Abstract
Purpose
Evaluation of potential tropic effects of vascular endothelial growth factor (VEGF) on the incorporation and differentiation of bone-marrow-derived stem cells (BMSCs) in a murine model of anterior ischemic optic neuropathy (AION).
Methods
In the first approach, small-sized subset of BMCs were isolated from GFP donors mice by counterflow centrifugal elutriation and depleted of hematopoietic lineages (Fr25lin-). These cells were injected into a peripheral vein (1 × 106 in 0.2 ml) or inoculated intravitreally (2 × 105) to syngeneic mice, with or without intravitreal injection of 5 μg/2μL VEGF, simultaneously with AION induction. In a second approach, hematopoietic cells were substituted by myelablative transplant of syngeseic GFP + bone marrow cells. After 3 months, progenitors were mobilized with granulocyte-macrophage colony-stimulating factor (GM-CSF) followed by VEGF inoculation into the vitreous body and AION induction . Engraftment and phenotype were examined by immunohistochemistry and FISH at 4 and 24 weeks post-transplantation, and VEGF receptors were determined by real time PCR.
Results
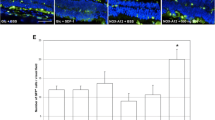
VEGF had no quantitative effect on incorporation of elutriated cells in the injured retina, yet it induced early expression of neuroal markers in cells incorporated in the RGC layer and promoted durable gliosis, most prominent perivascular astrocytes. These effects were mediated by VEGF-R1/Flt-1, which is constitutively expresses in the elutriated fraction of stem cells. Mobilization with GM-CSF limited the differentiation of bone marrow progenitors to microglia, which was also fostered by VEGF.
Conclusions
VEGF signaling mediated by Flt-1 induces early neural and sustained astrocytic differentiation of stem cells elutriated from adult bone-marrow, with significant contribution to stabilization retinal architecture following ischemic injury.





Similar content being viewed by others
References
Friedlander, M., Dorrell, M. I., Ritter, M. R., et al. (2007). Progenitor cells and retinal angiogenesis. Angiogenesis, 10(2), 89–101.
Liu, X., Li, Y., Liu, Y., et al. (2010). Endothelial progenitor cells (EPCs) mobilized and activated by neurotrophic factors may contribute to pathologic neovascularization in diabetic retinopathy. American Journal of Pathology, 176(1), 504–515.
McVicar, C. M., Colhoun, L. M., Abrahams, J. L., et al. (2010). Differential modulation of angiogenesis by erythropoiesis-stimulating agents in a mouse model of ischaemic retinopathy. PLoS One, 5(7), e11870.
Eglitis, M. A., & Mezey, E. (1997). Hematopoietic cells differentiate into both microglia and macroglia in the brains of adult mice. Proceedings of the National Academy of Sciences of the United States of America, 94, 4080–4085.
Brazelton, T. R., Rossi, F. M., Keshet, G. I., & Blau, H. M. (2000). From marrow to brain: Expression of neuronal phenotypes in adult mice. Science, 290, 1775–1779.
Mezey, E., Chandross, K. J., Harta, G., Maki, R. A., & McKercher, S. R. (2000). Turning blood into brain: Cells bearing neuronal antigens generated in vivo from bone marrow. Science, 290, 17791782.
Tomita, M., Adachi, Y., Yamada, H., et al. (2002). Bone marrow-derived stem cells can differentiate into retinal cells in injured rat retina. Stem Cells, 20, 279–283.
Mezey, E., Key, S., Vogelsang, G., Szalayova, I., Lange, G. D., & Crain, B. (2003). Transplanted bone marrow generates new neurons in human brains. Proceedings of the National Academy of Sciences of the United States of America, 100, 1364–1369.
Otani, A., Dorrell, M. I., Kinder, K., et al. (2004). Rescue of retinal degeneration by intravitreally injected adult bone marrow-derived lineage-negative hematopoietic stem cells. The Journal of Clinical Investigation, 114, 765–774.
Badami, C. D., Livingston, D. H., Sifri, Z. C., et al. (2007). Hematopoietic progenitor cells mobilize to the site of injury after trauma and hemorrhagic shock in rats. The Journal of Trauma, 63, 596–602.
