Abstract
Purpose of Review
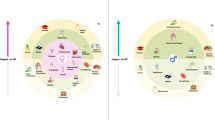
Heart failure (HF) is a significant cause of morbidity and mortality worldwide with unique phenotypes in men and women. Cardiomyopathy is a common cause of HF with distinct sex differences. This review focuses on sex differences in common types of cardiomyopathies.
Recent Findings
Genetic cardiomyopathies tend to affect men in part due to differences in X-chromosomes, while peripartum cardiomyopathy uniquely affects women of childbearing age. Moreover, women are more likely to develop cardiomyopathies related to emotional distress, inflammatory states, and certain cancer treatments. Sex hormones have been implicated in the pathogenesis of sex differences in cardiomyopathy. Data on sex differences in diagnosis, treatment response, and outcomes remain limited due to significant underrepresentation of women in studies. Finally, pregnancy may be pursued with close monitoring in most cases with notable exceptions, though official guidelines are limited.
Summary
Striking differences exist in HF between men and women, largely attributable to sex differences in the pathogenesis of cardiomyopathies, an important cause of HF. For many cardiomyopathies, presentation, disease trajectories, and treatment responses differ significantly between sexes, yet available data remain limited. Elucidating these sex-specific differences may refine screening, diagnosis, and treatment of cardiomyopathies. More studies are needed to aid in understanding the nuances between sexes which may enhance tailored preventive and therapeutic strategies for women and men.

Similar content being viewed by others
Abbreviations
- HF:
-
Heart failure
- HFpEF:
-
Heart failure with preserved ejection fraction
- HFrEF:
-
Heart failure with reduced ejection fraction
- LVH:
-
Left ventricular hypertrophy
- DCM:
-
Dilated cardiomyopathy
- CAD:
-
Coronary artery disease
- CMR:
-
Cardiac magnetic resonance
- PPCM:
-
Peripartum cardiomyopathy
- ECG:
-
Electrocardiogram
- BNP:
-
Brain natriuretic peptide
- GDMT:
-
Guideline-directed medical therapy
- ACE-I:
-
Ace inhibitor
- ARB:
-
Angiotensin receptor blocker
- LV:
-
Left ventricle
- ICD:
-
Implantable cardioverter defibrillator
- ACS:
-
Acute coronary syndrome
- HCM:
-
Hypertrophic cardiomyopathy
- BB:
-
Beta blocker
- SCD:
-
Sudden cardiac death
- RCM:
-
Restrictive cardiomyopathy
- AL:
-
Light chain
- ATTR:
-
Transthyretin amyloid
- AA:
-
Amyloid A
- HH:
-
Hereditary hemochromatosis
- ARVC:
-
Arrhythmogenic right ventricular cardiomyopathy
References
Disease GBD, Injury I, Prevalence C. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392(10159):1789–858.
Lloyd-Jones DM, Larson MG, Leip EP, Beiser A, D’Agostino RB, Kannel WB, et al. Lifetime risk for develo** congestive heart failure: the Framingham Heart Study. Circulation. 2002;106(24):3068–72.
Pfeffer MA, Shah AM, Borlaug BA. Heart failure with preserved ejection fraction in perspective. Circ Res. 2019;124(11):1598–617.
Sotomi Y, Hikoso S, Nakatani D, Mizuno H, Okada K, Dohi T, et al. Sex differences in heart failure with preserved ejection fraction. J Am Heart Assoc. 2021;10(5):e018574.
Regitz-Zagrosek V, Kararigas G. Mechanistic pathways of sex differences in cardiovascular disease. Physiol Rev. 2017;97(1):1–37.
Savji N, Meijers WC, Bartz TM, Bhambhani V, Cushman M, Nayor M, et al. The association of obesity and cardiometabolic traits with incident HFpEF and HFrEF. JACC Heart Fail. 2018;6(8):701–9.
Shah SJ, Lam CSP, Svedlund S, Saraste A, Hage C, Tan RS, et al. Prevalence and correlates of coronary microvascular dysfunction in heart failure with preserved ejection fraction: PROMIS-HFpEF. Eur Heart J. 2018;39(37):3439–50.
Williams D, Stout MJ, Rosenbloom JI, Olsen MA, Joynt Maddox KE, Deych E, et al. Preeclampsia predicts risk of hospitalization for heart failure with preserved ejection fraction. J Am Coll Cardiol. 2021;78(23):2281–90.
