Abstract
Purpose
Since the beginning of the COVID-19 pandemic, understanding the physiopathological mechanisms of its manifestations has been crucial to understand the disease and its implications. As the disease evolved, post-infection complications have arisen such as olfactory dysfunctions including parosmia in which odourants are perceived in a distorted or an unpleasant way.
Methods
In this article, we attempt to clarify these mechanisms and the role of human nasal epithelium in the development of post-COVID-19 parosmia.
Results
The mechanisms by which SARS-CoV-2 generates olfactory dysfunction have not been elucidated, and multiple theories have been proposed pointing to the sustentacular cells of the olfactory epithelium as the main probable target of the virus.
Conclusion
Establishing the main physiopathological mechanism of post-COVID-19 parosmia will set a path for further investigations and determine treatment and preventive options for patients who have been reported to be extensively affected in multiple aspects of their lives such as eating habits and mental health.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The coronavirus disease-19 (COVID-19) caused by the novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection was declared a pandemic in March 2020. When the infection was spread worldwide, olfactory dysfunction (OD) symptoms were spotted as a common condition in the absence of evident nasal blockage [1]. Smell is a special and vital sense with the olfactory receptor genes representing up to 1% of the mammalian genome. The ODs had been overlooked until the COVID-19 pandemic brought these to the focus along with their functional and emotional impact, although before the pandemic, the ODs prevailed in up to 23% of the population [2], [3]. An estimated 13.3 million people in the United States had previously reported that they were affected by some form of OD [4]. The prevalence of OD in COVID-19 patients is estimated to be 25–40% months after the onset of the disease and 15–28% by 6 months [1], [3]. Olfaction plays a key role in a person’s life, from the interpersonal aspects of human interaction to the survival of the individual. Parosmia directly affects the quality of life of patients as it disrupts daily life activities, such as eating, gardening, or social gatherings, causing distress and affecting not only their body and mental health but also vital functions that warn against potential hazards such as fires, leaking gas, or spoiled food. And it has also been linked to disruption of learning and memory processes [2], [4], [5].
Anatomy and Physiology of Smell
Olfaction is the process in which odour molecules or volatile compounds interact with bipolar olfactory sensory neurons (OSNs) at the olfactory epithelium (OE) in the nasal cavity. These odourants stimuli are then delivered to the first cranial nerve and finally to the glomerular layer of the olfactory bulb (OB), which differentiates, enhances detection, filters background odours, and receives inputs from the brain. There are about 350 types of receptors for odours, and the human brain interprets smell by following a combination of different signals [4], [6,7,8].
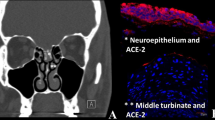
The OE is located in the olfactory cleft and is mainly composed of sustentacular cells, OSNs, microvillar cells, basal cells, and olfactory gland cells (Fig. 1) [9,10,11].
In the middle layer of the OE, around 25 million neuronal cell bodies of the primary OSNs are located with their dendrites extending to the apical surface; from this, 10–30 immotile cilia emerge that increase the surface area for odour interaction and generation of an action potential via G receptors to activate odourant transmission. On the apical layer, the sustentacular cells, that is, non-neural supporting cells enriched with cytochrome P450 family enzymes lie enclosing the cilia and contributing to the formation of mucus for odour interaction; detoxification; glucose, salt, and water balance. These cells also extend basally, regulating the basal cells in charge of replacing mature epithelial cells and maintaining the epithelium structure. The globose basal cells replace neurons in a continuous turnover throughout life, representing a reserve of stem cells along with horizontal basal cells that are activated by epithelial damage to repair or regenerate the olfactory cells by the action of neuroblasts from the subgranular zone of the dentate gyrus. Further, the mucus layer produced by Bowman’s glands is rich in odourant binding proteins (OBPs) that carry hydrophobic odourants to reversibly bind with the cilia of OSN [4], [9,10,11,12,13,14,15].