Asahara, T., Masuda, H., Takahashi, T., et al. (1999). Bone marrow origin of endothelial progenitor cells responsible for postnatal vasculogenesis in physiological and pathological neovascularization. Circulation Research, 85, 221–228.
Takahashi, T., Kalka, C., Masuda, H., et al. (1999). Ischemia- and cytokine-induced mobilization of bone marrow-derived endothelial progenitor cells for neovascularization. Nature Medicine, 5, 434–438.
Zou, H., Otani, A., Oishi, A., et al. (2010). Bone marrow-derived cells are differentially involved in pathological and physiological retinal angiogenesis in mice. Biochemical and Biophysical Research Communications, 391(2), 1268–1273.
Grant, M. B., May, W. S., Caballero, S., et al. (2002). Adult hematopoietic stem cells provide functional hemangioblast activity during retinal neovascularization. Nature Medicine, 8, 607–612.
Otani, A., Kinder, K., Ewalt, K., Otero, F. J., Schimmel, P., & Friedlander, M. (2002). Bone marrow-derived stem cells target retinal astrocytes and can promote or inhibit retinal angiogenesis. Nature Medicine, 8, 1004–1010.
Harris, J. R., Fisher, R., Jorgensen, M., Kaushal, S., & Scott, E. W. (2009). CD133 progenitor cells from the bone marrow contribute to retinal pigment epithelium repair. Stem Cells, 27, 457–466.
Sengupta, N., Caballero, S., Sullivan, S. M., et al. (2009). Regulation of adult hematopoietic stem cells fate for enhanced tissue-specific repair. Molecular Therapy, 17, 1594–1604.
Goldenberg-Cohen, N., Avraham-Lubin, B. R., Sadikov, T., Goldstein, R. S., & Askenasy, N. (2012). Primitive stem cells derived from bone marrow express glial and neuronal markers and support revascularization in injured retina exposed to ischemic and mechanical damage. Stem Cells and Development, 21(9), 1488–1500.
Bernstein, S. L., Guo, Y., Kelman, S. E., Flower, R. A., & Johnson, M. A. (2003). Functional and cellular responses in a novel rodent model of anterior ischemic optic neuropathy. Investigative Ophthalmology & Visual Science, 44, 4153–4162.
Goldenberg-Cohen, N., Guo, Y., Margolis, F., Cohen, Y., Miller, N. R., & Bernstein, S. L. (2005). Oligodendrocyte dysfunction after induction of experimental anterior optic nerve ischemia. Investigative Ophthalmology & Visual Science, 46, 2716–2725.
Miller, J. W., Adamis, A. P., Shima, D. T., et al. (1994). Vascular endothelial growth factor/vascular permeability factor is temporally and spatially correlated with ocular angiogenesis in a primate model. American Journal of Pathology, 145, 574–584.
Aiello, L. P., Pierce, E. A., Foley, E. D., et al. (1995). Suppression of retinal neovascularization in vivo by inhibition of vascular endothelial growth factor (VEGF) using soluble VEGF-receptor chimeric proteins. Proceedings of the National Academy of Sciences of the United States of America, 92, 10457–10461.
Pierce, E. A., Avery, R. L., Foley, E. D., Aiello, L. P., & Smith, L. E. H. (1995). Vascular endothelial growth factor/vascular permeability factor expression in a mouse model of retinal neovascularization. Proceedings of the National Academy of Sciences of the United States of America, 92, 905–909.
Okamoto, N., Tobe, T., Hackett, S. F., et al. (1997). Transgenic mice with increased expression of vascular endothelial growth factor in the retina: A new model of intraretinal and subretinal neovascularization. American Journal of Pathology, 151, 281–291.
Matsuzaki, H., Tamatani, M., Yamaguchi, A., et al. (2001). Vascular endothelial growth factor rescues hippocampal neurons from glutamate-induced toxicity: Signal transduction cascades. ASEB Journal, 15, 1218–1220.
Widenfalk, J., Lipson, A., Jubran, M., et al. (2003). Vascular endothelial growth factor improves functional outcome and decreases secondary degeneration in experimental spinal cord contusion injury. Neuroscience, 120, 951–960.
