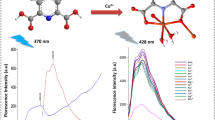
Abstract
Among the various essential trace elements for living organisms, the copper (Cu2+) ions are the most important. However, Cu2+ ions are vital for the human body and are associated with necessary physiological processes; insufficient or excessiveness has many hazardous effects on our bodies. In the present contribution, strategically, we have introduced a julolidine-coupled azine-based, 9,9'-((1E,1'E)-hydrazine-1,2-diylidene bis(methanylylidene)) bis(1,2,3,5,6,7-hexahydropyrido [3,2,1-ij] quinolin-8-ol) (HDBQ) reversible chromo-fluorogenic probe for specific detection of Cu2+ ions. Probe HDBQ exhibits observable orange colorimetric change from yellow, which is visible to the naked eye in daylight. The highly green fluorescence HDBQ becomes a non-fluorescent one with the incorporation of Cu2+ ions. Interestingly, the colorimetric change and non-fluorescent HDBQ-Cu2+ complex reverse to the original HDBQ in the presence of ethylenediamine tetraacetic acid (EDTA). The detection and quantification limit of HDBQ towards the detection of Cu2+ ions is found to be in the µM range, which is much lower than the limit (31.5 µM) recommended by WHO. We have also performed a colorimetric and fluorometric paper-based test strips-based experiment employing HDBQ for real-time on-site detection of Cu2+ ions. Using the reversibility characteristics of HDBQ for the consecutive addition of Cu2+ and EDTA, we have established the INHIBIT molecular logic gate. The present report brings a precise and sensitive probe for the detection of Cu2+ ions in real environmental and biological samples.















Similar content being viewed by others
Data Availability
No datasets were generated or analysed during the current study.
References
Lai CY, Trewyn BG, Jeftinija DM et al (2003) A mesoporous silica nanosphere-based carrier system with chemically removable CdS nanoparticle caps for stimuli-responsive controlled release of neurotransmitters and drug molecules. J Am Chem Soc 125:4451–4459. https://doi.org/10.1021/JA028650L/SUPPL_FILE/JA028650LSI20030120_115112.PDF
Sessler JL, Davis JM (2001) Sapphyrins: Versatile anion binding agents. Acc Chem Res 34:989–997. https://doi.org/10.1021/AR980117G/ASSET/IMAGES/LARGE/AR980117GF00009.JPEG
Prodi L (2005) Luminescent chemosensors: from molecules to nanoparticles. New J Chem 29:20–31. https://doi.org/10.1039/B411758A
Liu L, Dong X, **ao Y et al (2011) Two-photon excited fluorescent chemosensor for homogeneous determination of copper(II) in aqueous media and complicated biological matrix. Analyst 136:2139–2145. https://doi.org/10.1039/C0AN00933D
Huang J, Xu Y, Qian X (2009) A red-shift colorimetric and fluorescent sensor for Cu2+ in aqueous solution: unsymmetrical 4,5-diaminonaphthalimide with N-H deprotonation induced by metal ions. Org Biomol Chem 7:1299–1303. https://doi.org/10.1039/B818611A
Klein G, Kaufmann D, Schürch S, Reymond JL (2001) A fluorescent metal sensor based on macrocyclic chelation. Chem Commun 561–562. https://doi.org/10.1039/B100535I
Gunnlaugsson T, Glynn M, Tocci (née Hussey) GM et al (2006) Anion recognition and sensing in organic and aqueous media using luminescent and colorimetric sensors. Coord Chem Rev 250:3094–3117. https://doi.org/10.1016/J.CCR.2006.08.017
McDonald AJ, Dibble JP, Evans EGB, Millhauser GL (2014) A New Paradigm for Enzymatic Control of α-Cleavage and β-Cleavage of the Prion Protein. J Biol Chem 289:803–813. https://doi.org/10.1074/JBC.M113.502351
**ao G, Fan Q, Wang X, Zhou B (2013) Huntington disease arises from a combinatory toxicity of polyglutamine and copper binding. Proc Natl Acad Sci U S A 110:14995–15000. https://doi.org/10.1073/PNAS.1308535110/SUPPL_FILE/PNAS.201308535SI.PDF
Wang X, Wang X, Guo Z (2018) Metal-involved theranostics: An emerging strategy for fighting Alzheimer’s disease. Coord Chem Rev 362:72–84. https://doi.org/10.1016/J.CCR.2018.03.010
