Abstract
Currently, ascorbic acid (AA) is widely used as a skin whitening material, but, AA, an unstable hydrophilic molecule, cannot penetrate the skin easily, due to the hydrophobic character of the stratum corneum. Therefore, we conjugated AA with hydrated zinc oxide—an inorganic matrix with positive surface charge, to improve the stability of AA. The metal-conjugated-ascorbic acid (ZnAA) was then combined with yeast vacuole through the vacuolar membrane proteins that relate to metal transportation to create an enhanced vacuole that contained ZnAA. The characteristics of vacuole with ZnAA (ZnAA_Vac) were next examined by various tests that included X-ray diffraction (XRD), Fourier transform infrared spectroscopy (FT–IR), Field emission scanning electron microscopy (FE–SEM), and energy-dispersive X-ray (EDX) analysis. Furthermore, the ability of ZnAA_Vac to degrade melanin was confirmed in both melanoma cell line B16F10, and the artificial human skin MelanoDerm. The results showed that ZnAA_Vac possessed a higher depigmenting effect than the wild-type vacuole or ascorbic acid by reducing 75% of melanin color. Interestingly, ZnAA_Vac was found to be harmless, and did not cause any cytotoxicity to the cells. Overall, ZnAA_Vac is expected to provide a robust, harmless, and effective whitening agent for the skin.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Melanin is one of the main factors that regulate the pigmentation in human skin, besides carotenoids and hemoglobin [1]. There are two main types of melanin: pheomelanin (yellow/red soluble polymer) and epidermal melanin (black/brown insoluble polymer) [2, 3], which is the principal melanin synthesized in melanosome inside the melanocytes in the skin [4]. Even though melanin could protect the skin from the effect of DNA photodamage by absorbing and scattering UV rays, which reduces their penetration through the epidermis [5], overproduction of melanin could cause several skin pigmentation disorders, which include melasma, post-inflammatory melanoderma, and dark spot on the skin (freckles) [6]. In recent years, substantial investment has been made in the skin whitening cosmetics industry, and despite the impact of Sars-CoV-2, the global market for skin whitening products in 2020 was appraised at around 8.6 billion dollars, and by 2027, may reach 12.3 billion dollars [7]. Nevertheless, skin lightening agents could also harm human health, including inducing high blood pressure, kidney disease [8], dermatoses [9], or more seriously, cancer [10]. Thus, a novel bleaching agent is needed that could degrade melanin without adverse effects on human health.
Ascorbic acid (AA) is a natural water-soluble vitamin (vitamin C) and a nutrient that is necessary for several processes in the human body [11]. First, AA is a potent reducing agent and antioxidant that decreases endothelial permeability, enhances vascular function, and weakens cellular apoptosis in the pathological phase [12]. In addition, as an antioxidant, AA is an essential factor that protects the skin from free radical damage, pollutants, toxins [13], and is involved in a collagen synthesis, which is crucial for curing injury [14]. Interestingly, AA is also a depigmenting factor that can reduce melanin by inhibiting the tyrosinase—an important enzyme involved in the melanogenesis process [15]. Most mammals could synthesize AA in the liver, but the non-functional l-gulono-gamma lactone oxidase (GULO) gene means that human can only absorb vitamin C by oral supplementation [11, 16].
In this study, we report a novel potential whitening agent that combines AA and zinc nitrate hexahydrate with yeast vacuoles that we have invented. AA is an unstable hydrophilic molecule, and cannot penetrate the skin easily, due to the hydrophobic character of the stratum corneum [17]. Therefore, the conjugation of AA with other agents, such as hydrated zinc oxide (Zn(NO3)2·6H2O)—an inorganic matrix with positive surface charge, could improve the stability of AA to overcome its limitation in the field of cosmetic [18]. Here, we developed an encapsulating approach of AA from the work of Yang et al. with a modification using yeast vacuoles [18]. Lysosome-like-vacuole in yeast was previously discovered to degrade the melanin [19, 20], and the combination of yeast vacuole and AA could strengthen the original AA to create a powerful whitening agent. In this study, a synthetic material containing ascorbic acid and zinc ion was achieved by a coprecipitation reaction. The characteristics of vacuole with ZnAA (ZnAA_Vac) were next examined by various tests that included X-ray diffraction (XRD), Fourier transform infrared spectroscopy (FT–IR), Field emission scanning electron microscopy (FE–SEM), and energy-dispersive X-ray (EDX) analysis. And, the melanin reduction test was conducted with melanoma cell line B16F10 and the three-dimensional human skin model MelanoDerm from MatTek.
Interestingly, yeast vacuole is the primary site for zinc sequestration, and has two vacuolar zinc transporters Zrt3 and Zrc1, which are known to be involved in zinc’s transportation vacuole [21,22,23]. Therefore, zinc ion in the synthesis product would drive the conjugation zinc ion-ascorbic acid (ZnAA) to the vacuole, which technique could be used to carry drug [24, 25]. In summary, we have created a novel whitening agent that could reduce melanin using vitamin C, zinc ion, and yeast vacuoles. The nanoparticles were non-toxic, stable and suitable for human skin treatment.