Lau ES, Wang D, Roberts M, Taylor CN, Murugappan G, Shadyab AH, et al. Infertility and risk of heart failure in the women’s health initiative. J Am Coll Cardiol. 2022;79(16):1594–603.
Ho JE, Enserro D, Brouwers FP, Kizer JR, Shah SJ, Psaty BM, et al. Predicting heart failure with preserved and reduced ejection fraction: the International Collaboration on Heart Failure Subtypes. Circ Heart Fail. 2016;9(6).
Lam CSP, Arnott C, Beale AL, Chandramouli C, Hilfiker-Kleiner D, Kaye DM, et al. Sex differences in heart failure. Eur Heart J. 2019;40(47):3859–68.
Lannou S, Mansencal N, Couchoud C, Lassalle M, Dubourg O, Stengel B, et al. The public health burden of cardiomyopathies: insights from a nationwide inpatient study. J Clin Med. 2020;9(4):920.
Japp AG, Gulati A, Cook SA, Cowie MR, Prasad SK. The diagnosis and evaluation of dilated cardiomyopathy. J Am Coll Cardiol. 2016;67(25):2996–3010.
Cannata A, Fabris E, Merlo M, Artico J, Gentile P, Pio Loco C, et al. Sex differences in the long-term prognosis of dilated cardiomyopathy. Can J Cardiol. 2020;36(1):37–44.
Fairweather D, Cooper LT Jr, Blauwet LA. Sex and gender differences in myocarditis and dilated cardiomyopathy. Curr Probl Cardiol. 2013;38(1):7–46.
Cocker MS, Abdel-Aty H, Strohm O, Friedrich MG. Age and gender effects on the extent of myocardial involvement in acute myocarditis: a cardiovascular magnetic resonance study. Heart. 2009;95(23):1925–30.
Bozkurt B, Colvin M, Cook J, Cooper LT, Deswal A, Fonarow GC, et al. Current diagnostic and treatment strategies for specific dilated cardiomyopathies: a scientific statement from the American Heart Association. Circulation. 2016;134(23):e579–646.
Hammes SR, Levin ER. Minireview: recent advances in extranuclear steroid receptor actions. Endocrinology. 2011;152(12):4489–95.
Cavasin MA, Tao ZY, Yu AL, Yang XP. Testosterone enhances early cardiac remodeling after myocardial infarction, causing rupture and degrading cardiac function. Am J Physiol Heart Circ Physiol. 2006;290(5):H2043–50.
Pelliccia F, Limongelli G, Autore C, Gimeno-Blanes JR, Basso C, Elliott P. Sex-related differences in cardiomyopathies. Int J Cardiol. 2019;286:239–43.
Herman DS, Lam L, Taylor MR, Wang L, Teekakirikul P, Christodoulou D, et al. Truncations of titin causing dilated cardiomyopathy. N Engl J Med. 2012;366(7):619–28.
Vissing CR, Rasmussen TB, Dybro AM, Olesen MS, Pedersen LN, Jensen M, et al. Dilated cardiomyopathy caused by truncating titin variants: long-term outcomes, arrhythmias, response to treatment and sex differences. J Med Genet. 2021;58(12):832–41.
van Rijsingen IA, Nannenberg EA, Arbustini E, Elliott PM, Mogensen J, Hermans-van Ast JF, et al. Gender-specific differences in major cardiac events and mortality in lamin A/C mutation carriers. Eur J Heart Fail. 2013;15(4):376–84.
Argiro A, Ho C, Day SM, van der Velden J, Cerbai E, Saberi S, et al. Sex-related differences in genetic cardiomyopathies. J Am Heart Assoc. 2022;11(9):e024947.
Brambatti M, Caspi O, Maolo A, Koshi E, Greenberg B, Taylor MRG, et al. Danon disease: gender differences in presentation and outcomes. Int J Cardiol. 2019;286:92–8.
Lim KRQ, Sheri N, Nguyen Q, Yokota T. Cardiac involvement in dystrophin-deficient females: current understanding and implications for the treatment of dystrophinopathies. Genes (Basel). 2020;11(7):765.
Cheng Z, Fang Q. Danon disease: focusing on heart. J Hum Genet. 2012;57(7):407–10.
Kamdar F, Garry DJ. Dystrophin-deficient cardiomyopathy. J Am Coll Cardiol. 2016;67(21):2533–46.