Huster D, Lutsenko S (2007) Wilson disease: not just a copper disorder. Analysis of a Wilson disease model demonstrates the link between copper and lipid metabolism. Mol Biosyst 3:816–824. https://doi.org/10.1039/B711118P
Jung HS, Kwon PS, Lee JW et al (2009) Coumarin-derived cu2+-selective fluorescence sensor: Synthesis, mechanisms, and applications in living cells. J Am Chem Soc 131:2008–2012. https://doi.org/10.1021/JA808611D/SUPPL_FILE/JA808611D_SI_002.PDF
Merian E (1991) Metals and their compounds in the environment: Occurrence, analysis, and biological relevance
Forzani ES, Zhang H, Chen W, Tao N (2005) Detection of heavy metal ions in drinking water using a high-resolution differential surface plasmon resonance sensor. Environ Sci Technol 39:1257–1262. https://doi.org/10.1021/ES049234Z/SUPPL_FILE/ES049234ZSI20040825_125944.PDF
Soylak M, Narin I, Dogan M (1997) Trace Enrichment and Atomic Absorption Spectrometric Determination of Lead, Copper, Cadmium and Nickel in Drinking Water Samples by Use of an Activated Carbon Column. Anal Lett 30:2801–2810. https://doi.org/10.1080/00032719708001823
Ali A, Shen H, Yin X (1998) Simultaneous determination of trace amounts of nickel, copper and mercury by liquid chromatography coupled with flow-injection online derivatization and preconcentration. Anal Chim Acta 369:215–223. https://doi.org/10.1016/S0003-2670(98)00252-9
Liu Y, Liang P, Guo L (2005) Nanometer titanium dioxide immobilized on silica gel as sorbent for preconcentration of metal ions prior to their determination by inductively coupled plasma atomic emission spectrometry. Talanta 68:25–30. https://doi.org/10.1016/J.TALANTA.2005.04.035
Bobrowski A, Nowak K, Zarębski J (2005) Application of a bismuth film electrode to the voltammetric determination of trace iron using a Fe(III)-TEA-BrO 3- Catalytic system. Anal Bioanal Chem 382:1691–1697. https://doi.org/10.1007/S00216-005-3313-2/FIGURES/6
Valeur B, Berberan-Santos MN (2012) Molecular Fluorescence: Principles and Applications, Second Edition. Mol Fluoresc Princ Appl Second Ed. https://doi.org/10.1002/9783527650002
Mondal B, Kumar P, Ghosh P, Kalita A (2011) Fluorescence-based detection of nitric oxide in aqueous and methanol media using a copper(II) complex. Chem Commun 47:2964–2966. https://doi.org/10.1039/C0CC04054A
Yin S, Leen V, Van SS et al (2010) A highly sensitive, selective, colorimetric and near-infrared fluorescent turn-on chemosensor for Cu2+ based on BODIPY. Chem Commun 46:6329–6331. https://doi.org/10.1039/C0CC01772H
Jiao L, Li J, Zhang S et al (2009) A selective fluorescent sensor for imaging Cu2+ in living cells. New J Chem 33:1888–1893. https://doi.org/10.1039/B906441A
Qazi MA, Qureshi I, Memon S (2010) A highly copper selective chromogenic calix[4]arene derivative. New J Chem 34:2579–2586. https://doi.org/10.1039/C0NJ00396D
Hu L, Wang H, Fang B et al (2017) A reversible two-photon fluorescence probe for Cu(II) based on Schiff-base in HEPES buffer and in vivo imaging. Sensors Actuators B Chem 251:993–1000. https://doi.org/10.1016/J.SNB.2017.05.140
He X, **e Q, Fan J et al (2020) Dual-functional chemosensor with colorimetric/ratiometric response to Cu(II)/Zn(II) ions and its applications in bioimaging and molecular logic gates. Dye Pigment 177:108255. https://doi.org/10.1016/J.DYEPIG.2020.108255
Chandrasekhar V, Bag P, Pandey MD (2009) Phosphorus-supported multidentate coumarin-containing fluorescence sensors for Cu2+. Tetrahedron 65:9876–9883. https://doi.org/10.1016/J.TET.2009.09.040
Duan YW, Tang HY, Guo Y et al (2014) The synthesis and study of the fluorescent probe for sensing Cu2+ based on a novel coumarin Schiff-base. Chinese Chem Lett 25:1082–1086. https://doi.org/10.1016/J.CCLET.2014.05.001
Feng S, Gao Q, Gao X et al (2019) Fluorescent sensor for copper(II) ions based on coumarin derivative and its application in cell imaging. Inorg Chem Commun 102:51–56. https://doi.org/10.1016/J.INOCHE.2019.01.012
Bhorge YR, Tsai HT, Huang KF et al (2014) A new pyrene-based Schiff-base: A selective colorimetric and fluorescent chemosensor for detection of Cu(II) and Fe(III). Spectrochim Acta Part A Mol Biomol Spectrosc 130:7–12. https://doi.org/10.1016/J.SAA.2014.03.110
