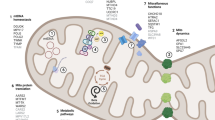
Abstract
Parkinson's disease (PD) is a prevalent and burdensome neurodegenerative disorder that has been extensively researched to understand its complex etiology, diagnosis, and treatment. The interplay between genetic and environmental factors in PD makes its pathophysiology difficult to comprehend, emphasizing the need for further investigation into genetic and epigenetic markers involved in the disease. Early diagnosis is crucial for optimal management of the disease, and the development of novel diagnostic biomarkers is ongoing. Although many efforts have been made in the field of recognition and interpretation of the mechanisms involved in the pathophysiology of the disease, the current knowledge about PD is just the tip of the iceberg. By scrutinizing genetic and epigenetic patterns underlying PD, new avenues can be opened for dissecting the pathology of the disorder, leading to more precise and efficient diagnostic and therapeutic approaches. This review emphasizes the importance of studying dysregulated cell signaling pathways and molecular processes associated with genes and epigenetic alterations in understanding PD, paving the way for the development of novel therapeutic strategies to combat this devastating disease.
Graphical Abstract

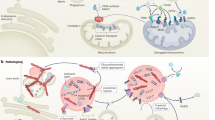
Epigenetic signaling cascade in PD. Figure was extracted from the Pathway Studio, Elsevier.




Similar content being viewed by others
References
Kalia LV, Lang AE (2015) Parkinson’s disease. Lancet 386(9996):896–912. https://doi.org/10.1016/S0140-6736(14)61393-3
Poewe W et al (2017) Parkinson disease. Nat Rev Dis Prim 3(1):1–21. https://doi.org/10.1038/nrdp.2017.13
Hipp MS, Kasturi P, Hartl FU (2019) The proteostasis network and its decline in ageing. Nat Rev Mol Cell Biol 20(7):421–435. https://doi.org/10.1038/s41580-019-0101-y
Skipper L, Liu J-J, Tan E-K (2006) Polymorphisms in candidate genes: implications for the current treatment of Parkinson’s disease. Expert Opin Pharmacother 7(7):849–855. https://doi.org/10.1517/14656566.7.7.849
Sharma A et al (2019) Common genetic variants associated with Parkinson’s disease display widespread signature of epigenetic plasticity. Sci Rep 9(1):18464. https://doi.org/10.1038/s41598-019-54865-w
Thomas B, Beal MF (2007) Parkinson’s disease. Hum Mol Genet 16(R2):R183–R194. https://doi.org/10.1093/hmg/ddm159
Puschmann A (2013) Monogenic Parkinson’s disease and parkinsonism: clinical phenotypes and frequencies of known mutations. Parkinsonism Relat Disord 19(4):407–415. https://doi.org/10.1016/j.parkreldis.2013.01.020
Schulte C, Gasser T (2011) Genetic basis of Parkinson’s disease: inheritance, penetrance, and expression. Appl Clin Genet:67–80. https://doi.org/10.2147/TACG.S11639
Smith L, Schapira AH (2022) GBA variants and Parkinson disease: mechanisms and treatments. Cells 11(8):1261. https://doi.org/10.3390/cells11081261
Gan-Or Z et al (2008) Genotype-phenotype correlations between GBA mutations and Parkinson disease risk and onset. Neurology 70(24):2277–2283. https://doi.org/10.1212/01.wnl.0000304039.11891.29
Sidransky E, Lopez G (2012) The link between the GBA gene and parkinsonism. The Lancet Neurol 11(11):986–998. https://doi.org/10.1016/S1474-4422(12)70190-4
Pedersen CC et al (2021) A systematic review of associations between common SNCA variants and clinical heterogeneity in Parkinson’s disease. npj. Parkinson's Dis 7(1):54. https://doi.org/10.1038/s41531-021-00196-5
Chittoor-Vinod VG, Nichols RJ, Schüle B (2021) Genetic and environmental factors influence the pleomorphy of LRRK2 parkinsonism. Int J Mol Sci 22(3):1045. https://doi.org/10.3390/ijms22031045
Lucchini RG et al (2020) Metal exposure and SNCA rs356219 polymorphism associated with Parkinson disease and parkinsonism. Front Neurol 11:556337. https://doi.org/10.3389/fneur.2020.556337
Chen B et al (2019) Interactions between iron and α-synuclein pathology in Parkinson’s disease. Free Radic Biol Med 141:253–260. https://doi.org/10.1016/j.freeradbiomed.2019.06.024
Yılmazer S et al (2021) Low Levels of LRRK2 Gene expression are associated with LRRK2 SNPs and contribute to Parkinson’s disease progression. Neuro Mol Med 23:292–304. https://doi.org/10.1007/s12017-020-08619-x
Lake J et al (2022) Coding and noncoding variation in LRRK2 and Parkinson’s disease risk. Mov Disord 37(1):95–105. https://doi.org/10.1002/mds.28787
Guadagnolo D et al (2021) Genotype-phenotype correlations in monogenic Parkinson disease: a review on clinical and molecular findings. Front Neurol 12:648588. https://doi.org/10.3389/fneur.2021.648588
Cilia R et al (2014) LRRK2 mutations in Parkinson’s disease: confirmation of a gender effect in the Italian population. Parkinsonism Relat Disord 20(8):911–914. https://doi.org/10.1016/j.parkreldis.2014.04.016
Jia R et al (2023) The relationship between iron and LRRK2 in a 6-OHDA-induced Parkinson’s disease model. Int J Mol Sci 24(4):3709. https://doi.org/10.3390/ijms24043709
Wu Y-R et al (2020) Rare VPS35 A320V variant in taiwanese Parkinson’s disease indicates disrupted CI-MPR sorting and impaired mitochondrial morphology. Brain Sci 10(11):783. https://doi.org/10.3390/brainsci10110783
Zimprich A et al (2011) A mutation in VPS35, encoding a subunit of the retromer complex, causes late-onset Parkinson disease. Am J Hum Genet 89(1):168–175. https://doi.org/10.1016/j.ajhg.2011.06.008
Lesage S et al (2012) Identification of VPS35 mutations replicated in French families with Parkinson disease. Neurology 78(18):1449–1450. https://doi.org/10.1212/WNL.0b013e318253d5f2
Shiner T et al (2021) The effect of GBA mutations and APOE polymorphisms on dementia with Lewy bodies in Ashkenazi Jews. J Alzheimers Dis 80(3):1221–1229. https://doi.org/10.3233/JAD-201295
Mallett V et al (2016) GBA p. T369M substitution in Parkinson disease: polymorphism or association? A meta-analysis. Neurology. Genetics 2(5). https://doi.org/10.1212/NXG.0000000000000104
Davis MY et al (2016) Association of GBA mutations and the E326K polymorphism with motor and cognitive progression in Parkinson disease. JAMA Neurol 73(10):1217–1224. https://doi.org/10.1001/jamaneurol.2016.2245
Ortega RA et al (2022) Differences in sex-specific frequency of glucocerebrosidase variant carriers and familial parkinsonism. Mov Disord 37(11):2217–2225. https://doi.org/10.1002/mds.29197
Ton ND et al (2020) Rare and novel variants of PRKN and PINK1 genes in Vietnamese patients with early-onset Parkinson’s disease. Mol Genet Genomic Med 8(10):e1463. https://doi.org/10.1002/mgg3.1463
Castelo Rueda MP et al (2021) Frequency of heterozygous parkin (PRKN) variants and penetrance of Parkinson’s disease risk markers in the population-based CHRIS cohort. Front Neurol 12:706145. https://doi.org/10.3389/fneur.2021.706145
Zhu W et al (2022) Heterozygous PRKN mutations are common but do not increase the risk of Parkinson’s disease. Brain 145(6):2077–2091. https://doi.org/10.1093/brain/awab456
Hayashida A et al (2021) The identified clinical features of Parkinson’s disease in homo-, heterozygous and digenic variants of PINK1. Neurobiol Aging 97:146. e1–146. e13. https://doi.org/10.1016/j.neurobiolaging.2020.06.017
