Abstract
Medullary thyroid carcinoma (MTC) is a C-cell-derived epithelial neuroendocrine neoplasm. With the exception of rare examples, most are well-differentiated epithelial neuroendocrine neoplasms (also known as neuroendocrine tumors in the taxonomy of the International Agency for Research on Cancer [IARC] of the World Health Organization [WHO]). This review provides an overview and recent evidence-based data on the molecular genetics, disease risk stratification based on clinicopathologic variables including molecular profiling and histopathologic variables, and targeted molecular therapies in patients with advanced MTC. While MTC is not the only neuroendocrine neoplasm in the thyroid gland, other neuroendocrine neoplasms in the thyroid include intrathyroidal thymic neuroendocrine neoplasms, intrathyroidal parathyroid neoplasms, and primary thyroid paragangliomas as well as metastatic neuroendocrine neoplasms. Therefore, the first responsibility of a pathologist is to distinguish MTC from other mimics using appropriate biomarkers. The second responsibility includes meticulous assessment of the status of angioinvasion (defined as tumor cells invading through a vessel wall and forming tumor-fibrin complexes, or intravascular tumor cells admixed with fibrin/thrombus), tumor necrosis, proliferative rate (mitotic count and Ki67 labeling index), and tumor grade (low- or high-grade) along with the tumor stage and the resection margins. Given the morphologic and proliferative heterogeneity in these neoplasms, an exhaustive sampling is strongly recommended. Routine molecular testing for pathogenic germline RET variants is typically performed in all patients with a diagnosis of MTC; however, multifocal C-cell hyperplasia in association with at least a single focus of MTC and/or multifocal C-cell neoplasia are morphological harbingers of germline RET alterations. It is of interest to assess the status of pathogenic molecular alterations involving genes other than RET like the MET variants in MTC families with no pathogenic germline RET variants. Furthermore, the status of somatic RET alterations should be determined in all advanced/progressive or metastatic diseases, especially when selective RET inhibitor therapy (e.g., selpercatinib or pralsetinib) is considered. While the role of routine SSTR2/5 immunohistochemistry remains to be further clarified, evidence suggests that patients with somatostatin receptor (SSTR)-avid metastatic disease may also benefit from the option of 177Lu-DOTATATE peptide radionuclide receptor therapy. Finally, the authors of this review make a call to support the nomenclature change of MTC to C-cell neuroendocrine neoplasm to align this entity with the IARC/WHO taxonomy since MTCs represent epithelial neuroendocrine neoplasms of endoderm-derived C-cells.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Thyroid cancer is the ninth most common cancer worldwide, with an estimated incidence of 586,202 new cases in 2020 [1]. Medullary thyroid carcinoma (MTC) accounts for only 1–2% of all thyroid cancers and it is associated with 8% of thyroid cancer-related deaths in the USA [2, 3]. However, the incidence of MTC in Asians is less than 1% of all thyroid cancers [4, 5]. MTC is a neuroendocrine neoplasm, arising from the C-cells of the thyroid. Its histological features differ from those of follicular cell-derived differentiated thyroid carcinomas, and are reflective of a more aggressive clinical outcome [2].
Advances in molecular biology over the past decades have led us to understand better how MTC develops and progresses by genetic and epigenetic alterations. Although contemporary tumor classification and risk stratification increasingly rely on molecular biomarkers that reflect genetic and epigenetic changes, histopathology retains its inherent value in the 2022 World Health Organization (WHO) classification of thyroid neoplasms [6]. The 2022 WHO classification adopted the prognostic International Medullary Thyroid Carcinoma Grading System (IMTCGS) among recently introduced histological grading systems which has been shown to facilitate the risk stratification and clinical management of patients with MTC [7].
MTC is primarily treated surgically. However, in case of recurrence or distant metastases, molecular testing is crucial to select patients for RET-targeted tyrosine kinase inhibitors and other targeted therapies [8, 9].
This review provides an overview and recent updates on the molecular genetics of MTC, disease risk stratification based on molecular profile and histopathology, and targeted molecular therapies in patients with advanced MTC.
Developmental Origin of C-Cells and Their Neoplasms
MTC is derived from parafollicular C-cells of the thyroid gland, which secrete calcitonin, a calcium-lowering hormone. The thyroid gland parenchyma is composed of endoderm-derived follicular cells and endodermal cells that migrate and/or associate with ultimobranchial/pharyngeal bodies originated parafollicular C-cells. The latter component comprises less than 0.1% of the thyroid epithelial cells [10, 11]. During the embryonic development of vertebrates, the ultimobranchial body is technically derived from the fifth pharyngeal pouch. However, its origin is practically the ventral recess of the fourth pharyngeal pouch because the fifth is rudimentary and merges with the fourth [12]. The fourth pharyngeal pouch gives rise to the superior parathyroid glands, the apex of the pyriform fossa, and laryngeal cartilages and muscles, as well as the ultimobranchial body [12]. The ultimobranchial body migrates caudally, invades the develo** thyroid gland, fuses to the thyroid primordium, and eventually gives rise to the parafollicular C-cells in mammals [10]. However, the ultimobranchial anlage of nonmammalian vertebrates and monotremes does not merge into thyroid primordium, instead remains as a separated organ, the ultimobranchial gland [12]. This ultimobranchial body origin concept supports that parafollicular C-cells are located mainly in the middle of the thyroid lobes and are very rare in the peripheral and isthmic areas [11]. Parafollicular C-cells cluster around the solid cell nests, which are considered to represent ultimobranchial body remnants [11]. Recent data on embryonic development proved that C-cells are of no neural crest origin and are of endodermal cells which migrate and/or associate with ultimobranchial/pharyngeal bodies [13, 14]. A minority of parafollicular C-cells may also be derived directly from endodermal follicular primordium progenitor cells [11].
MTC can present as a mixed tumor composed of MTC and follicular cell-derived thyroid carcinoma, including papillary thyroid carcinoma, follicular thyroid carcinoma, oncocytic thyroid carcinoma, poorly differentiated thyroid carcinoma, or anaplastic thyroid carcinoma [6]. The endodermal origin of parafollicular C-cells may explain the rare occurrence of follicular and C-cell-derived tumors within the same lesion [11]. The stem cell properties of ultimobranchial cells to differentiate into both C-cells and follicular cells contribute to the development of the mixed thyroid carcinomas [11]. Many clinical case reports support the common origin theory from an ultimobranchial stem cell. However, unlike the MTC component, the non-MTC tumor component shows co-expression of thyroglobulin and monoclonal PAX8 (especially C-terminus specific antibodies) and lacks expression for calcitonin, calcitonin gene-related peptide, monoclonal CEA, chromogranin-A, synaptophysin, and INSM1 [15,16,17,18]. The mixed tumors can develop lymph node metastases having both the cell types. Mixed carcinomas composed of MTC and papillary thyroid carcinoma have been reported in patients with pathogenic germline RET variants [19].
MTC and follicular cell-derived thyroid carcinoma components may indeed have different clonality. For example, BRAF p.V600E can be found in the papillary carcinoma component of a mixed MTC and papillary thyroid carcinoma (Fig. 1). The MTC component also harbors a similar molecular profile as that of the pure MTC.
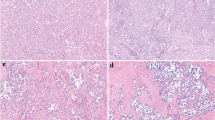
Mixed medullary and papillary thyroid carcinoma. A The papillary carcinoma component is intimately admixed with the medullary carcinoma and shows follicular growth with rudimentary papillae (arrow). B VE1 immunostaining highlights the classic papillary carcinoma component harboring the BRAF p.V600E variant
Amphicrine carcinoma expressing MTC and exocrine (mucin production) features in the same tumor cells has been reported in less than 10 cases in the thyroid gland [20]. However, evidence of mixed endocrine-exocrine differentiation in other thyroid neoplasms is largely lacking, with the exception of metaplastic trans-differentiation that occurs in salivary gland-type mucoepidermoid carcinoma of the thyroid [21].
Pathogenesis and Molecular Genetics
Up to 75% of all MTC cases develop sporadically. Sporadic MTCs are diagnosed at an average age of 45–55 years [2, 22]. In addition, 20–25% of cases of MTC are hereditary and associated with multiple endocrine neoplasia type 2 (MEN2) syndrome; occurring due to germline alterations in the RET proto-oncogene [22]. MTC occurs as a dominant presentation of the autosomal dominant tumor syndrome. It develops at an earlier age (10–20 years) in MEN2B (formerly known as MEN3) than in MEN2A (25–35 years), and at a variable age in isolated familial MTC [3, 23].
The RET proto-oncogene on chromosome 10q11.2 encodes a RET receptor tyrosine kinase that comprises an extracellular domain, a transmembrane domain, and an intracellular domain with tyrosine kinase activity (Fig. 2) [23]. Activating RET variants, usually point mutations or gene fusions, leads to constitutive activation of tyrosine kinase (Fig. 2). These genetic events are well-known oncogenic drivers in the development of thyroid cancer. RET point mutations are the most frequent driver events in MTC, whereas RET fusions are found in papillary thyroid carcinoma and poorly differentiated thyroid carcinoma [3, 23,24,25].
