.
Ahmad M, Rees RC, Ali SA. Escape from immunotherapy: Possible mechanisms that influence tumor regression/progression. Cancer Immunol Immunother. 2004;53(10):844–54.
Vago L, Perna SK, Zanussi M, Mazzi B, Barlassina C, Stanghellini MT, et al. Loss of mismatched HLA in leukemia after stem-cell transplantation. N Engl J Med. 2009;361(5):478–88.
Cornel AM, Mimpen IL, Nierkens S. MHC Class I downregulation in cancer: Underlying mechanisms and potential targets for cancer immunotherapy. Cancers (Basel). 2020;12(7):1760.
Barrett AJ, Le Blanc K. Immunotherapy prospects for acute myeloid leukaemia. Clin Exp Immunol. 2010;161(2):223–32.
Tratkiewicz JA, Szer J. Loss of natural killer activity as an indicator of relapse in acute leukaemia. Clin Exp Immunol. 1990;80(2):241–6.
Lowdell MW, Craston R, Samuel D, Wood ME, O’Neill E, Saha V, et al. Evidence that continued remission in patients treated for acute leukaemia is dependent upon autologous natural killer cells. Br J Haematol. 2002;117(4):821–7.
Pizzolo G, Trentin L, Vinante F, Agostini C, Zambello R, Masciarelli M, et al. Natural killer cell function and lymphoid subpopulations in acute non-lymphoblastic leukaemia in complete remission. Br J Cancer. 1988;58(3):368–72.
Rey J, Fauriat C, Kochbati E, Orlanducci F, Charbonnier A, D’Incan E, et al. Kinetics of cytotoxic lymphocytes reconstitution after induction chemotherapy in elderly AML patients reveals progressive recovery of normal phenotypic and functional features in NK cells. Front Immunol. 2017;8:64.
Minculescu L, Marquart HV, Friis LS, Petersen SL, Schiødt I, Ryder LP, et al. Early natural killer cell reconstitution predicts overall survival in T cell-replete allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2016;22(12):2187–93.
Dunbar EM, Buzzeo MP, Levine JB, Schold JD, Meier-Kriesche HU, Reddy V. The relationship between circulating natural killer cells after reduced intensity conditioning hematopoietic stem cell transplantation and relapse-free survival and graft-versus-host disease. Haematologica. 2008;93(12):1852–8.
Freud AG, Yokohama A, Becknell B, Lee MT, Mao HC, Ferketich AK, et al. Evidence for discrete stages of human natural killer cell differentiation in vivo. J Exp Med. 2006;203(4):1033–43.
Chretien AS, Fauriat C, Orlanducci F, Galseran C, Rey J, Bouvier Borg G, et al. Natural killer defective maturation is associated with adverse clinical outcome in patients with acute myeloid leukemia. Front Immunol. 2017;8:573.
Szczepanski MJ, Szajnik M, Welsh A, Foon KA, Whiteside TL, Boyiadzis M. Interleukin-15 enhances natural killer cell cytotoxicity in patients with acute myeloid leukemia by upregulating the activating NK cell receptors. Cancer Immunol Immunother. 2010;59(1):73–9.
Sanchez-Correa B, Gayoso I, Bergua JM, Casado JG, Morgado S, Solana R, et al. Decreased expression of DNAM-1 on NK cells from acute myeloid leukemia patients. Immunol Cell Biol. 2012;90(1):109–15.
Fauriat C, Just-Landi S, Mallet F, Arnoulet C, Sainty D, Olive D, et al. Deficient expression of NCR in NK cells from acute myeloid leukemia: Evolution during leukemia treatment and impact of leukemia cells in NCRdull phenotype induction. Blood. 2007;109(1):323–30.
Verheyden S, Bernier M, Demanet C. Identification of natural killer cell receptor phenotypes associated with leukemia. Leukemia. 2004;18(12):2002–7.
Sandoval-Borrego D, Moreno-Lafont MC, Vazquez-Sanchez EA, Gutierrez-Hoya A, López-Santiago R, Montiel-Cervantes LA, et al. Overexpression of CD158 and NKG2A inhibitory receptors and underexpression of NKG2D and NKp46 activating receptors on NK cells in acute myeloid leukemia. Arch Med Res. 2016;47(1):55–64.