Liu J et al (2020) Association between a DJ-1 polymorphism and the risk of Parkinson’s disease: a PRISMA-compliant systematic review and meta-analysis. J Int Med Res 48(8):0300060520947943. https://doi.org/10.1177/0300060520947943
Abou-Sleiman PM et al (2003) The role of pathogenic DJ-1 mutations in Parkinson’s disease. Ann Neurol 54(3):283–286. https://doi.org/10.1002/ana.10675
De Marco E et al (2010) DJ-1 is a Parkinson’s disease susceptibility gene in southern Italy. Clin Genet 77(2):183–188. https://doi.org/10.1111/j.1399-0004.2009.01310.x
Vilariño-Güell C et al (2009) ATP13A2 variability in Parkinson disease. Hum Mutat 30(3):406–410. https://doi.org/10.1002/humu.20877
Lin C et al (2008) Novel ATP13A2 variant associated with Parkinson disease in Taiwan and Singapore. Neurology 71(21):1727–1732. https://doi.org/10.1212/01.wnl.0000335167.72412.68
Marsili L, Mahajan A (2022) Clinical milestones in Parkinson’s disease: past, present, and future. J Neurol Sci:432. https://doi.org/10.1016/j.jns.2021.120082
Findley LJ (2007) The economic impact of Parkinson’s disease. Parkinsonism Relat Disord 13:S8–S12. https://doi.org/10.1016/j.parkreldis.2007.06.003
Seppi K et al (2019) Update on treatments for nonmotor symptoms of Parkinson’s disease—an evidence-based medicine review. Mov Disord 34(2):180–198. https://doi.org/10.1002/mds.27602
Henderson MX, Trojanowski JQ, Lee VM-Y (2019) α-Synuclein pathology in Parkinson’s disease and related α-synucleinopathies. Neurosci Lett 709:134316. https://doi.org/10.1016/j.neulet.2019.134316
Spehlmann R, Stahl SM (1976) Dopamine acetylcholine imbalance in Parkinson’s disease. Possible regenerative overgrowth of cholinergic axon terminals. Lancet 1(7962):724–726. https://doi.org/10.1016/s0140-6736(76)93095-6
Kassubek J (2014) Diagnostic procedures during the course of Parkinson’s disease. Basal Ganglia 4(1):15–18. https://doi.org/10.1016/j.baga.2014.02.001
Papuć E, Rejdak K (2020) Increased CSF NFL in non-demented Parkinson’s disease subjects reflects early white matter damage. Frontiers in Aging. Neuroscience:12. https://doi.org/10.3389/fnagi.2020.00128
Tolosa E et al (2021) Challenges in the diagnosis of Parkinson’s disease. The Lancet Neurol 20(5):385–397. https://doi.org/10.1016/S1474-4422(21)00030-2
Armstrong MJ, Okun MS (2020) Diagnosis and treatment of Parkinson disease: a review. Jama 323(6):548–560. https://doi.org/10.1001/jama.2019.22360
Tönges L et al (2022) Blood-based biomarker in Parkinson’s disease: potential for future applications in clinical research and practice. J Neural Transm (Vienna) 129(9):1201–1217. https://doi.org/10.1007/s00702-022-02498-1
Sabaei M et al (2023) Salivary levels of disease-related biomarkers in the early stages of Parkinson’s and Alzheimer’s disease: a cross-sectional study. IBRO Neurosci Rep 14:285–292. https://doi.org/10.1016/j.ibneur.2023.03.004
Van Heesbeen H, Smidt MPJF (2019) Entanglement of genetics and epigenetics in Parkinson’s disease. Front Neurosci 13:277. https://doi.org/10.3389/fnins.2019.00277
Cherubini M, Wade-Martins R (2018) Convergent pathways in Parkinson’s disease. Cell Tissue Res 373:79–90. https://doi.org/10.1007/s00441-017-2700-2
Klemann CJ et al (2017) Integrated molecular landscape of Parkinson’s disease. npj. Parkinson's Dis 3(1):14. https://doi.org/10.1038/s41531-017-0015-3
Cai Y et al (2021) The neurodevelopmental role of dopaminergic signaling in neurological disorders. Neurosci Lett 741:135540. https://doi.org/10.1016/j.neulet.2020.135540
Emamzadeh FN, Surguchov A (2018) Parkinson’s disease: biomarkers, treatment, and risk factors. Front Neurosci 12:612. https://doi.org/10.3389/fnins.2018.00612
Mishra A, Singh S, Shukla S (2018) Physiological and functional basis of dopamine receptors and their role in neurogenesis: possible implication for Parkinson’s disease. J Exp Neurosci 12:1179069518779829. https://doi.org/10.1177/1179069518779829
Blauwendraat C, Nalls MA, Singleton AB (2020) The genetic architecture of Parkinson’s disease. The Lancet Neurol 19(2):170–178. https://doi.org/10.1016/S1474-4422(19)30287-X
Hisahara S, Shimohama S (2011) Dopamine receptors and Parkinson’s disease. Int J Med Chem 2011. https://doi.org/10.1155/2011/403039
Majidinia M et al (2016) The roles of non-coding RNAs in Parkinson’s disease. Mol Biol Rep 43:1193–1204. https://doi.org/10.1007/s11033-016-4054-3
Alkanli N, Ay A (2019) The relationship between alpha-synuclein (SNCA) gene polymorphisms and development risk of Parkinson’s disease. In: Synucleins-Biochemistry and Role in Diseases. IntechOpen. https://doi.org/10.5772/intechopen.82808
Platt NJ et al (2012) Striatal dopamine transmission is subtly modified in human A53Tα-synuclein overexpressing mice. PLoS One 7(5):e36397. https://doi.org/10.1371/journal.pone.0036397
Hattori N, Mizuno Y (2004) Pathogenetic mechanisms of parkin in Parkinson’s disease. Lancet 364(9435):722–724. https://doi.org/10.1016/S0140-6736(04)16901-8
Kamienieva I, Duszyński J, Szczepanowska J (2021) Multitasking guardian of mitochondrial quality: Parkin function and Parkinson’s disease. Transl Neurodegen 10(1):1–18. https://doi.org/10.1186/s40035-020-00229-8
Vaughan RA, Foster JD (2013) Mechanisms of dopamine transporter regulation in normal and disease states. Trends Pharmacol Sci 34(9):489–496. https://doi.org/10.1016/j.tips.2013.07.005
Dolgacheva LP et al (2019) Role of DJ-1 in the mechanism of pathogenesis of Parkinson’s disease. J Bioenerg Biomembr 51:175–188. https://doi.org/10.1007/s10863-019-09798-4
Tozzi A et al (2018) Dopamine D2 receptor activation potently inhibits striatal glutamatergic transmission in a G2019S LRRK2 genetic model of Parkinson’s disease. Neurobiol Dis 118:1–8. https://doi.org/10.1016/j.nbd.2018.06.008
Zhou ZD et al (2022) The role of tyrosine hydroxylase–dopamine pathway in Parkinson’s disease pathogenesis. Cell Mol Life Sci 79(12):599. https://doi.org/10.1007/s00018-022-04574-x
Stott SR et al (2019) Loss of FBXO7 results in a Parkinson’s -like dopaminergic degeneration via an RPL23–MDM2–TP53 pathway. J Pathol 249(2):241–254. https://doi.org/10.1002/path.5312
Park J et al (2022) The role of histone modifications: from neurodevelopment to neurodiseases. Signal Transduct Target Ther 7(1):217. https://doi.org/10.1038/s41392-022-01078-9
Pasquini J, Brooks DJ, Pavese N (2021) The cholinergic brain in Parkinson’s disease. Movement Disord Clin Pract 8(7):1012–1026. https://doi.org/10.1002/mdc3.13319
Bohnen NI, Albin RL (2011) The cholinergic system and Parkinson disease. Behav Brain Res 221(2):564–573. https://doi.org/10.1016/j.bbr.2009.12.048
Creed RB et al (2019) Basal and evoked neurotransmitter levels in Parkin, DJ-1, PINK1 and LRRK2 knockout rat striatum. Neuroscience 409:169–179. https://doi.org/10.1016/j.neuroscience.2019.04.033
Myslivecek J (2021) Two players in the field: hierarchical model of interaction between the dopamine and acetylcholine signaling systems in the striatum. Biomedicines 9(1):25. https://doi.org/10.3390/biomedicines9010025
Liu S-Y et al (2018) The effect of LRRK2 mutations on the cholinergic system in manifest and premanifest stages of Parkinson’s disease: a cross-sectional PET study. The Lancet Neurol 17(4):309–316. https://doi.org/10.1016/S1474-4422(18)30032-2