The RET receptor tyrosine kinase signaling pathway and oncogenic signal activation. The extracellular domain of the RET receptor contains four cadherin-like domains and a cysteine-rich region. In a normal RET activation pathway, each of glial cell line-derived neurotrophic factors (GDNFs) interacts with a co-receptor of GDNF family receptor-α (GFRα) family ligands. The complex of GDNF-family ligands and co-receptors GFRα1-4 recruits two RET receptors closely and leads to the dimerization of RET receptors and autophosphorylation of tyrosine residues. However, oncogenic RET alterations have different activation mechanisms. Pathogenic RET variants involving the cysteine residues (indicated by asterisks) of the extracellular domain constitutively activate RET through the formation of intermolecular cysteine disulfide bridges leading to the dimerization of altered RET. Germline RET alterations at the cysteine residues cause multiple endocrine neoplasia type 2A (MEN2A) and familial medullary thyroid carcinoma (FMTC). RET variants affecting the tyrosine kinase residues (indicated by asterisks) of intracellular domains lead to a conformation change in the ATP binding pocket of RET and constitutively activate the monomeric form of RET. Germline pathogenic RET variants involving the intracellular tyrosine kinase domains lead to multiple endocrine neoplasia type 2B (MEN2B) or FMTC. Additionally, most RET fusion proteins are cytosolic without transmembrane domain and have constitutively active tyrosine kinase domains of RET receptors. RET gene fusions occur in papillary thyroid carcinoma (PTC) and poorly differentiated thyroid carcinoma (PDTC) but in MTC. Activation of normal RET or oncogenic RET signaling pathway promotes activation of downstream signaling pathways, including MAPK, PI3K/AKT, JAK2/STAT3, or PLCγ pathway
Germline Pathogenic RET Variants
Germline RET alterations are found in almost all patients with MEN2 and have a well-defined molecular genotype and clinical phenotype correlations [26]. Of the patients with MEN2A syndrome, about 95% have pathogenic germline RET alterations in codons 609, 611, 618, and 620 at exon 10 or 630 and 634 at exon 11 within the extracellular cysteine-rich domains (Fig. 3) [23]. Germline RET p.C634A is the most common genetic alteration in MEN2A patients [23, 27]. These gain-of-function variants lead to constitutive RET kinase dimerization by forming disulfide-bounded homodimers, resulting in ligand-independent activation of the RET tyrosine kinase receptor [26].
Relatively common RET gene alterations and their associated RET proteins in hereditary and sporadic medullary thyroid carcinomas (MTCs). Common RET variants are shown in different colors according to multiple endocrine neoplasia (MEN) subtypes, familial medullary thyroid carcinoma (FMTC), and sporadic MTC. Pathogenic variants affecting the extracellular cysteine-rich domain codons account for most cases of MEN2A and FMTC. Mutations of codons at exons 13–16 activate the RET receptor tyrosine kinase. Two ATP-competitive small-molecule inhibitors of RET, selpercatinib and pralsetinib, selectively inhibit the tyrosine kinase activity of wild-type and altered RET. They were approved by the Food and Drug Administration for treating patients with RET-altered MTC and RET fusion-positive follicular cell-derived thyroid cancers, and non-small cell lung cancers
Isolated familial MTC, a subtype of MEN2A, accounts for about 15% of all hereditary MTCs [23]. Most germline RET variants associated with isolated familial MTC affect extracellular codons other than codon 634 in the cysteine-rich domain located on exons 10 and 11 or intracellular non-cysteine codons 768, 790, and 791 at exon 13, codon 804 at exon 14, or codon 891 at exon 15 (Fig. 3) [23].
The MEN2B syndrome accounts for about 5% of all hereditary MTCs and is mainly associated with the germline pathogenic variant, RET p.M918T involving exon 16 within the intracellular tyrosine kinase domain [23]. The genetic variant at codon 918 leads to autophosphorylation of the kinase domain and activates the RET tyrosine kinase receptor as a dimerization-independent monomeric form (Fig. 3) [23].
Germline RET variants have been found in almost all MEN2 families. However, according to the three European studies, 1.1% (6/532) of patients with hereditary MTC had no alteration in the RET gene [27]. Moreover, not all patients with germline RET variants develop the MEN2 phenotype. For example, 1.2% of Italian MEN2 families with germline RET mutation did not clinically develop MTC [27]. In addition, rare examples of inherited MTCs that lack pathogenic germline RET variants were reported to harbor pathogenic germline MET variants [28]; therefore, evidence suggests that whole exome sequencing may identify additional genetic alterations involved in the pathogenesis of hereditary MTC.
Risk Stratification for Germline Pathogenic RET Variant Carriers
Specific germline pathogenic variants involving RET gene confer different cancer risks and aggressiveness. Therefore, current American Thyroid Association (ATA) guidelines stratify patients with germline RET variants into the highest, high, and moderate risk levels of aggressive MTC and recommend the appropriate timing of prophylactic thyroidectomy based on the specific RET alteration [2]. The highest risk category includes patients with MEN2B and RET p.M918T (Fig. 4). The high-risk category includes patients with the RET variants involving codon 634 and RET p.A883F. Patients with hereditary MTC and other germline pathogenic RET variants belong to the moderate-risk category.
Risk stratification of hereditary and sporadic medullary thyroid carcinoma (MTC) according to the molecular profiles. Current American Thyroid Association guidelines classify patients with pathogenic germline RET variants into 3 groups based on the aggressiveness of the MTC: highest risk includes patients with RET p.M918T variant, high risk includes patients with RET p.C634F/G/R/S/W/Y variants or p.A883F alteration, moderate risk includes patients with other pathogenic RET variants. Germline RET p.M918T and p.A883F variants mainly occur in multiple endocrine neoplasia type 2B (MEN2B), whereas germline RET alterations at codon C634 occur in MEN2A. In sporadic MTCs, patients with somatic RET p.M918T variant have the worst prognosis, whereas those with somatic RAS mutations have the best prognosis
Somatic Pathogenic RET Variants
Somatic pathogenic RET variants are found in about half of all patients with sporadic MTC (Fig. 4) [23, 26, 29]. The variant prevalence is lower in small MTCs and higher in patients with distant metastases [23, 30] and cervical lymph node metastases [Medullary Microcarcinoma Medullary microcarcinoma is a subtype of MTC measuring 10 mm or less in diameter [70]. There is no minimum size criterion. While some authors have shown tumors measuring 5 mm or less to have a better outcome compared to those with larger tumors [71], others documented smaller tumors to also have a variable risk of lymph node metastases and post-operative hypercalcitonemia [72, 73]. Kazaure et al. analyzed 310 patients with medullary microcarcinoma in the SEER registry from 1988 to 2007 [72]. Medullary microcarcinoma ≤ 5 mm had about 23% risk of lymph node metastasis, whereas the risk increased from 28% to 37% as tumor size increased from 6 mm to 10 mm [72]. In addition, Machens et al. found that with the increase in tumor size, more patients with medullary microcarcinoma had lymph node metastases from 13% in ≤ 2 mm tumors to 43% in 9– 10 mm [73]. A meta-analysis study showed that medullary microcarcinomas are associated with longer disease-free survival and a lower rate of extrathyroidal extension and lymph node metastases [74]. The prevalence of somatic RET p.M918T variant is also lower in microcarcinoma than in larger MTC [75]. However, there is no significant difference in the frequency of multifocality and distant metastases between microcarcinomas and larger tumors [74]. Patients having hereditary MTC diagnosed by screening have overall survival rates similar to the general population [63]. The excellent prognosis is attributed to an early diagnosis when the tumor is confined to the thyroid gland [2, 63]. Compared to hereditary MTCs, sporadic MTCs present at an older age and have higher lymph node recurrence [62]. However, whether MTC is sporadic or hereditary is not an independent prognostic factor [62, 76]. DSS and clinical outcomes are similar between hereditary MTCs and sporadic MTCs after adjusting for the tumor stage [76]. Calcitonin and carcinoembryonic antigen (CEA), two important hormones secreted by the C-cells, act as tumor markers for MTC. While calcitonin has diagnostic relevance, both are used in follow-up and have a role in predicting disease progression [2]. Niederle et al. demonstrated that basal serum calcitonin levels of > 85 pg/mL for females and > 100 pg/mL for males had 100% sensitivity for diagnosing lateral neck lymph node metastasis [77]. However, calcium-stimulated calcitonin levels did not improve preoperative diagnosis [77]. Basal serum calcitonin levels correlate with the extent of the disease progression. Machens et al. showed that thresholds of 20, 50, 200, and 500 pg/mL indicated lymph node metastasis at ipsilateral central and lateral neck, contralateral central neck, contralateral lateral neck, and upper mediastinum, respectively [78]. Through this study, the authors also deduced that bilateral compartment-oriented neck surgery could achieve biochemical cure in at least half of the patients with preoperative basal calcitonin levels of < 1000 pg/mL but in none of the patients with levels > 10,000 pg/mL [78]. Preoperative basal serum calcitonin level > 1000 pg/mL predicts failure to reach biochemical cure [79]. Preoperative serum CEA levels also correlate with the extent of lymph node involvement [80]. Doubling times of calcitonin and CEA levels, calculated by measuring them over multiple follow-up intervals, help in determining the tumor growth rate and are predictive of disease progression [81, 82]. The ATA recommends estimating doubling times of calcitonin and CEA in patients with detectable serum levels of calcitonin and CEA post-thyroidectomy [2]. The ATA advises against systemic therapy in patients with stable low-volume metastatic disease and serum calcitonin and CEA doubling times > 2 years. Systemic therapy should also not be administered to those without documented metastatic disease, even with increasing serum calcitonin and CEA levels [2]. Postoperative levels of calcitonin and CEA can predict tumor dedifferentiation. An increasing CEA level associated with a stable or declining calcitonin level is indicative of dedifferentiation and a worse prognosis [83]. MTCs with advanced disease and dedifferentiation often show normal or low calcitonin levels [84]. However, CEA levels may still be elevated, making CEA a more reliable biomarker in dedifferentiated MTCs. Procalcitonin produced by C-cells and MTC cells is very stable, and its concentration is unaffected by blood sampling and storage [85]. A recent meta-analysis suggested that owing to its high accuracy and better analytical characteristics, procalcitonin should replace calcitonin in the diagnosis and follow-up of MTC patients [86]. A high procalcitonin to calcitonin ratio predicts an increased risk of progressive disease and a shortened progression-free survival [87]. The high procalcitonin level is associated with larger primary tumor size, more lymph nodes involved, distant metastases, and lower biochemical cure rates [88]. The biochemical cure rates declined to no