Roos-Hesselink J, Baris L, Johnson M, De Backer J, Otto C, Marelli A, et al. Pregnancy outcomes in women with cardiovascular disease: evolving trends over 10 years in the ESC Registry Of Pregnancy And Cardiac disease (ROPAC). Eur Heart J. 2019;40(47):3848–55.
Davis MB, Arany Z, McNamara DM, Goland S, Elkayam U. Peripartum cardiomyopathy: JACC state-of-the-art review. J Am Coll Cardiol. 2020;75(2):207–21.
Hilfiker-Kleiner D, Kaminski K, Podewski E, Bonda T, Schaefer A, Sliwa K, et al. A cathepsin D-cleaved 16 kDa form of prolactin mediates postpartum cardiomyopathy. Cell. 2007;128(3):589–600.
Stapel B, Kohlhaas M, Ricke-Hoch M, Haghikia A, Erschow S, Knuuti J, et al. Low STAT3 expression sensitizes to toxic effects of beta-adrenergic receptor stimulation in peripartum cardiomyopathy. Eur Heart J. 2017;38(5):349–61.
Patten IS, Rana S, Shahul S, Rowe GC, Jang C, Liu L, et al. Cardiac angiogenic imbalance leads to peripartum cardiomyopathy. Nature. 2012;485(7398):333–8.
Damp J, Givertz MM, Semigran M, Alharethi R, Ewald G, Felker GM, et al. Relaxin-2 and soluble Flt1 levels in peripartum cardiomyopathy: results of the multicenter IPAC study. JACC Heart Fail. 2016;4(5):380–8.
Arany Z, Elkayam U. Peripartum Cardiomyopathy. Circulation. 2016;133(14):1397–409.
Sliwa K, Blauwet L, Tibazarwa K, Libhaber E, Smedema JP, Becker A, et al. Evaluation of bromocriptine in the treatment of acute severe peripartum cardiomyopathy: a proof-of-concept pilot study. Circulation. 2010;121(13):1465–73.
Elkayam U, Schafer A, Chieffo A, Lansky A, Hall S, Arany Z, et al. Use of Impella heart pump for management of women with peripartum cardiogenic shock. Clin Cardiol. 2019;42(10):974–81.
McNamara DM, Elkayam U, Alharethi R, Damp J, Hsich E, Ewald G, et al. Clinical outcomes for peripartum cardiomyopathy in North America: results of the IPAC Study (Investigations of Pregnancy-Associated Cardiomyopathy). J Am Coll Cardiol. 2015;66(8):905–14.
Mahowald MK, Basu N, Subramaniam L, Scott R, Davis MB. Long-term outcomes in peripartum cardiomyopathy. Open Cardiovasc Med J. 2019;13(1).
Davis MB, Walsh MN. Cardio-obstetrics. Circ Cardiovasc Qual Outcomes. 2019;12(2):e005417.
Elkayam U. Risk of subsequent pregnancy in women with a history of peripartum cardiomyopathy. J Am Coll Cardiol. 2014;64(15):1629–36.
Hilfiker-Kleiner D, Haghikia A, Masuko D, Nonhoff J, Held D, Libhaber E, et al. Outcome of subsequent pregnancies in patients with a history of peripartum cardiomyopathy. Eur J Heart Fail. 2017;19(12):1723–8.
Codsi E, Rose CH, Blauwet LA. Subsequent pregnancy outcomes in patients with peripartum cardiomyopathy. Obstet Gynecol. 2018;131(2):322–7.
Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer Statistics, 2021. CA Cancer J Clin. 2021;71(1):7–33.
Moslehi JJ. Cardiovascular toxic effects of targeted cancer therapies. N Engl J Med. 2016;375(15):1457–67.
Bansal N, Adams MJ, Ganatra S, Colan SD, Aggarwal S, Steiner R, et al. Strategies to prevent anthracycline-induced cardiotoxicity in cancer survivors. Cardiooncology. 2019;5:18.
Zamorano JL, Lancellotti P, Rodriguez Munoz D, Aboyans V, Asteggiano R, Galderisi M, et al. 2016 ESC Position Paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC Committee for Practice Guidelines: The Task Force for cancer treatments and cardiovascular toxicity of the European Society of Cardiology (ESC). Eur J Heart Fail. 2017;19(1):9–42.