Farag SS, Bacigalupo A, Eapen M, Hurley C, Dupont B, Caligiuri MA, et al. The effect of KIR ligand incompatibility on the outcome of unrelated donor transplantation: A report from the center for international blood and marrow transplant research, the European blood and marrow transplant registry, and the Dutch registry. Biol Blood Marrow Transplant. 2006;12(8):876–84.
Verheyden S, Demanet C. NK cell receptors and their ligands in leukemia. Leukemia. 2008;22(2):249–57.
Nguyen S, Beziat V, Dhedin N, Kuentz M, Vernant JP, Debre P, et al. HLA-E upregulation on IFN-gamma-activated AML blasts impairs CD94/NKG2A-dependent NK cytolysis after haplo-mismatched hematopoietic SCT. Bone Marrow Transplant. 2009;43(9):693–9.
Beldi-Ferchiou A, Caillat-Zucman S. Control of NK cell activation by immune checkpoint molecules. Int J Mol Sci. 2017;18(10):2129.
Goltz D, Gevensleben H, Grünen S, Dietrich J, Kristiansen G, Landsberg J, et al. PD-L1 (CD274) promoter methylation predicts survival in patients with acute myeloid leukemia. Leukemia. 2017;31(3):738–43.
Zhang Q, Bi J, Zheng X, Chen Y, Wang H, Wu W, et al. Blockade of the checkpoint receptor TIGIT prevents NK cell exhaustion and elicits potent anti-tumor immunity. Nat Immunol. 2018;19(7):723–32.
Sakamoto N, Ishikawa T, Kokura S, Okayama T, Oka K, Ideno M, et al. Phase I clinical trial of autologous NK cell therapy using novel expansion method in patients with advanced digestive cancer. J Transl Med. 2015;13:277.
Parkhurst MR, Riley JP, Dudley ME, Rosenberg SA. Adoptive transfer of autologous natural killer cells leads to high levels of circulating natural killer cells but does not mediate tumor regression. Clin Cancer Res. 2011;17(19):6287–97.
Guillerey C, Huntington ND, Smyth MJ. Targeting natural killer cells in cancer immunotherapy. Nat Immunol. 2016;17(9):1025–36.
Allison M, Mathews J, Gilliland T, Mathew SO. Natural killer cell-mediated immunotherapy for leukemia. Cancers (Basel). 2022;14(3):843.
Miller JS, Soignier Y, Panoskaltsis-Mortari A, McNearney SA, Yun GH, Fautsch SK, et al. Successful adoptive transfer and in vivo expansion of human haploidentical NK cells in patients with cancer. Blood. 2005;105(8):3051–7.
Franks SE, Wolfson B, Hodge JW. Natural born killers: NK cells in cancer therapy. Cancers (Basel). 2020;12(8):2131.
Rubnitz JE, Inaba H, Ribeiro RC, Pounds S, Rooney B, Bell T, et al. NKAML: A pilot study to determine the safety and feasibility of haploidentical natural killer cell transplantation in childhood acute myeloid leukemia. J Clin Oncol. 2010;28(6):955–9.
Handgretinger R, Lang P, André MC. Exploitation of natural killer cells for the treatment of acute leukemia. Blood. 2016;127(26):3341–9.
Huang J, Liu Y, Au BC, Barber DL, Arruda A, Schambach A, et al. Preclinical validation: LV/IL-12 transduction of patient leukemia cells for immunotherapy of AML. Mol Ther Methods Clin Dev. 2016;3:16074.
Shi Y, Dincheva-Vogel L, Ayemoba CE, Fung JP, Bergamaschi C, Pavlakis GN, et al. IL-15/IL-15Rα/CD80-expressing AML cell vaccines eradicate minimal residual disease in leukemic mice. Blood Adv. 2018;2(22):3177–92.
Zhao X, Cai L, Hu Y, Wang H. Cord-blood natural killer cell-based immunotherapy for cancer. Front Immunol. 2020;11: 584099.
