Abstract
Lung adenocarcinoma (LUAD) is one of the most prevalent and leading causes of cancer deaths globally, with limited diagnostic and clinically significant therapeutic targets. Identifying the genes and processes involved in develo** and progressing LUAD is crucial for develo** effective targeted therapeutics and improving patient outcomes. Therefore, the study aimed to explore the RNA sequencing data of LUAD from The Cancer Genome Atlas (TCGA) and gene expression profile datasets involving GSE10072, GSE31210, and GSE32863 from the Gene Expression Omnibus (GEO) databases. The differential gene expression and the downstream analysis determined clinically significant biomarkers using a network-based approach. These therapeutic targets predominantly enriched the dysregulation of mitotic cell cycle regulation and revealed the co-overexpression of Aurora-A Kinase (AURKA) and Targeting Protein for Xklp2 (TPX2) with high survival risk in LUAD patients. The hydrophobic residues of the AURKA–TPX2 interaction were considered as the target site to block the autophosphorylation of AURKA during the mitotic cell cycle. The tyrosine kinase inhibitor (TKI) dacomitinib demonstrated the strong binding potential to hinder TPX2, shielding the AURKA destabilization. This in silico study lays the foundation for repurposing targeted therapeutic options to impede the Protein–Protein Interactions (PPIs) in LUAD progression and aid in future translational investigations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lung cancer has emerged as the second most prevalent cancer and the leading cause of cancer-related death, posing a serious global health concern [1]. Tobacco smoking is the primary cause of lung cancer deaths worldwide, with men being more vulnerable than women [2]. Despite advances in research and therapy, LUAD remains a life-threatening malignancy that accounts for 40% of all lung cancer cases [3]. Despite advances in cancer treatment options, such as chemotherapy, immunotherapy, and non-invasive surgical resection, the 5-year overall survival (OS) rate for LUAD patients remains about 17.4% [4]. Therefore, it is imperative to understand the molecular mechanisms underlying the disease and identify key biomarkers to enable early detection and successful management of the disease. Technologies such as next-generation sequencing, microarrays, and proteomics have been instrumental in identifying biomarkers, but identifying key genes remains a challenge for develo** targeted therapies to improve patient outcomes [5]. Network pharmacology addresses the multiple key factors and targets that interact to govern associated complex pathways [6].
Recently, the clinical results of targeted therapy at the molecular level for LUAD patients were promising. However, the obstacle of drug resistance continues to impede patients’ overall cure. Precision oncology has improved treatment results and quality of life compared to conventional chemotherapy since the emergence of genomic medicine [7]. Recent progress in understanding pathways, advancements in technologies for identifying genetic abnormalities, and the emergence of novel drugs to inhibit these pathways have enabled healthcare professionals to customize treatment approaches [8]. Several significant targetable pathways in lung adenocarcinoma have been discovered, including the Epidermal Growth Factor Receptor (EGFR), PI3K/AKT/mTOR, and RAS-MAPK pathways [9, 10]. Targeting EGFR mutations is the primary approach for treating LUAD [11]. Identifying these genetic alterations is crucial in clinical practice across the globe. Despite this, novel oncogenic drivers have recently emerged, resulting in clinically effective therapeutics that are either approved or in development [12]. Recent studies revealed that overexpression, amplification, and exon-skip** mutations in novel molecular targets such as Mesenchymal–Epithelial Transition factor (MET) and Neurotrophic Tyrosine Kinase (NTRK) are associated with aggressiveness, metastasis, vascular invasion, and drug resistance ultimately impacting the poor prognosis of LUAD population [13, 14]. Numerous drugs that target these pathways have been developed and demonstrated therapeutic effects. Some of these, such as the EGFR inhibitors erlotinib and gefitinib and the PI3K/AKT/mTOR inhibitor everolimus, have now been supplanted as the first-line treatment [15, 16].
Network pharmacology addresses the multiple key factors and targets that interact to govern associated complex pathways [6]. This concept challenges the traditional notion of treating a single disease with a single medicine that targets a single biological target. Instead, it proposes a ‟multi-component, multi-target network” and is consistent with the complexity of compositions and the involvement of multiple targets [32]. Molecular docking provided a platform for repurposing 18 FDA-approved targeted cancer drugs and assessing their potential to target multiple targets. It demonstrated the inhibitory potential of FDA-targeted cancer drugs on the TPX2–AURKA interaction, aiding the experimental investigators to develop targeted therapeutic strategies and improve clinical outcomes.
Methodology
The study focuses on the analysis of transcriptome data from publicly accessible archives that pertain to LUAD patients. The aim was to identify clinically significant biomarkers employing a static network-based approach. FDA-approved anti-cancer drugs were repurposed for the uncovered target, revealing prospective therapeutic avenues through molecular interaction studies. Figure 1 depicts the overall workflow of the study.