more than 71%, 36%, 23%, and 10%, respectively, when the procalcitonin levels crossed thresholds of 1, 5, 10, and 50 ng/mL [88]. The serum levels also correlated with the lymph node compartments involved, which is essential for deciding the extent of required surgery [88]. Limited evidence suggests the utility of serum carbohydrate antigen 19–9 (CA19-9) as a marker of poor prognosis in MTC. Elisei et al. found elevated levels of CA19-9 in 16 of the 100 patients with advanced structural recurrent or persistent disease but in none of the 100 patients with biochemical cure [89]. Elevated serum CA19.9 was also a predictor of mortality [89]. In a more recent study, the same research group showed that serum CA19.9 positivity and doubling times of < 6 months and < 1 year were associated with mortality but not a progressive structural disease [90]. Importantly, the authors highlighted the cases of three patients having CA19.9 doubling times < 6 months. They died of disease after 6, 5, and 3 months, respectively, although there was no evidence of progressive disease [90]. Many studies have investigated different pathological parameters for their potential utility in the prognostication of MTC. However, none of the histological features, such as the predominant histologic pattern (solid, trabecular, or acinar), the predominant cytological appearance (plasmacytoid, fusocellular, small cells, oncocytic, clear cells), amount of amyloid deposited, presence or absence of calcification, C-cell hyperplasia, and multifocality, was prognostically relevant [68, 91]. C-cell hyperplasia is found in almost all prophylactic thyroidectomies for MTC and is considered the precursor of hereditary MTCs [92, 93]. Morphological features associated with germline RET alterations are C-cell hyperplasia in association with at least a single focus of MTC or medullary microcarcinoma and/or multifocal C-cell neoplasia (Fig. 5) [93]. These C-cells are neoplastic and have mild-to-moderate nuclear atypia and abundant cytoplasm that are identified in H&E-stained slides and confirmed by calcitonin immunostaining (Fig. 5). The criteria of C-cell hyperplasia remain not fully defined and problematic since the number of C-cells normally found in humans varies from 4–6 cells around a follicle to 50 cells per low-power (100 ×) field [70]. While C-cell hyperplasia may occur as a reactive (secondary) phenomenon (e.g., underlying follicular nodular disease, hyperparathyroidism, aging, medications), bilateral and multifocal C-cell hyperplasia in association with medullary (micro)carcinoma or multifocal medullary (micro)carcinoma should prompt genetic testing to rule out germline pathogenic RET variants. The distinction of medullary microcarcinoma from a nodular C-cell hyperplasia requires the demonstration of tumor invasion. Infiltrating tumor cells tend to result in desmoplastic tissue reaction and are often associated with irregular outlines at the periphery of the lesion, whereas C-cell hyperplasia lacks these histologic features [70]. The prognostic relevance of amphicrine phenotype remains uninvestigated primarily due to its rarity. Following a review of the limited literature available as case reports, a recent publication has suggested that the amphicrine morphology may imply a poor prognosis in MTC [20]. Angioinvasion is a reliable predictor of disease recurrence and DSS. By using pre-defined strict criteria for angioinvasion (tumor cells invading through a vessel wall and forming tumor-fibrin complexes or intravascular tumor cells admixed with fibrin/thrombus) (Fig. 6) [94], Erovic et al. found that 60% of the MTCs with angioinvasion developed metastases and 100% of them developed locoregional and/or distant metastasis [95]. Other studies also revealed the detrimental effect of angioinvasion on DSS in MTC patients [91]. Medullary thyroid carcinoma with angioinvasion (A) and lymphatic invasion (B) in surrounding thyroid parenchyma. This tumor metastasized to the cervical lymph nodes and lung. A Angioinvasion is characterized by thick-wall vascular spaces and intravascular tumor cells admixed with fibrin and thrombus. B Lymphatic tumor emboli are shown within the clear lumens of thin-wall lymphatic vessels lacking a muscular layer At the diagnosis of MTC, lymph node metastases are already present in 47–75% of patients [96]. In a recent study on 152 MTC patients from 2000 to 2020, lateral lymph node metastases and lymph node ratio (positive lymph nodes/retrieved lymph nodes) > 1/3 were independently associated with progressive disease [97]. The presence of contralateral nodal disease is also an indicator of poor outcomes [97]. A novel indicator is the log of the ratio between the number of positive nodes and the number of negative nodes [98]. The log odds of positive lymph nodes have been demonstrated to have better prognostic efficacy than lymph node status and the number of positive lymph nodes [98]. Multivariate analysis has confirmed the absence of regional metastases to predict long-term biochemical cure [63]. Kuo et al. found that older age, tumor size > 2 cm, regional spread (extrathyroidal extension or lymph node metastasis), and distant metastatic disease were independent risk factors for DSS [99]. Lymph node metastasis increased the risk of re-surgery. Central and lateral lymph node dissection at the time of initial surgery was associated with decreased recurrences [99]. Under the 8th edition of the AJCC staging system, MTCs with T1–3, central lymph node metastasis (N1a), and no distant metastasis (M0) are classified in stage III [69]. MTCs with lateral lymph node metastases (N1b) and without distant metastasis (M0) are classified in stage IV. The National Cancer Institute defines “histologic grade” as “a description of a tumor based on how abnormal the cancer cells and tissue look under a microscope and how quickly the cancer cells are likely to grow and spread” (https://www.cancer.gov/publications/dictionaries/cancer-terms/def/histologic-grade). The grading systems differ for each type of cancer. The commonly used parameters for grading cancers include proliferative activity (e.g., mitotic count and Ki67 index) and coagulative necrosis. The proliferative activity gives information regarding how rapidly the tumor cells are multiplying. This can be assessed by more than one method, the most common being calculation of mitoses. It has the advantage of easy applicability using routinely available hematoxylin and eosin (H&E)-stained sections. Traditionally, the number of mitotic figures is expressed per high power field (HPF). However, the method has its limitations; the major being differing fields of view across different microscopes, even at the same magnification. The problem has been further compounded by the increasing shift to digital pathology systems, where the fields are rectangular and the displayed area variable depending on the image viewer system. This would, beyond doubt, affect grading, especially in cases with borderline scores. To circumvent this, the fifth edition of the WHO classification of tumors recommends using standardized international (SI) units, first adopted in 2021 [100]. Mitoses now need to be counted per mm2 [101]; most grading schemes require documentation of mitotic figures per 2 mm2 based on a formal count from 10 mm2. Therefore, the area to be evaluated and the method used (“hotspot” or “average”; “conventional or phosphohistone-H3” assisted) also need mentioning [100]. The mitotic activity in a tumor can be expressed as mitotic count, mitotic index, or mitotic rate [100]. The mitotic count is the number of mitoses per mm2. It does not consider the size of neoplastic cells, the section thickness, and the tumor cell density; the latter being affected by the presence of intra-tumoral stroma like fibrosis and areas of necrosis. In comparison, the mitotic index is calculated by dividing the number of tumor cells in mitosis by the number of tumor cells counted [100]. This overcomes the concerns of cell size, size of the field of view, and intra-tumoral stroma, but is labor-intensive for implementation in routine clinical practice. However, the mitotic index can be assessed easily using digital pathology. The mitotic rate is the rate at which cells are entering the mitotic phase [100]. It is expressed as a percentage of the M phase entering cells counted per hour. It needs metaphase arrest or labeling of viable cells and is not feasible in routine practice. As there is interobserver variability in the identification of mitoses, immunohistochemistry for proteins expressed during mitosis has been suggested as an alternative to visual counting [101]. Ki67 immunohistochemistry is a method of assessing the proliferative activity of a tumor. It labels the nuclei in G1, G2, or S phase [100,99,102]. Immunohistochemistry for phosphoHistone-H3 antibody stains the phosphorylated nuclear histone protein, formed during late G2 to late anaphase, and has been shown to have better accuracy than the visual counting of mitosis and the Ki67 labeling index [100, 101, 103, 104]. Some of the other methods available for assessing tumor proliferative activity include immunohistochemistry for proliferating cell nuclear antigen, topoisomerase IIa, cyclin A, geminin, and minichromosome maintenance; flow cytometric evaluation for the S phase fraction; and incorporation methods like thymidine labeling index and bromodeoxyuridine labeling index. All have their advantages but are of limited clinical use [105]. Histologic grade integrating necrosis and proliferation index has been well-established for long as an important prognostic factor in most neuroendocrine neoplasms, particularly in the gastrointestinal tract and pancreas [106]. However, this concept has only recently been adapted to MTC. Since 2020, three grading systems have been proposed for MTC with prognostic implications. An initial proposal by a group from the Memorial Sloan Kettering Cancer Center (MSKCC, New York, NY, USA) introduced a two-tier grading system [107]. The authors retrospectively reviewed 144 MTCs for multiple clinicopathologic parameters and identified tumor necrosis and increased mitotic activity were the only independent predictors for DSS in multivariate analysis. Therefore, they defined low-grade MTCs as cases with a mitotic count < 5 mitoses per 10 HPFs (2 mm2) and no tumor necrosis. Cases with increased proliferation of ≥ 5 mitoses per 2 mm2 and/or presence of tumor necrosis were classified as high-grade (Fig. 7). Based on this grading system, 113 (78%) MTCs were classified as low-grade whereas the remaining 31 (22%) were considered as high-grade. Univariate survival analysis demonstrated that the MSKCC grading system predicted DSS well. The second system was proposed by a group from the Royal North Shore Hospital (Sydney, NSW, Australia) [108]. The authors evaluated multiple clinicopathologic parameters in 76 patients with MTCs and found that advanced age, absence of MEN2 syndrome, high Ki67 proliferative index, high mitotic count, and coagulative necrosis were significantly associated with worse overall survival in univariate analyses. A three-tier grading system was developed using a combination of Ki67 proliferative index, mitotic count, and the presence or absence of coagulative tumor necrosis (Figs. 7 and 8). Low-grade MTCs were defined as cases with low proliferative activity (< 3 mitoses per 2 mm2 and a Ki67 labeling index < 3%) and no tumor necrosis; intermediate-grade MTCs as cases with low proliferative activity but with tumor necrosis, or with intermediate proliferative activity (3–20 mitoses per 2 mm2 and/or 3–20% Ki67) and no