Materials and Methods
Cell Culture and Materials
B16F10 (KCLB 80080) cells were grown at 37 °C under 5% CO2. The cells were cultured in RPMI 1640 medium adding 10% NCS (Newborn calf serum, Cat No: 16010-159, GIBCO), 5 mM glutamine (Cat No: 56-85-9, Sigma Aldrich), 25 mM HEPES (Cat No: 7365-45-9, Sigma Aldrich) and 1% penicillin–streptomycin (Cat No: 15140-122, ThermoFisher). The media was exchanged every conducive day, and we treated ZnAA as the cells were grown at about 70%.
Saccharomyces cerevisiae s2805 (ATCC 208280) was kindly provided by the Korea Research Institute of Bioscience and Biotechnology (KRIBB). S. cerevisiae was grown to mid-log phase in YPD medium (1% yeast extract, 2% peptone, 2% glucose) at 30 °C in a shaking incubator at 180 rpm.
Encapsulation of Ascorbic Acid by Co Precipitation with Zinc Nitrate Hexahydrate (Zn(NO3)2·6H2O)
0.05 M zinc nitrate hexahydrate (Zn(NO3)2·6H2O, Cat No: 10196-18-6, Sigma Aldrich) was dissolved in distilled water (DW), then 0.1 M l-ascorbic acid (AA, Cat No: 50-81-7, Sigma Aldrich) was added in metal solution. The pH of the reaction solution was calibrated to pH 6.7 by adding NaOH with forceful stirring. To reduce the disintegration of AA and air contamination during the synthesis, nitrogen gas was flowed continuously through the reaction solvent. A white suspension appeared, and was kept at room temperature for 12 h, before being filtered and washed by DW. Finally, a vitamin C-hydrated zinc oxide hybrid was prepared, and called ZnAA [18, 26]. Figure 1 shows the synthetic process.
The encapsulation of ascorbic acid by coprecipitation with zinc nitrate hexahydrate (Zn(NO3)2·6H2O). Zn(NO3)2·6H2O was dissolved in distilled water, then l-ascorbic acid was added in metal solution, and the mixture was kept at room temperature for 12 h. then it was filtered and washed by DW, and freeze-dried
The characteristics of ZnAA were analyzed multi-purpose high performance X-ray diffractometer (XRD, X’PERT-PRO Powder, Malvern Panalytical, England) operating at 40 kV and 30 mA with Cu–Kα radiation (λ = 1.5404) for the fixed samples on a microscope slide. Furthermore, ZnAA was characterized by Fourier transform infrared spectroscopy (FT–IR, Perkin Elmer Spectrum GX, USA). Each FT–IR spectrum was collected at a resolution of 2 cm−1 from (4000 to 600) cm−1.
Vacuole Isolation from S. cerevisiae
Saccharomyces cerevisiae was cultured in YPD medium at 30 °C for 24 h. Cells were harvested and mixed with 0.1 M Tris-SO4 buffer (100 mM Tris-SO4 (pH 9.4) and 10 mM dithiothreitol (DTT)). This mixture was incubated at 30 °C (90 rpm) for 10 min, followed by centrifugation at 3000 rpm for 5 min. The supernatant was discarded, and the pellet was mixed with lyticase for 1 h to make the cell membrane flexible. After that, the mixture was centrifuged at 3000 rpm for 5 min, and the pellet was washed twice with Sorbitol K + phosphate buffer. For the next step, breaking buffer (20 mM Tris–Cl (pH 7.4), 0.6 mM Sorbitol, and 1 mM phenyl methane sulfonyl fluoride (PMSF)) were mixed with the pellet, followed by ultra-sonication at 40 W for 30 min (20 s on/10 s off pulse) on ice. The mixture was centrifuged at 3000 rpm for 10 min, and the supernatant was discarded. The pellet was remixed with breaking buffer, followed by a second ultra-sonication for 20 min (10 s on/10 s off pulse). After centrifugation at 500×g for 5 min, the supernatant was collected. The remaining pellet was subjected to a second centrifugation at 20,000×g for 30 min, and the pellet containing vacuoles from cells was collected [27].
Liquid Chromatography–Mass Spectrometry (LC–MS)
Saccharomyces cerevisiae was exposed individually to AA and ZnAA for 24 h. After exposing samples to the cells, the remaining AA and ZnAA in the medium would be determined. The analysis used column C18 (150 mm × 4.6 mm) which was maintained at 20 °C. The LC–MS data were collected, and processed by MassHunter software (Agilent).
Field Emission Scanning Electron Microscope (FE–SEM) with Energy-Dispersive X-ray (EDX) Spectroscopy
The morphology of ZnAA was observed by Field emission scanning electron microscopy (FE–SEM) (Carl Zeiss SUPRA, Germany). Samples were softened with DW, and were then prepared using the freeze-drying process [28]. While taking the image of ZnAA, electron was immediately analyzed by EDX spectroscopy.
Melanin Degradation Test
After being exposed to various samples, the melanoma cell line B16F10 was washed twice with Phosphate-buffered saline (PBS); after that, it was dissolved in NaOH with 10% DMSO, and heated at 80 °C for 1 h. The amount of melanin was determined by spectrophotometry at the absorbance of 400 nm [20].