Analysis of Differential Gene Expression
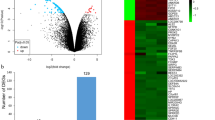
The study involved the analysis of transcriptomic profiling data from TCGA-LUAD with primary tumor and adjacent tumor normal samples [33]. The analysis also included three microarray datasets, GSE10072, GSE31210, and GSE32863 (https://www.ncbi.nlm.nih.gov/geo/). Each microarray dataset had different sample sizes and platforms. The analysis focused on gene expression profiles from human LUAD tissues and adjacent normal tissues, considering DNA methylation and smoking status. The edgeR (V3.36.0) and limma (V3.50.3) packages of R (V4.1.2) were used to identify the differentially expressed genes (DEGs) in tumor vs. normal samples. DEGs were determined based on P value < 0.05 and log2 fold change (log2FC) > 1, with false discovery rate control using the Benjamini & Hochberg method. The overlap** DEGs were screened for determining genes differentially expressed in all four LUAD gene expression datasets using a Venn diagram (https://bioinformatics.psb.ugent.be/webtools/Venn/) [34].
PPI Network Construction and Topological Analysis
The PPI network of the DEGs was determined using the String database (https://string-db.org/) at 5% confidence with a medium score, which aimed to exclude PPIs with low probability and enhance the reliability of the results. This approach facilitated increased coverage for a comprehensive understanding of protein interactions within the biological system, potentially encompassing less explored or transient interactions that may not be captured by high- and low-confidence networks [35]. Molecular Complex Detection (MCODE) was used to identify densely connected regions in a large PPI network. A cut-off degree of 10, cut-off node score of 0.2, K-core of 2, and a maximum depth of 100 were used as parameters. Further, CytoHubba identified the central nodes using the Maximal Clique Centrality (MCC) method [3).
PPI Network and Hub Genes
The String database generated a network of significant protein-coding genes with medium confidence. As a result, a network consists of 337 nodes and 2870 edges with a clustering coefficient of 0.435 (Supp Fig. 2a). MCODE identified six clusters of densely connected nodes within the network. Cluster 1, consisting of 62 nodes and 1881 edges, achieved a score of 61.672, indicating its significance compared to the scores of the other clusters (Supp Fig. 2b). The cluster identified the top hub genes using the MCC method (Fig. 4), which outperforms other centrality algorithms in accurately assessing the importance of nodes in terms of their network structure. We observed network interactions among these nodes, which supported their potential roles as key regulators in the network.
Gene Ontology and Functional Enrichment of Hub Genes
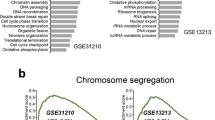
The number of hub genes enriched to the GO terms was determined based on fold enrichment at FDR < 0.05. The GO analysis uncovered the biological process (Fig. 5a), cellular component (Fig. 5b), and molecular function (Fig. 5c). A captivating revelation emerged as we uncovered the narrative of the dysregulated hub genes in LUAD was significantly related to cell cycle regulation, mitotic cell cycle, and cell division. The cellular components, such as the spindle, microtubule, and spindle pole, took the spotlight. Furthermore, these hub genes’ molecular functions have been correlated to Adenosine triphosphate (ATP) binding, microtubule binding, and tubulin binding. The hub genes enriched to the top 5 GO terms were involved in cell cycle regulation, mitotic cell cycle processing, regulation of signaling, and cell division, which was illustrated using a GO chord diagram (Fig. 5d).
Clinical Significance of the Hub Genes
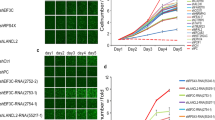
The KM plotter assessed the expression of hub genes and their relationship with OS risk in LUAD patients for 200 months (Fig. 6). The results revealed that the overexpression of these hub genes was associated with unfavorable OS rates in patients. The hub genes showed higher hazard ratios (HR) > 1 and log-rank P values < 0.05. The median survival expression in months was significantly higher in the low-expression cohort compared to the high-expression cohort (Table 2). The expression pattern of the hub genes in the selected gene expression datasets is listed in (Table 3). TPX2 and AURKA possessed higher expression cohorts in all four datasets with a high survival risk in the LUAD.
Molecular Docking
The analysis of molecular docking revealed that the drug molecules interacted with the binding pocket of the TPX2–AURKA interaction (Supp. Figs. 2 and 3). The complexes shown in Fig. 7 demonstrated the most favorable docking results, with a distance of < 3.5 Aº between the receptor’s binding pocket residues and the drug molecules. According to the findings, the compounds predominantly interacted with the target proteins through hydrogen bonds, electrostatic interactions, salt bridges, and hydrophobic interactions. The residues and the chains of the receptor involved in hydrogen bonding, electrostatic, and hydrophobic interactions, and the binding energies of the complexes are listed in Table 4. The binding energy of the leads to the target ranged from − 10.23 to − 5.61 kcal/mol. In contrast to ADP, Dacomitinib exhibited a higher binding affinity, establishing hydrogen bonds and negatively charged interactions and occupying the hydrophobic region of the TPX2-AURKA binding pocket.