tumor necrosis; and high-grade MTCs as cases with intermediate proliferative activity but with tumor necrosis, or with high proliferative activity (> 20 mitoses per 2 mm2 and/or > 20% Ki67) regardless of tumor necrosis status. Based on the Sydney grading system, 62 (81.5%) cases were low-grade, 9 (12%) were intermediate-grade, and 5 (6.5%) were high-grade. The survival analysis demonstrated low-grade MTCs with a mean overall survival of 195 months, followed by the intermediate-grade MTCs with a mean overall survival of 137 months, and then the high-grade MTCs with a mean overall survival of only 45 months. Comparison of histological grading methods in medullary thyroid carcinoma (MTC). Memorial Sloan Kettering Cancer Center (MSKCC) grading system is a two-tier system to differentiate high-grade MTC based on mitotic count and tumor necrosis [107]. Sydney grading system uses a three-tier grading system based on the mitotic count, tumor necrosis, and Ki67 index [108]. The international MTC grading system (IMTCGS) is a two-tier grading system based on the mitotic count, tumor necrosis, and Ki67 index [7]. The 2022 WHO classification adopted the IMTCGS as a prognostic variable. The area of 10 high-power fields (HPFs) is equivalent to 2 mm2 in most microscopes Although both grading systems have been validated to predict progression-free survival and DSS in an independent cohort of 44 sporadic MTCs from the Brigham and Women’s Hospital (Boston, MA, USA) [109], it was problematic to have two different grading systems for the clinical management. Therefore, a joint attempt has been made by both groups to create a third grading system (the international MTC grading system, IMTCGS) to unify the parameters that have been validated with consensus cutoffs [7]. In this multi-institutional study including a total of 327 MTC patients from five medical centers, a retrospective review of mitotic activity, Ki67 proliferative index, and necrosis using uniform criteria was conducted and a two-tier consensus grading system was developed. High-grade MTCs were defined as MTCs with at least one of the following features: mitotic index ≥ 5 per 2 mm2, Ki67 proliferative index ≥ 5%, or presence of tumor necrosis (Fig. 7). Based on this grading system, 246 (75%) MTCs were low-grade and 81 (25%) MTCs were high-grade. The IMTCGS was predictive of overall survival, DSS, distant metastasis-free survival, and locoregional recurrence-free survival. Several subsequent studies have validated the applications of the IMTCGS. Vissio et al. compared these three different grading systems using 151 MTC cases from two hospitals in Italy [110]. The results showed that although all three grading systems were significantly associated with disease recurrence, only the IMTCGS demonstrated a significant impact on DSS. In a recent reproducibility study, Williams et al. demonstrated that the IMTCGS had high interobserver agreement with an overall kappa value of 0.87 [111]. In addition, Nigam et al. found that IMTCGS high-grade patients demonstrated significantly faster calcitonin doubling times and majority of them were less than 2 years [112]. Although the IMTCGS is not a WHO-grading system, it has been endorsed by the 2022 WHO classification as a prognostic parameter [6, 113]. The pathologists are still required to document clearly the numeric value for mitotic count per 2 mm2 and the Ki67 labeling index irrespective of the tumor grade. Further prospective studies on this grading system are required for real-world application.Hereditary Versus Sporadic MTC
Circulating Serum Tumor Markers
Histologic Features
Angioinvasion
Lymph Node Metastasis
Proliferative Activity, Tumor Necrosis, and Grade
Progress in Systemic Therapy Options in MTCs
MTC is resistant to cytotoxic chemotherapy and does not respond to radioactive iodine and thyroid-stimulating hormone suppression therapy that is used for treating differentiated thyroid cancer. The evolving role of radiolabelled somatostatin analogs (e.g., 177Lu-DOTATATE) in the peptide receptor radionuclide therapy (PRRT) has been a focus of interest in somatostatin receptor (SSTR)-avid neuroendocrine neoplasms [114]. There is also emerging evidence that patients with metastatic SSTR-avid MTCs may benefit from PRRT [115]; however, access and indications vary in clinical practices.
Recently, a promising treatment strategy has been to inhibit RET kinase, which is highly expressed in MTC. Multi-targeted tyrosine kinase inhibitors were used for treating patients with advanced MTC before selective RET inhibitors became available (Table 1). However, multikinase inhibitors insufficiently inhibited RET and showed more off-target toxicities than selective RET inhibitors. Unlike the non-selective inhibitors, pathologists are required to confirm the RET mutation status for the use of selective RET inhibitors.
Non-selective RET Inhibitors
Two multikinase inhibitors, vandetanib and cabozantinib, have been approved by United States Food and Drug Administration (FDA) and European Medicines Agency (EMA) for the treatment of patients with a progressive metastatic MTC [9]. Vandetanib inhibits the activity of multiple receptor tyrosine kinases including vascular endothelial growth factor receptor (VEGFR)1/2/3, RET, and epidermal growth factor receptor (EGFR). The ZETA clinical trial defined that the use of vandetanib showed, compared to the control group, increased progression-free survival (30.5 vs. 19.3 months) and objective imaging response (45% vs. 13%), irrespective of RET mutation status, progression rate, disease location, and tumor extent at baseline [116].
Cabozantinib inhibits multiple tyrosine kinases including RET, VEGFR1/2/3, TIE-2, MET, FLT-3, and KIT [117]. Its efficiency was documented in the EXAM clinical trial, which reported an improved progression-free survival (11.2 vs. 4.0 months) and an objective imaging response (28% vs. 0%) were observed compared to placebo, irrespective of age, tumor burden, tumor location, progression rate, or prior tyro kinase inhibitor treatment [117]. Both drugs have been associated with disease stabilization in 30% and partial regression in 35% of cases; severe toxicities may occur, although manageable in most cases. However, the estimation of the benefit of these agents on overall survival is difficult as most patients have a long-life expectancy.
Selective RET Inhibitors
Recently, two novel selective RET inhibitors, selpercatinib (LOXO-292) and pralsetinib (BLU-667), have been approved for the same indications in patients with RET-aberrant thyroid cancer (RET-altered MTC and differentiated thyroid cancer with RET fusion) and non-small cell lung cancer [9]. Unlike previously targeted small molecules (vandetanib and cabozantinib) used in differentiated thyroid cancer and MTC, both selpercatinib and pralsetinib are highly selective inhibitors for RET alterations, including point mutations and gene fusions [118, 119]. Furthermore, as these small molecules are not primarily based on the inhibition of VEGFRs, the representative primary molecular target for anti-cancer therapy by small molecules, we can expect excellent drug durability and continuation of medications without severe adverse events [120].
Selpercatinib was rapidly approved in May 2020 by FDA for RET mutated MTCs or RET fusion-positive differentiated thyroid cancers, based on the results of the phase 1–2 clinical trial (LIBRETTO-001) [121]. The study enrolled RET-altered MTC cases with or without previous vandetanib or cabozantinib treatment. The reported results were an objective response rate (complete or partial response) of 73% and 1-year progression-free survival of 92% from previously untreated MTC patients (n = 88). In previously treated MTC patients, objective response rate was 69%, and 1-year progression-free survival was 82%.
Pralsetinib secured FDA approval in patients with advanced or metastatic RET-altered MTC and RET fusion-positive follicular cell-derived thyroid cancer in December 2020, based on a phase 1–2 clinical trial (ARROW) [9, 122]. The clinical trial reported an objective response rate of 71% and an estimated 1-year progression-free survival of 81% in treatment-naïve MTC RET-altered patients (n = 21) [122]. In previously treated RET-altered MTC patients (n = 55) with vandetanib or cabozantinib, an objective response rate of 60% and an estimated 1-year progression-free survival of 75% were obtained from the study results. In previously treated RET fusion-positive follicular cell-derived thyroid cancers, objective response rate of pralsetinib was 89%.
Most of the patients experienced several adverse events. However, most of them were less intense than the two non-selective (multikinase) inhibitors. The rates of discontinuation of selpercatinib and pralsetinib due to adverse events were 2% and 4% in the clinical trials, respectively. Therefore, selpercatinib and pralsetinib showed more potent efficacy and good safety profiles than multikinase inhibitors.
Although the survival benefits of selpercatinib and pralsetinib for advanced and metastatic RET-altered MTC have not been proved yet, ongoing phase 3 trial may have a high probability of a better outcome than non-selective RET inhibitors, cabozantinib or vandetanib.
A Case Report of Selective RET Inhibitor for Refractory MTC
A 63-year-old male patient presented with a 4.5-cm-sized nodule involving the left thyroid with multiple bilateral enlarged cervical lymph nodes. Fine needle aspiration was suspicious for MTC. The serum calcitonin level was 1017.0 pg/mL (normal value, < 10 pg/mL). He underwent total thyroidectomy with bilateral lymph node dissection. The pathology confirmed high-grade MTC with extrathyroidal extension and extensive bilateral lymph node metastasis. Subsequent lung computed tomography scan showed small multiple lung metastases. Germline variant study using blood showed c.2692G > T (p.D898Y) in RET exon15. The patient and his family have not demonstrated clinical features characteristic of MEN-related diseases. As multiple lung metastases were progressive with a maximal diameter of 13 mm and the doubling time of serum calcitonin levels showed 5.75 months, the patient was started on vandetanib, an approved first-line multikinase inhibitor, with a total dose of 300 mg per day. During the 2-year vandetanib treatment, the patient showed stable disease of multiple lung metastases. After that, however, the treatment dose was lowered to 100 mg daily due to frequent adverse events, such as QT prolongation on the electrocardiogram, gum bleeding, and moderate to severe hypertension or headache. Finally, he stopped the treatment because of a progressive disease of lung metastasis.
Without further treatment for metastatic lesions, the patient developed multiple spine metastasis and rapid progression of metastatic diseases at lung, parahilar, mediastinal, and lateral neck lymph nodes. Due to intolerable neck pain, immediate neck area radiation therapy was performed to relieve the pain. As there was no approved second-line tyrosine kinase inhibitor in MTC, potential candidate tyrosine kinase inhibitors were being searched as an early access through a Named Patient Program (NPP) and then selpercatinib was administered to the patient with dose of 120 mg two times per day.
Although the patient experienced 3 cm sized intracranial hemorrhage on right frontal cerebrum 1 year before selpercatinib treatment, he did not show any severe adverse events associated with selpercatinib, except one-time hypercalcemia and mild dry mouth, and showed partial response in nearly all metastatic lesions following 10-month treatment of selpercatinib (Fig. 9).
