Abstract
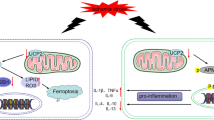
Nociceptive sensitization is accompanied by the upregulation of glycolysis in the central nervous system in neuropathic pain. Growing evidence has demonstrated glycolysis and angiogenesis to be related to the inflammatory processes. This study investigated whether fumagillin inhibits neuropathic pain by regulating glycolysis and angiogenesis. Fumagillin was administered through an intrathecal catheter implanted in rats with chronic constriction injury (CCI) of the sciatic nerve. Nociceptive, behavioral, and immunohistochemical analyses were performed to evaluate the effects of the inhibition of spinal glycolysis-related enzymes and angiogenic factors on CCI-induced neuropathic pain. Fumagillin reduced CCI-induced thermal hyperalgesia and mechanical allodynia from postoperative days (POD) 7 to 14. The expression of angiogenic factors, vascular endothelial growth factor (VEGF) and angiopoietin 2 (ANG2), increased in the ipsilateral lumbar spinal cord dorsal horn (SCDH) following CCI. The glycolysis-related enzymes, pyruvate kinase M2 (PKM2) and lactate dehydrogenase A (LDHA) significantly increased in the ipsilateral lumbar SCDH following CCI on POD 7 and 14 compared to those in the control rats. Double immunofluorescence staining indicated that VEGF and PKM2 were predominantly expressed in the astrocytes, whereas ANG2 and LDHA were predominantly expressed in the neurons. Intrathecal infusion of fumagillin significantly reduced the expression of angiogenic factors and glycolytic enzymes upregulated by CCI. The expression of hypoxia-inducible factor-1α (HIF-1α), a crucial transcription factor that regulates angiogenesis and glycolysis, was also upregulated after CCI and inhibited by fumagillin. We concluded that intrathecal fumagillin may reduce the expression of ANG2 and LDHA in neurons and VEGF and PKM2 in the astrocytes of the SCDH, further attenuating spinal angiogenesis in neuropathy-induced nociceptive sensitization. Hence, fumagillin may play a role in the inhibition of peripheral neuropathy-induced neuropathic pain by modulating glycolysis and angiogenesis.








Similar content being viewed by others
Data Availability
The data that support the findings of this study are available on request from the corresponding author.
References
Dahlhamer J, Lucas J, Zelaya C, Nahin R, Mackey S, DeBar L, Kerns R, Von Korff M et al (2018) Prevalence of chronic pain and high-impact chronic pain among adults - United States, 2016 MMWR Morb Mortal Wkly Rep 67(36):1001–1006
van Hecke O, Austin S, Khan RA, Smith BH, Torrance N (2014) Neuropathic pain in the general population: a systematic review of epidemiological studies. Pain 155(4):654–662
Alles SRA, Smith P (2018) Etiology and pharmacology of neuropathic pain. Pharmacol Rev 70(2):315–347
Finnerup NB, Attal N, Haroutounian S, McNicol E, Baron R, Dworkin RH, Gilron I, Haanpää M et al (2015) Pharmacotherapy for neuropathic pain in adults: a systematic review and meta-analysis. Lancet Neurol 14(2):162–173