Discussion
Lung adenocarcinoma is a prevalent form of lung cancer and a leading cause of cancer-related deaths [47]. As genomic and proteomic data become more accessible, accurately identifying target drugs has become increasingly important. Targeted therapies offer significant potential for effectively treating LUAD, making the identification of such therapies essential for develo** successful treatment approaches [48]. Therefore, the study involved identifying therapeutic targets by analyzing the transcriptomic datasets of the primary tumor in contrast to the adjacent tumor normal samples [49].
Our study used comprehensive in silico techniques to identify genes associated with LUAD by analyzing data from the TCGA and GEO databases. Unlike most previous studies, which focused on specific genetic events or cohort analysis, we used a broader approach. As a result, we discovered 337 overlap** DEGs. The association between important DEGs regarding their physical and functional relationships was determined using a PPI network [50]. The topology analysis of the network aided in determining the significant clusters within the network, focusing on densely interconnected regions [51]. The aim was to gain insights into the critical genes, their interrelationships, and their involvement in regulating cancer-related biological processes induced by aberrant DNA methylation status and smoking status of LUAD patients. The top 10 highly ranked hub genes were identified using the MCC centrality metric with participation in the largest cliques within the network, holding significant importance and being involved in critical biological processes that contribute to cancer progression [51, 52]. Gene ontology of the hub genes provided structured terms for describing molecular functions, biological processes, and cellular components [53].
The overexpressed hub genes were predominantly enriched to cell cycle regulation and mitotic cell cycle, along with cellular components, such as the spindle and molecular functions involving microtubule binding, tubulin binding, and ATP binding, holistically triggered the critical cellular events were involved in LUAD pathogenesis [54,55,56]. As a result, mitotic cell cycle regulation was disrupted, resulting in uncontrolled cell growth and elevated tumor development. The chromosome segregation during cell division was impaired, leading to genomic instability due to dysregulated microtubule dynamics, influencing cell motility and intracellular transport with alterations in the energy balance for regulating a wide range of cellular processes [57,58,59]. This intricate combination of overexpressed hub genes endorsed mitotic errors, genetic variations, and invasive attributes, contributing to LUAD aggressiveness [60]. However, the expression patterns of the eight hub genes varied, but AURKA and TPX2 were found co-overexpressed across the datasets.
The elevated levels of AURKA hindered the tumor suppressors through phosphorylation, impeded normal functioning, and triggered the activation of oncogenic factors, resulting in chromosomal instability [61]. TPX2 is crucial in ensuring accurate assembly of the mitotic spindle. In contrast, TPX2 was closely linked to the spindle pole during mitosis. TPX2, like other mitosis-regulating proteins, was associated with unfavorable prognoses and linked to enhanced proliferation, invasion, and migration capabilities [62]. TPX2 activated AURKA by attaching it to its N-terminal domain, which shielded AURKA from dephosphorylation. Therefore, the study demonstrated the significance of targeting co-overexpressed TPX2 and AURKA could present a promising and innovative therapeutic approach [63]. Moreover, experimental and structural studies have validated the interaction between TPX2 and AURKA at the mitotic spindle [64]. The implementation of KM plots is crucial in the process of selecting biomarkers that have the potential to predict both therapeutic response and clinical outcomes [65]. The study elucidated the survival risk associated with the expression patterns of hub genes in NSCLC patients over 200 months. The hub genes showed a lower median expression in cohorts with high expression levels, indicating their involvement in impaired cell cycle regulation. This dysregulation increased the survival risk in LUAD patients, as indicated by an HR > 1, signifying a higher level of risk [66]. The KM plots indicate a higher risk of high-expression cohorts of AURKA and TPX2, which has opened an avenue for targeting the AURKA-TPX2 complex to inhibit AURKA autophosphorylation in the progression of LUAD. This implies that PPI inhibitors targeting this specific interaction could potentially overcome the specificity challenges faced by ATP-based inhibitors to some extent [67].