Skin Whitening Test with Human Skin Model MelanoDerm
MelanoDerm (MatTek Corp., Ashland, NA) was used for skin whitening test as a viable three-dimensional human skin that includes normal melanocytes and keratinocytes. The cells are derived from African–American (MEL-B), Asian (MEL-A), or white (MEL-C) donors. MEL-B tissues were used in this study and were maintained in the NMM-113 medium. The MelanoDerm tissues were fed daily with 5 mL of fresh NMM-113 and various vacuole concentrations or ZnAA_Vac for 12 days [29].
Measurement of Melanin Content
Melanin content was measured as described in the previous work [29]. The skin tissue was taken out of the culture dish after 12 days, then moved in an Eppendorf tube and homogenized for 1 min in lysis buffer (20 mM Tris–Cl pH 7.4, 1 mM EDTA pH 8.8, 1 mM EGTA pH 8.5, 1% v/v Triton X-100). After homogenization, 20 μL protease K (5 mg/mL) was supplied, and the cells incubated at 45 °C overnight. The next day, 20 μL protease K was added for 4 h. Next, 50 μL 0.5 M sodium carbonate and 10 μL 30% H2O2 were added, and incubated at 80 °C for 30 min. After that, 100 μL of a 2 chloroform: 1 MtOH mixture was added, followed by centrifugation at 10,000×g for 10 min. The sample supernatant was used to determine melanin content by spectrophotometry at the absorbance of 405 nm.
Cell Viability
The cell viability test for MelanoDerm was described previously [30]. The tissues were washed twice by DPBS, before adding 300 μL MTT solution. All tissue samples were covered to protect from light and incubated 37 °C for 3 h. After being cleaned by sterilized gauze, the tissue samples were moved to a new plate, and then isopropanol and 0.04 M HCl were added. At the next step, the samples were stirred at 120 rpm without air in the anaerobic bag at RT for 2 h. Finally, the solutions were moved to an Eppendorf tube, and measured by ELISA plate reader on 96-well plates at the absorbance of 570 nm.
Data Analysis
All data were obtained from three independent samples, run simultaneously for error analysis, and the results are reported with the standard deviation. The data were analyzed using Sigma Plot (SPS, Chicago, IL. USA), and a p-value < 0.05 was considered significant.
Results
Characterizations of the Synthetic ZnAA
The properties of the synthesis product between ascorbic acid and zinc nitrate hexahydrate were analyzed by X-ray diffraction (XRD) and Fourier transform infrared spectroscopy (FT–IR).
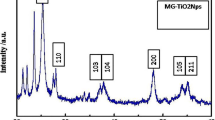
Figure 1 presents the encapsulating process of AA with zinc ion, while Fig. 2 provides the XRD patterns that show the crystalline structure through the specific diffraction peaks of ZnO (peaks (002), (110), and (201) observed at 2θ = (34.1, 58.8, and 69.2)°, respectively), as reported previously [31,32,33,34,35].
Figure 3 displays the characteristics of ZnAA by FT–IR and FE–SEM. Figure 3a shows that there were strong absorption bands in the ascorbic acid sample at (1686 and 1773) cm−1, which reflect the C=C and C=O stretching vibrations, respectively, while several bands from (1000 to 1500) cm−1 are related to C–O–C, CH2 scissoring, and other C–H modes, as reported previously [36, 37]. On the other hand, Fig. 3b depicts the FT–IR spectra of ZnAA in which a vibration band appears at 503 cm−1, indicating the formation of ZnO. Additionally, the peak at 3452 cm−1 shows O–H stretch, while peaks at (1397 and 1496) cm−1 are due to C–O–H bending vibrations. These FT–IR spectra results are similar to previous studies that analyzed the characteristics of ZnAA or ZnO [32, 33, 38, 39].
Characterizations of the synthetic ZnAA. The characteristics of ZnAA by FT–IR and FE–SEM. a FT–IR spectra of ascorbic acid; b FT–IR spectra of ZnAA; c the morphology of ZnAA by FE–SEM. ZnAA was characterized by Fourier transform infrared spectroscopy (FT–IR, Perkin Elmer Spectrum GX, USA). Each FT–IR spectrum was collected at a resolution of 2 cm−1 from (4000 to 600) cm−1. The morphology of ZnAA was observed by Field emission scanning electron microscopy (FE–SEM) (Carl Zeiss SUPRA, Germany)
Morphology of ZnAA with EDX Analysis
Figure 3C shows the morphology of ZnAA particle taken by FE–SEM, in which ZnAA is formed as a very tiny particle at (19–30) nm. Furthermore, Table 1 describes the ZnAA surface components using energy-dispersive X-ray (EDX) spectroscopy, where zinc ion is presented in the synthesis product, while not in the AA sample.