The OE undergoes neurogenesis to establish new connections with the OB; however, this process can be disrupted by environmental influence. For this restoration process, it is necessary that the glomeruli in the OB preserve their topography [6]. Rodent models have shown that Notch signalling is necessary for biotransformation enzyme expression in sustentacular cells; when this is disrupted, some degree of OSN death is observed. In addition, induced inflammation by the overexpression of tumour necrosis factor alpha (TNFɑ) and interleukin-6 (IL-6) in mouse models generate OD, suggesting that sustained inflammation can lead to OSN death impairing the functions of basal cells and epithelium regeneration [4], [8], [14], [16].
Parosmia
Parosmia is defined as a distorted smell perception in the presence of a familiar odour source or a fluctuant and reproducible qualitative OD in which the odours are perceived in a distorted or an unpleasant way [5], [17]. It has been described as a sequela of COVID-19 disease with an average onset of 3 months after initial SARS-CoV-2 infection and has been commonly linked to quantitative OD resolution, such as anosmia, in 57.1% of the cases or presented without any smell alteration in the course of the disease [2], [16], [18], [19]. It has been previously reported that some cases of OD can persist for up to 10 years, although a 12–24 month observation period is sufficient for chronic olfactory impairment to be classified as permanent. In addition, it has been recognized as a sign of recovery or a predictor of complete recovery in COVID-19 disease [3], [20].
According to a study by Raad et al., the primary triggers of unpleasant stimuli reported include coffee, citrus fruits, chicken, meat, onion, egg, garlic, rice, cucumber, tomato, fish, vegetables, nuts, dairy, pasta, soy, and even toothpaste [16], [21]. Studies have pointed out that the worst triggers are coffee, onion, meat, and fried foods; these have been linked due to their similar pathways that send out pyrazines and sulphur compounds after the Maillard reaction generated during its cooking, eliciting the ‘parosmia-like’ smell referred by respondents in a study by Parker et al. It has also been studied that the volatile profile of onion and garlic produces thiols and disulphides that trigger distortion. Moreover, the respondents in the study referred to a consistent metallic taste and nose and throat burn that exacerbated the problem. Some patients also reported sensitivity to particular molecules in fusion as some compounds tend to stand out generating an intense perception or a process of masking, thereby making some odourants more intense in the presence of other odourants [3].
In a study by Parker et al., some odourants were considered pleasant in spite of the instauration of parosmia, particularly rose in 50%, apple in 31%, and butter in 29% of respondents. It is hypothesized that partial regeneration of OSNs add up to higher or lower odourant thresholds with compounds or odourants presenting at concentrations closer than usual and changing the balance presenting the distortion [3].
Parosmia has been found to compromise mood, eating habits, and danger detection disrupting the patient’s health and social life as most experiences are disrupted by distorted smells. Despite a large proportion of patients recovering the sense of smell within weeks, it has been reported that 10% present persisting problems. The impact constitutes an altered relationship with appetite, weight changes, and people, thus impacting their mental health and linking parosmia to depression as daily routines and life are disturbed. This also leads to the inability of patients to describe the sensation or perceptions to others, causing notable frustration [3], [17], [18].
SARS-CoV-2 Viral Mechanism
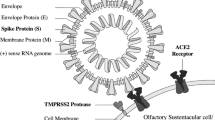
The causative agent of COVID-19, SARS-CoV-2, is a single-stranded RNA virus known to enter cells using the angiotensin-converting enzyme II (ACE2) as a receptor for binding to target cells and the co-factor enzyme transmembrane protease serine 2 (TMPRSS2). The entry of SARS-CoV-2 into the target cell is also mediated by spike (S) protein, a trimeric glycoprotein located in the viral capsid and made up of two subunits S1 and S2. The process depends on the binding of S1 surface unit to the S protein receptor of the host cell previously activated by ACE2. The S protein priming by TMPRSS2 protease lyses S1/S2 and S2’ sites in a process driven by the S2 subunit that allows viral and cellular membrane fusion. An alternate endosomal mechanism has been proposed where ACE2 binding is followed by virus internalization mediated by clathrin and pH-dependent cysteine protease cathepsin L [10], [14], [22,23,24]. Consequently, SARS-CoV-2 requires the joint expression of S protein receptor and proteases for proteolytic activation to cause infection [14].