A case of treatment with selpercatinib for refractory medullary thyroid carcinoma. Computerized tomography scans before and after 10 months of selpercatinib treatment (120 mg twice per day) show regression of left supraclavicular lymph node metastasis (A, B), subcarinal lymph node metastasis (C, D), and multiple lung metastases (E, F). Arrows indicate metastatic tumors
Conclusion
MTC is not the only neuroendocrine neoplasm in the thyroid gland. Other neuroendocrine neoplasms include intrathyroidal thymic neuroendocrine neoplasms, intrathyroidal parathyroid neoplasms, and thyroid paragangliomas as well as metastatic neuroendocrine neoplasms [18, 70]. The demonstration of diffuse CEA expression often helps distinguish various other calcitonin and/or calcitonin gene-related protein (CGRP)-producing neuroendocrine neoplasms from MTCs. Therefore, the first responsibility of a pathologist is to distinguish MTC from other mimics using appropriate immunohistochemical biomarkers. The second responsibility includes meticulous assessment of the status of angioinvasion, tumor necrosis, proliferative rate (mitotic count and Ki67 labeling index), and histologic grade (low- or high-grade) along with the tumor stage and the resection margins. Given the morphologic and proliferative heterogeneity in these neoplasms, an exhaustive sampling is strongly recommended. Routine molecular testing for pathogenic germline RET variants is typically performed in all patients with a diagnosis of MTC; however, C-cell hyperplasia in association with at least a single focus of MTC (or microcarcinoma) and/or multifocal C-cell neoplasia is morphological harbingers of germline RET alterations [93]. The status of somatic RET alterations should also be determined in all advanced/progressive or metastatic MTC, especially when selective RET inhibitor therapy is considered. While the role of routine SSTR2/5 immunohistochemistry requires further clarification, evidence also suggests that patients with SSTR-avid metastatic disease may benefit from 177Lu-DOTATATE PRRT. Finally, the authors of this review also make a call to support the nomenclature change of MTC to C-cell neuroendocrine neoplasm to align this entity with the IARC/WHO taxonomy since MTCs represent epithelial neuroendocrine neoplasms of endoderm-derived C-cells.
Availability of Data and Material
All data analyzed during this study are included in this published article.
Code Availability
Not applicable.
References
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71(3):209-49. https://doi.org/10.3322/caac.21660.
Wells SA, Jr., Asa SL, Dralle H, Elisei R, Evans DB, Gagel RF, et al. Revised American Thyroid Association guidelines for the management of medullary thyroid carcinoma. Thyroid. 2015;25(6):567-610. https://doi.org/10.1089/thy.2014.0335.
Barletta JA, Nose V, Sadow PM. Genomics and Epigenomics of Medullary Thyroid Carcinoma: From Sporadic Disease to Familial Manifestations. Endocr Pathol. 2021;32(1):35-43. https://doi.org/10.1007/s12022-021-09664-3.
Ahn HY, Chae JE, Moon H, Noh J, Park YJ, Kim SG. Trends in the Diagnosis and Treatment of Patients with Medullary Thyroid Carcinoma in Korea. Endocrinol Metab (Seoul). 2020;35(4):811-9. https://doi.org/10.3803/EnM.2020.709.
Howlader N, Noone AM, Krapcho M, Miller D, Brest A, Yu M, et al.: SEER Cancer Statistics Review, 1975–2016, National Cancer Institute. Bethesda, MD, based on November 2018 SEER data submission. https://seer.cancer.gov/archive/csr/1975_2016/ Accessed September 2022.
Baloch ZW, Asa SL, Barletta JA, Ghossein RA, Juhlin CC, Jung CK, et al. Overview of the 2022 WHO Classification of Thyroid Neoplasms. Endocr Pathol. 2022;33(1):27-63. https://doi.org/10.1007/s12022-022-09707-3.
Xu B, Fuchs TL, Ahmadi S, Alghamdi M, Alzumaili B, Bani MA, et al. International Medullary Thyroid Carcinoma Grading System: A Validated Grading System for Medullary Thyroid Carcinoma. J Clin Oncol. 2022;40(1):96-104. https://doi.org/10.1200/JCO.21.01329.
Roman-Gil MS, Pozas J, Rosero-Rodriguez D, Chamorro-Perez J, Ruiz-Granados A, Caracuel IR, et al. Resistance to RET targeted therapy in Thyroid Cancer: Molecular basis and overcoming strategies. Cancer Treat Rev. 2022;105:102372. https://doi.org/10.1016/j.ctrv.2022.102372.
Thein KZ, Velcheti V, Mooers BHM, Wu J, Subbiah V. Precision therapy for RET-altered cancers with RET inhibitors. Trends Cancer. 2021;7(12):1074-88. https://doi.org/10.1016/j.trecan.2021.07.003.
Grevellec A, Tucker AS. The pharyngeal pouches and clefts: Development, evolution, structure and derivatives. Semin Cell Dev Biol. 2010;21(3):325-32. https://doi.org/10.1016/j.semcdb.2010.01.022.
Nilsson M, Williams D. On the Origin of Cells and Derivation of Thyroid Cancer: C Cell Story Revisited. Eur Thyroid J. 2016;5(2):79-93. https://doi.org/10.1159/000447333.
Adams A, Mankad K, Offiah C, Childs L. Branchial cleft anomalies: a pictorial review of embryological development and spectrum of imaging findings. Insights Imaging. 2016;7(1):69-76. https://doi.org/10.1007/s13244-015-0454-5.
Abu-Bonsrah KD, Newgreen DF, Dottori M. Development of Functional Thyroid C Cell-like Cells from Human Pluripotent Cells in 2D and in 3D Scaffolds. Cells. 2021;10(11):2897. https://doi.org/10.3390/cells10112897.
Johansson E, Andersson L, Ornros J, Carlsson T, Ingeson-Carlsson C, Liang S, et al. Revising the embryonic origin of thyroid C cells in mice and humans. Development. 2015;142(20):3519-28. https://doi.org/10.1242/dev.126581.
Gucer H, Caliskan S, Kefeli M, Mete O. Do You Know the Details of Your PAX8 Antibody? Monoclonal PAX8 (MRQ-50) Is Not Expressed in a Series of 45 Medullary Thyroid Carcinomas. Endocr Pathol. 2020;31(1):33-8. https://doi.org/10.1007/s12022-019-09603-3.
Baloch Z, Mete O, Asa SL. Immunohistochemical Biomarkers in Thyroid Pathology. Endocr Pathol. 2018;29(2):91-112. https://doi.org/10.1007/s12022-018-9532-9.
Rindi G, Mete O, Uccella S, Basturk O, La Rosa S, Brosens LAA, et al. Overview of the 2022 WHO Classification of Neuroendocrine Neoplasms. Endocr Pathol. 2022;33(1):115-54. https://doi.org/10.1007/s12022-022-09708-2.
Asa SL, Mete O. Thyroid Neuroendocrine Neoplasms. In: Asa SL, La Rosa S, Mete O, editors. The Spectrum of Neuroendocrine Neoplasia. Cham: Springer International Publishing; 2021. p. 119-36.
Gurkan E, Gurbuz Y, Tarkun I, Canturk Z, Cetinarslan B. Mixed medullary-papillary carcinoma of the thyroid: report of two cases and review of the literature. Indian J Pathol Microbiol. 2014;57(4):598-602. https://doi.org/10.4103/0377-4929.142684.
Khandakar H, Agarwal S, Sharma MC, Kandasamy D, Bal C, Rathode Y, et al. Amphicrine Medullary Thyroid Carcinoma - a Case-Based Review Expanding on Its MUC Expression Profile. Endocr Pathol. 2022;33(3):378-87. https://doi.org/10.1007/s12022-022-09725-1.
Le HT, Nguyen TPX, Hirokawa M, Katoh R, Mitsutake N, Matsuse M, et al. Primary Thyroid Mucoepidermoid Carcinoma (MEC) Is Clinically, Prognostically, and Molecularly Different from Sclerosing MEC with Eosinophilia: A Multicenter and Integrated Study. Endocr Pathol. 2022. https://doi.org/10.1007/s12022-022-09741-1.
Lebbink CA, Links TP, Czarniecka A, Dias RP, Elisei R, Izatt L, et al. 2022 European Thyroid Association Guidelines for the management of pediatric thyroid nodules and differentiated thyroid carcinoma. Eur Thyroid J. 2022;11(6). https://doi.org/10.1530/ETJ-22-0146.
Salvatore D, Santoro M, Schlumberger M. The importance of the RET gene in thyroid cancer and therapeutic implications. Nat Rev Endocrinol. 2021;17(5):296-306. https://doi.org/10.1038/s41574-021-00470-9.
Cancer Genome Atlas Research N. Integrated genomic characterization of papillary thyroid carcinoma. Cell. 2014;159(3):676–90. https://doi.org/10.1016/j.cell.2014.09.050.
Chu YH, Sadow PM. Kinase Fusion-Related Thyroid Carcinomas: Towards Predictive Models for Advanced Actionable Diagnostics. Endocr Pathol. 2022;33(4):421-35. https://doi.org/10.1007/s12022-022-09739-9.
Romei C, Ciampi R, Elisei R. A comprehensive overview of the role of the RET proto-oncogene in thyroid carcinoma. Nat Rev Endocrinol. 2016;12(4):192-202. https://doi.org/10.1038/nrendo.2016.11.
Romei C, Mariotti S, Fugazzola L, Taccaliti A, Pacini F, Opocher G, et al. Multiple endocrine neoplasia type 2 syndromes (MEN 2): results from the ItaMEN network analysis on the prevalence of different genotypes and phenotypes. Eur J Endocrinol. 2010;163(2):301-8. https://doi.org/10.1530/EJE-10-0333.
Sponziello M, Benvenuti S, Gentile A, Pecce V, Rosignolo F, Virzi AR, et al. Whole exome sequencing identifies a germline MET mutation in two siblings with hereditary wild-type RET medullary thyroid cancer. Hum Mutat. 2018;39(3):371-7. https://doi.org/10.1002/humu.23378.
Grubbs EG, Williams MD, Scheet P, Vattathil S, Perrier ND, Lee JE, et al. Role of CDKN2C Copy Number in Sporadic Medullary Thyroid Carcinoma. Thyroid. 2016;26(11):1553-62. https://doi.org/10.1089/thy.2016.0224.
