Abstract
The overall purpose of this research work was to apply a microwave-assisted digestion process combined with ethanol organosolv as pretreatment for pistachio shells (PS) and cherry tree pruning (CTP) biomasses for cellulose production. This process would reduce the technical and environmental disadvantages of the accumulation of these agricultural wastes. A central composite design based on the Response Surface Methodology was applied to check the effect of reaction time, temperature, and concentration of ethanol acid solution. Adequate models have been obtained for the contents of cellulose and lignin of the treated solids as well as for solubilized cellulose percentage. Best conditions implied 67% ethanol and 30 min at 150 \(~\mathrm {^\circ }\)C, involving the production of a cellulose-enriched material (81.1% and 90.1% for treated CTP and PS, respectively). Finally, enzymatic hydrolysis of these materials was carried out, reaching glucose concentrations of 70 and 100 kg/m\(^{3}\) for CTP and PS, resulting in enzymatic hydrolysis yields at 12 h of 97.2% and 76.9%. These high values are one of the main novelties and strengths of this work, as show the ease of the process and can have a beneficial influence on its scale-up, reducing both costs and environmental impact.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In the framework of the current policy of sustainable development and circular economy, one of the main objectives is to advance in the maximum use of industrial agro-wastes, avoiding or reducing the need of high-cost primary resources while contributing to environment preservation. In this respect, the recovery of lignocellulose wastes for energy purposes to produce liquid biofuels can be an economic alternative, of feasible application in a biorefinery context, promoting the required valorization of widely available residual feedstocks.
Pistachio shells (PS) and cherry tree pruning (CTP) can be considered residual and renewable biomass in abundance worldwide from both agro-food industry and selective felling of cherry tree parts, respectively. According to the latest statistics data provided by the Food and Agriculture Organization of the United Nations [1], pistachio nut production stands at 1.13 million tons, with the USA and Iran being the largest producers. In addition, PS global production would be 0.51 million tons, approximately, as PS accounts for 45% of the nut [2]. Ministry of Agriculture, Fisheries and Food of Spain states that the emerging pistachio crop continues their productive expansion in Spain. In 2020, significant growth was recorded in pistachio plantation, 31% in rainfed and 20% in irrigated, reaching 48,000 of total cultivates hectares. On the other hand, cherry tree crop has also sharply increased in countries such as Turkey and the USA, which have doubled in the last 2 years. Annual CTP generates a large amount of biomass, which is often spread on the ground [3] causing serious phytosanitary problems, or atmospheric environmental impact if it is incinerated in an uncontrolled manner. Specifically, given the great scarcity of information from publications focused on CTP, the global and annual amount of this residue could be estimated from updated data in 1.43\(-\)3.11 million tons, considering a world harvested area of 4.45 10\(^5\) ha [1], with a medium tree density of 750 trees per ha [4] and CTP annual production in the range 4.29\(-\)9.32 kg per tree.
The structure of lignocellulosic materials, such as PS and CTP, is complex and recalcitrant, but it is possible to obtain solid waste biomass rich in decrystallized cellulose, if both the hemicellulose covering the cellulose and mainly the lignin fibers can be removed from the feedstock. To achieve this purpose, the processes must be capable to remove the hydrogen bonds that link the hemicellulose to the cellulose, and the ester and ether bonds that bind the lignin to both carbohydrate chains (cellulose and hemicellulose). Lignocellulose biomass disintegration can be achieved by using physical, chemical, physical-chemical, and biological pretreatments for matter deconstruction, although the lignocellulose biomass intricacy defines the type of pretreatment to be performed [5,6,7]. If the biomass pretreatment is sufficiently effective, assuming that depolymerization of amorphous cellulose occurs under conditions of lower severity than non amorphous one, it will lead to an increase in the surface area and porosity of the pretreated material. It allows to facilitate a subsequent cellulose hydrolytic process for glucose attainment, especially when enzymes are used since they are quite sensitive to substrate conditions (particle size, porosity, cellulose accessibility, etc.). Optimization of experimental parameters for both lignin and hemicellulosic components removal from PS and CTP could imply the reduction in the enzyme load required to carry out the cellulose enzymatic hydrolysis, decreasing overall costs in the second-generation ethanol production process.
Chemical pretreatments using compounds such as alkalis, acids, ionic liquids, organic solvents with low-boiling point (organosolv), and oxidizing agents are the current fractionation methods employed by the researchers to deconstruct lignocellulose materials; they improve lignin and/or hemicellulose removal and promote cellulose digestibility, reducing its polymerization degree and crystallinity and favoring enzymatic hydrolysis. In particular, the organosolv process, based on single organic compounds such as methanol, ethanol, carboxylic acids (formic, acetic and propionic acids), acetone, dimethyl sulfoxide, pyridine, pyrrolidinium, 1-methylimidazole, and tetrahydrofuran or binary solvents systems, offers an alternative for obtaining high purity cellulose, achieving simultaneously both polysaccharides hydrolysis and delignification [6].
Treatments with organic solvent could be used in combination with thermal methods to be applied directly for lignocellulose material disintegration. Microwave irradiation (MWI) combines non-conventional heating techniques with other specific effects resulting from microwaves action on biomass in aqueous solutions. Although MWI has been reported for biomass pretreatment, only in recent years, it has emerged to a greater extent as a possible way to reduce usual heating times (30–60 min) and temperatures (100–250\(~\mathrm {^\circ }\)C) [8], yielding a greater hemicellulose depolymerization compared to other support methods for lignocellulosic biomass disintegration, such as ultrasound technique [9]. Apart from the natural solution acidity as a consequence of the water dissociation at MWI experimental temperature (pH= 7.00 at 25\(~\mathrm {^\circ }\)C and pH=4.88 at 200\(~\mathrm {^\circ }\)C) [7], it could be catalyzed with extra-added acid in order to improve the process efficiency.
Despite some disadvantages, the mild thermal treatment of biomass is recognized as a very efficient process. Therefore, new approaches are being investigated. The presence of polar groups in the components of lignocellulosic biomass makes it a usable substrate for microwave heating technology [10].