The entry of SARS-CoV-2 activates the innate immune system by activating the pattern recognition receptors through toll-like receptor 7 (TLR7) or TLR3 with the generation of a viral replication phase with direct viral tissue damage; its extent determines the secondary phase where effector immune cells are recruited causing a local or systemic response that persists even after viral clearance. The generation of long-lasting symptoms has been related to chronic inflammation by persistent virus or viral antigens in tissues and unrepaired tissue damage. Patients presenting persistent alterations have shown an increase in IL-6, TNFɑ, and IL-1ß [25].
Prevalence
The prevalence of ODs such as parosmia has been studied broadly. A 434-patient survey conducted by Walker et al. reported that 43.1% of patients presented parosmia 6 months after their COVID-19 infection, which was supported by a study by Boscolo et al., Ohla et al. (longitudinal survey) and Parker et al., (prevalence of parosmia in the same time-frame of 6 months) [1,2,3], [5]. In addition, in a study by Parker et al., it was reported that 21% of patients lost smell suddenly, which was the primary symptom of the disease [3].
The longitudinal survey conducted by Ohla et al. also showed that the prevalence of parosmia rose from 10% during their baseline survey on patients with smell loss to 47% at follow-up, thus being more frequent in patients who reported < 80% smell recovery at follow-up, representing over 7 million patients worldwide [1].
As measured by Hopkins et al. study, the prevalence of parosmia after 6 months was found in 43.1% of people. In a study conducted by Schambeck et al., a large percentage discrepancy suggested that some patients may not be aware or have adapted to their altered sense of smell, hinting a large number of patients could present an OD [26], [27].
The prevalence of OD in a study by Callejón-Leblic et al. decreased from 82.4 to 45.1% in a 12-month follow-up of patients infected with the first SARS-CoV-2 variant between March and April 2020 in the South of Spain. This study also revealed a high dysfunction in the acute phase of the infection with an improvement in the course of the first year with persistence in varying recovery times and parosmia representing a prevalent long-lasting symptom after COVID-19 infection [27].
Damage Mechanism
The pathogenesis of parosmia after COVID-19 infection has not been elucidated, promoting the subsequent generation of encountered theories suggesting diverse disruptions in the olfactory pathway – a central deficit within brain integration centres or peripheral damage where alteration of OE formulates an incomplete stimuli transduction. The peripheral theory suggests that parosmia develops at the ephaptic neuronal transmission along with smell loss or incomplete characterization of the odourant as the neurons recover in late-onset cases (Fig. 2) [19], [28].
Central Mechanism
This shows the viral entry through the nose by a neurotropic effect onto the OE and advancing through the olfactory nerve into the central nervous system (CNS), resulting in neuronal damage by the immune and inflammatory response. Bryche et al. assay suggested that ACE2 receptors were expressed abundantly in the neurons of the OB with no viral detection after Syrian golden hamster inoculation that included the olfactory nerve bundles. In other experiments of SARS-CoV-2 inoculation in K18-hACE2, transgenic mice showed a 60-hour delay between inoculation and detection of the virus in the OB, therefore suggesting that viral replication occurs and accumulates in the OE before reaching the OB. Further, imaging studies such as magnetic resonance imaging have shown OB oedema and hyperintensity of olfactory tracts contrasting with the 18-Fluorodeoxyglucose positron emission tomography study, showing preserved activity in the secondary olfactory cortex in a patient who presented parosmia after anosmia recovery secondary to COVID-19 disease. As an alternate pathway, the trigeminal nerve forms a route of transmission to the CNS as its endings innervate the OE and branches to reach the OB [14], [20], [24], [29], [30].