Vacuolar Targeting of ZnAA in Saccharomyces cerevisiae
Saccharomyces cerevisiae was exposed individually to AA and ZnAA for 24 h. After exposing samples to the cells, to verify the existence of ZnAA in vacuole, the remaining ascorbic acid and ZnAA were measured by LC–MS. Table 2 shows that no ZnAA remained in the yeast cytosol and culture media, while (71 and 6.45) % AA remained in the media and cytosol, respectively. On the other hand, the 99.9% of ZnAA found in the vacuole affirmed that ZnAA was targeted to the vacuole as expected, while only 22.55% ascorbic acid was delivered to the vacuole. This phenomenon confirmed the role of zinc ion in the vacuolar targeting approach.
Melanin Reduction in Melanocytes by ZnAA-Vacuole
In this experiment, the vacuole that contained ZnAA was used to treat melanocytes for the melanin reduction test, and called ZnAA_Vac. ZnAA_Vac were isolated from S. cerevisiae, and 5% (wt/vol) vacuoles were exposed to melanocyte for 24 h. “Wild-type” vacuole was used to compare with the activity of ZnAA_Vac, while 2% ascorbic acid was the positive control, due to its depigmenting effect [40, 41].
The result in Fig. 4 shows no distinction in the melanin-reducing effect between 2% ascorbic acid, 5% vacuole, and vacuole that possessed 100 ppm ZnAA, which degraded approximatively 24% of melanin. Meanwhile, vacuole with 1000 ppm ZnAA degraded around 75.5% melanin; thus, 1000 ppm ZnAA provided a higher reducing ability. In fact, ZnAA without vacuole also showed a high effect of degrading melanin; however, it also caused high cytotoxicity that killed many cells (Fig. 4a).
Cell toxicity and melanin degradation test. a The cytotoxicity of ascorbic acid, vacuole and ZnAA_Vac was confirmed by MTT assay; b and, the melanin reduction test with vacuole and ZnAA_Vac. PBS was used as a negative control and ascorbic acid as a positive control. (n.s not significant; *p < 0.05; **p < 0.01)
Depigmentation Effect of ZnAA_Vac on Artificial Human Skin MelanoDerm
The depigmentation efficiency of ZnAA_Vac depicted in Fig. 5 was observed at day 0 and day 12 of the treatment. Figure 5a presents the effect of several vacuole’s concentrations from wild-type yeast on the artificial human skin after 12 h of treatment, while Fig. 5b shows the depigmenting effect of ZnAA_Vac. After treatment with (5 and 10)% vacuole or all samples exposed to ZnAA_Vac, the color reduction is clearly evident to the naked eye. Nevertheless, to precisely assess the depigmenting effect, melanin contents were measured after 12 days of treatment.
Skin whitening test with human skin model MelanoDerm. Plan view observation of MelanoDerm after being exposed to a various concentrations of vacuole; b vacuoles that possessed (100, 500, and 1000) ppm of ZnAA. c Melanin amount after 12 days of treatment with several concentrations of vacuole and ZnAA_Vac. The data were analyzed using Sigma Plot, and a p-value < 0.05 was considered significant. ZnAA_Vac 100 ppm, ZnAA_Vac 500 ppm, ZnAA_Vac 1000 ppm: vacuoles that contained ZnAA at (100, 500, and 1,000) ppm, respectively
Figure 5c shows that 2% ascorbic acid exhibited a high depigmenting ability by decreasing around 33% of melanin, while the vacuole's maximum effect was approximately 14% (vacuole (5 and 10)%). Regarding ZnAA_Vac, vacuole that contained 100 ppm ZnAA did not show a high depigmentation effect; however, the melanin degradation increased along with the augmentation of ZnAA dose. More precisely, ZnAA_Vac with 500 ppm ZnAA reduced 31% of melanin, while ZnAA_Vac with 1000 ppm ZnAA provided a superb ability by degrading 75% melanin after 12 days. These results were identical with the melanin reduction test in melanocytes, in which the ZnAA_Vac 1000 ppm had degraded 75.5% melanin (Fig. 4).
Discussion
In this study, we combine AA with yeast vacuoles that we have invented, and to improve skin penetration when used as a cosmetic, we conjugated AA with hydrated zinc oxide (Zn(NO3)2·6H2O). Through the X-ray diffraction (XRD) and Fourier transform infrared spectroscopy (FT–IR) analysis, it was confirmed that the synthetic ZnAA (zinc ion-ascorbic acid) is consistent with the previously reported properties of ZnAA or ZnO [27, 28, 34, 35]. The formation of ZnO from the chemical precipitation of zinc nitrate hexahydrate, sodium hydroxide, and ascorbic acid was commonly investigated before in which vitamin C had occupied an essential role in ZnO crystal formation [32, 33, 42]. The formation of ZnO can also be confirmed through the reduction of carbonic ion in EDX analysis. To combine ZnAA with yeast vacuoles by internalization into the vacuole, we incubated yeast with ZnAA. As mentioned before, the vacuole is the primary site for zinc sequestration, due to the two vacuolar zinc transporters Zrt3 and Zrc1; thus, the conjugation ZnAA is expected to agglomerate at the vacuole [21,22,23]. Recently, considering the hazardous nature of the chemical synthesis of nano particles, the current investigation is focusing on the green synthesis using eco-friendly biological extracts [43,44,45,46].