Ganguly A, Ghosh S, Kar S, Guchhait N (2015) Selective fluorescence sensing of Cu(II) and Zn(II) using a simple Schiff base ligand: Naked eye detection and elucidation of photoinduced electron transfer (PET) mechanism. Spectrochim Acta Part A Mol Biomol Spectrosc 143:72–80. https://doi.org/10.1016/J.SAA.2015.02.013
Akarasareenon W, Chanmungkalakul S, **aogang L, Rashatasakhon P (2023) Selective fluorescent sensors for copper(II) ion from julolidine hydrazone derivatives. J Photochem Photobiol A Chem 437:114422. https://doi.org/10.1016/J.JPHOTOCHEM.2022.114422
Pungut NAS, Heng MP, Saad HM et al (2021) From one to three, modifications of sensing behavior with solvent system: DFT calculations and real-life application in detection of multianalytes (Cu2+, Ni2+ and Co2+) based on a colorimetric Schiff base probe. J Mol Struct 1238:130453. https://doi.org/10.1016/J.MOLSTRUC.2021.130453
Chan WC, Saad HM, Sim KS et al (2022) A rapid turn-on dual functional rhodamine B probe for aluminum (III) and copper (II) that can be utilised as a molecular logic gate and in water analysis. J Mol Struct 1254:132337. https://doi.org/10.1016/J.MOLSTRUC.2022.132337
Wang Q, Lv H, Ding F et al (2021) Multifunctional chemosensor for tracing Ga(III), hypochlorite and pH change with bioimaging in living cells, Pseudomonas aeruginosa and Zebra fish. Sensors Actuators B Chem 345:130346. https://doi.org/10.1016/J.SNB.2021.130346
He X, Zheng Z, Zhang F et al (2020) Mitochondria-Targeted Chemosensor to Discriminately and Continuously Visualize HClO and H2S with Multiresponse Fluorescence Signals for in Vitro and in Vivo Bioimaging. ACS Appl Bio Mater 3:7886–7897. https://doi.org/10.1021/ACSABM.0C01029/ASSET/IMAGES/LARGE/MT0C01029_0008.JPEG
Okoye COB, Ugwu JN (2010) Impact of environmental cadmium, lead, copper and zinc on quality of goat meat in Nigeria. Bull Chem Soc Ethiop 24:133–138. https://doi.org/10.4314/bcse.v24i1.52975
Pettinari C, Tăbăcaru A, Galli S (2016) Coordination polymers and metal–organic frameworks based on poly(pyrazole)-containing ligands. Coord Chem Rev 307:1–31. https://doi.org/10.1016/J.CCR.2015.08.005
Klingele J, Dechert S, Meyer F (2009) Polynuclear transition metal complexes of metal⋯metal-bridging compartmental pyrazolate ligands. Coord Chem Rev 253:2698–2741. https://doi.org/10.1016/J.CCR.2009.03.026
Castro I, Barros WP, Calatayud ML et al (2016) Dicopper(II) pyrazolenophanes: Ligand effects on their structures and magnetic properties. Coord Chem Rev 315:135–152. https://doi.org/10.1016/J.CCR.2016.02.004
Dou Z, Yu J, Cui Y et al (2014) Luminescent metal-organic framework films as highly sensitive and fast-response oxygen sensors. J Am Chem Soc 136:5527–5530. https://doi.org/10.1021/JA411224J/SUPPL_FILE/JA411224J_SI_001.PDF
Reichardt C (1994) Solvatochromic dyes as solvent polarity indicators. Chem Rev 94:2319–2358. https://doi.org/10.1021/CR00032A005/ASSET/CR00032A005.FP.PNG_V03
Acknowledgements
MNR is highly indebted to DST-FIST, New Delhi, India, for instrumental support. MM and SA are thankful to the government of West Bengal, India, for providing them with the Swami Vivekananda Meritcum-Means Scholarship.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
Authors Arpita Maiti, Sabbir Ahamed and Manas Mahato wrote the main manuscript, prepared figures tables, and conceptualized it. Authors Vikas Kumar Dakua, Kanak Roy and Tanusree Ray reviewed all things.
Corresponding author
Ethics declarations
Ethical Approval
Authors have nothing to declare.
Ethics Statement
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Conflicts of Interest
There are no conflicts of interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Maiti, A., Ahamed, S., Mahato, M. et al. A Julolidine Coupled Azine-based Reversible Chromo-fluorogenic Probe for Specific Detection of Cu2+ Ions. J Fluoresc (2024). https://doi.org/10.1007/s10895-023-03577-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10895-023-03577-6