Heilmann AM, Subbiah V, Wang K, Sun JX, Elvin JA, Chmielecki J, et al. Comprehensive Genomic Profiling of Clinically Advanced Medullary Thyroid Carcinoma. Oncology. 2016;90(6):339-46. https://doi.org/10.1159/000445978.
Wang S, Wang B, **e C, Ye D. RET Proto-oncogene Gene Mutation Is Related to Cervical Lymph Node Metastasis in Medullary Thyroid Carcinoma. Endocr Pathol. 2019;30(4):297-304. https://doi.org/10.1007/s12022-019-09588-z.
Ciampi R, Romei C, Ramone T, Prete A, Tacito A, Cappagli V, et al. Genetic Landscape of Somatic Mutations in a Large Cohort of Sporadic Medullary Thyroid Carcinomas Studied by Next-Generation Targeted Sequencing. iScience. 2019;20:324–36. https://doi.org/10.1016/j.isci.2019.09.030.
Elisei R, Cosci B, Romei C, Bottici V, Renzini G, Molinaro E, et al. Prognostic significance of somatic RET oncogene mutations in sporadic medullary thyroid cancer: a 10-year follow-up study. J Clin Endocrinol Metab. 2008;93(3):682-7. https://doi.org/10.1210/jc.2007-1714.
Minna E, Romeo P, Dugo M, De Cecco L, Aiello A, Pistore F, et al. Medullary Thyroid Carcinoma Mutational Spectrum Update and Signaling-Type Inference by Transcriptional Profiles: Literature Meta-Analysis and Study of Tumor Samples. Cancers (Basel). 2022;14(8). https://doi.org/10.3390/cancers14081951.
Ciampi R, Romei C, Cosci B, Vivaldi A, Bottici V, Renzini G, et al. Chromosome 10 and RET gene copy number alterations in hereditary and sporadic Medullary Thyroid Carcinoma. Mol Cell Endocrinol. 2012;348(1):176-82. https://doi.org/10.1016/j.mce.2011.08.004.
Agrawal N, Jiao Y, Sausen M, Leary R, Bettegowda C, Roberts NJ, et al. Exomic sequencing of medullary thyroid cancer reveals dominant and mutually exclusive oncogenic mutations in RET and RAS. J Clin Endocrinol Metab. 2013;98(2):E364-9. https://doi.org/10.1210/jc.2012-2703.
Nikiforova MN, Wald AI, Roy S, Durso MB, Nikiforov YE. Targeted next-generation sequencing panel (ThyroSeq) for detection of mutations in thyroid cancer. J Clin Endocrinol Metab. 2013;98(11):E1852-60. https://doi.org/10.1210/jc.2013-2292.
Boichard A, Croux L, Al Ghuzlan A, Broutin S, Dupuy C, Leboulleux S, et al. Somatic RAS mutations occur in a large proportion of sporadic RET-negative medullary thyroid carcinomas and extend to a previously unidentified exon. J Clin Endocrinol Metab. 2012;97(10):E2031-5. https://doi.org/10.1210/jc.2012-2092.
Moura MM, Cavaco BM, Pinto AE, Leite V. High prevalence of RAS mutations in RET-negative sporadic medullary thyroid carcinomas. J Clin Endocrinol Metab. 2011;96(5):E863-8. https://doi.org/10.1210/jc.2010-1921.
Simbolo M, Mian C, Barollo S, Fassan M, Mafficini A, Neves D, et al. High-throughput mutation profiling improves diagnostic stratification of sporadic medullary thyroid carcinomas. Virchows Arch. 2014;465(1):73-8. https://doi.org/10.1007/s00428-014-1589-3.
Schilling T, Burck J, Sinn HP, Clemens A, Otto HF, Hoppner W, et al. Prognostic value of codon 918 (ATG-->ACG) RET proto-oncogene mutations in sporadic medullary thyroid carcinoma. Int J Cancer. 2001;95(1):62-6. https://doi.org/10.1002/1097-0215(20010120)95:1<62::aid-ijc1011>3.0.co;2-1.
Romei C, Casella F, Tacito A, Bottici V, Valerio L, Viola D, et al. New insights in the molecular signature of advanced medullary thyroid cancer: evidence of a bad outcome of cases with double RET mutations. J Med Genet. 2016;53(11):729-34. https://doi.org/10.1136/jmedgenet-2016-103833.
Vuong HG, Odate T, Ngo HTT, Pham TQ, Tran TTK, Mochizuki K, et al. Clinical significance of RET and RAS mutations in sporadic medullary thyroid carcinoma: a meta-analysis. Endocr Relat Cancer. 2018;25(6):633-41. https://doi.org/10.1530/ERC-18-0056.
Moura MM, Cavaco BM, Pinto AE, Domingues R, Santos JR, Cid MO, et al. Correlation of RET somatic mutations with clinicopathological features in sporadic medullary thyroid carcinomas. Br J Cancer. 2009;100(11):1777-83. https://doi.org/10.1038/sj.bjc.6605056.
Cote GJ, Evers C, Hu MI, Grubbs EG, Williams MD, Hai T, et al. Prognostic Significance of Circulating RET M918T Mutated Tumor DNA in Patients With Advanced Medullary Thyroid Carcinoma. J Clin Endocrinol Metab. 2017;102(9):3591-9. https://doi.org/10.1210/jc.2017-01039.
Shi X, Sun Y, Shen C, Zhang Y, Shi R, Zhang F, et al. Integrated proteogenomic characterization of medullary thyroid carcinoma. Cell Discov. 2022;8(1):120. https://doi.org/10.1038/s41421-022-00479-y.
Ji JH, Oh YL, Hong M, Yun JW, Lee HW, Kim D, et al. Identification of Driving ALK Fusion Genes and Genomic Landscape of Medullary Thyroid Cancer. PLoS Genet. 2015;11(8):e1005467. https://doi.org/10.1371/journal.pgen.1005467.
Hillier K, Hughes A, Shamberger RC, Shusterman S, Perez-Atayde AR, Wassner AJ, et al. A Novel ALK Fusion in Pediatric Medullary Thyroid Carcinoma. Thyroid. 2019;29(11):1704-7. https://doi.org/10.1089/thy.2019.0041.
Lu Y, Chan YT, Tan HY, Li S, Wang N, Feng Y. Epigenetic regulation in human cancer: the potential role of epi-drug in cancer therapy. Mol Cancer. 2020;19(1):79. https://doi.org/10.1186/s12943-020-01197-3.
Sponziello M, Durante C, Boichard A, Dima M, Puppin C, Verrienti A, et al. Epigenetic-related gene expression profile in medullary thyroid cancer revealed the overexpression of the histone methyltransferases EZH2 and SMYD3 in aggressive tumours. Mol Cell Endocrinol. 2014;392(1-2):8-13. https://doi.org/10.1016/j.mce.2014.04.016.
Wang N, Kjellin H, Sofiadis A, Fotouhi O, Juhlin CC, Backdahl M, et al. Genetic and epigenetic background and protein expression profiles in relation to telomerase activation in medullary thyroid carcinoma. Oncotarget. 2016;7(16):21332-46. https://doi.org/10.18632/oncotarget.7237.
Galuppini F, Censi S, Moro M, Carraro S, Sbaraglia M, Iacobone M, et al. MicroRNAs in Medullary Thyroid Carcinoma: A State of the Art Review of the Regulatory Mechanisms and Future Perspectives. Cells. 2021;10(4):955. https://doi.org/10.3390/cells10040955.
Pennelli G, Galuppini F, Barollo S, Cavedon E, Bertazza L, Fassan M, et al. The PDCD4/miR-21 pathway in medullary thyroid carcinoma. Hum Pathol. 2015;46(1):50-7. https://doi.org/10.1016/j.humpath.2014.09.006.
Aubert S, Berdelou A, Gnemmi V, Behal H, Caiazzo R, D’Herbomez M, et al. Large sporadic thyroid medullary carcinomas: predictive factors for lymph node involvement. Virchows Arch. 2018;472(3):461-8. https://doi.org/10.1007/s00428-018-2303-7.
Abraham D, Jackson N, Gundara JS, Zhao J, Gill AJ, Delbridge L, et al. MicroRNA profiling of sporadic and hereditary medullary thyroid cancer identifies predictors of nodal metastasis, prognosis, and potential therapeutic targets. Clin Cancer Res. 2011;17(14):4772-81. https://doi.org/10.1158/1078-0432.CCR-11-0242.
Romeo P, Colombo C, Granata R, Calareso G, Gualeni AV, Dugo M, et al. Circulating miR-375 as a novel prognostic marker for metastatic medullary thyroid cancer patients. Endocr Relat Cancer. 2018;25(3):217-31. https://doi.org/10.1530/ERC-17-0389.
Censi S, Bertazza L, Piva I, Manso J, Benna C, Iacobone M, et al. Serum miR-375 for Diagnostic and Prognostic Purposes in Medullary Thyroid Carcinoma. Front Endocrinol (Lausanne). 2021;12:647369. https://doi.org/10.3389/fendo.2021.647369.
Mian C, Pennelli G, Fassan M, Balistreri M, Barollo S, Cavedon E, et al. MicroRNA profiles in familial and sporadic medullary thyroid carcinoma: preliminary relationships with RET status and outcome. Thyroid. 2012;22(9):890-6. https://doi.org/10.1089/thy.2012.0045.
Cavedon E, Barollo S, Bertazza L, Pennelli G, Galuppini F, Watutantrige-Fernando S, et al. Prognostic Impact of miR-224 and RAS Mutations in Medullary Thyroid Carcinoma. Int J Endocrinol. 2017;2017:4915736. https://doi.org/10.1155/2017/4915736.
Rendl G, Manzl M, Hitzl W, Sungler P, Pirich C. Long-term prognosis of medullary thyroid carcinoma. Clin Endocrinol (Oxf). 2008;69(3):497-505. https://doi.org/10.1111/j.1365-2265.2008.03229.x.