The melanin reduction test of ZnAA_Vac, the vacuole that contained ZnAA, was conducted with melanoma cell line B16F10 and the three-dimensional human skin model MelanoDerm from MatTek. the highest depigmentation effect of ZnAA_Vac in both melanoma cell lines and artificial human skin cells demonstrated our precise approach when conjugating ascorbic acid, Zn ion, and vacuole. After being conjugated with the vacuole, ZnAA became an efficient and harmless whitening agent, while individual vacuole or ascorbic acid displayed a lower effect, and ZnAA without vacuole caused high cytotoxicity that killed a lot of cells. Therefore, the encapsulation approach of ZnAA with vacuoles could provide a safer and more robust agent for melanin degradation. According to recent studies, the use of biological compounds for the synthesis of inorganic nanoparticles has shown promising results such as cost-effective and environmentally friendly compared to the conventional nanoparticles synthesis pathway [47,48,49,50]. Previously, it was reported that a combination of tyrosine, zinc and vitamin C has been shown to increase the bioavailability of vitamin C 20-times vis-a-vis using just vitamin C [51]. Like these researches, many studies using ZnO to improve the biocompatibility and stability of AA have been reported. However, in this paper, the whitening efficacy of these complex nanoparticles was verified using melanocytes and artificial skin tissue for the first time, therefore it includes novel and meaningful results can be used in the pharmaceutical and cosmetic fields.
In this study, vacuole as a carrier to protect and enhance the activity of metal-conjugated-ascorbic acid was reported for the first time. Ascorbic acid synthesized with zinc ion was transported to vacuole through the vacuolar membrane protein, and then the vacuole that contained ZnAA was isolated, which we called ZnAA_Vac. ZnAA_Vac was found to be safe for the cell without cytotoxicity, and provided a considerable effect to degrade the melanin content in both melanocyte and artificial human skin cell.
Conclusions
In this study, vacuole as a carrier to protect and enhance the activity of metal-conjugated-ascorbic acid was reported for the first time. Ascorbic acid synthesized with zinc ion was transported to vacuole through the vacuolar membrane protein, and then the vacuole that contained ZnAA was isolated, which we called ZnAA_Vac. ZnAA_Vac was found to be safe for the cell without cytotoxicity, and provided a considerable effect to degrade the melanin content in both melanocyte and artificial human skin cells. The discovery of ZnAA_Vac could open up a new way to develop a state-of-the-art whitening agent that effectively reduce melanin, without damage to the skin, but in order to be commercialized, additional clinical and in vivo experiments must be conducted, and research on materialization is also required.
References
Lin, J. Y., & Fisher, D. E. (2007). Melanocyte biology and skin pigmentation. Nature, 445(7130), 843–850. https://doi.org/10.1038/nature05660
Lambert, M. W., Maddukuri, S., Karanfilian, K. M., Elias, M. L., & Lambert, W. C. (2019). The physiology of melanin deposition in health and disease. Clinics in Dermatology, 37(5), 402–417. https://doi.org/10.1016/j.clindermatol.2019.07.013
Pillaiyar, T., Namasivayam, V., Manickam, M., & Jung, S.-H. (2018). Inhibitors of melanogenesis: An updated review. Journal of medicinal chemistry, 61(17), 7395–7418. https://doi.org/10.1021/acs.jmedchem.7b00967
Karsten, A. E., & Smit, J. E. (2012). Modeling and verification of melanin concentration on human skin type. Photochemistry and photobiology, 88(2), 469–474. https://doi.org/10.1111/j.1751-1097.2011.01044.x
Brenner, M., & Hearing, V. J. (2008). The protective role of melanin against UV damage in human skin. Photochemistry and photobiology, 84(3), 539–549. https://doi.org/10.1111/j.1751-1097.2007.00226.x
Pillaiyar, T., Manickam, M., & Namasivayam, V. (2017). Skin whitening agents: Medicinal chemistry perspective of tyrosinase inhibitors. Journal of Enzyme Inhibition and Medicinal Chemistry, 32(1), 403–425. https://doi.org/10.1080/14756366.2016.1256882
Markets, R. A. (2020) Skin Lighteners - Global Market Trajectory & Analytics. Journal, https://www.researchandmarkets.com/reports/1056077/skin_lighteners_global_market_trajectory_and?utm_source=GNOM&utm_medium=PressRelease&utm_code=ncgpnq&utm_campaign=1416788+-%2412.3+Billion+Skin+Lighteners+Market+Outlook%2c+2027&utm_exec=joca220prd.
Okoye, O. (2019). Skin lightening and chronic kidney disease: A tale or a fact? Tropical Journal of Nephrology, 14(1), 15–23.