The proposed pathways of SARS-CoV-2 CNS entrance are axonal flow and axonal travel within ensheathing or transneuronal cells. On the other hand Neuropilin-1 (NRP-1) was detected in the mitral cells of OB, and as it expresses ACE2 and TMPRSS2 probably allowing SARS-CoV-2 entry, consequently working as a viral retrograde axonal transport facilitator, also causing interference to NRP-1 interaction with semaphorine-3 A (SEMA3A), an essential protein for neuronal development, therefore generating axonal damage and neuronal death perpetuating OD. Another viral entry proposed involves pericytes as a SARS-CoV-2 target causing blood–brain barrier disruption secondary to interleukin-1 (IL-1), IL-6, IL-12, interferon-y (INF-y), and TNFɑ release with microvascular damage following neurotoxicity and hypoperfusion to the OSNs, therefore generating neuronal death and causing OD [8], [11], [12], [20], [31]. Despite these theories, the presence of ACE and TMPRSS2 in human neurons, the key SARS-CoV-2 entry factors, has not been confirmed suggesting that direct infection of neuronal cells is unlikely or has to be further investigated [8], [11], [12], [15], [24], [26], [30], [32].
Peripheral Mechanism
The first proposed mechanism suggested that the inflammation of the olfactory cleft caused a mechanical obstruction of the airflow, generating a conductive disorder, not allowing the odourants to reach the OE, develo** a conductive loss, and not allowing the odours to be perceived in the right form. Although through the course of investigation imaging studies have shown a narrowing of the cleft in CT scans, this showed resolution after the infection course, rejecting the mechanism as the cause of persistent OD or parosmia after COVID-19 infection [8], [9], [20].
Another mechanism proposes that parosmia is generated secondary to an “inflammatory cycle” developed after the binding between SARS-Cov-2 and ACE2 in the OE with an unbalanced immune response followed by the release of cytokines, such as IL-6, thereby activating apoptotic pathways through the release of TNFɑ, promoting T-cell mediated inflammation, and inhibiting the sense of smell at the onset of COVID-19 as the initial viral insult. The presence of apoptotic mechanisms leads to the release of more cytokines and inflammatory factors, thus provoking inflammatory maintenance. This inflammation when prolonged can damage the OSNs and nasal stem cells impacting the perception of odours and also leading to disorganization, scarring, and atrophy of the OE. Therefore, the marked reduction of density and receptors explains the prolonged or inaccurate recovery in the OE, leading to parosmia [6], [9], [10], [14], [16].
In addition to the proposed mechanism, numerous ionized calcium-binding adapter molecule 1 (Iba1+) cells have been found in the olfactory mucosa of SARS-CoV-2-infected patients, suggesting that the infection is associated with olfactory mucosal inflammation and altered olfaction by an immune-cell mediated destruction as Iba1 + serves as a marker for monocyte/macrophage in the mucosa of infected animals. The presence of cleaved caspase-3 in infected and uninfected cells has also been detected, showing that infection induces cell death in the olfactory neuroepithelium [15], [29].
The rapid onset of OD in SARS-CoV-2 infection supports the theory that suggests the virus interaction with olfactory cell populations is the most probable entry pathway. As previously described, the SARS-CoV-2 virus and its S protein interact with ACE2 as a mediator of viral entry along with the action of TMPRSS2. Single-cell RNA sequencing, immunostaining, and hamster model assays have shown that human nasal sustentacular cells, horizontal basal cells, and Bowman’s gland cells co-express the ACE2 receptor and TMPRSS2 enzyme necessary for viral entrance; moreover, the virus has also been isolated in these cells by human autopsy and histology studies without finding the presence of ACE2 or TMPRSS2 in mature OSNs pointing to the sustentacular cells as the main probable viral target for OD development [13]. The recent study conducted by Schambeck et al. measuring antibody titres alongside the OD course also points the source to the supporting cells and pericytes after localized inflammation and cytokine release was measured that resulted in neuronal dysfunction, with the persistence being suggestive of secondary damage to the OSNs after sustentacular cell damage hence provoking a reduction of its nutrients, water supply, and denuding of OSN cilia. Thus, we can infer that the disruption of homeostasis and regeneration of ORNs in OE lead to OD [4], [10,11,12], [16], [24], [26], [28], [29], [33,34,35,36].