Skaper SD (2018) Neurotrophic factors: an overview. Neurotrophic Factors: Methods and Protocols:1–17. https://doi.org/10.1007/978-1-4939-7571-6_1
Palasz E et al (2020) BDNF as a promising therapeutic agent in Parkinson’s disease. Int J Mol Sci 21(3):1170. https://doi.org/10.3390/ijms21031170
Numakawa T, Odaka H (2021) Brain-derived neurotrophic factor signaling in the pathophysiology of Alzheimer’s disease: beneficial effects of flavonoids for neuroprotection. Int J Mol Sci 22(11):5719. https://doi.org/10.3390/ijms22115719
Rahmani F et al (2019) Plasma levels of brain-derived neurotrophic factor in patients with Parkinson disease: a systematic review and meta-analysis. Brain Res 1704:127–136. https://doi.org/10.1016/j.brainres.2018.10.006
Soman SK et al (2021) Cleaved PINK1 induces neuronal plasticity through PKA-mediated BDNF functional regulation. J Neurosci Res 99(9):2134–2155. https://doi.org/10.1002/jnr.24854
Feng C-W et al (2016) Neuroprotective effect of the marine-derived compound 11-dehydrosinulariolide through DJ-1-related pathway in in vitro and in vivo models of Parkinson’s disease. Mar Drugs 14(10):187. https://doi.org/10.3390/md14100187
López-Grueso MJ et al (2022) Deficiency of Parkinson’s related protein DJ-1 alters Cdk5 signalling and induces neuronal death by aberrant cell cycle re-entry. Cell Mol Neurobiol:1–13. https://doi.org/10.1007/s10571-022-01206-7
Mesa-Infante V et al (2022) Long-term exposure to GDNF induces dephosphorylation of Ret, AKT, and ERK1/2, and is ineffective at protecting midbrain dopaminergic neurons in cellular models of Parkinson’s disease. Mol Cell Neurosci 118:103684. https://doi.org/10.1016/j.mcn.2021.103684
Conway JA et al (2020) GDNF/RET signaling in dopamine neurons in vivo. Cell Tissue Res 382:135–146. https://doi.org/10.1007/s00441-020-03268-9
Yue P et al (2017) Intranasal administration of GDNF protects against neural apoptosis in a rat model of Parkinson’s disease through PI3K/Akt/GSK3β pathway. Neurochem Res 42:1366–1374. https://doi.org/10.1007/s11064-017-2184-1
Bondarenko O, Saarma M (2021) Neurotrophic factors in Parkinson’s disease: clinical trials, open challenges and nanoparticle-mediated delivery to the brain. Front Cell Neurosci 15:682597. https://doi.org/10.3389/fncel.2021.682597
Kramer ER, Liss B (2015) GDNF–Ret signaling in midbrain dopaminergic neurons and its implication for Parkinson disease. FEBS Lett 589(24):3760–3772. https://doi.org/10.1016/j.febslet.2015.11.006
Stepanova P et al (2020) Cerebral dopamine neurotrophic factor (CDNF) protects against quinolinic acid-induced toxicity in in vitro and in vivo models of Huntington’s disease. Sci Rep 10(1):19045. https://doi.org/10.1038/s41598-020-75439-1
Tenenbaum L (2021) CDNF: An innovative actor in disease-modifying approaches for Parkinson’s disease. Mol Ther 29(9):2634–2636. https://doi.org/10.1016/j.ymthe.2021.08.015
Stepanova P (2021) Effects of CDNF in experimental models of Huntington’s disease. DSHealth thesis series, Diss. University of Helsinki
Albert K et al (2021) Cerebral dopamine neurotrophic factor reduces α-synuclein aggregation and propagation and alleviates behavioral alterations in vivo. Mol Ther 29(9):2821–2840. https://doi.org/10.1016/j.ymthe.2021.04.035
Huttunen HJ et al (2021) First-in-man clinical trial of intraputamenal cdnf in Parkinson’s disease finds a consorted biomarker response in a subgroup of subjects. Age (years) 63(6.4):63.2–68.9
Chmielarz P, Saarma M (2020) Neurotrophic factors for disease-modifying treatments of Parkinson’s disease: gaps between basic science and clinical studies. Pharmacol Rep 72:1195–1217. https://doi.org/10.1007/s43440-020-00120-3
Tavakol S (2022) The twofold role of osteogenic small molecules in Parkinson’s disease therapeutics: crosstalk of osteogenesis and neurogenesis. Biomed Res Int 2022. https://doi.org/10.1155/2022/3813541
Komiya Y, Habas R (2008) Wnt signal transduction pathways. Organogenesis 4(2):68–75. https://doi.org/10.4161/org.4.2.5851
Vallée A, Vallée J-N, Lecarpentier Y (2021) Parkinson’s disease: potential actions of lithium by targeting the WNT/β-catenin pathway, oxidative stress, inflammation and glutamatergic pathway. Cells 10(2):230. https://doi.org/10.3390/cells10020230
Marchetti B et al (2020) Parkinson’s disease, aging and adult neurogenesis: Wnt/β-catenin signalling as the key to unlock the mystery of endogenous brain repair. Aging Cell 19(3):e13101. https://doi.org/10.1111/acel.13101
Chae W-J, Bothwell AL (2018) Canonical and non-canonical Wnt signaling in immune cells. Trends Immunol 39(10):830–847. https://doi.org/10.1016/j.it.2018.08.006
Berwick DC, Harvey K (2012) The importance of Wnt signalling for neurodegeneration in Parkinson’s disease. Biochem Soc Trans 40(5):1123–1128. https://doi.org/10.1042/BST20120122
Vallée A, Vallée J-N, Lecarpentier Y (2021) Potential role of cannabidiol in Parkinson’s disease by targeting the WNT/β-catenin pathway, oxidative stress and inflammation. Aging (Albany NY) 13(7):10796. https://doi.org/10.18632/aging.202951
Berwick DC, Harvey K (2014) The regulation and deregulation of Wnt signaling by PARK genes in health and disease. J Mol Cell Biol 6(1):3–12. https://doi.org/10.1093/jmcb/mjt037
Maiti P, Manna J, Dunbar GL (2017) Current understanding of the molecular mechanisms in Parkinson’s disease: targets for potential treatments. Transl Neurodegen 6:1–35. https://doi.org/10.1186/s40035-017-0099-z
Ching-Chi C et al (2020) (D620N) VPS35 causes the impairment of Wnt/β-catenin signaling cascade and mitochondrial dysfunction in a PARK17 knockin mouse model. Cell Death Dis:11(11). https://doi.org/10.1038/s41419-020-03228-9
Serafino A, Cozzolino M (2023) The Wnt/β-catenin signaling: a multifunctional target for neuroprotective and regenerative strategies in Parkinson’s disease. Neural Regen Res 18(2):306. https://doi.org/10.4103/1673-5374.343908
Liu X et al (2011) Genome-wide association study identifies candidate genes for Parkinson’s disease in an Ashkenazi Jewish population. BMC Med Genet 12:1–16. https://doi.org/10.1186/1471-2350-12-104
Long H-Z et al (2021) PI3K/AKT signal pathway: a target of natural products in the prevention and treatment of Alzheimer’s disease and Parkinson’s disease. Front Pharmacol 12:648636. https://doi.org/10.3389/fphar.2021.648636
Zhou Z et al (2022) Downregulation of PIK3CB involved in Alzheimer’s disease via apoptosis, axon guidance, and FoxO signaling pathway. Oxidative Med Cell Longev 2022. https://doi.org/10.1155/2022/1260161
Li Z et al (2016) Chronic inflammation links cancer and Parkinson’s disease. Front Aging Neurosci 8:126. https://doi.org/10.3389/fnagi.2016.00126
Kumar JS et al (2015) p38 MAPK and PI3K/AKT signalling cascades in Parkinson’s disease. Int J Mol Cell Med 4(2):67
Singh S, Singh TG (2020) Role of nuclear factor kappa B (NF-κB) signalling in neurodegenerative diseases: an mechanistic approach. Curr Neuropharmacol 18(10):918–935. https://doi.org/10.2174/1570159X18666200207120949
Tavakol S et al (2019) The impact of the particle size of curcumin nanocarriers and the ethanol on beta_1-integrin overexpression in fibroblasts: a regenerative pharmaceutical approach in skin repair and anti-aging formulations. DARU J Pharmaceut Sci 27:159–168. https://doi.org/10.1007/s40199-019-00258-3