Vashi, N. A., Patzelt, N., Wirya, S., Maymone, M. B., & Kundu, R. V. (2018). Dermatoses caused by cultural practices: Cosmetic cultural practices. Journal of the American Academy of Dermatology, 79(1), 19–30. https://doi.org/10.1016/j.jaad.2017.06.160
Burger, P., Landreau, A., Azoulay, S., Michel, T., & Fernandez, X. (2016). Skin whitening cosmetics: Feedback and challenges in the development of natural skin lighteners. Cosmetics, 3(4), 36. https://doi.org/10.3390/cosmetics3040036
van Gorkom, G. N., Lookermans, E. L., Van Elssen, C. H., & Bos, G. M. (2019). The effect of vitamin C (ascorbic acid) in the treatment of patients with cancer: A systematic review. Nutrients, 11(5), 977. https://doi.org/10.3390/nu11050977
Moskowitz, A., Andersen, L. W., Huang, D. T., Berg, K. M., Grossestreuer, A. V., Marik, P. E., Sherwin, R. L., Hou, P. C., Becker, L. B., & Cocchi, M. N. (2018). Ascorbic acid, corticosteroids, and thiamine in sepsis: A review of the biologic rationale and the present state of clinical evaluation. Critical Care, 22(1), 283. https://doi.org/10.1186/s13054-018-2217-4
Chambial, S., Dwivedi, S., Shukla, K. K., John, P. J., & Sharma, P. (2013). Vitamin C in disease prevention and cure: An overview. Indian Journal of Clinical Biochemistry, 28(4), 314–328. https://doi.org/10.1007/s12291-013-0375-3
Pielesz, A., Biniaś, D., Bobiński, R., Sarna, E., Paluch, J., & Waksmańska, W. (2017). The role of topically applied l-ascorbic acid in ex-vivo examination of burn-injured human skin. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, 185, 279–285. https://doi.org/10.1016/j.saa.2017.05.055
Serrano, G., Almudéver, P., Serrano, J.-M., Milara, J., Torrens, A., Expósito, I., & Cortijo, J. (2015). Phosphatidylcholine liposomes as carriers to improve topical ascorbic acid treatment of skin disorders. Clinical, Cosmetic and Investigational Dermatology. https://doi.org/10.2147/CCID.S90781
Van Gorkom, G. N., Klein Wolterink, R. G., Van Elssen, C. H., Wieten, L., Germeraad, W. T., & Bos, G. M. (2018). Influence of vitamin C on lymphocytes: An overview. Antioxidants, 7(3), 41.
Al-Niaimi, F., & Chiang, N. Y. Z. (2017). Topical vitamin C and the skin: Mechanisms of action and clinical applications. The Journal of Clinical and Aesthetic Dermatology, 10(7), 14. https://doi.org/10.3390/antiox7030041
Yang, J.-H., Lee, S.-Y., Han, Y.-S., Park, K.-C., & Choy, J.-H. (2003). Efficient transdermal penetration and improved stability of l-ascorbic acid encapsulated in an inorganic nanocapsule. Bulletin-Korean Chemical Society, 24(4), 499–503. https://doi.org/10.5012/bkcs.2003.24.4.499
Yoon, J., Kim, Y.-H., Ahn, J.-Y., Lee, H.-C., Oh, S.-J., Chung, B.-W., & Min, J. (2015). Melanin reduction by peroxidase activity in lysosome-related organelle extracts from hen egg whites, HeLa cells, and Saccharomyces cerevisiae. Molecular & Cellular Toxicology, 11(4), 441–447. https://doi.org/10.1007/s13273-015-0047-x
Park, D. J., Kim, Y.-H., & Min, J. (2017). The remarkable efficiency of melanin reduction by using functional organelle, lysosomes. Journal of Nanoscience and Nanotechnology, 17(7), 4638–4642. https://doi.org/10.1166/jnn.2017.13786
Simm, C., Lahner, B., Salt, D., LeFurgey, A., Ingram, P., Yandell, B., & Eide, D. J. (2007). Saccharomyces cerevisiae vacuole in zinc storage and intracellular zinc distribution. Eukaryotic cell, 6(7), 1166–1177. https://doi.org/10.1128/EC.00077-07
Kawamata, T., Horie, T., Matsunami, M., Sasaki, M., & Ohsumi, Y. (2017). Zinc starvation induces autophagy in yeast. Journal of Biological Chemistry, 292(20), 8520–8530. https://doi.org/10.1074/jbc.M116.762948
Zhao, Y.-Y., Cao, C.-L., Liu, Y.-L., Wang, J., Li, J., Li, S.-Y., & Deng, Y. (2020). Identification of the genetic requirements for zinc tolerance and toxicity in Saccharomyces cerevisiae. G3: Genes, Genomes, Genetics, 10(2), 479–488. https://doi.org/10.1534/g3.119.400933
Gujrati, V., Lee, M., Ko, Y.-J., Lee, S., Kim, D., Kim, H., Kang, S., Lee, S., Kim, J., & Jeon, H. (2016). Bioengineered yeast-derived vacuoles with enhanced tissue-penetrating ability for targeted cancer therapy. Proceedings of the National Academy of Sciences, 113(3), 710–715. https://doi.org/10.1073/pnas.1509371113
Choi, W., Heo, M. Y., Kim, S. Y., Wee, J.-H., Kim, Y.-H., & Min, J. (2020). Encapsulation of daunorubicin into Saccharomyces cerevisiae-derived lysosome as drug delivery vehicles for acute myeloid leukemia (AML) treatment. Journal of Biotechnology, 308, 118–123. https://doi.org/10.1016/j.jbiotec.2019.12.008