Recent advances in the development of fractionation processes have proposed the use of novel and improved solvents, such as organic co-solvent systems using \(\gamma \)-valerolactone (GVL) and tetrahydrofuran (THF), ionic liquid (IL), and deep eutectic solvent (DES) systems. Although these systems are effective in efficiently solubilizing lignin and/or hemicelluloses to obtain a highly digestible cellulosic fraction for enzymatic sugar production, the lignin resulting from most of them condensed, which decreased the enzymatic digestibility of the fractionated solids. In addition, the significantly higher costs of the solvents, together with the higher concentrations needed to be effective, require a very high chemical recovery efficiency that is very difficult to achieve for economical biorefinery operations [11].
In recent years, ethanol-water system has been a dominant single- or two-step biomass pretreatment, as ethanol is considered an environmental-friendly and easily recyclable solvent, with low toxicity and high volatility, apart from involving effective deconstruction of lignocellulosic biomass. Ethanol Organosolv (EOS) has been applied for biomass delignification not only by auto-catalized process, but also by acid-catalyzed pretreatments [12], in order to reduce, in this last case, both reaction time and operation temperature, as well as to facilitate enzymatic hydrolysis of remaining solid glucan-rich fraction. In this sense, several investigations that use aqueous ethanol solutions to facilitate obtaining separated streams of major lignocellulose biomass components have been performed from different cellulosic raw materials: spruce bark [13], black spruce, pinus pinaster [12], eucalyptus [6], olive tree pruning [14], beech [15], pine sawdust [16], wheat straw, mango seed husk [17], pine, poplar, walnut and rice husks [18], sugarcane bagasse, bamboo, rice straw, and corn stover/stalks [5], but quite scarce information is available for PS and CTP delignification. Specifically, one of the main novelties of this work is the use of an organosolv treatment under microwave conditions to obtain cellulose-rich materials that could be easily hydrolyzed enzymatically.
The objective of this work was to evaluate and optimize, using response surface methodology (RSM), the main experimental parameters (time, temperature, and ethanol-water ratio) affecting microwave-assisted EOS treatment of PS and CTP, by using sulfuric acid as catalyst in order to get a remaining solid with a high concentration of glucose. Apart from solid biomass characterizations and model confirmation experiments, enzymatic saccharification after selected thermochemical treatments was examined.
Materials and Methods
Materials: Feedstocks and Reagents
The pistachio shells were obtained from a factory situated in Jaén (southern Spain). The variety of the pistachio trees was Kerman/Peter. The material was also air-dried, milled, and screened to select the fraction of particles with a diameter between 0.600 and 0.800 mm and homogenized in a single lot.
The cherry tree pruning biomass was collected from Spanish 16 to 18 years old trees (located between 453 669-455 079 mE and 4 180 397-4 169 222 mN relative to UTM coordinates) after the fruit harvest; the samples were taken from fresh branches around 1.5 m above the ground. The variety of the cherry trees was Marvin (also called 4-70). The material was air-dried, milled, and screened to select the fraction of particles with a diameter between 0.425 and 0.600 mm and homogenized in a single lot.
Ethanol (96% v/v), sulfuric acid (72% w/w), and acetic acid (96% w/w) were purchased from Panreac (Spain). Standard sugars (glucose, xylose, and arabinose) were purchased from Sigma-Aldrich chemicals (Spain).
Buffer solution of citrate pH=5.00 was purchased from ITW Reagents (USA) and cellulase enzyme blend SAE0020 from Sigma-Aldrich (USA). This last product is a mixture of cellulases, \(\beta \)-glucosidases, and hemicellulase, for the application of degrading cellulose to fermentable sugars with a value \(\ge \) 1000 Units/g.
Pretreatment Procedure
The programmed experimental tests involved the application of organosolv pretreatments to the original biomasses using ethanol as solvent.
PS and CTP pretreatments were carried out by means of a Milestone - Ethos One microwave mineralizer, allowing treatments at high temperatures, with a proper agitation of the materials placed inside Teflon vessels (100 cm\(^3\)), which ensure thermal and chemical stability of the system. Pressure was an uncontrollable parameter since, although dependent on the composition of the solution, it rises as the temperature of the system increases [19].
Samples were prepared by mixing 2.5 g of dry biomass, placed in the special digestion vessels with 25 cm\(^3\) of ethanol-water solution, so that the solid/liquid ratio was fixed at a value of 1:10 (biomass dry weight/volume of organosolv) for all the experiments. Before the vessels were hermetically sealed, 1.8 cm\(^3\) of sulfuric acid were added to each one in order to catalyze the reaction, and a magnetic stirrer was introduced to homogenize the solution conveniently throughout the pretreatment period. The mineralizer made it possible to plan the pretreatment time and the temperature ramp (heating, holding, and cooling phases). The heating time was 5 min for all the tested experimental conditions, and the cooling was carried out by means of the forced ventilation system of the equipment close to environment temperature.
Once each pretreatment was completed, the resulting solution was carefully vacuum filtered through porous filter crucibles to recover all the solid fraction from the vessels. The remaining solid biomass obtained after the treatments was washed with distilled water and then placed in a drying oven at 50 \(\mathrm {^\circ }\)C. At the end of the thermal treatment, the filter crucibles were weighed to determine, by weight difference with respect to the tare of the plates, the amount of treated biomass recovered after each pretreatment. This solid fraction separated by filtration was then analyzed according to the procedure explained in “Analytical Methods.”
Enzymatic Hydrolysis
Pretreated samples at the optimized conditions were then submitted to enzymatic saccharification in 100 cm\(^3\) DURAN™original laboratory bottles (10% w/v solids loading), adding 45 mM of citrate buffer (pH 5.0). The experiments were carried out into an incubated bench-top orbital shaker, allowing to reach a thermal equilibrium for 1 h at 50 \(\mathrm {^\circ }\)C and 200 rpm shaking. After thermal equilibrium was established, the cellulase enzyme blend was added to the flasks at 0.3 g per gram of cellulose in the pretreated solid substrate, considering glucan digestion for 24 h.
The cellulase activity at the time of these tests was 159 FPU/ cm\(^{3}\), measured by the filter paper method as described by Adney and Baker [20]. The enzyme blend has a density of 1150 kg/m\(^{3}\), which let us to determine the enzymatic dosage. Furthermore, 0.1 cm\(^3\) of sodium azide (NaN\(_3\)) solution (5%) as a bacteriostatic agent was added to avoid bacterial activity. The hydrolysates were withdrawn periodically for glucose analysis.
The enzymatic hydrolysis yield (EHY) was calculated considering Eq. 1 where \(C_g\) is the glucose concentration (kg/m\(^{3}\)), \(V_H\) is the volume (cm\(^{3}\)) of the hydrolyzate, f is an anhidrous correction factor with a value of 0.9 for C6 monomers, and \(C_p\) is the cellulose content (g).