Peng C et al (2020) The NF-κB signaling pathway, the microbiota, and gastrointestinal tumorigenesis: recent advances. Front Immunol 11:1387. https://doi.org/10.3389/fimmu.2020.01387
Baltus THL et al (2021) Association of-94 ATTG insertion/deletion NFkB1 and c.* 126G> A NFkBIA genetic polymorphisms with oxidative and nitrosative stress biomarkers in Brazilian subjects with Parkinson’s disease. Neurosci Lett 740:135487. https://doi.org/10.1016/j.neulet.2020.135487
Dolatshahi M et al (2021) Nuclear factor-kappa B (NF-κB) in pathophysiology of Parkinson disease: diverse patterns and mechanisms contributing to neurodegeneration. Eur J Neurosci 54(1):4101–4123. https://doi.org/10.1111/ejn.15242
Hoenen C et al (2016) Alpha-synuclein proteins promote pro-inflammatory cascades in microglia: stronger effects of the A53T mutant. PLoS One 11(9):e0162717. https://doi.org/10.1371/journal.pone.0162717
Russo I et al (2015) Leucine-rich repeat kinase 2 positively regulates inflammation and down-regulates NF-κB p50 signaling in cultured microglia cells. J Neuroinflammation 12(1):1–13. https://doi.org/10.1186/s12974-015-0449-7
López de Maturana R et al (2016) Mutations in LRRK2 impair NF-κB pathway in iPSC-derived neurons. J Neuroinflammation 13:1–15. https://doi.org/10.1186/s12974-016-0761-x
Lin Z et al (2021) DJ-1 inhibits microglial activation and protects dopaminergic neurons in vitro and in vivo through interacting with microglial p65. Cell Death Dis 12(8):715. https://doi.org/10.1038/s41419-021-04002-1
Kopan R (2012) Notch signaling. Cold Spring Harb Perspect Biol 4(10):a011213. https://doi.org/10.1101/cshperspect.a011213
Tavakol S et al (2015) The effect of Noggin supplementation in Matrigel nanofiber-based cell culture system for derivation of neural-like cells from human endometrial-derived stromal cells. J Biomed Mater Res A 103(1):1–7. https://doi.org/10.1002/jbm.a.35079
Lasky JL, Wu H (2005) Notch signaling, brain development, and human disease. Pediatr Res 57(7):104–109. https://doi.org/10.1203/01.PDR.0000159632.70510.3D
Kapoor A, Nation DA (2021) Role of Notch signaling in neurovascular aging and Alzheimer’s disease. In: Seminars in cell & developmental biology. Elsevier. https://doi.org/10.1016/j.semcdb.2020.12.011
Ho RX-Y et al (2020) Loss of MINAR2 impairs motor function and causes Parkinson’s disease-like symptoms in mice. Brain. Communications 2(1):fcaa047. https://doi.org/10.1093/braincomms/fcaa047
Imai Y et al (2015) The Parkinson’s disease-associated protein kinase LRRK2 modulates notch signaling through the endosomal pathway. PLoS Genet 11(9):e1005503. https://doi.org/10.1371/journal.pgen.1005503
Desplats P et al (2012) α-Synuclein induces alterations in adult neurogenesis in Parkinson disease models via p53-mediated repression of Notch1. J Biol Chem 287(38):31691–31702. https://doi.org/10.1074/jbc.M112.354522
Yoon J-H et al (2017) Parkin mediates neuroprotection through activation of Notch1 signaling. Neuroreport 28(4):181–186. https://doi.org/10.1097/WNR.0000000000000726
Yang C, Qi Y, Sun Z (2021) The role of Sonic hedgehog pathway in the development of the central nervous system and aging-related neurodegenerative diseases. Front Mol Biosci 8:711710. https://doi.org/10.3389/fmolb.2021.711710
Carballo GB et al (2018) A highlight on Sonic hedgehog pathway. Cell Commun Signal 16:1–15. https://doi.org/10.1186/s12964-018-0220-7
Ketabforoush AHME et al (2023) Masitinib: the promising actor in the next season of the Amyotrophic Lateral Sclerosis treatment series. Biomed Pharmacother 160:114378. https://doi.org/10.1016/j.biopha.2023.114378
Schmidt S et al (2022) Primary cilia and SHH signaling impairments in human and mouse models of Parkinson’s disease. Nat Commun 13(1):4819. https://doi.org/10.1038/s41467-022-32229-9
Dhekne HS et al (2018) A pathway for Parkinson’s disease LRRK2 kinase to block primary cilia and Sonic hedgehog signaling in the brain. Elife 7:e40202. https://doi.org/10.7554/eLife.40202
Lashgari N-A et al (2021) The involvement of JAK/STAT signaling pathway in the treatment of Parkinson’s disease. J Neuroimmunol 361:577758. https://doi.org/10.1016/j.jneuroim.2021.577758
Hu X et al (2021) The JAK/STAT signaling pathway: from bench to clinic. Signal Transduct Target Ther 6(1):402. https://doi.org/10.1038/s41392-021-00791-1
Qin H et al (2016) Inhibition of the JAK/STAT pathway protects against α-synuclein-induced neuroinflammation and dopaminergic neurodegeneration. J Neurosci 36(18):5144–5159. https://doi.org/10.1523/JNEUROSCI.4658-15.2016
Choi D-J, Kwon J-K, Joe E-H (2018) A Parkinson’s disease gene, DJ-1, regulates astrogliosis through STAT3. Neurosci Lett 685:144–149. https://doi.org/10.1016/j.neulet.2018.08.025
Kim, J.-h., et al., DJ-1 facilitates the interaction between STAT1 and its phosphatase, SHP-1, in brain microglia and astrocytes: a novel anti-inflammatory function of DJ-1. Neurobiol Dis, 2013. 60: p. 1-10. DOI: https://doi.org/10.1016/j.nbd.2013.08.007
Hiew L-F et al (2021) TGF-β/smad signalling in neurogenesis: implications for neuropsychiatric diseases. Cells 10(6):1382. https://doi.org/10.3390/cells10061382
Muñoz MD, de la Fuente N, Sánchez-Capelo A (2020) TGF-β/Smad3 signalling modulates GABA neurotransmission: implications in Parkinson’s disease. Int J Mol Sci 21(2):590. https://doi.org/10.3390/ijms21020590
Corona JC, Duchen MR (2016) PPARγ as a therapeutic target to rescue mitochondrial function in neurological disease. Free Radic Biol Med 100:153–163. https://doi.org/10.1016/j.freeradbiomed.2016.06.023
Behl T et al (2021) Elucidating the neuroprotective role of PPARs in Parkinson’s disease: a neoteric and prospective target. Int J Mol Sci 22(18):10161. https://doi.org/10.3390/ijms221810161
Yakunin E et al (2014) The regulation of catalase activity by PPAR γ is affected by α-synuclein. Ann Clin Transl Neurol 1(3):145–159. https://doi.org/10.1002/acn3.38
Hindeya Gebreyesus H, Gebrehiwot Gebremichael T (2020) The potential role of astrocytes in Parkinson’s disease (PD). Med Sci 8(1):7. https://doi.org/10.3390/medsci8010007
Zhong Z et al (2021) Fecal microbiota transplantation exerts a protective role in MPTP-induced Parkinson’s disease via the TLR4/PI3K/AKT/NF-κB pathway stimulated by α-synuclein. Neurochem Res 46:3050–3058. https://doi.org/10.1007/s11064-021-03411-0
Wang S et al (2022) Functional cooperation of α-synuclein and tau is essential for proper corticogenesis. J Neurosci 42(37):7031–7046. https://doi.org/10.1523/JNEUROSCI.0396-22.2022
Harvey K, Outeiro TF (2019) The role of LRRK2 in cell signalling. Biochem Soc Trans 47(1):197–207. https://doi.org/10.1042/BST20180464
Wang Y et al (2021) LRRK2-NFATc2 pathway associated with neuroinflammation may be a potential therapeutic target for Parkinson’s disease. J Inflamm Res 14:2583. https://doi.org/10.2147/JIR.S301531
Ravinther AI et al (2022) Molecular pathways involved in LRRK2-linked Parkinson’s disease: a systematic review. Int J Mol Sci 23(19):11744. https://doi.org/10.3390/ijms231911744