Roman S, Lin R, Sosa JA. Prognosis of medullary thyroid carcinoma: demographic, clinical, and pathologic predictors of survival in 1252 cases. Cancer. 2006;107(9):2134-42. https://doi.org/10.1002/cncr.22244.
Abraham DT, Low TH, Messina M, Jackson N, Gill A, Chou AS, et al. Medullary thyroid carcinoma: long-term outcomes of surgical treatment. Ann Surg Oncol. 2011;18(1):219-25. https://doi.org/10.1245/s10434-010-1339-y.
Mathiesen JS, Kroustrup JP, Vestergaard P, Stochholm K, Poulsen PL, Rasmussen AK, et al. Survival and Long-Term Biochemical Cure in Medullary Thyroid Carcinoma in Denmark 1997-2014: A Nationwide Study. Thyroid. 2019;29(3):368-77. https://doi.org/10.1089/thy.2018.0564.
Gogna S, Goldberg M, Samson D, Gachabayov M, Felsenreich DM, Azim A, et al. Medullary Thyroid Cancer in Patients Older than 45-Epidemiologic Trends and Predictors of Survival. Cancers (Basel). 2020;12(11). https://doi.org/10.3390/cancers12113124.
Chen L, Wang Y, Zhao K, Wang Y, He X. Postoperative Nomogram for Predicting Cancer-Specific and Overall Survival among Patients with Medullary Thyroid Cancer. Int J Endocrinol. 2020;2020:8888677. https://doi.org/10.1155/2020/8888677.
Modigliani E, Cohen R, Campos JM, Conte-Devolx B, Maes B, Boneu A, et al. Prognostic factors for survival and for biochemical cure in medullary thyroid carcinoma: results in 899 patients. The GETC Study Group. Groupe d’etude des tumeurs a calcitonine. Clin Endocrinol (Oxf). 1998;48(3):265–73. https://doi.org/10.1046/j.1365-2265.1998.00392.x.
Kebebew E, Ituarte PH, Siperstein AE, Duh QY, Clark OH. Medullary thyroid carcinoma: clinical characteristics, treatment, prognostic factors, and a comparison of staging systems. Cancer. 2000;88(5):1139-48. https://doi.org/10.1002/(sici)1097-0142(20000301)88:5<1139::aid-cncr26>3.0.co;2-z.
Schroder S, Bocker W, Baisch H, Burk CG, Arps H, Meiners I, et al. Prognostic factors in medullary thyroid carcinomas. Survival in relation to age, sex, stage, histology, immunocytochemistry, and DNA content. Cancer. 1988;61(4):806–16. https://doi.org/10.1002/1097-0142(19880215)61:4<806::aid-cncr2820610428>3.0.co;2-g.
Amin MB, Edge S, Greene F, Byrd DR, Brookland RK, Washington MK, et al. AJCC Cancer Staging Manual. 8th ed. New York, NY: Springer; 2017. p. 891-901.
Livolsi VA. Neuroendocrine Tumors of the Thyroid and Their Mimics. Endocr Pathol. 2021;32(2):211-21. https://doi.org/10.1007/s12022-021-09672-3.
Pillarisetty VG, Katz SC, Ghossein RA, Tuttle RM, Shaha AR. Micromedullary thyroid cancer: how micro is truly micro? Ann Surg Oncol. 2009;16(10):2875-81. https://doi.org/10.1245/s10434-009-0595-1.
Kazaure HS, Roman SA, Sosa JA. Medullary thyroid microcarcinoma. Cancer 2012; 118(3): 620-627. https://doi.org/10.1002/cncr.26283.
Machens A, Dralle H. Biological Relevance of Medullary Thyroid Microcarcinoma. The Journal of Clinical Endocrinology & Metabolism 2012; 97(5):1547-1553. https://doi.org/10.1210/jc.2011-2534.
Kim JH, Pyo JS, Cho WJ. Clinicopathological Significance and Prognosis of Medullary Thyroid Microcarcinoma: A Meta-analysis. World J Surg. 2017;41(10):2551-8. https://doi.org/10.1007/s00268-017-4031-6.
Romei C, Ugolini C, Cosci B, Torregrossa L, Vivaldi A, Ciampi R, et al. Low prevalence of the somatic M918T RET mutation in micro-medullary thyroid cancer. Thyroid. 2012;22(5):476-81. https://doi.org/10.1089/thy.2011.0358.
Raue F, Bruckner T, Frank-Raue K. Similar Stage-dependent Survival and Outcome in Sporadic and Hereditary Medullary Thyroid Carcinoma. J Clin Endocrinol Metab. 2021;106(9):e3582-e91. https://doi.org/10.1210/clinem/dgab326.
Niederle MB, Scheuba C, Riss P, Selberherr A, Koperek O, Niederle B. Early Diagnosis of Medullary Thyroid Cancer: Are Calcitonin Stimulation Tests Still Indicated in the Era of Highly Sensitive Calcitonin Immunoassays? Thyroid. 2020;30(7):974-84. https://doi.org/10.1089/thy.2019.0785.
Machens A, Dralle H. Biomarker-based risk stratification for previously untreated medullary thyroid cancer. J Clin Endocrinol Metab. 2010;95(6):2655-63. https://doi.org/10.1210/jc.2009-2368.
Machens A, Lorenz K, Dralle H. Prediction of biochemical cure in patients with medullary thyroid cancer. Br J Surg. 2020;107(6):695-704. https://doi.org/10.1002/bjs.11444.
Machens A, Ukkat J, Hauptmann S, Dralle H. Abnormal carcinoembryonic antigen levels and medullary thyroid cancer progression: a multivariate analysis. Arch Surg. 2007;142(3):289–93; discussion 94. https://doi.org/10.1001/archsurg.142.3.289.
Laure Giraudet A, Al Ghulzan A, Auperin A, Leboulleux S, Chehboun A, Troalen F, et al. Progression of medullary thyroid carcinoma: assessment with calcitonin and carcinoembryonic antigen doubling times. Eur J Endocrinol. 2008;158(2):239-46. https://doi.org/10.1530/EJE-07-0667.
Ito Y, Miyauchi A, Kihara M, Kudo T, Miya A. Calcitonin doubling time in medullary thyroid carcinoma after the detection of distant metastases keenly predicts patients’ carcinoma death. Endocr J. 2016;63(7):663-7. https://doi.org/10.1507/endocrj.EJ16-0140.
Giovanella L, Crippa S, Cariani L. Serum calcitonin-negative medullary thyroid carcinoma: role of CgA and CEA as complementary markers. Int J Biol Markers. 2008;23(2):129-31. https://doi.org/10.1177/172460080802300212.
Frank-Raue K, Machens A, Leidig-Bruckner G, Rondot S, Haag C, Schulze E, et al. Prevalence and clinical spectrum of nonsecretory medullary thyroid carcinoma in a series of 839 patients with sporadic medullary thyroid carcinoma. Thyroid. 2013;23(3):294-300. https://doi.org/10.1089/thy.2012.0236.
Meisner M, Tschaikowsky K, Schnabel S, Schmidt J, Katalinic A, Schuttler J. Procalcitonin--influence of temperature, storage, anticoagulation and arterial or venous asservation of blood samples on procalcitonin concentrations. Eur J Clin Chem Clin Biochem. 1997;35(8):597-601. https://doi.org/10.1515/cclm.1997.35.8.597.
Giovanella L, Garo ML, Ceriani L, Paone G, Campenni A, D’Aurizio F. Procalcitonin as an Alternative Tumor Marker of Medullary Thyroid Carcinoma. J Clin Endocrinol Metab. 2021;106(12):3634-43. https://doi.org/10.1210/clinem/dgab564.
Walter MA, Meier C, Radimerski T, Iten F, Kranzlin M, Muller-Brand J, et al. Procalcitonin levels predict clinical course and progression-free survival in patients with medullary thyroid cancer. Cancer. 2010;116(1):31-40. https://doi.org/10.1002/cncr.24738.
Machens A, Lorenz K, Dralle H. Utility of serum procalcitonin for screening and risk stratification of medullary thyroid cancer. J Clin Endocrinol Metab. 2014;99(8):2986-94. https://doi.org/10.1210/jc.2014-1278.
Elisei R, Lorusso L, Piaggi P, Torregrossa L, Pellegrini G, Molinaro E, et al. Elevated level of serum carbohydrate antigen 19.9 as predictor of mortality in patients with advanced medullary thyroid cancer. Eur J Endocrinol. 2015;173(3):297–304. https://doi.org/10.1530/EJE-15-0304.
Lorusso L, Romei C, Piaggi P, Fustini C, Molinaro E, Agate L, et al. Ca19.9 Positivity and Doubling Time Are Prognostic Factors of Mortality in Patients with Advanced Medullary Thyroid Cancer with No Evidence of Structural Disease Progression According to Response Evaluation Criteria in Solid Tumors. Thyroid. 2021;31(7):1050–5. https://doi.org/10.1089/thy.2020.0060.
Rios A, Rodriguez JM, Acosta JM, Balsalobre MD, Torregrosa N, Sola J, et al. Prognostic value of histological and immunohistochemical characteristics for predicting the recurrence of medullary thyroid carcinoma. Ann Surg Oncol. 2010;17(9):2444-51. https://doi.org/10.1245/s10434-010-1021-4.
Etit D, Faquin WC, Gaz R, Randolph G, Delellis RA, Pilch BZ. Histopathologic and Clinical Features of Medullary Microcarcinoma and C-Cell Hyperplasia in Prophylactic Thyroidectomies for Medullary Carcinoma: A Study of 42 Cases. Arch Pathol Lab Med. 2008;132(11):1767-73. https://doi.org/10.5858/132.11.1767.
Mete O, Hannah-Shmouni F, Kim R, Stratakis CA. Inherited Neuroendocrine Neoplasms. In: Asa SL, La Rosa S, Mete O, editors. The Spectrum of Neuroendocrine Neoplasia. Cham: Springer International Publishing; 2021. p. 409-59.
Mete O, Asa SL. Pathological definition and clinical significance of vascular invasion in thyroid carcinomas of follicular epithelial derivation. Mod Pathol. 2011;24(12):1545-52. https://doi.org/10.1038/modpathol.2011.119.