Analytical Methods
Chemical Characterization
The composition of both original biomasses and solid residues obtained after the different pretreatments was determined according to the following procedures: moisture (TAPPI T 12 os-75), ash (TAPPI T 15 os-58) and lignin, hemicellulose and cellulose from the NREL methodology [21].
Concentrations of D-glucose, D-xylose, L-arabinose, and acetic acid were determined by high performance liquid cromatography (HPLC), using a Dionex UltiMate 3000 (Thermo Scientifc, Sunnyvale, CA, USA) equipped with a Bio-Rad Aminex HPX-87 H column (Biorad, CA, USA) (\(300 \times 7.8\) mm). The flow rate and the temperature were set at 0.6 cm\(^3\)/min and 50 \(\mathrm {^\circ }\)C, respectively. The detector was a refractive index one, ERC RefractoMax 520 (Thermo Scientific, Waltham, MA, USA), set at 50 \(\mathrm {^\circ }\)C. The mobile phase was a 0.01 N H\(_2\)SO\(_4\) water solution. Instrumental controls, data acquisition, and chromatographic integration were performed by using Dionex Chromeleon software employing an ESTD method.
Extractives were evaluated gravimetrically using a two-step secuencial extraction process by Soxhlet in order to remove water soluble material (inorganic compounds, non structural sugars, and nitrogenous substances, among others) and ethanol soluble material (chlorophyll, waxes, or minor components), according to a procedure adapted by Sluiter et al. [21].
Scanning Electron Microscopy (SEM)
A field emission scanning electron microscope (Merlin, Carl Zeiss) was used to observe the surface morphology of both materials, PS and CTP. So, the effects of the pretreatment on the morphological features of the fibers were evaluated by comparing the micrographs of untreated and pretreated biomasses. The samples were placed on the stub, dried in an oven at 60 \(\mathrm {^\circ }\)C for 2 h, and coated with gold by using a vacuum sputter coater (model Q150T ES, Quorum Technologies). The analysis of the samples was performed at an accelerating voltage of 15 kV.
Fourier Transformed Infrared (FTIR) Spectroscopy
The identification of functional groups in the biomass samples and the chemical changes produced after the hydrolysis treatments were assessed by Fourier Transformed Infrared (FTIR) spectroscopy (Tensor 27 spectrophotometer, Bruker). Prior to the analysis, the samples were milled and forced to pass a 0.5 mm sieve (Ultra Centrifugal Mill ZM 200, Retsch). The FTIR spectra were recorded in 3600–800 cm\(^{-1}\) range, with a resolution of 1.9 cm\(^{-1}\) and an accumulation of 32 scans. All the analyses were carried out in the Attenuated Total Reflection (ATR) mode.
Experimental Design and Statistical Analysis
The MWI-assisted pretreatment assays were performed considering a face-centered central composite design, with 17 runs including three replicates in the central point. Table 1 shows the resulting matrix for the design of experiments (DOE), including both the coded and real values of the three independent variables. Response surface methodology (RSM) was applied to determine the best experimental conditions for the dependent variables: reaction time (t), ranged from 5.9 to 34.1 min, reaction temperature (T), according to the 75-186\(~\mathrm {^\circ }\)C interval, and organosolvent (ethanol) concentration (c), varying from 26.5 to 73.5% (w/v).
The following variables have been chosen as response parameters of the model: on the one hand, the percentages of cellulose (cel) and lignin (lig), whose results were obtained from the direct characterization of the remaining solids recovered after pretreatment of the original biomasses, under the conditions established in the experimental design. On the other hand, the percentages of cellulose removed (\(Y_{c}\)), calculated as difference between the mass of cellulose in the original and pretreated PS and CTP biomasses, referred to the original ones (Eq. 2).
where \(m_0\) is the cellulose mass of the original biomass and \(m_{cel}\) is the cellulose mass of the pretreated biomass.
The numerical and graphical analysis of the results were performed by means of both MODDE v8.0 (Unimetrics AB, USA) and Design-Expert v13.0 (Stat-Ease Inc., USA) software. The relationship between the response functions and the coded variables was described by a second-degree polynomial (Eq. 3), where Y is the specific response function, \(x_i\) and \(x_j\) are independent variables, \(\beta _0\) is a constant, and \(\beta _i\), \(\beta _{ii}\), and \(\beta _{ij}\) are the linear, quadratic, and interactive coefficients, respectively. Analysis of variance (ANOVA) was the method to select relevant factors and determine the model’s coefficients.
Results and Discussion
Feedstocks Chemical Composition
The experimental results about the composition of the raw materials are shown in Table 2.
The composition of PS and CTP before EOS pretreatment, including the main fractions such as cellulose, hemicellulose, and lignin, expressed all the results as percentages by weight of dry original matter. The main component for CTP is clearly cellulose (37.6%), whereas the minority constituent is hemicellulose (16.9%). However, for PS, both cellulose and hemicellulose represent quite similar compositions, close to 31%. Analogue contents of lignin, about 21% and 23% for PS and CTP, respectively, were found.
According to an exhaustive bibliographic review, only some publications collect information on the chemical composition of PS biomass as a recoverable feedstock by biochemical processes and hardly any publications on CTP in this regard. Some of these publications reveal lignin composition data, either as acid-insoluble lignin or simply as lignin, in close agreement with those obtained in this research [22,23,24], whereas other authors show quite lower values (14.0% [25] or 16.5% [26]). Furthermore, the cellulose composition data found in this work is at an intermediate value between lower ones (as glucans) by Ozbek et al. [22] (24.3% as average) and higher results as those reported in the articles of the rest of the aforementioned research studies (38.1\(-\)56.5% range). Hemicellulose or xylans content is in line with the values published [22, 23, 25] (30.0\(-\)35.9%) although Nazem et al. [26] reported 25.5%. No ash or volatile matter was detected in the starting PS, while the content of aqueous and ethanolic extracts (12.7%) was in the same order of magnitude as those found in residues such as olive-tree pruning waste [27]. On the other hand, the composition results of the three fractions for CTP are slightly lower than the partial data offered in the only two publications found (43–44% cellulose, 30% hemicellulose, and 26.2% lignin [28]. The amounts of extracts are around 20%, and no ash was detected in this waste biomass.