Sarvaria A, Jawdat D, Madrigal JA, Saudemont A. Umbilical cord blood natural killer cells, their characteristics, and potential clinical applications. Front Immunol. 2017;8:329.
Dolstra H, Roeven MWH, Spanholtz J, Hangalapura BN, Tordoir M, Maas F, et al. Successful transfer of umbilical cord blood CD34(+) hematopoietic stem and progenitor-derived NK cells in older acute myeloid leukemia patients. Clin Cancer Res. 2017;23(15):4107–18.
Shah N, Li L, McCarty J, Kaur I, Yvon E, Shaim H, et al. Phase I study of cord blood-derived natural killer cells combined with autologous stem cell transplantation in multiple myeloma. Br J Haematol. 2017;177(3):457–66.
Gunesch JT, Angelo LS, Mahapatra S, Deering RP, Kowalko JE, Sleiman P, et al. Genome-wide analyses and functional profiling of human NK cell lines. Mol Immunol. 2019;115:64–75.
Zhang J, Zheng H, Diao Y. Natural killer cells and current applications of chimeric antigen receptor-modified NK-92 cells in tumor immunotherapy. Int J Mol Sci. 2019;20(2):317.
Suck G, Odendahl M, Nowakowska P, Seidl C, Wels WS, Klingemann HG, et al. NK-92: An “off-the-shelf therapeutic” for adoptive natural killer cell-based cancer immunotherapy. Cancer Immunol Immunother. 2016;65(4):485–92.
Boyiadzis M, Agha M, Redner RL, Sehgal A, Im A, Hou JZ, et al. Phase 1 clinical trial of adoptive immunotherapy using “off-the-shelf” activated natural killer cells in patients with refractory and relapsed acute myeloid leukemia. Cytotherapy. 2017;19(10):1225–32.
Kang S, Gao X, Zhang L, Yang E, Li Y, Yu L. The advances and challenges of NK cell-based cancer immunotherapy. Curr Oncol. 2021;28(2):1077–93.
Kaito Y, Sugimoto E, Nakamura F, Tsukune Y, Sasaki M, Yui S, et al. Immune checkpoint molecule DNAM-1/CD112 axis is a novel target for NK-cell therapy in acute myeloid leukemia. Haematologica. 2023;109(4):1107–20.
Matsubara H, Niwa A, Nakahata T, Saito MK. Induction of human pluripotent stem cell-derived natural killer cells for immunotherapy under chemically defined conditions. Biochem Biophys Res Commun. 2019;515(1):1–8.
Romee R, Rosario M, Berrien-Elliott MM, Wagner JA, Jewell BA, Schappe T, et al. Cytokine-induced memory-like natural killer cells exhibit enhanced responses against myeloid leukemia. Sci Transl Med. 2016;8(357):357ra123.
Cooper MA, Elliott JM, Keyel PA, Yang L, Carrero JA, Yokoyama WM. Cytokine-induced memory-like natural killer cells. Proc Natl Acad Sci U S A. 2009;106(6):1915–9.
Bednarski JJ, Zimmerman C, Berrien-Elliott MM, Foltz JA, Becker-Hapak M, Neal CC, et al. Donor memory-like NK cells persist and induce remissions in pediatric patients with relapsed AML after transplant. Blood. 2022;139(11):1670–83.
Porter DL, Levine BL, Kalos M, Bagg A, June CH. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N Engl J Med. 2011;365(8):725–33.
Maude SL, Frey N, Shaw PA, Aplenc R, Barrett DM, Bunin NJ, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med. 2014;371(16):1507–17.
Malmberg KJ, Carlsten M, Björklund A, Sohlberg E, Bryceson YT, Ljunggren HG. Natural killer cell-mediated immunosurveillance of human cancer. Semin Immunol. 2017;31:20–9.
Lanier LL. Up on the tightrope: Natural killer cell activation and inhibition. Nat Immunol. 2008;9(5):495–502.
Shimasaki N, Jain A, Campana D. NK cells for cancer immunotherapy. Nat Rev Drug Discov. 2020;19(3):200–18.
Klingemann H. Are natural killer cells superior CAR drivers? Oncoimmunology. 2014;3: e28147.