Silber JH, Jakacki RI, Larsen RL, Goldwein JW, Barber G. Increased risk of cardiac dysfunction after anthracyclines in girls. Med Pediatr Oncol. 1993;21(7):477–9.
Lipshultz SE, Lipsitz SR, Mone SM, Goorin AM, Sallan SE, Sanders SP, et al. Female sex and higher drug dose as risk factors for late cardiotoxic effects of doxorubicin therapy for childhood cancer. N Engl J Med. 1995;332(26):1738–43.
Wang L, Tan TC, Halpern EF, Neilan TG, Francis SA, Picard MH, et al. Major cardiac events and the value of echocardiographic evaluation in patients receiving anthracycline-based chemotherapy. Am J Cardiol. 2015;116(3):442–6.
Cote GM, Sawyer DB, Chabner BA. ERBB2 inhibition and heart failure. N Engl J Med. 2012;367(22):2150–3.
Xue J, Jiang Z, Qi F, Lv S, Zhang S, Wang T, et al. Risk of trastuzumab-related cardiotoxicity in early breast cancer patients: a prospective observational study. J Breast Cancer. 2014;17(4):363–9.
Onitilo AA, Engel JM, Stankowski RV. Cardiovascular toxicity associated with adjuvant trastuzumab therapy: prevalence, patient characteristics, and risk factors. Ther Adv Drug Saf. 2014;5(4):154–66.
Nolan M, Oikonomou EK, Silversides CK, Hines MR, Thompson KA, Campbell BA, et al. Impact of cancer therapy-related cardiac dysfunction on risk of heart failure in pregnancy. JACC CardioOncol. 2020;2(2):153–62.
Napp LC, Cammann VL, Jaguszewski M, Szawan KA, Wischnewsky M, Gili S, et al. Coexistence and outcome of coronary artery disease in Takotsubo syndrome. Eur Heart J. 2020;41(34):3255–68.
Ghadri JR, Wittstein IS, Prasad A, Sharkey S, Dote K, Akashi YJ, et al. International expert consensus document on Takotsubo syndrome (part I): clinical characteristics, diagnostic criteria, and pathophysiology. Eur Heart J. 2018;39(22):2032–46.
Ueyama T, Kasamatsu K, Hano T, Tsuruo Y, Ishikura F. Catecholamines and estrogen are involved in the pathogenesis of emotional stress-induced acute heart attack. Ann N Y Acad Sci. 2008;1148:479–85.
Singh T, Khan H, Gamble DT, Scally C, Newby DE, Dawson D. Takotsubo syndrome: pathophysiology, emerging concepts, and clinical implications. Circulation. 2022;145(13):1002–19.
Templin C, Ghadri JR, Diekmann J, Napp LC, Bataiosu DR, Jaguszewski M, et al. Clinical features and outcomes of Takotsubo (stress) cardiomyopathy. N Engl J Med. 2015;373(10):929–38.
Khera R, Light-McGroary K, Zahr F, Horwitz PA, Girotra S. Trends in hospitalization for takotsubo cardiomyopathy in the United States. Am Heart J. 2016;172:53–63.
Sobue Y, Watanabe E, Ichikawa T, Koshikawa M, Yamamoto M, Harada M, et al. Physically triggered Takotsubo cardiomyopathy has a higher in-hospital mortality rate. Int J Cardiol. 2017;235:87–93.
Sliwa K, van der Meer P, Petrie MC, Frogoudaki A, Johnson MR, Hilfiker-Kleiner D, et al. Risk stratification and management of women with cardiomyopathy/heart failure planning pregnancy or presenting during/after pregnancy: a position statement from the Heart Failure Association of the European Society of Cardiology Study Group on Peripartum Cardiomyopathy. Eur J Heart Fail. 2021;23(4):527–40.
Elliott P, McKenna WJ. Hypertrophic cardiomyopathy. Lancet. 2004;363(9424):1881–91.
Maron BJ, Maron MS. Hypertrophic cardiomyopathy. Lancet. 2013;381(9862):242–55.
Siontis KC, Ommen SR, Geske JB. Sex, survival, and cardiomyopathy: differences between men and women with hypertrophic cardiomyopathy. J Am Heart Assoc. 2019;8(21):e014448.
O’Mahony C, Jichi F, Ommen SR, Christiaans I, Arbustini E, Garcia-Pavia P, et al. International external validation study of the 2014 European Society of Cardiology Guidelines on Sudden Cardiac Death Prevention in Hypertrophic Cardiomyopathy (EVIDENCE-HCM). Circulation. 2018;137(10):1015–23.