The variations in the composition of both materials could be due to taking the data directly from the mentioned publications without clearly specifying the calculation basis (dry or wet biomass) used. For example, data offered by Ozbek et al. [22] are quite close to those ones for this research, especially as regards xylan and lignin content, with results expressed on a dry basis. Finally, it should be noted that the subspecies of pistachio, the climate, and the method of analysis could partly explain the differences in the composition of the raw materials studied [23].
Organosolv Pretreatment
EOS pretreatment experiments were performed by considering both PS and CTP feedstocks under defined conditions, according to the experimental design shown in Table 1. The exhaustive characterization of the solids remaining from the pretreatments assays had made it possible to assess the cellulose and lignin content, expressed as a percentage on a dry basis (Table 3). After chemical attack with microwave-assisted ethanol, the remaining solids exhibited cellulose contents ranging from 35 to 93% for PS and from 38 to 86% for CTP biomass, depending on the used treatment conditions. On the other hand, for both materials under study, lignin content did not exceed 50% (except in experiment 4, carried out at one of the most severe conditions: 180\(~\mathrm {^\circ }\)C, 33.3% w/v of acidified ethanol solution and 30 min of treatment time). Likewise, the parameter \(Y_c\) related to the percentage of cellulose removed from the original material shows the maximum value for the same experiment 4. For this experiment, the higher amount of cellulose dissolved from the original biomass (57.5% and 60.5% for CTP and PS, respectively) resulted in a treated solid with the lower cellulose content (37.8% and 35.9%, respectively) and the higher lignin content (60.5% and 53.6%).
Therefore, the use of a microwave-assisted digester to carry out the EOS treatment has made it possible to obtain remaining solids with substantial cellulose enrichment in all cases; for some conditions, data are even higher than those reported in previous studies with traditional EOS process applied to other types of biomasses [14, 29]. For example, in the case of assay 6, where neither too high temperatures (150\(~\mathrm {^\circ }\)C) nor excessive treatment times (30 min) were used, significant changes in the chemical composition of the lignocellulosic biomass were observed before and after treatment (Fig. 1). In this case, with the EOS pretreatment, it was possible to obtain a remaining biomass material with more than twice the cellulose and almost half the lignin content compared to the original biomass. The delignification percentages for assay 6 (76% for CTP and 93% for PS) were highly remarkable. However, it is obvious that there is a decrease in the mass obtained after the different pretreatments, but this basically coincides with the lost of hemicellulose and lignin that occurs as a consequence of the EOS treatment. In any case, the solid yields vary in the 31–41%.
Choi et al. [6] performed a traditional ethanol organosolv pretreatment on eucalyptus biomass (140\(~\mathrm {^\circ }\)C, 10 min, and 1% (w/v) H\(_2\)SO\(_4\)) resulting in 51.5% of lignin removal, a value slightly lower than that obtained in the current study for both CTP and PS biomasses, which could be due to more severe conditions in experiment 6 and/or to differential effect of MW radiation combined with organosolv treatment.
Anyway, although the EOS pretreatment assays involved an evident improvement of the final biomass residue composition to be subjected to subsequent enzymatic hydrolysis, it was necessary to optimize the independent variables, with significant impact, planned in the experimental design to ensure proper pretreatment effectiveness. The results presented in Table 3 were the starting point to determine the second order models using RSM. In addition to the cellulose and lignin contents of the pretreated biomass, the percentage of cellulose removed with respect to the starting biomass has also been used as model’s dependent variable, since this parameter must be not excessively high, as it is an awared factor for the economic consideration of the process.
Significance of the statistical models, at 95% of confidence level, was researched throughout ANOVA analysis from results of experimental design under planned conditions, considering the examined response variables for PS and CTP treated biomasses. In this sense, p-values confirmed all model’s significance (p-value < 0.0001) and the regression coefficient, R\(^2\), represented the goodness-of-fit between experimental and predicted data, as shown in Fig. 2, with R\(^2\) values, in general, above 0.97 (Table 4).
Models obtained for treated PS biomass (Table 4) included not only the linear terms for reaction time, temperature, and ethanol concentration, but also terms representing the interactions between different variables and pure quadratic parameters. With respect to treated CTP solids, the models included the interactions between time and temperature as the only ones contemplated by the mathematical equations for the three responses (\(\% ~cel\), % lig, and \(Y_c\)), while they have not pure quadratic terms because they are not statistically significant (p-value>0.05). In general, an increase in the concentration of the ethanol solution for EOS treatment favored PS and CTP solids with a higher cellulose and lower lignin contents. Furthermore, it could not be ensured that an increase in both time and temperature yields the lowest cellulose content in the treated CTP material, since the interaction between these variables must be taken into account. It could occur exclusively when individual effects of the mentioned variables were considered.
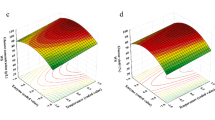
Figure 3a–c for PS and d–f for CTP collects three-dimensional graphical representations illustrating how the model responses in terms of % cel (a) and (d), % lig (b) and (e), and \(Y_c\) (c) and (f) when modifying simultaneously two of the input parameters (reaction time and temperature), kee** constant the third independent variable (50%, w/v of ethanol-water solution concentration, corresponding to the central point), according to the input parameters range provided for the experimental design (Table 1).
CTP biomass plots show the highest percentage of cellulose in two experimental conditions involving either high temperature (180\(~\mathrm {^\circ }\)C) and low time (10 min) or, on the contrary, low temperature (150\(~\mathrm {^\circ }\)C) and high time (30 min): 74.4% and 72.9%, respectively. If the selected ethanol concentrations were fixed at (-1) and (+1) coded values, the highest cellulose contents would have been obtained at the same experimental conditions, but the cellulose percentages would be lower (62.5%) or higher (86.2%) than those obtained at the center point, respectively.
On the other hand, the treated solids with the highest percentage of lignin (43.3%) (Fig. 3b) were achieved for high values of both parameters (180\(~\mathrm {^\circ }\)C and 30 min), while the treated materials with lower lignin content (23.13%) were obtained in conditions of lower severity (150\(~\mathrm {^\circ }\)C and 10 min). Finally, with respect to the percentage of solubilized cellulose (Fig. 3c), a higher value (48.2%) was reached for high values of temperature (180\(~\mathrm {^\circ }\)C) and time (30 min): it may hinder subsequent enzymatic hydrolysis, decreasing the overall process yields.