Erovic BM, Kim D, Cassol C, Goldstein DP, Irish JC, Asa SL, et al. Prognostic and predictive markers in medullary thyroid carcinoma. Endocr Pathol. 2012;23(4):232-42. https://doi.org/10.1007/s12022-012-9225-8.
Qu N, Shi RL, Lu ZW, Liao T, Wen D, Sun GH, et al. Metastatic lymph node ratio can further stratify risk for mortality in medullary thyroid cancer patients: A population-based analysis. Oncotarget. 2016;7(40):65937-45. https://doi.org/10.18632/oncotarget.11725.
Wu X, Li B, Zheng C. Clinical Characteristics, Surgical Management, and Prognostic Factors of Medullary Thyroid Carcinoma: A Retrospective, Single-Center Study. Technol Cancer Res Treat. 2022;21:15330338221078435. https://doi.org/10.1177/15330338221078435.
Tang J, Jiang S, Gao L, ** X, Zhao R, Lai X, et al. Construction and Validation of a Nomogram Based on the Log Odds of Positive Lymph Nodes to Predict the Prognosis of Medullary Thyroid Carcinoma After Surgery. Ann Surg Oncol. 2021;28(8):4360-70. https://doi.org/10.1245/s10434-020-09567-3.
Kuo EJ, Sho S, Li N, Zanocco KA, Yeh MW, Livhits MJ. Risk Factors Associated With Reoperation and Disease-Specific Mortality in Patients With Medullary Thyroid Carcinoma. JAMA Surg. 2018;153(1):52-9. https://doi.org/10.1001/jamasurg.2017.3555.
Cree IA, Tan PH, Travis WD, Wesseling P, Yagi Y, White VA, et al. Counting mitoses: SI(ze) matters! Mod Pathol. 2021;34(9):1651-7. https://doi.org/10.1038/s41379-021-00825-7.
Cree IA. From Counting Mitoses to Ki67 Assessment: Technical Pitfalls in the New WHO Classification of Endocrine and Neuroendocrine Tumors. Endocr Pathol. 2022;33(1):3-5. https://doi.org/10.1007/s12022-021-09701-1.
Agarwal S, Bychkov A, Jung CK. Emerging Biomarkers in Thyroid Practice and Research. Cancers (Basel). 2021;14(1). https://doi.org/10.3390/cancers14010204.
Lea D, Gudlaugsson EG, Skaland I, Lillesand M, Soreide K, Soreide JA. Digital Image Analysis of the Proliferation Markers Ki67 and Phosphohistone H3 in Gastroenteropancreatic Neuroendocrine Neoplasms: Accuracy of Grading Compared With Routine Manual Hot Spot Evaluation of the Ki67 Index. Appl Immunohistochem Mol Morphol. 2021;29(7):499-505. https://doi.org/10.1097/PAI.0000000000000934.
Kim JY, Jeong HS, Chung T, Kim M, Lee JH, Jung WH, et al. The value of phosphohistone H3 as a proliferation marker for evaluating invasive breast cancers: A comparative study with Ki67. Oncotarget. 2017;8(39):65064-76. https://doi.org/10.18632/oncotarget.17775.
Ibrahim A, Lashen A, Toss M, Mihai R, Rakha E. Assessment of mitotic activity in breast cancer: revisited in the digital pathology era. J Clin Pathol. 2022;75(6):365-72. https://doi.org/10.1136/jclinpath-2021-207742.
Kloppel G. Classification and pathology of gastroenteropancreatic neuroendocrine neoplasms. Endocr Relat Cancer. 2011;18 Suppl 1:S1-16. https://doi.org/10.1530/ERC-11-0013.
Alzumaili B, Xu B, Spanheimer PM, Tuttle RM, Sherman E, Katabi N, et al. Grading of medullary thyroid carcinoma on the basis of tumor necrosis and high mitotic rate is an independent predictor of poor outcome. Mod Pathol. 2020;33(9):1690-701. https://doi.org/10.1038/s41379-020-0532-1.
Fuchs TL, Nassour AJ, Glover A, Sywak MS, Sidhu SB, Delbridge LW, et al. A Proposed Grading Scheme for Medullary Thyroid Carcinoma Based on Proliferative Activity (Ki-67 and Mitotic Count) and Coagulative Necrosis. Am J Surg Pathol. 2020;44(10):1419-28. https://doi.org/10.1097/PAS.0000000000001505.
Najdawi F, Ahmadi S, Capelletti M, Dong F, Chau NG, Barletta JA. Evaluation of grade in a genotyped cohort of sporadic medullary thyroid carcinomas. Histopathology. 2021;79(3):427-36. https://doi.org/10.1111/his.14370.
Vissio E, Maletta F, Fissore J, Osella Abate S, Retta F, Brizzi MP, et al. External Validation of Three Available Grading Systems for Medullary Thyroid Carcinoma in a Single Institution Cohort. Endocr Pathol. 2022;33(3):359-70. https://doi.org/10.1007/s12022-022-09719-z.
Williams JF, Zhao M, Najdawi F, Ahmadi S, Hornick JL, Wong KS, et al. Grading of Medullary Thyroid Carcinoma: an Interobserver Reproducibility Study. Endocr Pathol. 2022;33(3):371-7. https://doi.org/10.1007/s12022-022-09718-0.
Nigam A, Xu B, Spanheimer PM, Ganly I, Tuttle RM, Wong RJ, et al. Tumor Grade Predicts for Calcitonin Doubling Times and Disease-Specific Outcomes After Resection of Medullary Thyroid Carcinoma. Thyroid. 2022;32(10):1193-200. https://doi.org/10.1089/thy.2022.0217.
Jung CK, Bychkov A, Kakudo K. Update from the 2022 World Health Organization Classification of Thyroid Tumors: A Standardized Diagnostic Approach. Endocrinol Metab (Seoul). 2022;37(5):703-18. https://doi.org/10.3803/EnM.2022.1553.
Hofland J, Brabander T, Verburg FA, Feelders RA, de Herder WW. Peptide Receptor Radionuclide Therapy. J Clin Endocrinol Metab. 2022;107(12):3199-208. https://doi.org/10.1210/clinem/dgac574.
Parghane RV, Naik C, Talole S, Desmukh A, Chaukar D, Banerjee S, et al. Clinical utility of (177) Lu-DOTATATE PRRT in somatostatin receptor-positive metastatic medullary carcinoma of thyroid patients with assessment of efficacy, survival analysis, prognostic variables, and toxicity. Head Neck. 2020;42(3):401-16. https://doi.org/10.1002/hed.26024.
Wells SA, Jr., Robinson BG, Gagel RF, Dralle H, Fagin JA, Santoro M, et al. Vandetanib in patients with locally advanced or metastatic medullary thyroid cancer: a randomized, double-blind phase III trial. J Clin Oncol. 2012;30(2):134-41. https://doi.org/10.1200/JCO.2011.35.5040.
Elisei R, Schlumberger MJ, Muller SP, Schoffski P, Brose MS, Shah MH, et al. Cabozantinib in progressive medullary thyroid cancer. J Clin Oncol. 2013;31(29):3639-46. https://doi.org/10.1200/JCO.2012.48.4659.
Saltiki K, Simeakis G, Karapanou O, Alevizaki M. MANAGEMENT OF ENDOCRINE DISEASE: Medullary thyroid cancer: from molecular biology and therapeutic pitfalls to future targeted treatment perspectives. Eur J Endocrinol. 2022;187(3):R53-R63. https://doi.org/10.1530/EJE-22-0312.
Ma LX, Espin-Garcia O, Bedard PL, Stockley T, Prince R, Mete O, et al. Clinical Application of Next-Generation Sequencing in Advanced Thyroid Cancers. Thyroid. 2022;32(6):657-66. https://doi.org/10.1089/thy.2021.0542.
Belli C, Anand S, Gainor JF, Penault-Llorca F, Subbiah V, Drilon A, et al. Progresses Toward Precision Medicine in RET-altered Solid Tumors. Clin Cancer Res. 2020;26(23):6102-11. https://doi.org/10.1158/1078-0432.CCR-20-1587.
Wirth LJ, Sherman E, Robinson B, Solomon B, Kang H, Lorch J, et al. Efficacy of Selpercatinib in RET-Altered Thyroid Cancers. N Engl J Med. 2020;383(9):825-35. https://doi.org/10.1056/NEJMoa2005651.
Subbiah V, Hu MI, Wirth LJ, Schuler M, Mansfield AS, Curigliano G, et al. Pralsetinib for patients with advanced or metastatic RET-altered thyroid cancer (ARROW): a multi-cohort, open-label, registrational, phase 1/2 study. Lancet Diabetes Endocrinol. 2021;9(8):491-501. https://doi.org/10.1016/S2213-8587(21)00120-0.
Funding
This research was supported by a grant (HI21C0940) from the Korean Health Technology R&D Project, Ministry of Health and Welfare, Republic of Korea. This research was also supported by a grant (NRF-2020R1F1A1070028) from the Basic Science Research Program through the National Research Foundation of Korea funded by the Ministry of Science and ICT.
Author information
Authors and Affiliations
Contributions
Conceptualization: CKJ; writing — initial draft preparation: CKJ, SA, J-FH, D-JL; writing — review and editing: CKJ, SA, AB, J-FH, D-JL, and OM; funding acquisition: CKJ; resources: CKJ.
Corresponding author
Ethics declarations
Ethics Approval
This study was performed according to the principles of the Declaration of Helsinki. Approval was granted by the Catholic Medical Center, The Catholic University of Korea (XC21ENDI0031K).
Consent for Publication
All the authors approved for the publication of this paper.
Conflict of Interest
Dr. Ozgur Mete is the editor-in-chief of Endocrine Pathology. This article was handled by an independent senior editor. Other authors have no conflict of interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jung, C.K., Agarwal, S., Hang, JF. et al. Update on C-Cell Neuroendocrine Neoplasm: Prognostic and Predictive Histopathologic and Molecular Features of Medullary Thyroid Carcinoma. Endocr Pathol 34, 1–22 (2023). https://doi.org/10.1007/s12022-023-09753-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12022-023-09753-5