Figtree GA, Kindmark A, Lind L, Grundberg E, Speller B, Robinson BG, et al. Novel estrogen receptor alpha promoter polymorphism increases ventricular hypertrophic response to hypertension. J Steroid Biochem Mol Biol. 2007;103(2):110–8.
Lind JM, Chiu C, Ingles J, Yeates L, Humphries SE, Heather AK, et al. Sex hormone receptor gene variation associated with phenotype in male hypertrophic cardiomyopathy patients. J Mol Cell Cardiol. 2008;45(2):217–22.
Rowin EJ, Maron MS, Wells S, Patel PP, Koethe BC, Maron BJ. Impact of sex on clinical course and survival in the contemporary treatment era for hypertrophic cardiomyopathy. J Am Heart Assoc. 2019;8(21):e012041.
Lakdawala NK, Olivotto I, Day SM, Han L, Ashley EA, Michels M, et al. Associations between female sex, sarcomere variants, and clinical outcomes in hypertrophic cardiomyopathy. Circ Genom Precis Med. 2021;14(1):e003062.
Butters A, Lakdawala NK, Ingles J. Sex Differences in hypertrophic cardiomyopathy: interaction with genetics and environment. Curr Heart Fail Rep. 2021;18(5):264–73.
Maron MS, Olivotto I, Betocchi S, Casey SA, Lesser JR, Losi MA, et al. Effect of left ventricular outflow tract obstruction on clinical outcome in hypertrophic cardiomyopathy. N Engl J Med. 2003;348(4):295–303.
Kim M, Kim B, Choi YJ, Lee HJ, Lee H, Park JB, et al. Sex differences in the prognosis of patients with hypertrophic cardiomyopathy. Sci Rep. 2021;11(1):4854.
Ommen SR, Mital S, Burke MA, Day SM, Deswal A, Elliott P, et al. 2020 AHA/ACC guideline for the diagnosis and treatment of patients with hypertrophic cardiomyopathy: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2020;142(25):e558–631.
Desai MY, Wolski K, Owens A, Naidu SS, Geske JB, Smedira NG, et al. Study design and rationale of VALOR-HCM: evaluation of mavacamten in adults with symptomatic obstructive hypertrophic cardiomyopathy who are eligible for septal reduction therapy. Am Heart J. 2021;239:80–9.
Olivotto I, Oreziak A, Barriales-Villa R, Abraham TP, Masri A, Garcia-Pavia P, et al. Mavacamten for treatment of symptomatic obstructive hypertrophic cardiomyopathy (EXPLORER-HCM): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2020;396(10253):759–69.
Ho CY, Mealiffe ME, Bach RG, Bhattacharya M, Choudhury L, Edelberg JM, et al. Evaluation of mavacamten in symptomatic patients with nonobstructive hypertrophic cardiomyopathy. J Am Coll Cardiol. 2020;75(21):2649–60.
Pereira NL, Grogan M, Dec GW. Spectrum of restrictive and infiltrative cardiomyopathies: part 1 of a 2-part series. J Am Coll Cardiol. 2018;71(10):1130–48.
Muchtar E, Dispenzieri A, Magen H, Grogan M, Mauermann M, McPhail ED, et al. Systemic amyloidosis from A (AA) to T (ATTR): a review. J Intern Med. 2021;289(3):268–92.
AbouEzzeddine OF, Davies DR, Scott CG, Fayyaz AU, Askew JW, McKie PM, et al. Prevalence of transthyretin amyloid cardiomyopathy in heart failure with preserved ejection fraction. JAMA Cardiol. 2021;6(11):1267–74.
Caponetti AG, Rapezzi C, Gagliardi C, Milandri A, Dispenzieri A, Kristen AV, et al. Sex-related risk of cardiac involvement in hereditary transthyretin amyloidosis: insights from THAOS. JACC Heart Fail. 2021;9(10):736–46.
Kroi F, Fischer N, Gezin A, Hashim M, Rozenbaum MH. Estimating the gender distribution of patients with wild-type transthyretin amyloid cardiomyopathy: a systematic review and meta-analysis. Cardiol Ther. 2021;10(1):41–55.
