Abstract
Cucurbit[n]urils (CB[n], n = 5–8) are the most important class of host molecules that are widely applied in various important applications. Until now, no sensitive sensor has been developed exclusively for detecting cucurbiturils. Here, we have utilized the supramolecular assembly of acriflavine and graphene oxide (ACF-GO) as a fluorescence sensor for the detection of cucurbituril family members such as CB[5], CB[6] and CB[7] with the discrimination of fluorescence intensities. Among them, CB[7] displayed the highest sensitivity, which can be detected as low as nano-molar concentration, and that allowed us to make a facile fluorescence method of detection for CB[7] and other CBs.
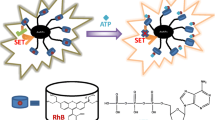
Graphical abstract
Supramolecular assembly of acriflavine on the graphene oxide turn-off the fluorescence of acriflavine and upon interaction with cucurbit[7]uril, that released the acriflavine to the solution and turn-on the fluorescence, which allowed to detect the CB[7] to the lowest of nano-molar concentration.










Similar content being viewed by others
References
Lee J W, Samal S, Selvapalam N, Kim H J and Kim K 2003 Cucurbituril homologues and derivatives: new opportunities in supramolecular chemistry Acc. Chem. Res. 36 621
Flinn A, Hough G C, Stoddart J F and Williams D J 1992 Decamethylcucurbit[5]uril Angew. Chem. Int. Ed. 31 1475
Isobe H, Sato S and Nakamura E 2002 Synthesis of disubstituted cucurbit[6]uril and its rotaxane derivative Org. Lett. 4 1287
Stancl A, Svec J and Sindelar V 2011 Novel supramolecular hosts based on linear and cyclic oligomers of glycoluril Isr. J. Chem. 51 592
Miyahara Y, Goto K, Oka M and Inazu T 2004 Remarkably facile ring-size control in macrocyclization: synthesis of hemicucurbit[6]uril and hemicucurbit[12]uril Angew. Chem. Int. Ed. 43 5019
Kim K, Selvapalam N, Ko Y H, Park K M, Kim D and Kim J 2007 Functionalized cucurbiturils and their applications Chem. Soc. Rev. 36 267
Barrow S J, Kasera S, Rowland M J, del Barrio J and Scherman O A 2015 Cucurbituril-based molecular recognition Chem. Rev. 115 12320
Cao L, Šekutor M, Zavalij P Y, Mlinarić-Majerski K, Glaser R and Isaacs L 2014 Cucurbit[7]uril⋅guest pair with an attomolar dissociation constant Angew. Chem. Int. Ed. 53 988
Cheng G, Luo J, Liu Y, Chen X, Wu Z and Chen T 2020 Cucurbituril-oriented nanoplatforms in biomedical applications ACS Appl. Bio Mater. 12 8211
Quan J, Zhang X, Ding Y, Li S, Qiu Y, Wang R and Zhou X 2021 Cucurbit[7]uril as a broad-spectrum antiviral agent against diverse RNA viruses Virol. Sin. 36 1165
Jon S Y, Selvapalam N, Oh D H, Kang J K, Kim S Y, Jeon Y J, et al. 2003 Facile synthesis of cucurbit[n]uril derivatives via direct functionalization: expanding utilization of cucurbit[n]uril J. Am. Chem. Soc. 125 10186
Kim E, Kim D, Jung H, Lee J, Paul S, Selvapalam N, et al. 2010 Facile, template-free synthesis of stimuli-responsive polymer nanocapsules for targeted drug delivery Angew. Chem. Int. Ed. 26 4405
Baek K, Xu D, Murray J, Kim S and Kim K 2016 Permselective 2D-polymer-based membrane tuneable by host-guest chemistry Chem. Commun. 52 9676
Pemberton B C, Singh R K, Johnson A C, Jockusch S, Da Silva J P, Ugrinov A, et al. 2011 Supramolecular photocatalysis: insights into cucurbit[8]uril catalyzed photodimerization of 6-methylcoumarin Chem. Commun. 47 6323
Lee T C and Scherman O A 2010 Formation of dynamic aggregates in water by cucurbit[5]uril capped with gold nanoparticles Chem. Commun. 46 2438
Prakash R, Usha G, Piramuthu L and Selvapalam N 2017 Facile detection of cucurbit[7]uril by rhodamine B-decorated nanoparticles Chem. Lett. 46 1300
Prakash R, Usha G, Sivaranjana P, Karpagalakshmi K, Piramuthu L and Selvapalam N 2018 Graphene oxide based fluorescence sensor for cucurbit[7]uril New J. Chem. 42 13038
Usha G, Prakash R, Karpagalakshmi K, Ramalakshmi S, Piramuthu L, Yang C and Selvapalam N 2020 Supramolecular assembly of acriflavine on graphene oxide for the sensing of adenosine phosphates Anal. Sci. 36 1365
Usha G, Prakash R, Karpagalakshmi K, Ramalakshmi S, Piramuthu L, Yang C and Selvapalam N 2019 A graphene oxide-based fluorescent sensor for surfactants Anal. Methods 11 5826
Day A, Arnold A P, Blanch R J and Snushall B 2001 Controlling factors in the synthesis of cucurbituril and its homologues J. Org. Chem. 66 8094
Kim J, Jung I S, Kim S Y, Lee E, Kang J K, Sakamoto S, et al. 2000 New cucurbituril homologues: syntheses, isolation, characterization, and X-ray crystal structures of cucurbit[n]uril (n = 5, 7, and 8) J. Am. Chem. Soc. 122 540
Li D, He X, Zhao L, Li H, Zhang X, Chen J, et al. 2022 Ultrafast charge transfer dynamics of Rhodamine B with graphene oxide J. Chem. Phys. 157 214701
Nau W M and Mohanty J Taming fluorescent dyes with cucurbituril Int. J. Photoenergy 7 133
Montes-Navajas P, Corma A and Garcia H 2008 Complexation and fluorescence of tricyclic basic dyes encapsulated in cucurbiturils ChemPhysChem 9 713
Kemp S, Wheate N J, Stootman F H and Aldrich-Wright J R 2007 The host-guest chemistry of proflavine with cucurbit[6,7,8]urils Supramol. Chem. 19 475
Acknowledgements
This work is financially supported by SERB, India, under the Early Career Research Award scheme (ECR/2015/000318). KK and RP thanks KARE for offering a University PhD fellowship. GU thanks the SERB for funding her research. All authors thank Dr S Shasi Anand, the Vice President of KARE, for constant research support and encouragement.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Karpagalakshmi, K., Prakash, R., Usha, G. et al. A nano-molar sensitive fluorescence sensor for cucurbit[7]uril. J Chem Sci 136, 43 (2024). https://doi.org/10.1007/s12039-024-02285-3
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12039-024-02285-3