The study focused on determining the inhibitory potential of FDA-approved cancer drugs to overcome the need to target dysregulated AURKA-TPX2 complex in lung adenocarcinoma. TKIs have been extensively used to treat various cancers [68]. They have been developed to attenuate the enzymatic activity of mutant tyrosine kinases that contribute to the malignant traits of cells by blocking the ATP-binding sites [69]. The molecular docking study demonstrated the binding potential of the second-generation EGFR-tyrosine kinase in contrast to the ADP, which served as control. The catalytic activity of AURKA involves ATP hydrolysis to release ADP and bind to the receptor, releasing energy to facilitate autophosphorylation [70]. Dacomitinib interacted with AURKA at TYR197, LYS271, and GLU211 and TPX2 at PHE19 and GLU25 with non-covalent interactions and possessed strong binding affinity with the complex [71]. The hydrogen bond formation of dacomitinib with the receptor at TYR197 demonstrated strong evidence of exerting pharmacological actions on TPX2, shielding the dephosphorylation at the tyrosine residues during the mitotic cell cycle due to the dysregulation of protein tyrosine phosphatase [32]. The findings revealed that screened drugs occupied the hydrophobic residues of the receptor’s interaction pocket and illustrated the potential to impede the AURKA–TPX2 interaction in LUAD progression [46]. It is widely recognized in computational drug development that integrating a drug into healthcare necessitates multiple modifications and advancements [72]. These drugs could evolve as promising therapeutic agents for inhibiting the dysregulated protein–protein interactions in lung adenocarcinoma through rigorous in vitro and clinical investigations.
Conclusions
The study determined that 337 DEGs were differentially expressed across the transcriptomic datasets of LUAD samples. The downstream analysis of the DEGs using a network-based approach determined that the densely interconnected nodes were predominantly involved in suppressing tumor suppressors, dysregulated mitotic cell cycle, and driving genomic instability due to impaired chromosomal segregation during cell division. These events were endorsed due to the co-overexpression of AURKA and TPX2 across the LUAD samples. The survival analysis of these hub genes revealed their clinical significance to be recognized as a critical therapeutic target, which has broadened the knowledge for targeting the AURKA-TPX2 complex in LUAD progression. The FDA-approved cancer-targeted drugs revealed the strong binding potential to the hydrophobic residues of the AURKA–TPX2 interaction pocket. Dacomitinib overperformed in the molecular docking studies, held with hydrogen and electrostatic interactions with both the chains and occupying the interaction pocket of the receptor. This study demonstrated an innovative targeted therapeutic strategy and addressed the knowledge gap on the pharmacological potential of FDA-approved cancer drugs in disrupting the AURKA–TPX2 interaction. Consequently, further in vitro evaluations and clinical studies of these drugs, coupled with structural modifications, would enhance drug-like properties and overcome the acquired drug resistance in LUAD patients, which holds the potential to develop a promising novel targeted therapeutic approach.
Data Availability
All authors ensure that data and materials are available and transparent.
References
Siegel, R. L., Miller, K. D., Sandeep, N. W., & Ahmedin, J. (2023). Cancer statistics, 2023. CA: A Cancer Journal for Clinicians, 73(1), 17–48.
Safiri, S., Nejadghaderi, S. A., Abdollahi, M., Carson-Chahhoud, K., Kaufman, J. S., Bragazzi, N. L., Moradi-Lakeh, M., Mansournia, M. A., Sullman, M. J. M., Almasi-Hashiani, A., Taghizadieh, A., Collins, G. S., & Kolahi, A.-A. (2022). Global, regional, and national burden of cancers attributable to tobacco smoking in 204 countries and territories, 1990–2019. Cancer Medicine, 11(13), 2662.
Denisenko, T. V., Budkevich, I. N., & Zhivotovsky, B. (2018). Cell death-based treatment of lung adenocarcinoma. Cell Death & Disease, 9(2), 1–14.
Luo, J., & Du, X. (2021). A promising prognostic signature for lung adenocarcinoma (LUAD) patients basing on 6 hypoxia-related genes. Medicine (United States), 100(50), E28237.
Sears, C. R., & Mazzone, P. J. (2020). Biomarkers in lung cancer. Clinics in Chest Medicine, 41(1), 115–127.
Lee, H. S., Lee, I. H., Kang, K., Park, S. I., Moon, S. J., Lee, C. H., & Lee, D. Y. (2021). A network pharmacology study on the molecular mechanisms of FDY003 for breast cancer treatment. Evidence-based Complementary and Alternative Medicine, 2021, 3919143.
Yuan, M., Huang, L. L., Chen, J. H., Wu, J., & Xu, Q. (2019). The emerging treatment landscape of targeted therapy in non-small-cell lung cancer. Signal Transduction and Targeted Therapy. https://doi.org/10.1038/s41392-019-0099-9
Herbst, R. S., Morgensztern, D., & Boshoff, C. (2018). The biology and management of non-small cell lung cancer. Nature., 553, 446–454.
Wu, J., & Lin, Z. (2022). Non-small cell lung cancer targeted therapy: drugs and mechanisms of drug resistance. International Journal of Molecular Sciences, 23(23), 15056.
Chang, K. T., Ju, J. A., & Vitolo, M. I. (2022). Noncoding RNAs and modulation of the EGFR/ERK pathway by circRNA C190 in non-small cell lung cancer. ExRNA, 4, 1–5.