Rapezzi C, Riva L, Quarta CC, Perugini E, Salvi F, Longhi S, et al. Gender-related risk of myocardial involvement in systemic amyloidosis. Amyloid. 2008;15(1):40–8.
Zampieri M, Argiro A, Allinovi M, Tassetti L, Zocchi C, Gabriele M, et al. Sex-related differences in clinical presentation and all-cause mortality in patients with cardiac transthyretin amyloidosis and light chain amyloidosis. Int J Cardiol. 2022;351:71–7.
Baughman RP, Lower EE, du Bois RM. Sarcoidosis. Lancet. 2003;361(9363):1111–8.
Pour-Ghaz I, Kayali S, Abutineh I, Patel J, Roman S, Nayyar M, et al. Cardiac sarcoidosis: pathophysiology, diagnosis, and management. Hearts. 2021;2(2):234–50.
Nery PB, Beanlands RS, Nair GM, Green M, Yang J, McArdle BA, et al. Atrioventricular block as the initial manifestation of cardiac sarcoidosis in middle-aged adults. J Cardiovasc Electrophysiol. 2014;25(8):875–81.
Duvall C, Pavlovic N, Rosen N, Wand AL, Griffin J, Okada D, et al. Sex differences in presentation and outcomes of cardiac sarcoidosis. J Cardiac Fail. 2022;28(5):S16.
Kalra R, Malik S, Chen KA, Ogugua F, Athwal PSS, Elton AC, et al. Sex differences in patients with suspected cardiac sarcoidosis assessed by cardiovascular magnetic resonance imaging. Circ Arrhythm Electrophysiol. 2021;14(9):e009966.
Nakasuka K, Ishibashi K, Hattori Y, Mori K, Nakajima K, Nagayama T, et al. Sex-related differences in the prognosis of patients with cardiac sarcoidosis treated with cardiac resynchronization therapy. Heart Rhythm. 2022.
Hadid V, Patenaude V, Oddy L, Abenhaim HA. Sarcoidosis and pregnancy: obstetrical and neonatal outcomes in a population-based cohort of 7 million births. J Perinat Med. 2015;43(2):201–7.
Brissot P, Pietrangelo A, Adams PC, de Graaff B, McLaren CE, Loreal O. Haemochromatosis. Nat Rev Dis Primers. 2018;4:18016.
Steinberg KK, Cogswell ME, Chang JC, Caudill SP, McQuillan GM, Bowman BA, et al. Prevalence of C282Y and H63D mutations in the hemochromatosis (HFE) gene in the United States. JAMA :J Am Med Assoc. 2001;285(17):2216–22.
Muchtar E, Blauwet LA, Gertz MA. Restrictive cardiomyopathy: genetics, pathogenesis, clinical manifestations, diagnosis, and therapy. Circ Res. 2017;121(7):819–37.
Kowdley KV, Brown KE, Ahn J, Sundaram V. ACG Clinical Guideline: hereditary hemochromatosis. Am J Gastroenterol. 2019;114(8):1202–18.
Zacharski LR, Ornstein DL, Woloshin S, Schwartz LM. Association of age, sex, and race with body iron stores in adults: analysis of NHANES III data. Am Heart J. 2000;140(1):98–104.
Hou Y, Zhang S, Wang L, Li J, Qu G, He J, et al. Estrogen regulates iron homeostasis through governing hepatic hepcidin expression via an estrogen response element. Gene. 2012;511(2):398–403.
Qian Y, Yin C, Chen Y, Zhang S, Jiang L, Wang F, et al. Estrogen contributes to regulating iron metabolism through governing ferroportin signaling via an estrogen response element. Cell Signal. 2015;27(5):934–42.
Pereira NL, Grogan M, Dec GW. Spectrum of restrictive and infiltrative cardiomyopathies: part 2 of a 2-part series. J Am Coll Cardiol. 2018;71(10):1149–66.
European Association For The Study Of The L. EASL clinical practice guidelines for HFE hemochromatosis. J Hepatol. 2010;53(1):3–22.
Benveniste MF, Gomez D, Carter BW, Betancourt Cuellar SL, Shroff GS, Benveniste APA, et al. Recognizing radiation therapy-related complications in the chest. Radiographics. 2019;39(2):344–66.
Belzile-Dugas E, Eisenberg MJ. Radiation-induced cardiovascular disease: review of an underrecognized pathology. J Am Heart Assoc. 2021;10(18):e021686.