According to the mechanism previously described involving the sustentacular cells targeted by SARS-CoV-2 followed by cytokine and apoptotic factors release and secondary epithelium and OSNs damage, parosmia may develop as an aberrant neuronal regeneration secondary to the recovery from the initial viral insult, as the OE regeneration process is rarely complete after severe viral insults that generate a patchy and thin epithelium structure on metaplastic squamous epithelia causing immune cell infiltration, decreased cilia, reduced OSN, and sustentacular cells, thereby leading to alteration of genome organization of olfactory receptor clusters in OSNs and generating a delayed restoration originating prevalent OD [5], [13,14,15,16], [37].
Evidence suggests that SARS-CoV-2 virus targets the OE sustentacular cells that express the necessary receptors for viral entry. A recent study showed that supporting cells in mouse OE express TLR3, consequently activating NF-kB and triggering events of the innate immune response and recruiting leukocytes along with the epithelium’s macrophages and a dysregulated inflammatory reaction that cause the downregulation of OSN expression and its signals [2], [11], [14], [20].
The mismatch in the rewiring of OB during neurogenesis secondary to the SARS-CoV-2 infection of the basal stem cells and the diverse OSN recovery time-frames and altered expression may generate a cross-wiring between the regenerating OSNs and the glomeruli or OB, therefore generating the OD [1], [3], [6], [11], [16]. The neuronal damage in the neuroepithelium and OB can also alter the receptor-specific targets and generate aberrant patterns and dysfunction as the unresolved inflammation of OB does not recover the original functions [6], [16].
Pathophysiological mechanisms proposed for parosmia. The mechanisms proposed are divided in central advocating to the SARS-CoV-2 neurotropism after nasal entry via axonal flow or travel or trigeminal nerve as an alternative pathway for its direct relation with olfactory epithelium and olfactory bulb and pericytes infection with blood-brain barrier disruption terminating in neuronal damage secondary to central nervous system (CNS) invasion and peripheral focusing on the sustentacular cells of the olfactory epithelium as the main viral target releasing cytokines such as IL-6 and TNFɑ, therefore causing an indirect damage to OSN altering stimuli transduction and interpretation
Treatment
Even though the pathophysiological mechanism involved in parosmia development remains unclear, olfactory training is the predominant intervention applied after its onset. Topical intranasal corticosteroids have been trialled with little evidence along with vitamin A, as retinoic acid has an effect on the regulation of neurogenesis and regeneration, along with supplements of alpha-lipoic acid and omega-3 fatty acids [2], [4], [8], [10]. Other off-label therapies that have been used include sodium valproate, gabapentin, pregabalin, zinc gluconate, intranasal insulin, minocycline, vitamin B, and caroverin [5], [10].
As stem cell activation is altered, the use of platelet-rich plasma (PRP) as anti-inflammatory and pro-regenerative has been proposed by upregulating growth factors such as transforming growth factor and vascular endothelial growth factor to diminish neurodegeneration [16].