**, K., Zhu, Y., Sun, Y., Mao, X. O., **e, L., & Greenberg, D. A. (2002). Vascular endothelial growth factor (VEGF) stimulates neurogenesis in vitro and in vivo. Proceedings of the National Academy of Sciences of the United States of America, 99, 11946–11950.
Sun, Y. E., Martinowich, K., & Ge, W. (2003). Making and repairing the mammalian brain–signaling toward neurogenesis and gliogenesis. Seminars in Cell & Developmental Biology, 14, 161–168.
Schänzer, A., Wachs, F. P., Wilhelm, D., et al. (2004). Direct stimulation of adult neural stem cells in vitro and neurogenesis in vivo by vascular endothelial growth factor. Brain Pathology, 14, 237–248.
Asahara, T., Takahashi, T., Masuda, H., et al. (1999). VEGF contributes to postnatal neovascularization by mobilizing bone marrow-derived endothelial progenitor cells. EMBO Journal, 18, 3964–3972.
Kwak, N., Okamoto, N., Wood, J. M., & Campochiaro, P. A. (2000). VEGF is major stimulator in model of choroidal neovascularization. Investigative Ophthalmology & Visual Science, 41, 3158–3164.
Tomita, M., Yamada, H., Adachi, Y., et al. (2004). Choroidal neovascularization is provided by bone marrow cells. Stem Cells, 22, 21–26.
Krum, J. M., Mani, N., & Rosenstein, J. M. (2002). Angiogenic and astroglial responses to vascular endothelial growth factor administration in adult rat brain. Neuroscience, 110, 589–604.
Shen, J., Yang, X., **ao, W. H., Hackett, S. F., Sato, Y., & Campochiaro, P. A. (2006). Vasohibin is up-regulated by VEGF in the retina and suppresses VEGF receptor 2 and retinal neovascularization. The FASEB Journal, 20, 723–725.
Hashimoto, T., Zhang, X. M., Chen, B. Y., & Yang, X. J. (2006). VEGF activates divergent intracellular signaling components to regulate retinal progenitor cell proliferation and neuronal differentiation. Development, 133, 2201–2210.
Wada, T., Haigh, J. J., Ema, M., et al. (2006). Vascular endothelial growth factor directly inhibits primitive neural stem cell survival but promotes definitive neural stem cell survival. Journal of Neuroscience, 26, 6803–6812.
Nishiguchi, K. M., Nakamura, M., Kaneko, H., Kachi, S., & Terasaki, H. (2007). The role of VEGF and VEGFR2/Flk1 in proliferation of retinal progenitor cells in murine retinal degeneration. Investigative Ophthalmology & Visual Science, 48, 4315–4320.
Zhang, F., Tang, Z., Hou, X., et al. (2009). VEGF-B is dispensable for blood vessel growth but critical for their survival, and VEGF-B targeting inhibits pathological angiogenesis. Proceedings of the National Academy of Sciences of the United States of America, 106, 6152–6157.
Purpura, K. A., George, S. H., Dang, S. M., Choi, K., Nagy, A., & Zandstra, P. A. (2008). Soluble Flt-1 regulates Flk-1 activation to control hematopoietic and endothelial development in an oxygen-responsive manner. Stem Cells, 26, 2832–2842.
Jang, Y. Y., Collector, M. I., Baylin, S. B., Diehl, A. M., & Sharkis, S. J. (2004). Hematopoietic stem cells convert into liver cells within days without fusion. Nature Cell Biology, 6, 532–539.
Krause, D. S., Theise, N. D., Collector, M. I., et al. (2001). Multi-organ, multi-lineage engraftment by a single bone marrow-derived stem cell. Cell, 105, 369–377.
Iskovich, S., Goldenberg-Cohen, N., Stein, J., Yaniv, I., Fabian, I., & Askenasy, N. (2012). Elutriated stem cells derived from the adult bone marrow differentiate into insulin producing cells in vivo and reverse chemical diabetes. Stem Cells and Development, 21(1):86–96.