Chikere, C., Faisal, N., Lin, P., & Fernandez, C. (2019). Zinc oxide nanoparticles modified-carbon paste electrode used for the electrochemical determination of Gallic acid. Journal, 1310, 012008. https://doi.org/10.1088/1742-6596/1310/1/012008
Nguyen, N.-T., Park, R.-M., Kim, Y.-H., & Min, J. (2018). Detection and discrimination of Shigella sonnei and Shigella flexneri based on vacuolar responses in Saccharomyces cerevisiae. Journal of biotechnology, 287, 1–7. https://doi.org/10.1016/j.jbiotec.2018.09.009
Baskin, T. I., Orr, T. J., Jercinovic, M., & Yoshida, M. (2014). Sample preparation for scanning electron microscopy: The surprising case of freeze drying from tertiary butanol. Microscopy Today, 22(3), 36–39. https://doi.org/10.1017/S1551929514000522
Park, D. J., Jeon, G., Bang, S. H., Kim, S. Y., Wee, J.-H., Kim, Y.-H., & Min, J. (2020). Cellular lysosomes’ activity for melanin reduction on artificial skin tissue. Molecular Biotechnology, 62(3), 185–191. https://doi.org/10.1007/s12033-019-00235-w
Fischer, A. H., Jacobson, K. A., Rose, J., & Zeller, R. (2008). Hematoxylin and eosin staining of tissue and cell sections. Cold Spring Harbor Protocols. https://doi.org/10.1101/pdb.prot4986
Yusof, N. A. A., Zain, N. M., & Pauzi, N. (2019). Synthesis of chitosan/zinc oxide nanoparticles stabilized by chitosan via microwave heating. Bulletin of Chemical Reaction Engineering & Catalysis, 14(2), 450–458. https://doi.org/10.9767/bcrec.14.2.3319.450-458
Ghahramaninezhad, M., & Niknam Shahrak, M. (2019). Facile and efficient self-template synthesis of core-coronal-shell ZnO@ ZIF-8 nanohybrid using ascorbic acid and its application for arsenic removal. Inorganic Chemistry Research. https://doi.org/10.22036/icr.2019.191125.1051
Kumar, H. N., Mohana, N. C., Nuthan, B., Ramesha, K., Rakshith, D., Geetha, N., & Satish, S. (2019). Phyto-mediated synthesis of zinc oxide nanoparticles using aqueous plant extract of Ocimum americanum and evaluation of its bioactivity. SN Applied Sciences, 1(6), 651. https://doi.org/10.1007/s42452-019-0671-5
Sadollahkhani, A., Kazeminezhad, I., Lu, J., Nur, O., Hultman, L., & Willander, M. (2014). Synthesis, structural characterization and photocatalytic application of ZnO@ ZnS core–shell nanoparticles. RSC Advances, 4(70), 36940–36950. https://doi.org/10.1039/C4RA05247A
Cho, S., Jang, J.-W., Park, H. J., Jung, D.-W., Jung, A., Lee, J. S., & Lee, K.-H. (2012). A method for synthesizing ZnO–carbonaceous species nanocomposites, and their conversion to quasi-single crystal mesoporous ZnO nanostructures. RSC Advances, 2(2), 566–572. https://doi.org/10.1039/C1RA00661D
Ahmed, I., Haque, A., Bhattacharyya, S., Patra, P., Plaisier, J. R., Perissinotto, F., & Bal, J. K. (2018). Vitamin C/stearic acid hybrid monolayer adsorption at air–water and air–solid interfaces. ACS Omega, 3(11), 15789–15798. https://doi.org/10.1021/acsomega.8b02235
Panicker, C. Y., Varghese, H. T., & Philip, D. (2006). FT-IR, FT-Raman and SERS spectra of Vitamin C. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, 65(3–4), 802–804. https://doi.org/10.1016/j.saa.2005.12.044
Nagaraju, G., Prashanth, S., Shastri, M., Yathish, K., Anupama, C., & Rangappa, D. (2017). Electrochemical heavy metal detection, photocatalytic, photoluminescence, biodiesel production and antibacterial activities of Ag–ZnO nanomaterial. Materials Research Bulletin, 94, 54–63. https://doi.org/10.1016/j.materresbull.2017.05.043
Lefatshe, K., Muiva, C. M., & Kebaabetswe, L. P. (2017). Extraction of nanocellulose and in-situ casting of ZnO/cellulose nanocomposite with enhanced photocatalytic and antibacterial activity. Carbohydrate Polymers, 164, 301–308. https://doi.org/10.1016/j.carbpol.2017.02.020
Yussif, N. M., Rahman, A. R. A., & ElBarbary, A. (2019). Minimally invasive non-surgical locally injected vitamin C versus the conventional surgical depigmentation in treatment of gingival hyperpigmentation of the anterior esthetic zone: A prospective comparative study. Clinical Nutrition Experimental, 24, 54–65. https://doi.org/10.1016/j.yclnex.2018.12.003
Sanadi, R. M., & Deshmukh, R. S. (2020). The effect of vitamin C on melanin pigmentation—A systematic review. Journal of Oral and Maxillofacial Pathology, 24(2), 374. https://doi.org/10.4103/jomfp.JOMFP_207_20