Kim S, Lee M, Choi YK (2020) The role of a neurovascular signaling pathway involving hypoxia-inducible factor and notch in the function of the central nervous system. Biomol Ther 28(1):45. https://doi.org/10.4062/biomolther.2019.119
Lei R et al (2019) Inactivating the ubiquitin ligase Parkin suppresses cell proliferation and induces apoptosis in human keloids. J Cell Physiol 234(9):16601–16608. https://doi.org/10.1002/jcp.28332
Gao W et al (2017) Up-regulation of caveolin-1 by DJ-1 attenuates rat pulmonary arterial hypertension by inhibiting TGFβ/Smad signaling pathway. Exp Cell Res 361(1):192–198. https://doi.org/10.1016/j.yexcr.2017.10.019
Pandey S et al (2020) An in silico analysis of deleterious single nucleotide polymorphisms and molecular dynamics simulation of disease linked mutations in genes responsible for neurodegenerative disorder. J Biomol Struct Dyn 38(14):4259–4272. https://doi.org/10.1080/07391102.2019.1682047
Hounjet J, Vooijs M (2021) The role of intracellular trafficking of notch receptors in ligand-independent notch activation. Biomolecules 11(9):1369. https://doi.org/10.3390/biom11091369
Huang Y et al (2022) UCHL1 promoted polarization of M1 macrophages by regulating the PI3K/AKT signaling pathway. J Inflamm Res:735–746. https://doi.org/10.2147/JIR.S343487
Wang X et al (2023) Ubiquitin C-terminal hydrolase-L1: a new cancer marker and therapeutic target with dual effects. Oncol Lett 25(3):1–12. https://doi.org/10.3892/ol.2023.13709
Zhong J et al (2012) UCHL1 acts as a colorectal cancer oncogene via activation of the β-catenin/TCF pathway through its deubiquitinating activity. Int J Mol Med 30(2):430–436. https://doi.org/10.3892/ijmm.2012.1012
Ghosh P, Saadat A (2021) Neurodegeneration and epigenetics: a review. Neurologia. https://doi.org/10.1016/j.nrl.2021.01.016
Al Aboud NM, Tupper C, Jialal I (2018) Genetics, epigenetic mechanism. StatPearls Publishing, Treasure Island (FL)
Moore LD, Le T, Fan G (2013) DNA methylation and its basic function. Neuropsychopharmacology 38(1):23–38. https://doi.org/10.1038/npp.2012.112
Miranda-Morales E et al (2017) Implications of DNA methylation in Parkinson’s disease. Front Mol Neurosci 10:225. https://doi.org/10.3389/fnmol.2017.00225
Neidhart M (2015) DNA methylation and complex human disease. Academic Press
Cacabelos R, Tellado I, Cacabelos P (2019) The epigenetic machinery in the life cycle and pharmacoepigenetics. In: Pharmacoepigenetics. Elsevier, pp. 1–100
Bohnsack JP, Pandey SC (2021) Histone modifications, DNA methylation, and the epigenetic code of alcohol use disorder. In: International review of neurobiology. Elsevier, pp. 1–62. https://doi.org/10.1016/bs.irn.2020.08.005
Wüllner U et al (2016) DNA methylation in Parkinson’s disease. J Neurochem 139:108–120. https://doi.org/10.1111/jnc.13646
Coppede F (2012) Genetics and epigenetics of Parkinson’s disease. Sci World J 2012:489830. https://doi.org/10.1100/2012/489830
Razali K et al (2022) Integrating nutriepigenomics in Parkinson’s disease management: new promising strategy in the omics era. IBRO Neurosci Rep 13:364–372. https://doi.org/10.1016/j.ibneur.2022.10.003
Manna I et al (2021) Roles of non-coding RNAs as novel diagnostic biomarkers in Parkinson’s disease. J Parkinsons Dis 11(4):1475–1489. https://doi.org/10.3233/JPD-212726
Sun P, Hamblin MH, Yin K-J (2022) Non-coding RNAs in the regulation of blood–brain barrier functions in central nervous system disorders. Fluids Barriers CNS 19(1):1–39. https://doi.org/10.1186/s12987-022-00317-z
Bertrand-Lehouillier V, Legault L-M, McGraw S (2019) Endocrine epigenetics, epigenetic profiling and biomarker identification. Elsevier. https://doi.org/10.1016/B978-0-12-801238-3.65830-0
Watson CN, Belli A, Di Pietro V (2019) Small non-coding RNAs: new class of biomarkers and potential therapeutic targets in neurodegenerative disease. Front Genet:364. https://doi.org/10.3389/fgene.2019.00364
Zhang H et al (2022) The role of non-coding RNAs in the pathogenesis of Parkinson’s disease: recent advancement. Pharmaceuticals 15(7):811. https://doi.org/10.3390/ph15070811
Isakova A et al (2020) A mouse tissue atlas of small noncoding RNA. Proc Natl Acad Sci 117(41):25634–25645. https://doi.org/10.1073/pnas.2002277117
Aalto AP, Pasquinelli AE (2012) Small non-coding RNAs mount a silent revolution in gene expression. Curr Opin Cell Biol 24(3):333–340. https://doi.org/10.1016/j.ceb.2012.03.006
Srijyothi L et al (2018) Roles of non-coding RNAs in transcriptional regulation. In: Transcriptional and Post-transcriptional regulation, p. 55. https://doi.org/10.5772/intechopen.76125
O'Brien J et al (2018) Overview of microRNA biogenesis, mechanisms of actions, and circulation. Front Endocrinol 9:402. https://doi.org/10.3389/fendo.2018.00402
Li S et al (2022) MicroRNAs play a role in Parkinson’s disease by regulating microglia function: from pathogenetic involvement to therapeutic potential. Front Mol Neurosci 14:744942. https://doi.org/10.3389/fnmol.2021.744942
Zhao N et al (2014) Serum microRNA-133b is associated with low ceruloplasmin levels in Parkinson’s disease. Parkinsonism Relat Disord 20(11):1177–1180. https://doi.org/10.1016/j.parkreldis.2014.08.016
Cressatti M, Schipper HM (2022) Dysregulation of a heme oxygenase–synuclein axis in Parkinson disease. NeuroSci 3(2):284–299. https://doi.org/10.3390/neurosci3020020
Goh SY et al (2019) Role of MicroRNAs in Parkinson’s disease. Int J Mol Sci 20(22):5649. https://doi.org/10.3390/ijms20225649
Ramaswamy P et al (2020) Clinical application of circulating microRNAs in Parkinson’s disease: the challenges and opportunities as diagnostic biomarker. Ann Indian Acad Neurol 23(1):84. https://doi.org/10.4103/aian.AIAN_440_19
Zhang J et al (2019) miR-let-7a suppresses α-Synuclein-induced microglia inflammation through targeting STAT3 in Parkinson’s disease. Biochem Biophys Res Commun 519(4):740–746. https://doi.org/10.1016/j.bbrc.2019.08.140
Tatura R et al (2016) Parkinson’s disease: SNCA-, PARK2-, and LRRK2-targeting microRNAs elevated in cingulate gyrus. Parkinsonism Relat Disord 33:115–121. https://doi.org/10.1016/j.parkreldis.2016.09.028
McMillan KJ et al (2017) Loss of microRNA-7 regulation leads to α-synuclein accumulation and dopaminergic neuronal loss in vivo. Mol Ther 25(10):2404–2414. https://doi.org/10.1016/j.ymthe.2017.08.017
Zhang J et al (2022) LncRNA miR-17-92a-1 cluster host gene (MIR17HG) promotes neuronal damage and microglial activation by targeting the microRNA-153-3p/alpha-synuclein axis in Parkinson’s disease. Bioengineered 13(2):4493–4516. https://doi.org/10.1080/21655979.2022.2033409
Su C, Yang X, Lou J (2016) Geniposide reduces α-synuclein by blocking microRNA-21/lysosome-associated membrane protein 2A interaction in Parkinson disease models. Brain Res 1644:98–106. https://doi.org/10.1016/j.brainres.2016.05.011
Minones-Moyano E et al (2011) MicroRNA profiling of Parkinson’s disease brains identifies early downregulation of miR-34b/c which modulate mitochondrial function. Hum Mol Genet 20(15):3067–3078. https://doi.org/10.1093/hmg/ddr210
Kabaria S et al (2015) Inhibition of miR-34b and miR-34c enhances α-synuclein expression in Parkinson’s disease. FEBS Lett 589(3):319–325. https://doi.org/10.1016/j.febslet.2014.12.014