Denis, M. G., & Bennouna, J. (2020). Osimertinib for front-line treatment of locally advanced or metastatic egfr-mutant nsclc patients: Efficacy, acquired resistance and perspectives for subsequent treatments. Cancer Management and Research, 12, 12593–12602.
Rebuzzi, S. E., Zullo, L., Rossi, G., Grassi, M., Murianni, V., Tagliamento, M., Prelaj, A., Coco, S., Longo, L., DalBello, M. G., Alama, A., Dellepiane, C., Bennicelli, E., Malapelle, U., & Genova, C. (2021). Novel emerging molecular targets in non-small cell lung cancer. International Journal of Molecular Sciences, 22(5), 1–25.
Salgia, R., Sattler, M., Scheele, J., Stroh, C., & Felip, E. (2020). The promise of selective MET inhibitors in non-small cell lung cancer with MET exon 14 skip**. Cancer Treatment Reviews, 87(April), 102022.
Elfving, H., Broström, E., Moens, L. N. J., Almlöf, J., Cerjan, D., Lauter, G., Nord, H., Mattsson, J. S. M., Ullenhag, G. J., Strell, C., Backman, M., La Fleur, L., Brunnström, H., Botling, J., & Micke, P. (2021). Evaluation of NTRK immunohistochemistry as a screening method for NTRK gene fusion detection in non-small cell lung cancer. Lung Cancer, 151, 53–59.
Lee, C. K., Davies, L., Wu, Y.-L., Mitsudomi, T., Inoue, A., Rosell, R., Zhou, C., Nakagawa, K., Thongprasert, S., Fukuoka, M., Lord, S., Marschner, I., Tu, Y.-K., Gralla, R. J., Gebski, V., Mok, T., & Yang, J. C. H. (2017). Gefitinib or erlotinib vs chemotherapy for EGFR mutation-positive lung cancer: individual patient data meta-analysis of overall survival. Journal of the National Cancer Institute, 109(6), 1–9.
Du, L., Li, X., Zhen, L., Chen, W., Mu, L., Zhang, Y., & Song, A. (2018). Everolimus inhibits breast cancer cell growth through PI3K/AKT/mTOR signaling pathway. Molecular Medicine Reports, 17(5), 7163–7169.
Zhang, Y. Z., Yang, J. Y., Wu, R. X., Fang, C., Lu, H., Li, H. C., Li, D. M., Zuo, H. L., Ren, L. P., Liu, X. Y., Xu, R., Wen, J. H., Huang, H. D., Hong, R., & Chen, Q. J. (2021). Network pharmacology–based identification of key mechanisms of **huang Pill in the treatment of triple-negative breast cancer stem cells. Frontiers in Pharmacology, 12(October), 1–16.
Hua, Y., Dai, X., Xu, Y., **ng, G., Liu, H., Lu, T., Chen, Y., & Zhang, Y. (2022). Drug repositioning: Progress and challenges in drug discovery for various diseases. European Journal of Medicinal Chemistry, 234, 114239.
Rabie, A. M. (2021). Two antioxidant 2,5-disubstituted-1,3,4-oxadiazoles (CoViTris2020 and ChloViD2020): Successful repurposing against COVID-19 as the first potent multi-target anti-SARS-CoV-2 drugs. New Journal of Chemistry, 45(2), 761–771.
Correia, A. S., Gärtner, F., & Vale, N. (2021). Drug combination and repurposing for cancer therapy: The example of breast cancer. Heliyon, 7(1), e05948.
Santos, R., Ursu, O., Gaulton, A., Bento, A. P., Donadi, R. S., Bologa, C. G., Karlsson, A., Lazikani, B. A., Hersey, A., Oprea, T. I., & Overington, J. P. (2016). A comprehensive map of molecular drug targets. Nature Reviews Drug Discovery, 16(1), 19–34.
Kannan, M. P., Sreeraman, S., Somala, C. S., Kushwah, R. B., Mani, S. K., Sundaram, V., & Thirunavukarasou, A. (2023). Advancement of targeted protein degradation strategies as therapeutics for undruggable disease targets. Future Medicinal Chemistry, 15(10), 867–883.
Arkin, M. R., Tang, Y., & Wells, J. A. (2014). Small-molecule inhibitors of protein-protein interactions: progressing toward the reality. Chemistry & Biology, 21(9), 1102–1114.
Lu, H., Zhou, Q., He, J., Jiang, Z., Peng, C., Tong, R., & Shi, J. (2020). Recent advances in the development of protein–protein interactions modulators: mechanisms and clinical trials. Signal Transduction and Targeted Therapy. https://doi.org/10.1038/s41392-020-00315-3
Ivanov, A. A., Khuri, F. R., & Fu, H. (2013). Targeting protein-protein interactions as an anti-cancer strategy. Trends in Pharmacological Sciences, 34(7), 393–400.