Conclusion
Parosmia is a prevalent post-viral OD that affects patients who had SARS-CoV-2 infection after their recovery. Doctors should not overlook this as it impacts multiple aspects of patients’ lives such as eating, mental health, and social life. Research is ongoing and much remains to be studied; however, currently, diverse pathophysiological mechanisms have been proposed to explain parosmia development with neuroinvasion being less supported in spite of neurotropism, atrophy, and hypometabolism being reported, as the direct link between the virus and neurons has not been established, therefore pointing their damage to be indirect and associated to alterations in functions at the OE level, from which inflammation and damage to the enclosed sustentacular cells and olfactory receptor neurons and their secondary abnormal regeneration and decreased expression are the most supported, not rejecting the likelihood of a mechanism not mutually exclusive, combining alterations at different levels of the olfactory pathway. Further work with mechanism models is needed to understand how diverse molecules generate distortions in the integration of odourant perception to assess preventive measures and an adequate evidence-based treatment, therefore improving the prognosis for patients with this condition. On the contrary, studies with larger samples are also needed to completely elucidate the prevalence and impact of parosmia, and multiple questions remain unanswered as the role of immune infiltration in the OE, epithelium desquamation process, whether alternative viral entry pathways are plausible as NRP1, and if persistent olfactory impairment could be a marker of a long-term increase in neurodegenerative disorders opening multiple opportunities for investigation in the future as ODs progress in time.
References
Ohla K, Veldhuizen M, Green T et al (Apr 2022) A follow-up on quantitative and qualitative olfactory dysfunction and other symptoms in patients recovering from COVID-19 smell loss. Rhinology J 60(6):207–217. https://doi.org/10.4193/RHIN21.415
Boscolo-Rizzo P, Polesel J, Vaira LA (2022) Smell and taste dysfunction after covid-19. BMJ BMJ Publishing Group. https://doi.org/10.1136/bmj.o1653
Parker JK, Methven L, Pellegrino R, Smith BC, Gane S, Kelly CE (Apr 2022) Emerging pattern of post-COVID-19 parosmia and its effect on food perception. Foods 11(7). https://doi.org/10.3390/foods11070967
Choi R, Gupta R, Finlay JB, Goldstein BJ (2022) “Olfactory dysfunction and COVID-19,” Operative Techniques in Otolaryngology - Head and Neck Surgery, vol. 33, no. 2, pp. 141–146, Jun https://doi.org/10.1016/j.otot.2022.04.010
Walker A, Kelly C, Pottinger G, Hopkins C (Apr 2022) Parosmia—a common consequence of covid-19. BMJ 377. https://doi.org/10.1136/BMJ-2021-069860
di Stadio A, D’Ascanio L, la Mantia I, Ralli M, Brenner MJ (2022) Parosmia after COVID-19: olfactory training, neuroinflammation and distortions of smell. Eur Rev Med Pharmacol Sci 26(1):1–3. https://doi.org/10.26355/eurrev_202201_27739
Saniasiaya J, Narayanan P (2022) “Parosmia post COVID-19: an unpleasant manifestation of long COVID syndrome,” Postgraduate Medical Journal, vol. 98, no. e2, p. 1, https://doi.org/10.1136/postgradmedj-2021-139855
Mastrangelo A, Bonato M, Cinque P (Mar 2021) Smell and taste disorders in COVID-19: from pathogenesis to clinical features and outcomes. Neurosci Lett 748. https://doi.org/10.1016/j.neulet.2021.135694
Najafloo R, Majidi J, Asghari A et al (2021) Mechanism of anosmia caused by symptoms of COVID-19 and emerging treatments. ACS Chem Neurosci 12:3795–3805. no. 20, American Chemical Society https://doi.org/10.1021/acschemneuro.1c00477
Othman BA, Maulud S, Jalal P et al (2022) “Olfactory dysfunction as a post-infectious symptom of SARS-CoV-2 infection,” Annals of Medicine and Surgery, vol. 75. Elsevier Ltd, Mar 01, https://doi.org/10.1016/j.amsu.2022.103352
Cooper KW, Brann D, Farruggia M et al (2020) “COVID-19 and the chemical senses: supporting players take center stage,” Neuron, vol. 107, no. 2, Cell Press, pp. 219–233, Jul 22, https://doi.org/10.1016/j.neuron.2020.06.032
Wei G, Gu J, Gu Z et al (2022) Olfactory dysfunction in patients with coronavirus disease 2019: a review. Front Neurol 12. Frontiers Media S.A.https://doi.org/10.3389/fneur.2021.783249
Meunier N, Briand L, Jacquin-Piques A, Brondel L, Pénicaud L (2021) COVID 19-induced smell and taste impairments: putative impact on physiology. Front Physiol 11. Frontiers Media S.A.https://doi.org/10.3389/fphys.2020.625110
Glezer I, Bruni-Cardoso A, Schechtman D, Malnic B (2021) Viral infection and smell loss: the case of COVID-19. J Neurochem 157(4):930–943. John Wiley and Sons Inc https://doi.org/10.1111/jnc.15197
Xu W, Sunavala-Dossabhoy G, Spielman AI (2022) Chemosensory loss in COVID-19. ” Oral Diseases. John Wiley and Sons Inc. https://doi.org/10.1111/odi.14300.