Weng HR, Taing K, Chen L, Penney A (2023) EZH2 methyltransferase regulates neuroinflammation and neuropathic pain. Cells 12(7):1058
Wen ZH, Huang S, Kuo HM, Chen CT, Chen NF, Chen WF, Tsui KH et al (2021) Fumagillin attenuates spinal angiogenesis, neuroinflammation, and pain in neuropathic rats after chronic constriction injury. Biomedicines 9(9):1187
Afridi R, Kim J, Rahman MH, Suk K (2020) Metabolic regulation of glial phenotypes: implications in neuron-glia interactions and neurological disorders. Front Cell Neurosci 14:20
Martínez-Cuesta MÁ, Blanch-Ruiz M, Ortega-Luna R, Sánchez-López A, Álvarez Á (2020) Structural and functional basis for understanding the biological significance of P2X7 receptor. Int J Mol Sci 21(22):8454
Brandes RP, Rezende F (2021) Glycolysis and inflammation: partners in crime! Circ Res 129(1):30–32
Victor VM, Nuñez C, D’Ocón P, Taylor CT, Esplugues JV, Moncada S (2009) Regulation of oxygen distribution in tissues by endothelial nitric oxide. Circ Res 104:1178–1183
Doyle TM, Salvemini D (2021) Mini-review: mitochondrial dysfunction and chemotherapy-induced neuropathic pain. Neurosci Lett 760:136087
Lim TK, Rone M, Lee S, Antel JP, Zhang J (2015) Mitochondrial and bioenergetics dysfunction in trauma-induced painful peripheral neuropathy. Mol Pain 11:58
Lewis JE, Gilmour K, Moorhead MJ, Perry SF, Markham MR (2014) Action potential energetics at the organismal level reveal a trade-off in efficiency at high firing rates. J Neurosci 34(1):197–201
Wang B, Liu S, Fan B, Xu X, Chen Y, Lu R, Xu Z, Liu X (2018) PKM2 is involved in neuropathic pain by regulating ERK and STAT3 activation in rat spinal cord. J Headache Pain 19(1):7
Ludman T, Melemedjian O (2019) Bortezomib-induced aerobic glycolysis contributes to chemotherapy-induced painful peripheral neuropathy. Mol Pain 15:1744806919837429
Melkonian EA, Schury MP (2022) Biochemistry, Anaerobic Glycolysis In: StatPearls, Treasure Island (FL): StatPearls Publishing; 2024 Jan
Soto-Heredero G, Gómez de Las Heras MM, Gabandé-Rodríguez E, Oller J, Mittelbrunn M (2020) Glycolysis - a key player in the inflammatory response. Febs j 287(16):3350–3369
Valvona CJ et al (2016) The regulation and function of lactate dehydrogenase A: therapeutic potential in brain tumor. Brain Pathol 26(1):3–17
Yang W, Lu Z (2015) Pyruvate kinase M2 at a glance. J Cell Sci 128:1655–1660
Jafary F, Ganjalikhany M, Moradi A, Hemati M, Jafari S (2019) Novel peptide inhibitors for lactate dehydrogenase A (LDHA): a survey to inhibit LDHA activity via disruption of protein-protein interaction. Sci Rep 9(1):4686
Laughton JD, Charnay Y, Belloir B, Pellerin L, Magistretti PJ, Bouras C (2000) Differential messenger RNA distribution of lactate dehydrogenase LDH-1 and LDH-5 isoforms in the rat brain. Neuroscience 96(3):619–625
Damasceno LEA, Prado DS, Veras FP, Fonseca MM, Toller-Kawahisa JE, Rosa MH, Públio GA, Martins TV, et al. (2020) PKM2 promotes Th17 cell differentiation and autoimmune inflammation by fine-tuning STAT3 activation. J Exp Med, 217(10).