Díaz-Eufracio, B. I., Naveja, J. J., & Medina-Franco, J. L. (2018). Protein-protein interaction modulators for epigenetic therapies. Advances in protein chemistry and structural biology, 110, 65–84.
Souers, A. J., Leverson, J. D., Boghaert, E. R., Ackler, S. L., Catron, N. D., Chen, J., Dayton, B. D., Ding, H., Enschede, S. H., Fairbrother, W. J., Huang, D. C. S., Hymowitz, S. G., **, S., Khaw, S. L., Kovar, P. J., Lam, L. T., Lee, J., Maecker, H. L., Marsh, H. L., & Elmore, S. W. (2013). ABT-199, a potent and selective BCL-2 inhibitor, achieves antitumor activity while sparing platelets. Nature Medicine, 19(2), 202–208.
Vu, B., Wovkulich, P., Pizzolato, G., Lovey, A., Ding, Q., Jiang, N., Liu, J.-J., Zhao, C., Glenn, K., Wen, Y., Tovar, C., Packman, K., Vassilev, L., & Graves, B. (2013). Discovery of RG7112: A small-molecule MDM2 inhibitor in clinical development. ACS Medicinal Chemistry Letters, 4(5), 466–469.
Chen, X., Liu, P., Wang, Q., Li, Y., Fu, L., Fu, H., Zhu, J., Chen, Z., Zhu, W., **e, C., & Lou, L. (2018). DCZ3112, a novel Hsp90 inhibitor, exerts potent antitumor activity against HER2-positive breast cancer through disruption of Hsp90-Cdc37 interaction. Cancer Letters, 434, 70–80.
Murarka, S., Martín-Gago, P., Schultz-Fademrecht, C., Al Saabi, A., Baumann, M., Fansa, E. K., Ismail, S., Nussbaumer, P., Wittinghofer, A., & Waldmann, H. (2017). Development of pyridazinone chemotypes targeting the PDEδ prenyl binding site. Chemistry: A European Journal, 23(25), 6083–6093.
Chauhan, J., Wang, H., Yap, J. L., Sabato, P. E., Hu, A., Prochownik, E. V., & Fletcher, S. (2014). Discovery of methyl 4′-Methyl-5-(7-nitrobenzo[c][1,2,5]oxadiazol-4-yl)-[1,1′-biphenyl]-3-carboxylate, an improved small-molecule inhibitor of c-myc–max dimerization. ChemMedChem, 9(10), 2274–2285.
Du, R., Huang, C., Liu, K., Li, X., & Dong, Z. (2021). Targeting AURKA in cancer: molecular mechanisms and opportunities for cancer therapy. Molecular Cancer, 20(1), 1–27.
Campbell, J. D., Alexandrov, A., Kim, J., Wala, J., Berger, A. H., Pedamallu, C. S., Shukla, S. A., Guo, G., Brooks, A. N., Murray, B. A., Imielinski, M., Hu, X., Ling, S., Akbani, R., Rosenberg, M., Cibulskis, C., Ramachandran, A., Collisson, E. A., Kwiatkowski, D. J., … Meyerson, M. (2016). Distinct patterns of somatic genome alterations in lung adenocarcinomas and squamous cell carcinomas. Nature Genetics, 48(6), 607–616.
Wang, F., Su, Q., & Li, C. (2022). Identidication of novel biomarkers in non-small cell lung cancer using machine learning. Scientific Reports, 12(1), 1–15.
Bozhilova, L. V., Whitmore, A. V., Wray, J., Reinert, G., & Deane, C. M. (2019). Measuring rank robustness in scored protein interaction networks. BMC Bioinformatics, 20(1), 1–14.
Cai, Y., Mei, J., **ao, Z., Xu, B., Jiang, X., Zhang, Y., & Zhu, Y. (2019). Identification of five hub genes as monitoring biomarkers for breast cancer metastasis in silico. Hereditas, 156(1), 20.
Li, T., Gao, X., Han, L., Yu, J., & Li, H. (2018). Identification of hub genes with prognostic values in gastric cancer by bioinformatics analysis. World Journal of Surgical Oncology, 16(1), 1–12.
Ge, S. X., Jung, D., Jung, D., & Yao, R. (2020). ShinyGO: A graphical gene-set enrichment tool for animals and plants. Bioinformatics, 36(8), 2628–2629.
Sekaran, T. S. G., Kedilaya, V. R., Kumari, S. N., Shetty, P., & Gollapalli, P. (2021). Exploring the differentially expressed genes in human lymphocytes upon response to ionizing radiation: A network biology approach. Radiation Oncology Journal, 39(1), 48.
Udhaya Kumar, S., Thirumal Kumar, D., Bithia, R., Sankar, S., Magesh, R., Sidenna, M., Doss, C. G. P., & Zayed, H. (2020). Analysis of differentially expressed genes and molecular pathways in familial hypercholesterolemia involved in atherosclerosis: A systematic and bioinformatics approach. Frontiers in Genetics, 11, 1–16.