Nair VD, Ge Y (2016) Alterations of miRNAs reveal a dysregulated molecular regulatory network in Parkinson’s disease striatum. Neurosci Lett 629:99–104. https://doi.org/10.1016/j.neulet.2016.06.061
Zhang L et al (2021) MiR-30c-5p/ATG5 axis regulates the progression of Parkinson’s disease. Front Cell Neurosci 15:644507. https://doi.org/10.3389/fncel.2021.644507
Yao L et al (2019) MicroRNA-124 regulates the expression of p62/p38 and promotes autophagy in the inflammatory pathogenesis of Parkinson’s disease. FASEB J 33(7):8648–8665. https://doi.org/10.1096/fj.201900363R
Yao L et al (2018) MicroRNA-124 regulates the expression of MEKK3 in the inflammatory pathogenesis of Parkinson’s disease. J Neuroinflammation 15:1–19. https://doi.org/10.1186/s12974-018-1053-4
Fan Y et al (2020) LncRNA BDNF-AS promotes autophagy and apoptosis in MPTP-induced Parkinson’s disease via ablating microRNA-125b-5p. Brain Res Bull 157:119–127. https://doi.org/10.1016/j.brainresbull.2020.02.003
Zeng R et al (2019) MicroRNA-135b alleviates MPP+-mediated Parkinson’s disease in in vitro model through suppressing FoxO1-induced NLRP3 inflammasome and pyroptosis. J Clin Neurosci 65:125–133. https://doi.org/10.1016/j.jocn.2019.04.004
**e X et al (2017) miR-145-5p/Nurr1/TNF-α signaling-induced microglia activation regulates neuron injury of acute cerebral ischemic/reperfusion in rats. Front Mol Neurosci 10:383. https://doi.org/10.3389/fnmol.2017.00383
Li H et al (2020) MicroRNA-150 serves as a diagnostic biomarker and is involved in the inflammatory pathogenesis of Parkinson’s disease. Mol Genet Genomic Med 8(4):e1189. https://doi.org/10.1002/mgg3.1189
Thome AD et al (2016) microRNA-155 regulates alpha-synuclein-induced inflammatory responses in models of Parkinson disease. J Neurosci 36(8):2383–2390. https://doi.org/10.1523/JNEUROSCI.3900-15.2016
Caggiu E et al (2018) Differential expression of miRNA 155 and miRNA 146a in Parkinson’s disease patients. eNeurologicalSci 13:1–4. Back to cited text, (49). https://doi.org/10.1016/j.ensci.2018.09.002
Liu Y, Song Y, Zhu X (2017) MicroRNA-181a regulates apoptosis and autophagy process in Parkinson’s disease by inhibiting p38 mitogen-activated protein kinase (MAPK)/c-Jun N-terminal kinases (JNK) signaling pathways. Med Sci Monitor: Int Med J Exp Clin Res 23:1597. https://doi.org/10.12659/msm.900218
Sun Q et al (2019) MicroRNA-190 alleviates neuronal damage and inhibits neuroinflammation via Nlrp3 in MPTP-induced Parkinson’s disease mouse model. J Cell Physiol 234(12):23379–23387. https://doi.org/10.1002/jcp.28907
Ren Y et al (2019) MicroRNA-195 triggers neuroinflammation in Parkinson’s disease in a Rho-associated kinase 1-dependent manner. Mol Med Rep 19(6):5153–5161. https://doi.org/10.3892/mmr.2019.10176
Chiu C-C et al (2019) Upregulated expression of microRNA-204-5p leads to the death of dopaminergic cells by targeting DYRK1A-mediated apoptotic signaling cascade. Front Cell Neurosci 13:399. https://doi.org/10.3389/fncel.2019.00399
Tang C-Z et al (2019) Overexpression of microRNA-301b accelerates hippocampal microglia activation and cognitive impairment in mice with depressive-like behavior through the NF-κB signaling pathway. Cell Death Dis 10(4):316. https://doi.org/10.1038/s41419-019-1522-4
Feng Y et al (2021) Effective inhibition of miR-330/SHIP1/NF-κB signaling pathway via miR-330 sponge repolarizes microglia differentiation. Cell Biol Int 45(4):785–794. https://doi.org/10.1002/cbin.11523
He Q et al (2017) Downregulation of miR-7116-5p in microglia by MPP+ sensitizes TNF-α production to induce dopaminergic neuron damage. Glia 65(8):1251–1263. https://doi.org/10.1002/glia.23153
Su L et al (2018) A meta-analysis of public microarray data identifies biological regulatory networks in Parkinson’s disease. BMC Med Genet 11(1):1–22. https://doi.org/10.1186/s12920-018-0357-7
Chatterjee P et al (2017) Biological networks in Parkinson’s disease: an insight into the epigenetic mechanisms associated with this disease. BMC Genomics 18:1–17. https://doi.org/10.1186/s12864-017-4098-3
Sibley CR et al (2012) Silencing of Parkinson’s disease-associated genes with artificial mirtron mimics of miR-1224. Nucleic Acids Res 40(19):9863–9875. https://doi.org/10.1093/nar/gks712
Rorbach G, Unold O, Konopka BM (2018) Distinguishing mirtrons from canonical miRNAs with data exploration and machine learning methods. Sci Rep 8(1):1–13. https://doi.org/10.1038/s41598-018-25578-3
Titov II, Vorozheykin PS (2018) Comparing miRNA structure of mirtrons and non-mirtrons. BMC Genomics 19:91–102. https://doi.org/10.1186/s12864-018-4473-8
Fyfe I (2020) MicroRNAs—diagnostic markers in Parkinson disease? Nat Rev Neurol 16(2):65–65. https://doi.org/10.1038/s41582-019-0305-y
Nies YH et al (2021) MicroRNA dysregulation in Parkinson’s disease: a narrative review. Front Neurosci 15:660379. https://doi.org/10.3389/fnins.2021.660379
Hu B et al (2020) Therapeutic siRNA: state of the art. Signal Transduct Target Ther 5(1):101. https://doi.org/10.1038/s41392-020-0207-x
Snead NM, Rossi JJ (2010) Biogenesis and function of endogenous and exogenous siRNAs. Wiley Interdiscip Rev: RNA 1(1):117–131. https://doi.org/10.1002/wrna.14
Neumeier J, Meister G (2021) siRNA specificity: RNAi mechanisms and strategies to reduce off-target effects. Front Plant Sci 11:526455. https://doi.org/10.3389/fpls.2020.526455
Deng H et al (2005) Small interfering RNA targeting the PINK1 induces apoptosis in dopaminergic cells SH-SY5Y. Biochem Biophys Res Commun 337(4):1133–1138. https://doi.org/10.1016/j.bbrc.2005.09.178
Titze-de-Almeida R et al (2019) Suppressing nNOS enzyme by small-interfering RNAs protects SH-SY5Y cells and nigral dopaminergic neurons from 6-OHDA injury. Neurotox Res 36:117–131. https://doi.org/10.1007/s12640-019-00043-9
Helmschrodt C et al (2017) Polyethylenimine nanoparticle-mediated siRNA delivery to reduce α-Synuclein expression in a model of Parkinson’s disease. Mol Ther-Nucleic Acids 9:57–68. https://doi.org/10.1016/j.omtn.2017.08.013
Yap YJ et al (2021) Development of ATP13A2-deficient in vitro model for PARK9 Parkinson’s disease. Curr Signal Transduct Ther 16(3):280–291. https://doi.org/10.2174/1574362416666210325112850
Prommahom A, Dharmasaroja P (2021) Effects of eEF1A2 knockdown on autophagy in an MPP+-induced cellular model of Parkinson’s disease. Neurosci Res 164:55–69. https://doi.org/10.1016/j.neures.2020.03.013
Acharya R, Chakraborty M, Chakraborty J (2019) Prospective treatment of Parkinson’s disease by a siRNA–LDH nanoconjugate. MedChemComm 10(2):227–233. https://doi.org/10.1039/c8md00501j
Cortes H et al (2017) Nanotechnology as potential tool for siRNA delivery in Parkinson’s disease. Curr Drug Targets 18(16):1866–1879. https://doi.org/10.2174/1389450118666170321130003
Kuo M-C et al (2021) The role of noncoding RNAs in Parkinson’s disease: biomarkers and associations with pathogenic pathways. J Biomed Sci 28(1):1–28. https://doi.org/10.1186/s12929-021-00775-x
Zhang T, Wong G (2022) Dysregulation of human somatic piRNA expression in Parkinson’s disease subtypes and stages. Int J Mol Sci 23(5):2469. https://doi.org/10.3390/ijms23052469
Shen L et al (2021) Dysregulation of MicroRNAs and PIWI-interacting RNAs in a Caenorhabditis elegans Parkinson’s disease model overexpressing human α-Synuclein and influence of tdp-1. Front Neurosci 15:600462. https://doi.org/10.3389/fnins.2021.600462