Cho, S., Jeong, H., Park, D.-H., Jung, S.-H., Kim, H.-J., & Lee, K.-H. (2010). The effects of vitamin C on ZnO crystal formation. CrystEngComm, 12(3), 968–976. https://doi.org/10.1039/B916750A
Chia, S. R., Ahmad, M., Sultana, S., Zafar, M., Asif, S., Bokhari, A., Nomanbhay, S., Mubashir, M., Khoo, K. S., & Show, P. L. (2022). Green synthesis of biodiesel from Citrus medica seed oil using green nanoparticles of copper oxide. Fuel, 323, 124285. https://doi.org/10.1016/j.fuel.2022.124285
Ahmad, M., Asif, S., Klemeš, J. J., Mubashir, M., Bokhari, A., Sultana, S., Mukhtarf, A., Zafara, M., Bazmi, A. A., Ullah, S., Khan, M. S., Koyande, A. K., Mofijur, M., & Show, P. L. (2022). Conversion of the toxic and hazardous Zanthoxylum armatum seed oil into methyl ester using green and recyclable silver oxide nanoparticles. Fuel, 310, 122296. https://doi.org/10.1016/j.fuel.2021.122296
Karim, S. S., Murtaza, Z., Farrukh, S., Umer, M. A., Ali, S. S., Younas, M., & Show, P. L. (2022). Future advances and challenges of nanomaterial-based technologies for electromagnetic interference-based technologies: A review. Environmental Research, 205, 112402. https://doi.org/10.1016/j.envres.2021.112402
Mubashir, M., Ashena, R., Bokhari, A., Mukhtar, A., Saqib, S., Ali, A., Saidur, R., Khoo, K. S., Ng, H. S., Karimi, F., Karaman, C., & Show, P. L. (2022). Effect of process parameters over carbon-based ZIF-62 nano-rooted membrane for environmental pollutants separation. Chemosphere, 291, 133006. https://doi.org/10.1016/j.chemosphere.2021.133006
Chan, S. S., Low, S. S., Chew, K. W., Ling, T. C., Rinklebe, J., Juan, J. C., Ng, P. E., & Show, P. L. (2022). Prospects and environmental sustainability of phyconanotechnology: A review on algae-mediated metal nanoparticles synthesis and mechanism. Environmental Research, 212, 113140. https://doi.org/10.1016/j.envres.2022.113140
Lau, Z. L., Low, S. S., Ezeigwe, E. R., Chew, K. W., Chai, W. S., Bhatnagar, A., Yap, Y. J., & Show, P. L. (2022). A review on the diverse interactions between microalgae and nanomaterials: Growth variation, photosynthesis performance and toxicity. Bioresource Technology, 351, 127048. https://doi.org/10.1016/j.biortech.2022.127048
Mubashir, M., Fong, Y. Y., Leng, C. T., Keong, L. K., & Jusoh, N. (2021). Nanocomposite catalytic membranes for energy production: Advances and challenges. Handbook of Nanotechnology Applications. https://doi.org/10.1016/B978-0-12-821506-7.00010-7
Ali, A., Mubashir, M., Abdulrahman, A., & Phelan, P. E. (2022). Ultra-permeable intercalated metal-induced microporous polymer nano-dots rooted smart membrane for environmental remediation. Chemosphere, 306, 135482. https://doi.org/10.1016/j.chemosphere.2022.135482
Traikovich, & Steven, S. (1999). Use of topical ascorbic acid and its effects on Photo damaged skin topography. Archives of Otorhinolaryngology-Head & Neck Surgery, 125(10), 1091–1098. https://doi.org/10.1001/archotol.125.10.1091
Acknowledgements
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (Grant No. 2020R1A6A1A06046235).
Author information
Authors and Affiliations
Contributions
GJ, HC, D-JP and N-TN have contributed equally to this work. GJ: Conceptualization, Methodology, Investigation, Visualization, Writing—original draft, Writing—review & editing, HC: Methodology, Investigation, Visualization, Writing—original draft, Writing—review & editing, DJP: Conceptualization, Investigation, Methodology, Visualization, Writing—review & editing, N-TN: Investigation, Writing—review & editing, YHK: Supervision, Writing—review & editing, JM: Supervision, Methodology, Writing—review & editing. The manuscript was written through the contributions of all authors. All authors have given approval to the final version of the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
Gyeongchan Jeon, Hyo** Choi, Dong-Jun Park, Ngoc-Tu Nguyen, Yang-Hoon Kim and Jiho Min declare that they have no conflict of interest.
Informed Consent
Informed consent was obtained from all individual participants included in the study
Research Involving Human Participants and/or Animals
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jeon, G., Choi, H., Park, DJ. et al. Melanin Treatment Effect of Vacuoles-Zinc Oxide Nanoparticles Combined with Ascorbic Acid. Mol Biotechnol 65, 1119–1128 (2023). https://doi.org/10.1007/s12033-022-00608-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12033-022-00608-8