Pearl-Yafe, M., Stein, J., Yolcu, E. S., et al. (2007). Fas transduces dual apoptotic and trophic signals in hematopoietic progenitors. Stem Cells, 25, 3194–3203.
Iskovich, S., Goldenberg-Cohen, N., Stein, J., Yaniv, I., Farkas, D. L., & Askenasy, N. (2011). β-cell neogenesis: experimental considerations in stem cell differentiation. Stem Cells and Development, 20, 569–582.
Vroemen, M., Weidner, N., & Blesch, A. (2005). Loss of gene expression in lentivirus- and retrovirus-transduced neural progenitor cells is correlated to migration and differentiation in the adult spinal cord. Experimental Neurology, 195, 127–139.
Krum, J. M., Mani, N., & Rosenstein, J. M. (2008). Roles of the endogenous VEGF receptors flt-1 and flk-1 in astroglial and vascular remodeling after brain injury. Experimental Neurology, 212, 108–117.
Lee, S. S., Ghosn, C., Yu, Z., et al. (2010). Vitreous VEGF clearance is increased after vitrectomy. Investigative Ophthalmology & Visual Science, 51, 2135–2138.
Shibuya, M., & Claesson-Welsh, L. (2006). Signal transduction by VEGF receptors in regulation of angiogenesis and lymphangiogenesis. Experimental Cell Research, 312, 549–560.
Wehner, T., Böntert, M., Eyüpoglu, I., et al. (2003). Bone marrow-derived cells expressing green fluorescent protein under the control of the glial fibrillary acidic protein promoter do not differentiate into astrocytes in vitro and in vivo. Journal of Neuroscience, 23, 5004–5011.
Xu, H., Chen, M., Mayer, E. J., Forrester, J. V., & Dick, A. D. (2007). Turnover of resident retinal microglia in the normal adult mouse. Glia, 55, 1189–1198.
Kezic, J., & McMenamin, P. G. (2008). Differential turnover rates of monocyte-derived cells in varied ocular tissue microenvironments. Journal of Leukocyte Biology, 84, 721–729.
Hess, D. C., Abe, T., Hill, W. D., et al. (2004). Hematopoietic origin of microglial and perivascular cells in brain. Experimental Neurology, 186, 134–144.
Simard, A. R., & Rivest, S. (2004). Bone marrow stem cells have the ability to populate the entire central nervous system into fully differentiated parenchymal microglia. The FASEB Journal, 18, 998–1000.
Kaneko, H., Nishiguchi, K. M., Nakamura, M., Kachi, S., & Terasaki, H. (2008). Characteristics of bone marrow–derived microglia in the normal and injured retina. Investigative Ophthalmology & Visual Science, 49, 4162–4168.
Schilling, M., Besselmann, M., Leonhard, C., Mueller, M., Ringelstein, E. B., & Kiefer, R. (2003). Microglial activation precedes and predominates over macrophage infiltration in transient focal cerebral ischemia: A study in green fluorescent protein transgenic bone marrow chimeric mice. Experimental Neurology, 183, 25–33.
Tanaka, R., Komine-Kobayashi, M., Mochizuki, H., et al. (2003). Migration of enhanced green fluorescent protein expressing bone marrow-derived microglia/macrophage into the mouse brain following permanent focal ischemia. Neuroscience, 117, 531–539.
Hisatomi, T., Sakamoto, T., Sonoda, K. H., et al. (2003). Clearance of apoptotic photoreceptors: Elimination of apoptotic debris into the subretinal space and macrophage-mediated phagocytosis via phosphatidylserine receptor and integrin αvβ3. American Journal of Pathology, 162, 1869–1879.
Djukic, M., Mildner, A., Schmidt, H., et al. (2006). Circulating monocytes engraft in the brain, differentiate into microglia and contribute to the pathology following meningitis in mice. Brain, 129, 2394–2403.
Schabitz, W. R., Krüger, C., Pitzer, C., et al. (2008). A neuroprotective function for the hematopoietic protein granulocyte-macrophage colony stimulating factor (GM-CSF). Journal of Cerebral Blood Flow and Metabolism, 28(1), 29–43.