Hou J, Wei H, Liu B (2022) iPiDA-GCN: Identification of piRNA-disease associations based on Graph Convolutional Network. PLoS Comput Biol 18(10):e1010671. https://doi.org/10.1371/journal.pcbi.1010671
Huang X, Wong G (2021) An old weapon with a new function: PIWI-interacting RNAs in neurodegenerative diseases. Transl Neurodegen 10(1):1–21. https://doi.org/10.1186/s40035-021-00233-6
Wang B-G et al (2020) The role of transfer RNA-derived small RNAs (tsRNAs) in digestive system tumors. J Cancer 11(24):7237. https://doi.org/10.7150/jca.46055
Liu B et al (2021) Deciphering the tRNA-derived small RNAs: origin, development, and future. Cell Death Dis 13(1):24. https://doi.org/10.1038/s41419-021-04472-3
Li S, Xu Z, Sheng J (2018) tRNA-derived small RNA: a novel regulatory small non-coding RNA. Genes 9(5):246. https://doi.org/10.3390/genes9050246
Tian H, Hu Z, Wang C (2022) The therapeutic potential of tRNA-derived small RNAs in neurodegenerative disorders. Aging Dis 13(2):389. https://doi.org/10.14336/AD.2021.0903
Magee R, Londin E, Rigoutsos I (2019) TRNA-derived fragments as sex-dependent circulating candidate biomarkers for Parkinson’s disease. Parkinsonism Relat Disord 65:203–209. https://doi.org/10.1016/j.parkreldis.2019.05.035
Elkordy A et al (2018) Stress-induced tRNA cleavage and tiRNA generation in rat neuronal PC12 cells. J Neurochem 146(5):560–569. https://doi.org/10.1111/jnc.14321
Wei C-W et al (2018) The role of long noncoding RNAs in central nervous system and neurodegenerative diseases. Front Behav Neurosci 12:175. https://doi.org/10.3389/fnbeh.2018.00175
Plewka P, Raczynska KD (2022) Long intergenic noncoding RNAs affect biological pathways underlying autoimmune and neurodegenerative disorders. Mol Neurobiol 59(9):5785–5808. https://doi.org/10.1007/s12035-022-02941-0
Ye Y et al (2018) A lincRNA-p21/miR-181 family feedback loop regulates microglial activation during systemic LPS-and MPTP-induced neuroinflammation. Cell Death Dis 9(8):803. https://doi.org/10.1038/s41419-018-0821-5
Kraus TF et al (2017) Altered long noncoding RNA expression precedes the course of Parkinson’s disease—a preliminary report. Mol Neurobiol 54(4):2869–2877. https://doi.org/10.1007/s12035-016-9854-x
Xu, X., et al., LincRNA-p21 inhibits cell viability and promotes cell apoptosis in Parkinson’s disease through activating α-Synuclein expression. Biomed Res Int, 2018. 2018. DOI: https://doi.org/10.1155/2018/8181374
He X et al (2022) Ghrelin alleviates 6-hydroxydopamine-induced neurotoxicity in SH-SY5Y cells. Neural Regen Res 17(1):170. https://doi.org/10.4103/1673-5374.314314
Lu D, Xu A-D (2016) Mini review: circular RNAs as potential clinical biomarkers for disorders in the central nervous system. Front Genet 7:53. https://doi.org/10.3389/fgene.2016.00053
D’Ambra E, Capauto D, Morlando M (2019) Exploring the regulatory role of circular RNAs in neurodegenerative disorders. Int J Mol Sci 20(21):5477. https://doi.org/10.3390/ijms20215477
Jia E et al (2020) Transcriptomic profiling of circular RNA in different brain regions of Parkinson’s disease in a mouse model. Int J Mol Sci 21(8):3006. https://doi.org/10.3390/ijms21083006
Wang M et al (2021) CircRNA 001372 reduces inflammation in propofol-induced neuroinflammation and neural apoptosis through PIK3CA/Akt/NF-κB by miRNA-148b-3p. J Investig Surg 34(11):1167–1177. https://doi.org/10.1080/08941939.2020.1771639
Doxakis E (2022) Insights into the multifaceted role of circular RNAs: implications for Parkinson’s disease pathogenesis and diagnosis. npj. Parkinson's Disease 8(1):7. https://doi.org/10.1038/s41531-021-00265-9
Kong F et al (2021) RNA-sequencing of peripheral blood circular RNAs in Parkinson disease. Medicine 100(23). https://doi.org/10.1097/MD.0000000000025888
Morais P, Adachi H, Yu Y-T (2021) Spliceosomal snRNA epitranscriptomics. Front Genet 12:652129. https://doi.org/10.3389/fgene.2021.652129
Shen S et al (2021) Long non-coding RNA small nucleolar RNA host gene 14, a promising biomarker and therapeutic target in malignancy. Front Cell Dev Biol 9:746714. https://doi.org/10.3389/fcell.2021.746714
Yan L, Li L, Lei J (2021) Long noncoding RNA small nucleolar RNA host gene 12/microRNA-138-5p/nuclear factor I/B regulates neuronal apoptosis, inflammatory response, and oxidative stress in Parkinson’s disease. Bioengineered 12(2):12867–12879. https://doi.org/10.1080/21655979.2021.2005928
Theotoki EI et al (2020) Dicing the disease with dicer: the implications of dicer ribonuclease in human pathologies. Int J Mol Sci 21(19):7223. https://doi.org/10.3390/ijms21197223
Armeev GA, Gribkova AK, Shaytan AK (2022) Nucleosomes and their complexes in the cryoEM era: trends and limitations. Front Mol Biosci:9. https://doi.org/10.3389/fmolb.2022.1070489
Tamaru H (2010) Confining euchromatin/heterochromatin territory: jumonji crosses the line. Genes Dev 24(14):1465–1478. https://doi.org/10.1101/gad.1941010
Shilatifard A (2006) Chromatin modifications by methylation and ubiquitination: implications in the regulation of gene expression. Annu Rev Biochem 75:243–269. https://doi.org/10.1146/annurev.biochem.75.103004.142422
Pavlou MAS, Outeiro TF (2017) Epigenetics in Parkinson’s disease. In: Neuroepigenomics in aging and disease. Springer, pp. 363–390. https://doi.org/10.1007/978-3-319-53889-1_19
Wang R et al (2020) Imbalance of lysine acetylation contributes to the pathogenesis of Parkinson’s disease. Int J Mol Sci 21(19). https://doi.org/10.3390/ijms21197182
Harrison IF, Smith AD, Dexter DT (2018) Pathological histone acetylation in Parkinson’s disease: neuroprotection and inhibition of microglial activation through SIRT 2 inhibition. Neurosci Lett 666:48–57. https://doi.org/10.1016/j.neulet.2017.12.037
Toker L et al (2021) Genome-wide histone acetylation analysis reveals altered transcriptional regulation in the Parkinson’s disease brain. Mol Neurodegener 16(1):31. https://doi.org/10.1186/s13024-021-00450-7
Shukla S, Tekwani BL (2020) Histone deacetylases inhibitors in neurodegenerative diseases, neuroprotection and neuronal differentiation. Front Pharmacol 11:537. https://doi.org/10.3389/fphar.2020.00537
Formisano L et al (2015) MS-275 inhibits aroclor 1254–induced SH-SY5Y neuronal cell toxicity by preventing the formation of the HDAC3/REST complex on the synapsin-1 promoter. J Pharmacol Exp Ther 352(2):236–243. https://doi.org/10.1124/jpet.114.219345
Mazzocchi M et al (2021) LMK235, a small molecule inhibitor of HDAC4/5, protects dopaminergic neurons against neurotoxin-and α-synuclein-induced degeneration in cellular models of Parkinson’s disease. Mol Cell Neurosci 115:103642. https://doi.org/10.1016/j.mcn.2021.103642
Mazzocchi M et al (2019) Gene co-expression analysis identifies histone deacetylase 5 and 9 expression in midbrain dopamine neurons and as regulators of neurite growth via bone morphogenetic protein signaling. Front Cell Dev Biol 7:191. https://doi.org/10.3389/fcell.2019.00191
Li B et al (2021) Acetylation of NDUFV1 induced by a newly synthesized HDAC6 inhibitor HGC rescues dopaminergic neuron loss in Parkinson models. Iscience 24(4):102302. https://doi.org/10.1016/j.isci.2021.102302