Zhao Y, Xu H (2022) Microglial lactate metabolism as a potential therapeutic target for Alzheimer’s disease. Mol Neurodegener 17(1):36
Azoitei N, Becher A, Steinestel K, Rouhi A, Diepold K, Genze F, Simmet T, Seufferlein T (2016) PKM2 promotes tumor angiogenesis by regulating HIF-1α through NF-κB activation. Mol Cancer 15:3
Lin H, Muramatso R, Maedera N, Tsunematsu H, Hamaguchi M, Koyama Y, Kuroda M, Ono K et al (2018) Extracellular lactate dehydrogenase a release from damaged neurons drives central nervous system angiogenesis. EBioMedicine 27:71–85
Sung C-S, Cheng H-J, Chen N-F, Tang S-H, Kuo H-M, Sung P-J, Chen W-F, Wen Z-H (2023) Antinociceptive effects of aaptamine, a sponge component, on peripheral neuropathy in rats. Mar Drugs 21(2):113
Dudley A, Griffioen A (2005) Pathological angiogenesis: mechanisms and therapeutic strategies. Angiogenesis 26(3):313–347
Lapel M, Weston P, Strassheim D, Karoor V, Burns N, Lyubchenko T, Paucek P, Stenmark K, Gerasimovskaya E (2017) Glycolysis and oxidative phosphorylation are essential for purinergic receptor-mediated angiogenic responses in vasa vasorum endothelial cells. Am J Physiol Cell Physiol 312(1):C56–C70
Jackson JR, Seed M, Kircher CH, Willoughby DA, Winkler JD (1997) The codependence of angiogenesis and chronic inflammation. FASEB J 11:457–465
Costa C, Incio J, Soares R (2007) Angiogenesis and chronic inflammation: cause or consequence? Angiogenesis 10:149–166
Chae S-S, Kamoun WS, Farrar CT, Kirkpatrick ND, Niemeyer E, de Graff AMA, Sorensen AG, Munn LL, Jain RK, Fukumura D (2010) Angiopoietin-2 interferes with anti-VEGFR2-induced vessel normalization and survival benefit in mice bearing gliomas. Clin Cancer Res 16:3618–3627
Holash J, Maisonpierre PC, Compton D, Boland P, Alexander CR, Zagzag D, Yancopoulos GD, Wiegand SJ (1999) Vessel cooption, regression, and growth in tumors mediated by angiopoietins and VEGF. Science 284(5422):1994–1998
Hashizume H, Falcón BL, Kuroda T, Baluk P, Coxon A, Yu D, Bready JV, Oliner JD, McDonald DM (2010) Complementary actions of inhibitors of angiopoietin-2 and VEGF on tumor angiogenesis and growth. Cancer Res 70:2213–2223
Greenberg DA, ** K (2005) From angiogenesis to neuropathology. Nature 438:954–959
Lefkove B, Govindarajan B, Arbiser JL (2007) Fumagillin: an anti-infective as a parent molecule for novel angiogenesis inhibitors. Expert Rev Anti Infect Ther 5(4):573–579
Pan D, Sanyal N, Schmieder AH, Senpan A, Kim B, Yang X, Hu G, Allen JS et al (2012) Antiangiogenic nanotherapy with lipase-labile Sn-2 fumagillin prodrug. Nanomedicine (Lond) 7(10):1507–1519
Zhou HF, Yan H, Hu Y, Springer LE, Yang X, Wickline SA, Pan D, Lanza GM et al (2014) Fumagillin prodrug nanotherapy suppresses macrophage inflammatory response via endothelial nitric oxide. ACS Nano 8(7):7305–7317
Vatte S, Ugale R (2023) HIF-1, an important regulator in potential new therapeutic approaches to ischemic stroke. Neurochem Int 170:105605
Xu X, Yang M, Zhang B, Dong J, Zhuang Y, Ge Q, Niu F, Liu B (2023) HIF-1α participates in secondary brain injury through regulating neuroinflammation. Transl Neurosci 14(1):20220272
Kierans SJ, Taylor CT (2021) Regulation of glycolysis by the hypoxia-inducible factor (HIF): implications for cellular physiology. J Physiol 599(1):23–37