The same cellulose percentage trend occurred for PS-treated biomass (Fig. 3d). Thus, the maximum percentages of this fraction in the recovered materials were obtained under the same conditions, although treated PS exhibited slightly higher results, specifically 11% or 15% higher than for treated CTP, under the two referred conditions. In addition, the same severity conditions as those mentioned for treated CTP resulted in PS treated solids recovery with lower cellulose content (63.7%). Finally, it is worth noting that higher temperatures and times (180\(~\mathrm {^\circ }\)C and 30 min) resulted in both higher lignin content (44.2%) and cellulose loss (66.7%), while less drastic treatment conditions yielded lower values for both lignin (22.1%) and solubilized cellulose (11.4%) contents for PS biomass.
The simultaneous optimization of the responses obtained from the proposed models was finally investigated for the two biomasses, providing as the best condition in both cases corresponds to the pretreatment with a duration of 30 min, at a temperature of 150\(~\mathrm {^\circ }\)C, and with an ethanol concentration of 67%. In this situation, pretreated solids were obtained from CTP and PS with quite high cellulose contents (81.1% and 90.6%, respectively). Correspondingly, these cellulose-rich substrates showed a low percentage of lignin (13.58% and 4.4%), and the selected conditions removed scarce amount of cellulose from the original materials (12.8 and 6.5%, respectively).
Scanning Electron Microscopic Observation
Morphology of PS and CTP original biomasses and the corresponding solid materials recovered after EOS pretreatment, at the best experimental conditions according to a mathematical optimization process, were visualized using scanning electron microscopy (SEM) technique (magnification of 2000) to explore surface structural changes (Fig. 4). The application of the pretreatment contributed to deconstruct both biomasses, much more compact before altering their morphology by the action of the combined organosolv-microwave pretreatment. Photomicrography of original CTP biomass (Fig. 4a) apparently exhibited a more fibrous structure than PS image (Fig. 4c), which visually appeared to be more cohesive and smoother. Anyway, the application of EOS pretreatment to both lignocellulose materials led to an increased formation of gaps and cracks on the structure (Fig. 4b and d), as a consequence of the rupture of bonds among the main constituent of the fibers. Therefore, a partial solubilization of its structural components (mainly hemicellulose, lignin, and extracts) occurs, causing more fragile materials.
This result suggested that both biomasses were well extracted and the remaining fibers may be in a good form for cellulase enzyme penetration and accessibility into the cellulose structure.
Fourier-Transformed Infrared Spectroscopy Analysis
The residual solid phases obtained in optimized conditions for microwave-assisted organosolv pretreatments as well as original biomasses, CTP and PS, were characterized by Fourier Transform Infrared Spectroscopy (FTIR) in order to identify changes in functional groups, present in cellulose, hemicellulose, and lignin fractions (Table 5). Figure 5 shows the spectra, ranged from 3600 to 800 cm\(^{-1}\) at a resolution of 1.9 cm\(^{-1}\), for both raw and treated biomasses.
Adsorption peaks in the 3600–3000 cm\(^{-1}\) region are usually due to various O-H and N-H group extension vibrations. O-H linkages tend to appear at higher wavenumbers and often to be broader than the N-bands. Therefore, the broad peak shape above 3270 cm\(^{-1}\) could correspond to the O-H bond, probably as a consequence of stretching vibration of hydroxyl group of hydrogen bond, with possible slight overlap of the N-H group. This band undergoes a slight flattening after organosolv treatment, which may be due to the removal of considerable fraction of hemicellulose and part of lignin in the residual solid. In principle, considering the similar moisture content of the analyzed samples (6–8%), the slight attenuation of this band is not attributed to hydrogen bond O-H stretching vibration, indicative of the fibers’ hydrophilic tendency [30].
One of the most significant changes in the bands of the spectra in Fig. 5 can be observed at 1705 cm\(^{-1}\), corresponding to the stretching vibration of the C=O due to carbonyl bonds of acetyl groups from xylans. In addition, the notable decrease in the peak corresponding to 1220 cm\(^{-1}\) would imply the cleavage of C-O stretching bonds from hemicellulose acetyl groups. Therefore, the thermochemical attacks onto the two original biomasses have resulted in a significant reduction of these spectral bands, as a consequence of the hemicellulose fraction hydrolysis. It involved its nearly complete removal from both CTP and PS biomasses, in view of the faint signal offered at the characteristic wavelengths of this above mentioned fraction. The vibration of aliphatic C-H bonds is found, approximately, in the region 2900–2800 cm\(^{-1}\), and it is frequently splitting into more than one peak, due to symmetric or asymmetric elongation of the C-H bond. The peaks appearing at 2850 cm\(^{-1}\) and 2800 cm\(^{-1}\) are assigned to aliphatic C-H bond vibration, probably determining the existence of open or ring CH\(_2\) and CH\(_3\) from cellulose, lignin, or hemicellulose fractions in biomass or even associated with C-H bond with stretching and blending vibrations of CH\(_3\), CH\(_2\), CHO, and CH functional groups of cellulose, according to Table 5 references [30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46]. Both peaks in the pretreated PS have undergone a significant decrease with respect to the corresponding natural biomass, while for CTP, less significant visual change is observed. On the other hand, in the spectral band 1009–1170 cm\(^{-1}\), there are other peaks that may highlight the presence of cellulose in the treated lignocellulosic materials, even compared to the original ones (CTP and PS). Chihaoui et al. [47] observed a band in the 1080–1170 cm\(^{-1}\) range, associated to vibrations of the pyranose ring, corresponding to 1085 cm\(^{-1}\) in PS and CTP. With regard to the peaks appearing at 1140 cm\(^{-1}\) (Fig. 5), they may be indicative of the presence of cellulose due to the anti-symmetric vibrational stretching of the C-O-C group of the ring by \(\beta \)-(1,4) glycosidic bonds, while peaks at 1036 cm\(^{-1}\) correspond to stretching C-O. Although Dávila et al. [44] and Biswas et al. [33] pointed out that at 1036 cm\(^{-1}\), approximately, the hemicellulosic biopolymer could also have a contribution to the absorbance of the peak at that wavelength, the observed peaks for both PS and CTP could be primary related to the fraction composed only of glucose as polysaccharide, considering that the optimized treatment conditions could have disintegrated most of hemicellulose. The wavelength’s range 1270–1670 cm\(^{-1}\) is related to a vibration band corresponding to the lignin fraction of the biomass. For instance, the peaks located at 1430 cm\(^{-1}\) and 1590 cm\(^{-1}\) would be associated to the (Ar)-C=C stretching pair in aromatic molecules. Furthermore, it is possible that the peak at 1290 cm\(^{-1}\) could appear as a consequence of the presence of guaiacyl units. In general, a slight absorbance decrease in the spectral region comprising wavelengths in the aforementioned region is visually observed, especially in the band with maximum absorption at 1590 cm\(^{-1}\), that could be evidence of the delignification process suffered by the starting materials when subjected to the optimized microwave-assisted chemical treatments. Anyway, for the peak at 879 cm\(^{-1}\), which other authors [35] associate to the C-H bond of the aromatic ring of lignin, no relevant changes were observed.