Johnston TH et al (2013) RGFP109, a histone deacetylase inhibitor attenuates L-DOPA-induced dyskinesia in the MPTP-lesioned marmoset: a proof-of-concept study. Parkinsonism Relat Disord 19(2):260–264. https://doi.org/10.1016/j.parkreldis.2012.07.001
Kim T et al (2019) HDAC inhibition by valproic acid induces neuroprotection and improvement of PD-like behaviors in LRRK2 R1441G transgenic mice. Exp Neurobiol 28(4):504. https://doi.org/10.5607/en.2019.28.4.504
Li Y et al (2022) Histone deacetylases as epigenetic targets for treating Parkinson’s disease. Brain Sci 12(5):672. https://doi.org/10.3390/brainsci12050672
Sharma S, Taliyan R (2015) Targeting histone deacetylases: a novel approach in Parkinson’s disease. Parkinson’s Dis 2015. https://doi.org/10.1155/2015/303294
Miller JL, Grant PA (2012) The role of DNA methylation and histone modifications in transcriptional regulation in humans. Epigenetics: Dev Dis:289–317. https://doi.org/10.1007/978-94-007-4525-4_13
Basavarajappa BS, Subbanna S (2021) Histone methylation regulation in neurodegenerative disorders. Int J Mol Sci 22(9):4654. https://doi.org/10.3390/ijms22094654
Guhathakurta S et al (2021) Targeted attenuation of elevated histone marks at SNCA alleviates α-synuclein in Parkinson’s disease. EMBO Mol Med 13(2):e12188. https://doi.org/10.15252/emmm.202012188
Heinemann B et al (2014) Inhibition of demethylases by GSK-J1/J4. Nature 514(7520):E1–E2. https://doi.org/10.1038/nature13688
Mu M-D et al (2020) Therapeutic effect of a histone demethylase inhibitor in Parkinson’s disease. Cell Death Dis 11(10):927. https://doi.org/10.1038/s41419-020-03105-5
Cao J, Yan Q (2012) Histone ubiquitination and deubiquitination in transcription, DNA damage response, and cancer. Front Oncol 2:26. https://doi.org/10.3389/fonc.2012.00026
Buneeva O, Medvedev A (2022) Atypical ubiquitination and Parkinson’s disease. Int J Mol Sci 23(7):3705. https://doi.org/10.3390/ijms23073705
Ben Yehuda A et al (2017) Ubiquitin accumulation on disease associated protein aggregates is correlated with nuclear ubiquitin depletion, histone de-ubiquitination and impaired DNA damage response. PLoS One 12(1):e0169054. https://doi.org/10.1371/journal.pone.0169054
Navarro-Yepes J et al (2016) Inhibition of protein ubiquitination by paraquat and 1-methyl-4-phenylpyridinium impairs ubiquitin-dependent protein degradation pathways. Mol Neurobiol 53:5229–5251. https://doi.org/10.1007/s12035-015-9414-9
Li S, Le W, Deng H (2022) Genetic and epigenetic mechanisms of Parkinson’s disease. Front Neurosci 16. https://doi.org/10.3389/fnins.2022.842709
Zhu D, Zhang Y, Wang S (2021) Histone citrullination: a new target for tumors. Mol Cancer 20(1):1–17. https://doi.org/10.1186/s12943-021-01373-z
Mastronardi FG et al (2006) Increased citrullination of histone H3 in multiple sclerosis brain and animal models of demyelination: a role for tumor necrosis factor-induced peptidylarginine deiminase 4 translocation. J Neurosci 26(44):11387–11396. https://doi.org/10.1523/JNEUROSCI.3349-06.2006
Witalison E, Thompson PR, Hofseth LJ (2015) Protein arginine deiminases and associated citrullination: physiological functions and diseases associated with dysregulation. Curr Drug Targets 16(7):700–710. https://doi.org/10.2174/1389450116666150202160954
Petrozziello T et al (2020) Neuroinflammation and histone H3 citrullination are increased in X-linked Dystonia Parkinsonism post-mortem prefrontal cortex. Neurobiol Dis 144:105032. https://doi.org/10.1016/j.nbd.2020.105032
Ou Z et al (2021) Global trends in the incidence, prevalence, and years lived with disability of Parkinson’s disease in 204 countries/territories from 1990 to 2019. Front Public Health 9:776847. https://doi.org/10.3389/fpubh.2021.776847
Ammal Kaidery N, Tarannum S, Thomas B (2013) Epigenetic landscape of Parkinson’s disease: emerging role in disease mechanisms and therapeutic modalities. Neurotherapeutics 10:698–708. https://doi.org/10.1007/s13311-013-0211-8
Mahdieh N, Rabbani B (2013) An overview of mutation detection methods in genetic disorders. Iran J Pediatr 23(4):375
Gibney E, Nolan C (2010) Epigenetics and gene expression. Heredity 105(1):4–13. https://doi.org/10.1038/hdy.2010.54
Huang Y et al (2022) Parkinson’s disease: from genetics to molecular dysfunction and targeted therapeutic approaches. Genes Dis. https://doi.org/10.1016/j.gendis.2021.12.015
Tan MM et al (2021) Genome-wide association studies of cognitive and motor progression in Parkinson’s disease. Mov Disord 36(2):424–433. https://doi.org/10.1002/mds.28342
Lanore A et al (2022) Does the expression and epigenetics of genes involved in monogenic forms of parkinson’s disease influence sporadic forms? Genes 13(3):479. https://doi.org/10.3390/genes13030479
Over L, Brüggemann N, Lohmann K (2021) Therapies for genetic forms of Parkinson’s disease: systematic literature review. J Neuromusc Dis 8(3):341–356. https://doi.org/10.3233/JND-200598
Rathore AS et al (2021) Epigenetic modulation in Parkinson’s disease and potential treatment therapies. Neurochem Res 46(7):1618–1626. https://doi.org/10.1007/s11064-021-03334-w
Menon S et al (2022) Alpha-synuclein targeting therapeutics for Parkinson’s disease and related synucleinopathies. Front Neurol:809. https://doi.org/10.3389/fneur.2022.852003
Navarro-Romero A, Montpeyó M, Martinez-Vicente M (2020) The emerging role of the lysosome in Parkinson’s disease. Cells 9(11):2399. https://doi.org/10.3390/cells9112399
Hegarty SV, Sullivan AM, O'Keeffe GW (2016) The epigenome as a therapeutic target for Parkinson’s disease. Neural Regen Res 11(11):1735. https://doi.org/10.4103/1673-5374.194803
Paccosi E, Proietti-De-Santis L (2023) Parkinson’s disease: from genetics and epigenetics to treatment, a miRNA-based strategy. Int J Mol Sci 24(11):9547. https://doi.org/10.3390/ijms24119547
Pardo-Moreno T et al (2023) Current treatments and new, tentative therapies for Parkinson’s disease. Pharmaceutics 15(3):770. https://doi.org/10.3390/pharmaceutics15030770
Acknowledgements
The authors acknowledge the Cellular and Molecular Research Center, Iran, University of Medical Sciences, Tehran, Iran, for the finding assistance.
Funding
This work was supported by the Cellular and Molecular Research Center, Iran University of Medical Sciences, Tehran, Iran (grant number 98-4-20-13245 and 96-03-117-31785).
Author information
Authors and Affiliations
Contributions
Shayesteh Kokabi Hamidpour: writing and editing epigenetic section. Mobina Amiri: writing the cell signaling and genetic section. Arsh Haj Mohamad Ebrahim Ketabforoush: writing the introduction. Saeede Saeedi: writing the epigenic section. Abdolhamid Angaji: conceptualization. Shima Tavakol: conceptualization, methodology, supervision, writing—editing and review, and funding acquisition.
Corresponding author
Ethics declarations
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hamidpour, S.K., Amiri, M., Ketabforoush, A.H.M.E. et al. Unraveling Dysregulated Cell Signaling Pathways, Genetic and Epigenetic Mysteries of Parkinson’s Disease. Mol Neurobiol (2024). https://doi.org/10.1007/s12035-024-04128-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12035-024-04128-1