Liu, K., Kang, M., Li, J., Qin, W., & Wang, R. (2019). Prognostic value of the mRNA expression of members of the HSP90 family in non-small cell lung cancer. Experimental and Therapeutic Medicine, 17(4), 2657.
Lánczky, A., & Győrffy, B. (2021). Web-based survival analysis tool tailored for medical research (KMplot): Development and implementation. J Med Internet Res, 23(7), e27633.
Trott, O., & Olson, A. J. (2009). AutoDock vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. Journal of Computational Chemistry. https://doi.org/10.1002/jcc.21334
Tian, W., Chen, C., Lei, X., Zhao, J., & Liang, J. (2018). CASTp 3.0: Computed atlas of surface topography of proteins. Nucleic Acids Research, 46(W1), W363–W367.
Haredi Abdelmonsef, A. (2019). Computer-aided identification of lung cancer inhibitors through homology modeling and virtual screening. Egyptian Journal of Medical Human Genetics, 20(1), 1–14.
Bhardwaj, V. K., & Purohit, R. (2020). Targeting the protein-protein interface pocket of Aurora-A-TPX2 complex: Rational drug design and validation. Journal of Biomolecular Structure and Dynamics, 39(11), 3882–3891. https://doi.org/10.1080/07391102.2020.1772109
Bhimji, S. S., & Wallen, J. M. (2023). Lung adenocarcinoma. StatPearls. Retrieved from https://www.ncbi.nlm.nih.gov/books/NBK519578/
Chan, B. A., & Hughes, B. G. M. (2015). Targeted therapy for non-small cell lung cancer: Current standards and the promise of the future. Translational Lung Cancer Research., 4, 36–54.
Kaya, I. H., Al-Harazi, O., Kaya, M. T., & Colak, D. (2022). Integrated analysis of transcriptomic and genomic data reveals blood biomarkers with diagnostic and prognostic potential in non-small cell lung cancer. Frontiers in Molecular Biosciences, 9, 1.
Suratanee, A., & Plaimas, K. (2018). Network-based association analysis to infer new disease-gene relationships using large-scale protein interactions. PLoS ONE, 13(6), e0199435.
Ashok, G., Miryala, S. K., Anbarasu, A., & Ramaiah, S. (2021). Integrated systems biology approach using gene network analysis to identify the important pathways and new potential drug targets for Neuroblastoma. Gene Reports, 23, 101101.
Habib, I., Anjum, F., Mohammad, T., Sulaimani, M. N., Shafie, A., Almehmadi, M., Yadav, D. K., Sohal, S. S., & Hassan, M. I. (2022). Differential gene expression and network analysis in head and neck squamous cell carcinoma. Molecular and Cellular Biochemistry, 477(5), 1361–1370.
Thomas, P. D. (2017). The gene ontology and the meaning of biological function. Methods in molecular biology (Clifton, NJ), 1446, 15.
Cao, S., **ao, S., Zhang, J., & Li, S. (2023). Identification of the cell cycle characteristics of non-small cell lung cancer and its relationship with tumor immune microenvironment, cell death pathways, and metabolic reprogramming. Frontiers in Endocrinology, 14, 1147366.
Zadra, I., Jimenez-Delgado, S., Anglada-Girotto, M., Segura-Morales, C., Compton, Z. J., Janke, C., Serrano, L., Ruprecht, V., & Vernos, I. (2022). Chromosome segregation fidelity requires microtubule polyglutamylation by the cancer downregulated enzyme TTLL11. Nature Communications, 13(1), 1–16.
Nobili, S., Lapucci, A., Landini, I., Coronnello, M., Roviello, G., & Mini, E. (2020). Role of ATP-binding cassette transporters in cancer initiation and progression. Seminars in Cancer Biology, 60, 72–95.
Čermák, V., Dostál, V., Jelínek, M., Libusová, L., Kovář, J., Rösel, D., & Brábek, J. (2020). Microtubule-targeting agents and their impact on cancer treatment. European Journal of Cell Biology, 99(4), 151075.
Ke, R., Xu, Q., Li, C., Luo, L., & Huang, D. (2018). Mechanisms of AMPK in the maintenance of ATP balance during energy metabolism. Cell Biology International, 42(4), 384–392.
Bunning, A. R., & Gupta, M. L. (2023). The importance of microtubule-dependent tension in accurate chromosome segregation. Frontiers in Cell and Developmental Biology, 11, 1096333.
Levine, M. S., & Holland, A. J. (2018). The impact of mitotic errors on cell proliferation and tumorigenesis. Genes & Development, 32(9–10), 620.