Verification of the Model
The generated models were verified by carrying out three experimental tests under the optimal conditions provided by the software, which involved positive results. To verify the models obtained, it is necessary for the results of these tests to be within a confidence interval of 95%. In this sense, the experiment carried out at the best conditions, 30 min at a temperature of 150\(~\mathrm {^\circ }\)C and with an ethanol concentration of 67%, was repeated to confirm the results (Table 6).
In the case of PS, the percentages of cellulose, lignin, and hemicellulose are within the validity interval in absolute values established by the statistical program (86.3\(-\)100.0%; 2.3\(-\)9.0% and 3.9\(-\)9.2%, respectively). On the other hand, with respect to the selected CTP treated biomass, the agreement of the experimental and simulation model data is even more consistent than the PS prediction; therefore, the results offered by the CTP model are, a priori, within the interval proposed to confirm its validity at 95% of confidence level (78.2\(-\)93.1%; 5.8\(-\)20.5%; 0.5\(-\)23.6%, respectively).
Enzymatic Hydrolysis
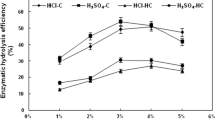
Solid resulting of EOS microwave assisted pretreatment under optimized conditions for PS and CTP original biomasses were submitted to an enzymatic hydrolysis, in order to convert glucans into glucose. Enzymatic hydrolysis of both raw PS and CTP was carried out, but no significant yields were obtained (data not shown), as the structure of these biomasses prevents the enzymes from accessing them. However, once pretreated, the resulting solids, mainly composed of cellulose, are in very favorable conditions for enzymatic hydrolysis. Figure 6 shows the evolution of the glucose concentration during the enzyme treatment for both treated biomasses. It can be seen that in both cases, the process reached the maximum concentration of monomer near 12 h (100 kg/m\(^{3}\) for PS and 70 kg/m\(^{3}\) for CTP). On the other hand, the obtained values of recovered glucose from PS were higher than the ones obtained from CTP treated material.
Regarding enzymatic hydrolysis yields (EHY), it should be noted that their evolution over time follows a very similar trend to that for glucose concentration, reaching the maximum values at 12 h, 97.2% and 76.9% for PS and CTP, respectively. The values reached in this work are higher than those indicated for other biomasses and treatments (62% for wheat straw treated with ionic liquids [48], 69% for organosolv process on sawdust [49], 72% for acid pretreated bamboo residues [50]). The high hydrolysis efficiency observed is one of the main strengths of the present research.
Although the nature of the residual solids obtained after microwave-assisted EOS treatment in relation to the characteristics of the structural cellulose fibers has certainly conditioned the course of the enzymatic hydrolysis, the difference between the lignin content of the cellulose-enriched solids for PS and CTP (see experiment 6, Table 3) could also explain the different behavior of the EHY. The presence of a higher amount of lignin in the CTP solid could have hindered the access of enzymes to cellulose to a greater extent, providing lower glucose yields from CTP C6-carbohydrate polymer than those obtained from PS biomass. Thus, the justification for the high yields obtained may be due to two facts; firstly, the high percentages of cellulose obtained after treatment and secondly the fact that this cellulose has an easily accessible structure.
Conclusions
An evaluation of fermentable sugars production from PS and CTP for glucose production using an microwave-assited EOS technique was performed. The best conditions for both biomasses were 30 min, 150\(\mathrm {^\circ }\)C, and 67% ethanol. The results obtained allow to consider these biomasses as a promising feedstock for glucose production. Moreover, one of the main novelties and strengths of this work is the high enzymatic yields obtained, through an easy pretreatment that may have a beneficial influence on its scale-up. So, these agricultural biomasses can generate an important benefit as the proposed process can reduce both costs and environmental impact.