Karamali K, Elliott M, Hopkins C (2022) “COVID-19 related olfactory dysfunction. ” Current opinion in Otolaryngology and Head and Neck surgery, vol 30. Lippincott Williams and Wilkins, pp 19–25. 1 https://doi.org/10.1097/MOO.0000000000000783.
Watson DLB, Campbell M, Hopkins C, Smith B, Kelly C, Deary V (Sep 2021) Altered smell and taste: anosmia, parosmia and the impact of long Covid-19. PLoS ONE 16(9). https://doi.org/10.1371/journal.pone.0256998
Olofsson JK, Ekesten F, Nordin S (Dec 2022) Olfactory distortions in the general population. Sci Rep 12(1). https://doi.org/10.1038/s41598-022-13201-5
Lerner DK, Garvey K, Arrighi-Allisan A et al (Mar 2022) Clinical features of parosmia associated with COVID-19 infection. Laryngoscope 132(3):633–639. https://doi.org/10.1002/lary.29982
Xydakis MS, Albers M, Holbrook E et al (2021) Post-viral effects of COVID-19 in the olfactory system and their implications. Lancet Neurol 20(01):753–761. no. 9, Lancet Publishing Group https://doi.org/10.1016/S1474-4422(21)00182-4
Raad N, Ghorbani J, Safavi Naeini A, Tajik N, Karimi-Galougahi M (Oct 2021) Parosmia in patients with COVID-19 and olfactory dysfunction. Int Forum Allergy Rhinology 11(10):1497–1500. https://doi.org/10.1002/alr.22818
Hoffmann M, Kleine-Weber H, Schroeder S et al (Apr 2020) SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 181(2):271. https://doi.org/10.1016/J.CELL.2020.02.052
Zhou P, Yang X, Wang X et al (Mar 2020) A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 579(7798):270–273. https://doi.org/10.1038/S41586-020-2012-7
Araújo L, Arata V, Figueiredo RG (Sep 2021) Olfactory disorders in post-acute COVID-19 syndrome. Sinusitis 5(2):116–122. https://doi.org/10.3390/sinusitis5020012
Merad M, Blish CA, Sallusto F, Iwasaki A “The immunology and immunopathology of COVID-19” [Online]. Available: https://www.science.org
Schambeck SE, Crowell CS, Wagner KI et al (Nov 2021) Phantosmia, parosmia, and dysgeusia are prolonged and late-onset symptoms of covid-19. J Clin Med 10(22). https://doi.org/10.3390/jcm10225266
Callejón-Leblic MA, Martín-Jiménez D, Moreno-Luna R et al (2022) “Analysis of prevalence and predictive factors of long-lasting olfactory and gustatory dysfunction in COVID-19 patients,” Life, vol. 12, no. 8, https://doi.org/10.3390/life12081256
Lee JC, Nallani R, Cass L, Bhalla V, Chiu AG, Villwock JA (May 2021) A systematic review of the neuropathologic findings of post-viral olfactory dysfunction: implications and novel insight for the COVID-19 pandemic. Am J Rhinol Allergy 35(3):323–333. https://doi.org/10.1177/1945892420957853
de Melo GD, Lazarini F, Levallois S et al (Jun 2021) COVID-19-related anosmia is associated with viral persistence and inflammation in human olfactory epithelium and brain infection in hamsters. Sci Transl Med 13(596):8396. https://doi.org/10.1126/SCITRANSLMED.ABF8396/SUPPL_FILE/ABF8396_SM.PDF