Miralaei, N., Majd, A., Ghaedi, K., Peymani, M., & Safaei, M. (2021). Integrated pan-cancer of AURKA expression and drug sensitivity analysis reveals increased expression of AURKA is responsible for drug resistance. Cancer Medicine, 10(18), 6428.
Wang, J., Zheng, H., He, H., Meng, S., Han, Y., Su, Z., Yan, H., & Zhang, Y. (2022). TPX2 serves as a cancer susceptibility gene and is closely associated with the poor prognosis of endometrial cancer. Genetics Research. https://doi.org/10.1155/2022/5401106
Asteriti, I. A., Daidone, F., Colotti, G., Rinaldo, S., Lavia, P., Guarguaglini, G., & Paiardini, A. (2017). Identification of small molecule inhibitors of the Aurora-A/TPX2 complex. Oncotarget, 8(19), 32117.
Bayliss, R., Sardon, T., Vernos, I., & Conti, E. (2003). Structural basis of Aurora-A activation by TPX2 at the mitotic spindle. Molecular Cell, 12(4), 851–862.
Zhu, C., Menyhart, O., Gyorffy, B., & He, X. (2019). The prognostic association of SPAG5 gene expression in breast cancer patients with systematic therapy. BMC Cancer, 19(1), 1–12.
Rich, J. T., Neely, J. G., Paniello, R. C., Voelker, C. C. J., Nussenbaum, B., & Wang, E. W. (2010). A practical guide to understanding Kaplan–Meier curves. Otolaryngology Head and Neck Surgery: Official Journal of American Academy of Otolaryngology-Head and Neck Surgery, 143(3), 331.
Bhullar, K. S., Lagarón, N. O., McGowan, E. M., Parmar, I., Jha, A., Hubbard, B. P., & Rupasinghe, H. P. V. (2018). Kinase-targeted cancer therapies: progress, challenges and future directions. Molecular Cancer. https://doi.org/10.1186/s12943-018-0804-2
Krchniakova, M., Skoda, J., Neradil, J., Chlapek, P., & Veselska, R. (2020). Repurposing tyrosine kinase inhibitors to overcome multidrug resistance in cancer: A focus on transporters and lysosomal sequestration. International Journal of Molecular Sciences, 21(9), 3157.
Pottier, C., Fresnais, M., Gilon, M., Jérusalem, G., Longuespée, R., & Sounni, N. E. (2020). Tyrosine kinase inhibitors in cancer: Breakthrough and challenges of targeted therapy. Cancers, 12(3), 731.
Janeček, M., Rossmann, M., Sharma, P., Emery, A., Huggins, D. J., Stockwell, S. R., Stokes, J. E., Tan, Y. S., Almeida, E. G., Hardwick, B., Narvaez, A. J., Hyvönen, M., Spring, D. R., McKenzie, G. J., & Venkitaraman, A. R. (2016). Allosteric modulation of AURKA kinase activity by a small-molecule inhibitor of its protein-protein interaction with TPX2. Scientific Reports, 6, 1–12.
Todsaporn, D., Mahalapbutr, P., Poo-arporn, R. P., Choowongkomon, K., & Rungrotmongkol, T. (2022). Structural dynamics and kinase inhibitory activity of three generations of tyrosine kinase inhibitors against wild-type, L858R/T790M, and L858R/T790M/C797S forms of EGFR. Computers in Biology and Medicine, 147, 105787.
Choudhuri, S., Yendluri, M., Poddar, S., Li, A., Mallick, K., Mallik, S., & Ghosh, B. (2023). Recent advancements in computational drug design algorithms through machine learning and optimization. Kinases and Phosphatases, 1(2), 117–140.
Acknowledgements
The authors thank the organizers and management of CompBio22 for providing the Jetstream2 HPC facility and the Manipal Academy of Higher Education for providing Dr. TMA Pai scholarship to carry out this work.
Funding
Open access funding provided by Manipal Academy of Higher Education, Manipal.
Author information
Authors and Affiliations
Contributions
AM contributed to Conceptualization; Methodology; Software; and Data curation; Formal analysis; Investigation; Validation; Visualization; Writing of the original draft; Writing, reviewing, and editing of the manuscript. YPH contributed to Software; Data curation; Formal analysis; Investigation; Validation; Visualization; and Writing of the original draft. MKS contributed to Conceptualization; Methodology; Supervision; and Writing, reviewing, and editing of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors do not have any conflict of interest to declare.
Informed Consent
No informed consent is applicable to this study.
Research Involving Human/Animal Rights
No human or animal rights are applicable to this study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mukherjee, A., Yadav, P.H. & Mukunthan, K.S. Unveiling Potential Targeted Therapeutic Opportunities for Co-Overexpressed Targeting Protein for Xklp2 and Aurora-A Kinase in Lung Adenocarcinoma. Mol Biotechnol (2023). https://doi.org/10.1007/s12033-023-00879-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12033-023-00879-9