Availability of Data and Materials
The data generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
FAO. Food and Agriculture Organization of the United Nations (2020) Crops and livestock products. https://www.fao.org/faostat/en/#data/QCL. Accessed 12 June 2023
Robles E, Izaguirre N, Martin A, Moschou D, Labidiurl J (2021) Assessment of bleached and unbleached nanofibers from pistachio shells for nanopaper making. Molecules 5:1371. https://doi.org/10.3390/molecules26051371
Willig G, Brunissen F, Brunois F, Godon B, Magro C et al (2022) Phenolic compounds extracted from cherry tree (Prunus avium) branches: impact of the process on cosmetic properties. Antioxidants 5:813. https://doi.org/10.3390/antiox11050813
Shen J, Zhang P, Chang Y, Zhang L, Hao Y et al (2021) The environmental performance of greenhouse versus open-field cherry production systems in China. Sustain Prod Consum 28:736–748. https://doi.org/10.1016/j.spc.2021.07.002
Baruah J, Nath BK, Sharma R, Kumar S, Deka RC et al (2018) Recent trends in the pretreatment of lignocellulosic biomass for value-added products. Front Energy Res 6:141. https://doi.org/10.3389/fenrg.2018.00141
Choi JH, Jang SK, Kim JH, Park SY, Kim JC et al (2019) Simultaneous production of glucose, furfural and ethanol organosolv lignin for total utilization of high recalcitrant biomass by organosolv pretreatment. Renew Energy 150:952–960. https://doi.org/10.1016/j.renene.2018.05.052
Li H, Qu Y, Yang Y, Chang S, Xu J (2016) Microwave irradiation - a green and efficient way to pretreat biomass. Bioresour Technol 199:34–41. https://doi.org/10.1016/j.biortech.2015.08.099
Vaidya AA, Murton KD, Smith DA, Dedual G (2022) A review on organosolv pretreatment of softwood with a focus on enzymatic hydrolysis of cellulose. Biomass Convers Biorefin 12:5427–5442. https://doi.org/10.1007/s13399-022-02373-9
Ríos-González LJ, Medina-Morales MA, Rodriguez JA, Romero-Galarza A, Dávila D et al (2021) Comparison of dilute acid pretreatment of agave assisted by microwave versus ultrasound to enhance enzymatic hydrolysis. Bioresour Technol 319:124049. https://doi.org/10.1016/j.niortech.2020.124099
Arshanitsa A, Akishin Y, Zile E, Dizhbite T, Solodovnik V et al (2016) Microwave treatment combined with conventional heating of plant biomass pellets in a rotated reactor as a high rate process for solid biofuel manufacture. Renew Energ 91:386–396. https://doi.org/10.1016/j.renene.2016.01.080
Wadaa Y, Fujii S, Tsubakid S (2021) Activation of chemical reactions on solid catalysts under microwave irradiation. In: Török B, Schäfer C (ed) Non-traditional activation methods in green and sustainable applications. Elsevier, pp 27–69. https://doi.org/10.1016/B978-0-12-819009-8.00011-6
Rui Moreira R, Mendes CVT, Banaco BF, Carvalho MGVS, Portugal A (2020) New insights in the fractionation of Pinus pinaster wood: sequential autohydrolysis, soda ethanol organosolv and acidic precipitation. Ind Crops Prod 152:112499. https://doi.org/10.1016/j.indcrop.2020.112499
Hrůzová K, Matsakas L, Rova U, Christakopoulos P (2021) Organosolv fractionation of spruce bark using ethanol-water mixtures: towards a novel bio-refinery concept. Bioresour Technol 341. https://doi.org/10.1016/j.biortech.2021.125855
Díaz MJ, Huijgen WJJ, van der Laan RR, Reith JH, Cara C et al (2011) Organosolv pretreatment of olive tree biomass for fermentable sugars. Holzforschung 65(2):177–183. https://doi.org/10.1515/hf.2011.030
Ibrahim Q, Kruse A (2020) Prehydrolysis and organosolv delignification process for the recovery of hemicellulose and lignin from beech wood. Bioresour Technol Rep 11:100506. https://doi.org/10.1016/j.biteb.2020.100506
Li H, Cai X, Wang Z, Xu C (2020) Cost-effective production of organosolv lignin from woody biomass using ethanol-water mixed solvent at mild conditions. J Supercrit Fluids 158:104745. https://doi.org/10.1016/j.supflu.2019.104745
Bello F, Chimphango A (2021) Optimization of lignin extraction from alkaline treated mango seed husk by high shear homogenization-assisted organosolv process using response surface methodology. Int J Biol Macromol 167:1379–1392. https://doi.org/10.1016/j.ijbiomac.2020.11.092
Sheng Y, Ma Z, Wang X, Han Y (2022) Ethanol organosolv lignin from different agricultural residues: toward basic structural units and antioxidant activity. Food Chem 376:131895. https://doi.org/10.1016/j.foodchem.2021.131895
Alio MA, Tugui OC, Vial C, Agnès Pons A (2019) Microwave-assisted Organosolv pretreatment of a sawmill mixed feedstock for bioethanol production in a wood biorefinery. Bioresour Technol 276:170–176. https://doi.org/10.1016/j.biortech.2018.12.078
Adney B, Baker J (1996) Measurement of cellulase activities NREL/TP-510-42628. National Renewable Energy Laboratory USA. https://www.nrel.gov/docs/gen/fy08/42628.pdf. Accessed 10 June 2023
Sluiter A, Ruiz R, Scarlata C, Sluiter J, Templeton D (2008) Determination of extractives in biomass NREL/TP-510-42619. National Renewable Energy Laboratory USA. https://www.nrel.gov/docs/gen/fy08/42619.pdf. Accessed 10 June 2023
Özbek HN, Yanık DK, Fadıloğlu S, Gögüs F (2020) Effect of microwave-assisted alkali pre-treatment on fractionation of pistachio shell and enzymatic hydrolysis of cellulose-rich residues. J Chem Technol Biotechnol 96(2):521–531. https://doi.org/10.1002/jctb.6569
Kasiri N, Fathi M (2018) Production of cellulose nanocrystals from pistachio shells and their application for stabilizing pickering emulsions. Int J Biol Macromol 106:1023–1031. https://doi.org/10.1016/j.ijbiomac.2017.08.112
Orooji Y, Han N, Nezafat Z, Shafiei N, Shen Z et al (2022) Valorisation of nuts biowaste: prospects in sustainable bio(nano)catalysts and environmental applications. J Clean Prod 347:131220. https://doi.org/10.1016/j.jclepro.2022.131220
Houfani AA, Anders N, Spiess AC, Baldrian P, Benallaoua S (2020) Insights from enzymatic degradation of cellulose and hemicellulose to fermentable sugars-a review. Biomass Bioenergy 134:105481. https://doi.org/10.1016/j.biombioe.2020.105481
Nazem MA, Zare MH, Shirazian S (2020) Preparation and optimization of activated nano-carbon production using physical activation by water steam from agricultural wastes. RSC Adv 10(3):1463–1475. https://doi.org/10.1039/c9ra07409k
Peinado S, Mateo S, Sánchez S, Moya AJ (2019) Effectiveness of sodium borohydride treatment on acid hydrolyzates from olive-tree pruning biomass for bioethanol production. BioEnergy Res 12(2):302–311. https://doi.org/10.1007/s12155-019-09979-4