Hsieh YL, Chou LW, Chang PL, Yang CC, Kao MJ, Hong CZ (2012) Low-level laser therapy alleviates neuropathic pain and promotes function recovery in rats with chronic constriction injury: possible involvements in hypoxia-inducible factor 1α (HIF-1α). J Comp Neurol 520(13):2903–2916
He J, Qin Z, Chen X, He W, Li D, Zhang L, Le Y, **ong Q et al (2022) HIF-1α ameliorates diabetic neuropathic pain via parkin-mediated mitophagy in a mouse model. Biomed Res Int 16:5274375
Lin YC, Huang SY, Jean YH, Chen WF, Sung CS, Kao ES, Wang HM, Chakraborty C et al (2011) Intrathecal lemnalol, a natural marine compound obtained from formosan soft coral, attenuates nociceptive responses and the activity of spinal glial cells in neuropathic rats. Behav. Pharmacol 22:739–750
Bennett GJ, **e YK (1988) A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain 33:87–107
Huang SY, Sung C, Chen WF, Chen CH, Feng CW, Yang SN, Hung HC, Chen NF, Lin PR et al (2015) Involvement of phosphatase and tensin homolog deleted from chromosome 10 in rodent model of neuropathic pain. J Neuroinflammation 12:59
Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL (1994) Quantitative assessment of tactile allodynia in the rat paw. J. Neurosci 53:55–63
Manders EMM, Verbeek FJ, Aten JA (1993) Measurement of co-localization of objects in dual-colour confocal images. J Microsc 169(3):375–382
Sung CS, Cherng CH, Wen ZH, Chang WK, Huang SY, Lin SL, Chan KH, Wong CS (2012) Minocycline and fluorocitrate suppress spinal nociceptive signaling in intrathecal IL-1beta-induced thermal hyperalgesic rats. Glia 60:2004–2007
Kiguchi N, Kobayashi Y, Kadowaki Y, Fukazawa Y, Saika F, Kishioka S (2014) Vascular endothelial growth factor signaling in injured nerves underlies peripheral sensitization in neuropathic pain. J Neurochem 129(1):169–178
Breier G (2000) Functions of the VEGF/VEGF receptor system in the vascular system. Semin Thromb Hemost 26:553–559
Carmeliet P (2005) Angiogenesis in life, disease and medicine. Nature 438:932–936
Seabrook TJ, Littlewood-Evans A, Brinkmann V, Pollinger B, Schnell C, Hiestand PC (2010) Angiogenesis is present in experimental autoimmune encephalomyelitis and pro-angiogenic factors are increased in multiple sclerosis lesions. J. Neuroinflamm 7:95
Johnson EA, Guignet M, Dao TL, Hamilton TA, Kan RK (2015) Interleukin-18 expression increases in response to neurovascular damage following soman-induced status epilepticus in rats. J. Inflamm 12:43
Gantenbein AR, Sándor PS (2006) Physiological parameters as biomarkers migraine. Headache 46:1069–1074
Montoro CI, Duschek S, de Guevara CM, Reyes Del Paso GA (2016) Patterns of cerebral blood flow modulation during painful stimulation in fibromyalgia: a transcranial doppler sonography study. Pain Med 17:2256–2267
Kusumbe AP, Ramasamy S, Adams RH (2014) Coupling of angiogenesis and osteogenesis by a specific vessel subtype in bone. Nature 507:323–328
Kamenaga T, Kuroda Y, Nagai K, Tsubosaka M, Takashima Y, Kikuchi K, Fujita M, Ikuta K et al (2021) Cryopreserved human adipose-derived stromal vascular fraction maintains fracture healing potential via angiogenesis and osteogenesis in an immunodeficient rat model. Stem Cell Res Ther 12:110
Veith AP, Henderson K, Spencer A, Sligar AD, Baker AB (2019) Therapeutic strategies for enhancing angiogenesis in wound healing. Adv Drug Deliv Rev 146:97–125