Christodoulou I, Ho WJ, Marple A, Ravich JW, Tam A, Rahnama R, et al. Engineering CAR-NK cells to secrete IL-15 sustains their anti-AML functionality but is associated with systemic toxicities. J Immunother Cancer. 2021;9(12): e003894.
Morgan MA, Kloos A, Lenz D, Kattre N, Nowak J, Bentele M, et al. Improved activity against acute myeloid leukemia with chimeric antigen receptor (CAR)-NK-92 cells designed to target CD123. Viruses. 2021;13(7):1365.
Salman H, Pinz KG, Wada M, Shuai X, Yan LE, Petrov JC, et al. Preclinical targeting of human acute myeloid leukemia using CD4-specific chimeric antigen receptor (CAR) T cells and NK cells. J Cancer. 2019;10(18):4408–19.
Ureña-Bailén G, Dobrowolski JM, Hou Y, Dirlam A, Roig-Merino A, Schleicher S, et al. Preclinical evaluation of CRISPR-edited CAR-NK-92 cells for off-the-shelf treatment of AML and B-ALL. Int J Mol Sci. 2022;23(21):12828.
Du Z, Ng YY, Zha S, Wang S. piggyBac system to co-express NKG2D CAR and IL-15 to augment the in vivo persistence and anti-AML activity of human peripheral blood NK cells. Mol Ther Methods Clin Dev. 2021;23:582–96.
Tang X, Yang L, Li Z, Nalin AP, Dai H, Xu T, et al. First-in-man clinical trial of CAR NK-92 cells: Safety test of CD33-CAR NK-92 cells in patients with relapsed and refractory acute myeloid leukemia. Am J Cancer Res. 2018;8(6):1083–9.
Cronk RJ, Zurko J, Shah NN. Bispecific chimeric antigen receptor T cell therapy for B cell malignancies and multiple myeloma. Cancers (Basel). 2020;12(9):2523.
Hope KJ, ** L, Dick JE. Acute myeloid leukemia originates from a hierarchy of leukemic stem cell classes that differ in self-renewal capacity. Nat Immunol. 2004;5(7):738–43.
Sutherland HJ, Blair A, Zapf RW. Characterization of a hierarchy in human acute myeloid leukemia progenitor cells. Blood. 1996;87(11):4754–61.
Schmidt P, Raftery MJ, Pecher G. Engineering NK cells for CAR therapy-recent advances in gene transfer methodology. Front Immunol. 2020;11: 611163.
Davis ZB, Vallera DA, Miller JS, Felices M. Natural killer cells unleashed: Checkpoint receptor blockade and BiKE/TriKE utilization in NK-mediated anti-tumor immunotherapy. Semin Immunol. 2017;31:64–75.
Allan DSJ, Chakraborty M, Waller GC, Hochman MJ, Poolcharoen A, Reger RN, et al. Systematic improvements in lentiviral transduction of primary human natural killer cells undergoing ex vivo expansion. Mol Ther Methods Clin Dev. 2021;20:559–71.
Mensali N, Dillard P, Hebeisen M, Lorenz S, Theodossiou T, Myhre MR, et al. NK cells specifically TCR-dressed to kill cancer cells. EBioMedicine. 2019;40:106–17.
Ågerstam H, Karlsson C, Hansen N, Sandén C, Askmyr M, von Palffy S, et al. Antibodies targeting human IL1RAP (IL1R3) show therapeutic effects in xenograft models of acute myeloid leukemia. Proc Natl Acad Sci U S A. 2015;112(34):10786–91.
Koerner SP, André MC, Leibold JS, Kousis PC, Kübler A, Pal M, et al. An Fc-optimized CD133 antibody for induction of NK cell reactivity against myeloid leukemia. Leukemia. 2017;31(2):459–69.
Vasu S, He S, Cheney C, Gopalakrishnan B, Mani R, Lozanski G, et al. Decitabine enhances anti-CD33 monoclonal antibody BI 836858-mediated natural killer ADCC against AML blasts. Blood. 2016;127(23):2879–89.