Španić N, Jambreković V, Klarić M (2016) Basic chemical composition of wood as a parameter in raw material selection for biocomposite production. Cel Chem Technol 52(3–4):163–168
Buratti C, Foschini D, Barbanera M, Fantozzi F (2018) Fermentable sugars production from peach tree prunings: response surface model optimization of NaOH alkaline pretreatment. Biomass Bioenergy 112:128–137. https://doi.org/10.1016/j.biombioe.2017.12.032
Selvakumar P, Adane AA, Zelalem T, Hunegnaw BM, Karthik V et al (2022) Optimization of binary acids pretreatment of corncob biomass for enhanced recovery of cellulose to produce bioethanol. Fuel 321:124060. https://doi.org/10.1016/j.fuel.2022.124060
Bedoui A, Souissi-Najar S, Idris SS, Rahman NA, Ouederni A (2021) Thermal behaviour of impregnated olive stones with phosphoric acid via TGA-MS. C R Chim 24:149–162. https://doi.org/10.5802/crchim.118
Souza LSS, Pereira AM, dos Santos-Farias MA, Oliveira RL, Duvoisin S, Quaresma JNN (2020) Valorization of andiroba (Carapa guianensis Aubl.) residues through optimization of alkaline pretreatment to obtain fermentable sugars. Bioresources 15:894–909. https://doi.org/10.15376/biores.15.1.894-909
Biswas S, Rahaman T, Gupta P, Mitra R, Dutta S et al (2022) Cellulose and lignin profiling in seven, economically important bamboo species of India by anatomical, biochemical, FTIR spectroscopy and thermogravimetric analysis. Biomass Bioenergy 158:106362. https://doi.org/10.1016/j.biombioe.2022.106362
Xu K, Shi Z, Lyu J, Zhang Q, Zhong T et al (2020) Effects of hydrothermal pretreatment on nano-mechanical property of switchgrass cell wall and on energy consumption of isolated lignin-coated cellulose nanofibrils by mechanical grinding. Ind Crops Prod 149:112317. https://doi.org/10.1016/j.indcrop.2020.112317
Al-Ghouti MA, Dib SS (2020) Utilization of nano-olive stones in environmental remediation of methylene blue from water. J Environ Health Sci Eng 18:63–77. https://doi.org/10.1007/s40201-019-00438-y
Gandolfi S, Ottolina G, Riva S, Fantoni GP, Patel I (2013) Complete chemical analysis of Carmagnola hemp hurds and structural features of its components. Bioresources 8(2):2641–2656. https://doi.org/10.15376/biores.8.2.2641-2656
Cheng B, Zhang X, Lin Q, **n F, Sun R et al (2018) A new approach to recycle oxalic acid during lignocellulose pretreatment for xylose production. Biotechnol Biofuels 11:324. https://doi.org/10.1186/s13068-018-1325-3
Sim SF, Mohamed M, Lu NA, Lu MI, Sarman NSP, Samsudin Siti Nor Sihariddh SNS (2012) Computer-assisted analysis of Fourier transform infrared (FTIR) spectra for characterization of various treated and untreated agriculture biomass. Bioresources 7(4):5367–5380
Arpaci SS, Tomak ED, Ermeydan MA, Ibrahim Yildirim I (2021) Natural weathering of sixteen wood species: changes on surface properties. Polym Degrad Stab 183:109415. https://doi.org/10.1016/j.polymdegradstab.2020.109415
Deng Z, **a A, Liao Q, Zhu X, Huang Y, Fu Q (2019) Laccase pretreatment of wheat straw: effects of the physicochemical characteristics and the kinetics of enzymatic hydrolysis. Biotechnol Biofuels 12:159. https://doi.org/10.1186/s13068-019-1499-3
Arun V, Perumal EM, Prakash KA, Rajesh M, Tamilarasan K (2020) Sequential fractionation and characterization of lignin and cellulose fiber from waste rice bran. J Environ Chem Eng 8:104124. https://doi.org/10.1016/j.jece.2020.104124
Dhar P, Sugimura K, Yoshioka M, Yoshinaga A, Kamitakahara H (2021) Synthesis-property-performance relationships of multifunctional bacterial cellulose composites fermented in situ alkali lignin medium. Carbohydr Polym 252:117114. https://doi.org/10.1016/j.carbpol.2020.117114
Wang P, Fu Y, Shao Z, Zhang F, Qin M (2016) Structural changes to aspen wood lignin during autohydrolysis pretreatment. Bioresources 2:4086–4103
Dávila I, Remón J, Gullón P, Labidi J, Budarin V (2019) Production and characterization of lignin and cellulose fractions obtained from pretreated vine shoots by microwave assisted alkali treatment. Biores Technol 289:121726. https://doi.org/10.1016/j.biortech.2019.121726
Ganguly P, Sengupta S, Das P, Bhowal A (2020) Valorization of food waste: extraction of cellulose, lignin and their application in energy use and water treatment. Fuel 280. https://doi.org/10.1016/j.fuel.2020.118581
Zhuang J, Li M, Pu Y, Ragauskas A, Yoo C (2020) Observation of potential contaminants in processed biomass using Fourier transform infrared spectroscopy. App Sci 10(12):4345. https://doi.org/10.3390/app10124345
Chihaoui B, Tarrés Q, Delgado-Aguilar M, Mutjé P, Boufi S (2022) Lignin-containing cellulose fibrils as reinforcement of plasticized PLA biocomposites produced by melt processing using PEG as a carrier. Ind Crops Prod 175:114287. https://doi.org/10.1016/j.indcrop.2021.114287
Bernardo J, Gírio F, Łukasik R (2019) The effect of the chemical character of ionic liquids on biomass pre-treatment and posterior enzymatic hydrolysis. Molecules 24(4):808. https://doi.org/10.3390/molecules24040808
Assabjeu AC, Noubissié E, Desobgo SCZ, Ali A (2020) Optimization of the enzymatic hydrolysis of cellulose of triplochiton scleroxylon sawdust in view of the production of bioethanol. Sci Afr 8. https://doi.org/10.1016/j.sciaf.2020.e00438
Lin W, Chen D, Yong Q, Huang C, Huang S (2019) Improving enzymatic hydrolysis of acid-pretreated bamboo residues using amphiphilic surfactant derived from dehydroabietic acid. Biores Technol 293:122055. https://doi.org/10.1016/j.biortech.2019.122055
Funding
Funding for open access publishing: Universidad de Jaén/CBUA. The authors have no competing interests to declare that are relevant to the content of this article. In the same way, the authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Luca Corsi, Soledad Mateo, Francesco Spaccini, and Alberto J. Moya. The first draft of the manuscript was written by Alberto J. Moya and Cinzia Buratti and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics Approval and Consent to Participate and Publicate
All authors consent to participate in the publication of the manuscript and so approve it.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Corsi, L., Mateo, S., Spaccini, F. et al. Optimization of Microwave-Assisted Organosolv Pretreatment for Enzymatic Hydrolysis of Cherry Tree Pruning and Pistachio Shells: a Step to Bioethanol Production. Bioenerg. Res. 17, 294–308 (2024). https://doi.org/10.1007/s12155-023-10647-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12155-023-10647-x