Tas SW, Maracle C, Balogh E, Szekanecz Z (2016) Targeting of proangiogenic signalling pathways in chronic inflammation. Nat Rev Rheumatol 2016 12(2):111–122
Tsivelekas K, Evangelopoulos DS, Pallis D, Benetos IS, Papadakis SA, Vlamis J, Pneumaticos SG (2022) Angiogenesis in spinal cord injury: progress and treatment. Cureus 30(5):e25475
Cao Y, Langer R, Ferrara N (2023) Targeting angiogenesis in oncology, ophthalmology and beyond. Nat Rev Drug Discov 22:476–495
Yang Y, Torbey M (2020) Angiogenesis and blood-brain barrier permeability in vascular remodeling after stroke. Curr Neuropharmacol 18:1250–1265
Tsuji-Tamura K, Ogawa M (2018) Morphology regulation in vascular endothelial cells. Inflamm Regen 38:25
Evans CE, Iruela-Arispe M, Zhao YY (2021) Mechanisms of endothelial regeneration and vascular repair and their application to regenerative medicine. Am J Pathol 191:52–65
Apte RS, Chen D, Ferrara N (2019) VEGF in signaling and disease: beyond discovery and development. Cell 176:1248–1264
Tsuji-Tamura K, Ogawa M (2016) Inhibition of the PI3K-Akt and mTORC1 signaling pathways promotes the elongation of vascular endothelial cells. J Cell Sci 129:1165–1178
Zhang Y, Liu J, Zou T, Qi Y, Yi B, Dissanayaka WL, Zhang C (2021) DPSCs treated by TGF-β1 regulate angiogenic sprouting of three-dimensionally co-cultured HUVECs and DPSCs through VEGF-Ang-Tie2 signaling. Stem Cell Res Ther 12(1):281
Augustin HG, Koh G, Thurston G, Alitalo K (2009) Control of vascular morphogenesis and homeostasis through the angiopoietin-Tie system. Nat Rev Mol Cell Biol 10(3):165–177
Fiedler U, Reiss Y, Scharpfenecker M, Grunow V, Koidl S, Thurston G, Gale NW, Witzenrath M, Rosseau S, Suttorp N, Sobke A, Herrmann M, Preissner KT, Vajkoczy P, Augustin HG (2006) Angiopoietin-2 sensitizes endothelial cells to TNF-alpha and has a crucial role in the induction of inflammation. Nat Med 12(2):235–239
Imhof BA, Aurrand-Lions M (2006) Angiogenesis and inflammation face off. Nat Med 12(2):171–172
Kim H, Koh G (2011) Ang2, the instigator of inflammation. Blood 118(18):4767–4768
Li Z, Korhonen EA, Merlini A, Strauss J, Wihuri E, Nurmi H, Antila S, Paech J et al (2020) Angiopoietin-2 blockade ameliorates autoimmune neuroinflammation by inhibiting leukocyte recruitment into the CNS. J Clin Invest 130(4):1977–1990
Koh YJ, Kim H-Z, Hwang S-I, Lee JE, Oh N, Jung K, Kim M, Kim KE et al (2010) Double antiangiogenic protein, DAAP, targeting VEGF-A and angiopoietins in tumor angiogenesis, metastasis, and vascular leakage. Cancer Cell 18(2):171–184
Devanney NA, Stewart A, Gensel JC (2020) Microglia and macrophage metabolism in CNS injury and disease: the role of immunometabolism in neurodegeneration and neurotrauma. Exp Neurol 329:113310
Miyamoto K, Ishikura K, Kume K, Ohsawa M (2019) Astrocyte-neuron lactate shuttle sensitizes nociceptive transmission in the spinal cord. Glia 67(1):27–36
Liu P, Chen T, Tan F, Tian J, Zheng L, Deng Y, Chen J, Chi X (2020) Dexmedetomidine alleviated neuropathic pain in dorsal root ganglion neurons by inhibition of anaerobic glycolysis activity and enhancement of ROS tolerance. Biosci Rep 40(5):BSR20191994