Ylikoski J, Markkanen M, Mäkitie A (2020) “Pathophysiology of the COVID-19–entry to the CNS through the nose,” Acta Oto-Laryngologica, vol. 140, no. 10, Taylor and Francis Ltd., pp. 886–889, Oct 01, https://doi.org/10.1080/00016489.2020.1773533
Khan S, Gomes J (2020) “Neuropathogenesis of SARS-CoV-2 infection,” Elife, vol. 9, pp. 1–9, Jul https://doi.org/10.7554/eLife.59136
Bilinska K, Butowt R (2020) Anosmia in COVID-19: a bumpy road to establishing a cellular mechanism. ACS Chem Neurosci 11(05):2152–2155. no. 15, American Chemical Society https://doi.org/10.1021/acschemneuro.0c00406
Hou YJ, Okuda K, Edwards C et al (Jul 2020) SARS-CoV-2 reverse genetics reveals a variable infection gradient in the respiratory tract. Cell 182(2):429–446 no. e14. https://doi.org/10.1016/J.CELL.2020.05.042/ATTACHMENT/36CE948B-608F-445B-99E9-C5EB06ACEBAB/MMC1.PDF
Sungnak W, Huang N, Bécavin C et al (Apr 2020) SARS-CoV-2 entry factors are highly expressed in nasal epithelial cells together with innate immune genes. Nat Med 26(5):681–687. https://doi.org/10.1038/s41591-020-0868-6
Brann DH, Tsukahara T, Weinreb C et al (Jul 2020) Non-neuronal expression of SARS-CoV-2 entry genes in the olfactory system suggests mechanisms underlying COVID-19-associated anosmia. Sci Adv 6(31):5801–5832. https://doi.org/10.1126/SCIADV.ABC5801/SUPPL_FILE/PAPV4.PDF
Bryche B, St Albin A, Murri S et al (Oct 2020) Massive transient damage of the olfactory epithelium associated with infection of sustentacular cells by SARS-CoV-2 in golden syrian hamsters. Brain Behav Immun 89:579–586. https://doi.org/10.1016/j.bbi.2020.06.032
Doty RL “The mechanisms of smell loss after SARS-CoV-2 infection,”The Lancet Neurology, vol. 20, no. 9, Lancet Publishing Group, pp.693–695, Sep 01, 2021, https://doi.org/10.1016/S1474-4422(21)00202-7
Author information
Authors and Affiliations
Contributions
AXP, JLM, JOF and JPO planned the Rapid Review. AXP, JLM, JOF and JPO contributed to the literature search. All authors wrote and revised the first draft. AXP and JLM: Conceptualization, Data Curation, Visualization, Writing - review & editing, designed and made the figures. All authors revised the manuscript on the basis of editorial and reviewer comments. All authors reviewed and approved the final draft of the manuscript.
Corresponding author
Ethics declarations
Declarations
We declare that there are no financial or non-financial interests directly or indirectly related to our work, and no funding was received in assisting with the preparation of this manuscript. All authors certify that they have no affiliations or involvement with any organization in the subject matter or materials discussed in the manuscript.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Andrea, XP., Joceline, LM., Jose, OF. et al. Human Nasal Epithelium Damage as the Probable Mechanism Involved in the Development of Post-COVID-19 Parosmia. Indian J Otolaryngol Head Neck Surg 75 (Suppl 1), 458–464 (2023). https://doi.org/10.1007/s12070-023-03559-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12070-023-03559-x