Navarro-Sobrino, M., Rosell, A., Penalba, A., et al. (2009). Role of endogenous granulocyte-macrophage colony stimulating factor following stroke and relationship to neurological outcome. Current Neurovascular Research, 6(4), 246–251.
Schallenberg, M., Charalambous, P., & Thanos, S. (2009). GM-CSF regulates the ERK1/2 pathways and protects injured retinal ganglion cells from induced death. Experimental Eye Research, 89(5), 665–677.
Tsai, R. K., Chang, A. H., Sheu, M. M., & Huang, Z. L. (2010). Anti-apoptotic effects of human granulocyte colony-stimulating factor (G-CSF) on retinal ganglion cells after optic nerve crush are PI3K/AKT-dependent. Experimental Eye Research, 90, 537–545.
Kong, T., Choi, J. K., Park, H., et al. (2009). Reduction in programmed cell death and improvement in functional outcome of transient focal cerebral ischemia after administration of granulocyte-macrophage colony-stimulating factor in rats. Laboratory investigation. Journal of Neurosurgery, 111(1), 155–163.
Sasahara, M., Otani, A., Oishi, A., et al. (2008). Activation of bone marrow-derived microglia promotes photoreceptor survival in inherited retinal degeneration. American Journal of Pathology, 172, 1693–1703.
Cuthbertson, R. A., & Lang, R. A. (1989). Developmental ocular disease in GM-CSF transgenic mice is mediated by autostimulated macrophages. Developmental Biology, 134(1), 119–129.
Tsai, R. K., Chang, C. H., & Wang, H. Z. (2008). Neuroprotective effects of recombinant human granulocyte colony-stimulating factor (G-CSF) in neurodegeneration after optic nerve crush in rats. Experimental Eye Research, 87, 242–250.
Frank, T., Schlachetzki, J. C., Göricke, B., et al. (2009). Both systemic and local application of granulocyte-colony stimulating factor (G-CSF) is neuroprotective after retinal ganglion cell axotomy. BMC Neuroscience, 10, 49.
Bu, P., Basith, B., Stubbs, E. B., Jr., & Perlman, J. I. (2010). Granulocyte colony-stimulating factor facilitates recovery of retinal function following retinal ischemic injury. Experimental Eye Research, 91, 104–106.
Englund-Johansson, U., Mohlin, C., Liljekvist-Soltic, I., Ekstrom, P., & Johansson, K. (2010). Human neural progenitor cells promote photoreceptor survival in retinal explants. Experimental Eye Research, 90(2), 292–299.
Taneera, J., Rosengree, A., Renstrom, E., Nygren, J. M., Serup, P., Rorsman, P., & Jacobsen, S. E. (2006). Failure of transplanted bone marrow cells to adopt a pancreatic beta-cell fate. Diabetes, 55, 290–296.
Jung, K. H., Chu, K., Lee, S. T., Kim, S. J., Sinn, D. I., Kim, S. U., Kim, M., & Roh, J. K. (2006). Granulocyte colony-stimulating factor stimulates neurogenesis via vascular endothelial growth factor with STAT activation. Brain Research, 1073–1074, 190–201.
Kruger, C., Laage, R., Pitzer, C., Schabitz, W. R., & Schneider, A. (2007). The hematopoietic factor GM-CSF (granulocyte-macrophage colony-stimulating factor) promotes neuronal differentiation of adult neural stem cells in vitro. BMC Neuroscience, 8, 88.
Liu, H., Jia, D., Fu, J., et al. (2009). Effects of granulocyte colony-stimulating factor on the proliferation and cell-fate specification of neural stem cells. Neuroscience, 164, 1521–1530.
Love, R. (2003). GM-CSF induced arteriogenesis: A potential treatment for stroke? Lancet Neurology, 2(8), 458.
Eubank, T. D., Roberts, R., Galloway, M., Wang, Y., Cohn, D. E., & Marsh, C. B. (2004). GM-CSF induces expression of soluble VEGF receptor-1 from human monocytes and inhibits angiogenesis in mice. Immunity, 21(6), 831–842.