Koukourakis MI, Giatromanolaki A, Sivridis E, Bougioukas G, Didilis V, Gatter KC, Harris AL, Tumour and Angiogenesis Research Group (2003) Lactate dehydrogenase-5 (LDH-5) overexpression in non-small-cell lung cancer tissues is linked to tumour hypoxia, angiogenic factor production and poor prognosis. Br J Cancer 89(5):877–885
Giatromanolaki A, Sivridis E, Gatter KC, Turley H, Harris AL, Koukourakis MI, Angiogenesis Research Group (2006) Lactate dehydrogenase 5 (LDH-5) expression in endometrial cancer relates to the activated VEGF/VEGFR2(KDR) pathway and prognosis. Gynecol Oncol 103(3):912–918
Liu Y, Guo J, Liu Y, Wang K, Ding W, Wang H, Liu X, Zhou S, Lu XC, Yang HB, Xu C, Gao W, Zhou L et al (2018) Nuclear lactate dehydrogenase A senses ROS to produce α-hydroxybutyrate for HPV-induced cervical tumor growth. Nat Commun 9(1):4429
Teixeira-Santos L, Albino-Teixeira A, Pinho D (2020) Neuroinflammation, oxidative stress and their interplay in neuropathic pain: focus on specialized pro-resolving mediators and NADPH oxidase inhibitors as potential therapeutic strategies. Pharmacol Res 162:105280
Dai CQ, Guo Y, Chu XY (2020) Neuropathic pain: the dysfunction of Drp1, mitochondria, and ROS homeostasis. Neurotox Res 38(3):553–563
Hirschhaeuser F, Sattler U, Mueller-Klieser W (2011) Lactate: a metabolic key player in cancer. Cancer Res 71(22):6921–6925
Porporato PE, Payen V, De Saedeleer CJ, Préat V, Thissen JP, Feron O, Sonveaux P (2012) Lactate stimulates angiogenesis and accelerates the healing of superficial and ischemic wounds in mice. Angiogenesis 2012 15(4):581–592
Yuen TJ, Silbereis JC, Griveau A, Chang SM, Daneman R, Fancy SPJ, Zahed H, Maltepe E et al (2014) Oligodendrocyte-encoded HIF function couples postnatal myelination and white matter angiogenesis. Cell 158(2):383–396
Guruceaga X, Perez-Cuesta U, Abad-Diaz de Cerio A, Gonzalez O, Alonso RM, Hernando FL, Ramirez-Garcia A, Rementeria A (2019) Fumagillin, a mycotoxin of aspergillus fumigatus: biosynthesis, Biological activities, detection, and applications,. Toxins (Basel) 12(1)
Castro BM, Prieto M, Silva LC (2014) Ceramide: a simple sphingolipid with unique biophysical properties. Prog Lipid Res 54:53–67
Merrill AH Jr, Wang E, Vales TR, Smith ER, Schroeder JJ, Menaldino DS, Alexander C, Crane HM et al (1996) Fumonisin toxicity and sphingolipid biosynthesis. Adv Exp Med Biol 392:297–306
Riley RT, Wang E, Schroeder JJ, Smith ER, Plattner RD, Abbas H, Yoo HS, Merrill AH Jr (1996) Evidence for disruption of sphingolipid metabolism as a contributing factor in the toxicity and carcinogenicity of fumonisins. Nat Toxins 4(1):3–15
Hannun YA, Bell R (1989) Functions of sphingolipids and sphingolipid breakdown products in cellular regulation. Science 243:500–507
Veronica Kalhori KK, Asghar MY, Bergelin N, Jaakkola P (2013) Sphingosine-1-phosphate as a regulator of hypoxia-induced factor-1α in thyroid follicular carcinoma cell. PLoS One 8(6):e66189
Krock BL, Skuli N, Simon MC (2011) Hypoxia-induced angiogenesis: good and evil. Genes Cancer 2(12):1117–1133
Sanmarco LM, Rone JM, Polonio CM, Lahore GF, Giovannoni F, Ferrara K, Gutierrez-Vazquez C, Li N, Sokolovska A, Plasencia A, Akl CF, Nanda P, Heck ES, Li Z et al (2023) Lactate limits CNS autoimmunity by stabilizing HIF-1α in dendritic cells. Nature 620(7975):881–889