Krupka C, Lichtenegger FS, Köhnke T, Bögeholz J, Bücklein V, Roiss M, et al. Targeting CD157 in AML using a novel Fc-engineered antibody construct. Oncotarget. 2017;8(22):35707–17.
Mani R, Rajgolikar G, Nunes J, Zapolnik K, Wasmuth R, Mo X, et al. Fc-engineered anti-CD33 monoclonal antibody potentiates cytotoxicity of membrane-bound interleukin-21 expanded natural killer cells in acute myeloid leukemia. Cytotherapy. 2020;22(7):369–76.
Khan M, Arooj S, Wang H. NK cell-based immune checkpoint inhibition. Front Immunol. 2020;11:167.
Coles SJ, Wang EC, Man S, Hills RK, Burnett AK, Tonks A, et al. CD200 expression suppresses natural killer cell function and directly inhibits patient anti-tumor response in acute myeloid leukemia. Leukemia. 2011;25(5):792–9.
Vey N, Bourhis JH, Boissel N, Bordessoule D, Prebet T, Charbonnier A, et al. A phase 1 trial of the anti-inhibitory KIR mAb IPH2101 for AML in complete remission. Blood. 2012;120(22):4317–23.
Godal R, Bachanova V, Gleason M, McCullar V, Yun GH, Cooley S, et al. Natural killer cell killing of acute myelogenous leukemia and acute lymphoblastic leukemia blasts by killer cell immunoglobulin-like receptor-negative natural killer cells after NKG2A and LIR-1 blockade. Biol Blood Marrow Transplant. 2010;16(5):612–21.
Ruggeri L, Urbani E, André P, Mancusi A, Tosti A, Topini F, et al. Effects of anti-NKG2A antibody administration on leukemia and normal hematopoietic cells. Haematologica. 2016;101(5):626–33.
Lanuza PM, Pesini C, Arias MA, Calvo C, Ramirez-Labrada A, Pardo J. Recalling the biological significance of immune checkpoints on NK cells: A chance to overcome LAG3, PD1, and CTLA4 inhibitory pathways by adoptive NK cell transfer? Front Immunol. 2019;10:3010.
Sanchez-Correa B, Valhondo I, Hassouneh F, Lopez-Sejas N, Pera A, Bergua JM, et al. DNAM-1 and the TIGIT/PVRIG/TACTILE axis: Novel immune checkpoints for natural killer cell-based cancer immunotherapy. Cancers (Basel). 2019;11(6):877.
Berger R, Rotem-Yehudar R, Slama G, Landes S, Kneller A, Leiba M, et al. Phase I safety and pharmacokinetic study of CT-011, a humanized antibody interacting with PD-1, in patients with advanced hematologic malignancies. Clin Cancer Res. 2008;14(10):3044–51.
Davids MS, Kim HT, Bachireddy P, Costello C, Liguori R, Savell A, et al. Ipilimumab for patients with relapse after allogeneic transplantation. N Engl J Med. 2016;375(2):143–53.
Gallois A, Silva I, Osman I, Bhardwaj N. Reversal of natural killer cell exhaustion by TIM-3 blockade. Oncoimmunology. 2014;3(12): e946365.
Xu L, Huang Y, Tan L, Yu W, Chen D, Lu C, et al. Increased Tim-3 expression in peripheral NK cells predicts a poorer prognosis and Tim-3 blockade improves NK cell-mediated cytotoxicity in human lung adenocarcinoma. Int Immunopharmacol. 2015;29(2):635–41.
Author information
Authors and Affiliations
Contributions
YK and YI wrote the manuscript. YI supervised the project. All the authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
YI received research funding from JSPS KAKENHI (Grant Number 20K08726), Takeda Pharma, and Bristol Myers Squibb. YI received an honorarium from Bristol Myers Squibb, Takeda Pharma, Janssen Pharmaceutical, and Sanofi. The remaining authors declare no competing financial interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Kaito, Y., Imai, Y. Evolution of natural killer cell-targeted therapy for acute myeloid leukemia. Int J Hematol 120, 34–43 (2024). https://doi.org/10.1007/s12185-024-03778-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12185-024-03778-0