Lancellotti P, Nkomo VT, Badano LP, Bergler-Klein J, Bogaert J, Davin L, et al. Expert consensus for multi-modality imaging evaluation of cardiovascular complications of radiotherapy in adults: a report from the European Association of Cardiovascular Imaging and the American Society of Echocardiography. Eur Heart J Cardiovasc Imaging. 2013;14(8):721–40.
Chang HM, Okwuosa TM, Scarabelli T, Moudgil R, Yeh ETH. Cardiovascular complications of cancer therapy: best practices in diagnosis, prevention, and management: part 2. J Am Coll Cardiol. 2017;70(20):2552–65.
Corrado D, Link MS, Calkins H. Arrhythmogenic right ventricular cardiomyopathy. N Engl J Med. 2017;376(1):61–72.
Corrado D, Thiene G. Arrhythmogenic right ventricular cardiomyopathy/dysplasia: clinical impact of molecular genetic studies. Circulation. 2006;113(13):1634–7.
Groeneweg JA, Bhonsale A, James CA, te Riele AS, Dooijes D, Tichnell C, et al. Clinical presentation, long-term follow-up, and outcomes of 1001 arrhythmogenic right ventricular dysplasia/cardiomyopathy patients and family members. Circ Cardiovasc Genet. 2015;8(3):437–46.
Bhonsale A, Groeneweg JA, James CA, Dooijes D, Tichnell C, Jongbloed JD, et al. Impact of genotype on clinical course in arrhythmogenic right ventricular dysplasia/cardiomyopathy-associated mutation carriers. Eur Heart J. 2015;36(14):847–55.
Rigato I, Bauce B, Rampazzo A, Zorzi A, Pilichou K, Mazzotti E, et al. Compound and digenic heterozygosity predicts lifetime arrhythmic outcome and sudden cardiac death in desmosomal gene-related arrhythmogenic right ventricular cardiomyopathy. Circ Cardiovasc Genet. 2013;6(6):533–42.
Akdis D, Saguner AM, Shah K, Wei C, Medeiros-Domingo A, von Eckardstein A, et al. Sex hormones affect outcome in arrhythmogenic right ventricular cardiomyopathy/dysplasia: from a stem cell derived cardiomyocyte-based model to clinical biomarkers of disease outcome. Eur Heart J. 2017;38(19):1498–508.
Wallace R, Calkins H. Risk stratification in arrhythmogenic right ventricular cardiomyopathy. Arrhythmia Electrophysiol Rev. 2021;10(1):26.
Marcus FI, McKenna WJ, Sherrill D, Basso C, Bauce B, Bluemke DA, et al. Diagnosis of arrhythmogenic right ventricular cardiomyopathy/dysplasia: proposed modification of the task force criteria. Circulation. 2010;121(13):1533–41.
Choudhary N, Tompkins C, Polonsky B, McNitt S, Calkins H, 3rd Mark Estes NA, et al. Clinical presentation and outcomes by sex in arrhythmogenic right ventricular cardiomyopathy: findings from the North American ARVC Registry. J Cardiovasc Electrophysiol. 2016;27(5):555–62.
Corrado D, Wichter T, Link MS, Hauer RN, Marchlinski FE, Anastasakis A, et al. Treatment of arrhythmogenic right ventricular cardiomyopathy/dysplasia: an International Task Force Consensus Statement. Circulation. 2015;132(5):441–53.
Maron BJ, Udelson JE, Bonow RO, Nishimura RA, Ackerman MJ, Estes NA 3rd, et al. Eligibility and disqualification recommendations for competitive athletes with cardiovascular abnormalities: Task Force 3: Hypertrophic Cardiomyopathy, Arrhythmogenic Right Ventricular Cardiomyopathy and Other Cardiomyopathies, and Myocarditis: a scientific statement from the American Heart Association and American College of Cardiology. Circulation. 2015;132(22):e273–80.
Funding
E.S.L is supported by the NIH K23-HL159243 and the American Heart Association (853922).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Christy N. Taylor, MD, MPH, and Emily S. Lau, MD, MPH, declare they have no relevant conflicts of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Women and Heart Disease
Rights and permissions
About this article
Cite this article
Taylor, C.N., Lau, E.S. Sex Differences in Cardiomyopathy. Curr Cardiovasc Risk Rep 16, 159–170 (2022). https://doi.org/10.1007/s12170-022-00700-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12170-022-00700-3