Schneeloch, E., Mies, G., Busch, H. J., Buschmann, I. R., & Hossmann, K. A. (2004). Granulocyte-macrophage colony-stimulating factor-induced arteriogenesis reduces energy failure in hemodynamic stroke. Proceedings of the National Academy of Sciences of the United States of America, 101(34), 12730–12735.
Hossmann, K. A., & Buschmann, I. R. (2005). Granulocyte-macrophage colony-stimulating factor as an arteriogenic factor in the treatment of ischaemic stroke. Expert Opinion on Biological Therapy, 5(12), 1547–1556.
Sacramento, C. B., Cantagalli, V. D., Grings, M., et al. (2009). Granulocyte-macrophage colony-stimulating factor gene based therapy for acute limb ischemia in a mouse model. The Journal of Gene Medicine, 11(4), 345–353.
Sharkis, S. J., Collector, M. I., Barber, J. P., Vala, M. S., & Jones, R. J. (1995). Phenotypic and functional characterization of the hematopoietic stem cell. Stem Cells, 15(Suppl 1), 41–45.
Ratajczak, J., Zuba-Surma, A., Paczkowska, E., Kucia, M., Nowacki, P., & Ratajczak, M. Z. (2011). Stem cells for neural regeneration - a potential application of very small embryonic-like stem cells. Journal of Physiology and Pharmacology, 62, 3–12.
Jang, Y. Y., & Sharkis, S. J. (2005). Stem cell plasticity: A rare cell, not a rare event. Stem Cell Reviews, 1, 45–51.
Quesenberry, P. J., Dooner, G., Dooner, M., & Colvin, G. (2005). The stem cell continuum: considerations on the heterogeneity and plasticity of marrow stem cells. Stem Cell Reviews, 1, 29–36.
Zipori, D. (2005). The stem state: Plasticity is essential, whereas self-renewal and hierarchy are optional. Stem Cells, 23, 719–726.
Acknowledgments
Thanks are due to Dr. Saul Sharkis and Dr. Michael Collector for their outstanding support and conceptual contribution to this study. Funding was provided by generous grants from the Leah and Edward M. Frankel Trust for bone marrow transplantation and the Zanvyl and Isabelle Krieger Fund, Baltimore, MD, USA; the Israel Science Foundation (NGC, 1371/08); the Eldor-Metzner Clinician Scientist Award, Chief Scientist, (NGC, 3-3741); Chief Scientist and the Lirot Foundation (NGC, 3-4538), Israel Ministry of Health; Pilot Grant from the North American Neuro-Ophthalmology Society; The Adams Super Center for Brain Research, Tel Aviv University, Israel (NGC); the Clair and Ameddee Maratier Fund to prevent blindness, Tel Aviv University, Israel (NGC, BRAL); Walter Friendliest and Herman Shudder Fund, Tel Aviv University, Israel (NGC), The Lions (BRAL) and Mazritzky Fund (TS), Tel Aviv University, Israel.
The results of this study were presented in part at the Meeting of the Association for Research in Vision and Ophthalmology, Fort Lauderdale, Florida, USA (ARVO); the Meeting of the Israel Society for Eye and Vision Research, Neve Ilan, Israel (ISVER); the Meeting of the North American Neuro-Ophthalmology Society (NANOS), Tucson, AZ; the Meeting of the International Society for Stem Cell Research (ISSCR), Philadelphia, PA; the Meeting of the Federation of the Israel Societies for Experimental Biology (ILANIT/FISEB), Eilat, Israel.
This work was submitted as partial fulfillment of the requirements for a PhD degree of B.R. Avraham-Lubin and MSc degree of T. Sadikov, Tel Aviv University.
Disclosure of potential conflict of interest
The authors have no potential conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Bat-Chen R. Avraham-Lubin and Nitza Goldenberg-Cohen contributed equally to this study
Rights and permissions
About this article
Cite this article
Avraham-Lubin, BC.R., Goldenberg-Cohen, N., Sadikov, T. et al. VEGF Induces Neuroglial Differentiation in Bone Marrow-Derived Stem Cells and Promotes Microglia Conversion Following Mobilization with GM-CSF. Stem Cell Rev and Rep 8, 1199–1210 (2012). https://doi.org/10.1007/s12015-012-9396-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12015-012-9396-1