Pucino V, Certo M, Bulusu V, Cucchi D, Goldmann K, Pontarini E, Haas R, Smith J et al (2019) Lactate Buildup at the site of chronic inflammation promotes Disease by inducing CD4(+) T cell metabolic rewiring. Cell Metab 30(6):1055-1074e1058
Wang ZH, Peng WB, Zhang P, Yang XP, Zhou Q (2021) Lactate in the tumour microenvironment: from immune modulation to therapy. EBioMedicine 73:103627
Chaoguang Y, Pan R-Y, Guan F, Yuan Z (2024) Lactate metabolism in neurodegenerative diseases. Neural Regen Res 19(1):69–74
Qi W, Keenan HA, Li Q, Ishikado A, Kannt A, Sadowski T, Yorek MA, Wu I-H, Lockhart S et al (2017) Pyruvate kinase M2 activation may protect against the progression of diabetic glomerular pathology and mitochondrial dysfunction. Nat Med 23(6):753–762
Guo D, Gu J, Jiang H, Ahmed A, Zhang Z, Gu Y (2016) Inhibition of pyruvate kinase M2 by reactive oxygen species contributes to the development of pulmonary arterial hypertension. J Mol Cell Cardiol 91:179–187
Palsson-McDermott EM, Curtis AM, Goel G, Lauterbach MA, Sheedy FJ, Gleeson LE, van den Bosch MW, Quinn SR et al (2015) Pyruvate kinase M2 regulates Hif-1α activity and IL-1β induction and is a critical determinant of the warburg effect in LPS-activated macrophages. Cell Metab 21(1):65–80
Chen CC, Chen Y, Hsiao HY, Chang C, Chern Y (2013) Neurovascular abnormalities in brain disorders: highlights with angiogenesis and magnetic resonance imaging studies. J Biomed Sci 20:47–55
Feng Y et al (2022) Pyruvate kinase M2 (PKM2) improve symptoms of post-ischemic stroke depression by activating VEGF to mediate the MAPK/ERK pathway. Brain Behav 12(1):e2450
Dai M et al (2023) LDHA as a regulator of T cell fate and its mechanisms in disease. Biomed Pharmacother 158:114164
Kong E et al (2022) Glycometabolism reprogramming of glial cells in central nervous system: novel target for neuropathic pain. Front Immunol 13:861290
Grammas P (2011) Neurovascular dysfunction, inflammation and endothelial activation: implications for the pathogenesis of Alzheimer’s disease. J Neuroinflammation 8:26
Acknowledgements
The illustrations (Fig. 8) were created using BioRender.com.
Funding
This study was supported by National Science and Technology Council, Taiwan, 109-2314-B-075-045-MY3, and Taipei Veterans General Hospital, Taiwan, V113C-056.
Author information
Authors and Affiliations
Contributions
ZHW and CSS designed the experiments; ZHW and CSS supervised the design and course of the experiments; HJC, SYH and SHT performed the experiments; SYH, WNT, NFC, FWS and CSS performed the analyses and interpretation of the experiments; ZHW, ZSW and CSS wrote the manuscript; ZHW and CSS reviewed and edited the manuscript. All of the authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics Approval
All animal experiments were approved by the Institutional Animal Care and Use Committee of National Sun Yat-sen University (Approval No. IACUC-111-37).
Consent to Participate
Not applicable.
Consent for Publication
Consent for publication was obtained from the participants.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wen, ZH., Wu, ZS., Cheng, HJ. et al. Intrathecal Fumagillin Alleviates Chronic Neuropathy-Induced Nociceptive Sensitization and Modulates Spinal Astrocyte-Neuronal Glycolytic and Angiogenic Proteins. Mol Neurobiol (2024). https://doi.org/10.1007/s12035-024-04254-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12035-024-04254-w