Abstract
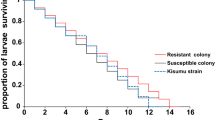
Biochemical synthetic pyrethroids, deltamethrin are presently used insecticides for the control of mosquito vector-borne diseases in worldwide. Mosquito re-emergence with diseases becoming a serious problem due to development of insecticide resistance. The comprehensive knowledge on the underlying mechanisms of resistance against deltamethrin is required for implementation of an efficient vector control programme. The assessment of the biological fitness of a mosquito strain exposed to insecticide pressure is extremely vital because it provides information on the development of resistance. In the present study, the adult stage of malaria vector, Anopheles stephensi, was designated for the study of deltamethrin resistance (F40 generations). The non-blood-fed, laboratory-reared females to sub-lethal doses of deltamethrin (0.004%, 0.005%, 0.007%, or 0.01%) exposed to every generation for up to F40. The adult mosquito susceptibility was performed by WHO standard method for evaluation. After 24 h, mortality was recorded in both treated and control groups. Therefore, the biological fitness characteristics such as feeding, fecundity, hatchability, egg retention, immature duration, adult emergence, and adult life span were studied to assess the exposed deltamethrin under selection pressure as compared to the unexposed (control) population. The laboratory selection of An. stephensi exposed deltamethrin over the generations were diminished its biological fitness. Information on biological fitness including reproductive potential of mosquito strain under selection pressure against deltamethrin is incredibly necessary because it would facilitate in resistance management. Baseline information gives in this experiment will guide for future studies on the susceptibilities of wild malaria mosquito populations in India.


Similar content being viewed by others
Data availability
All data and materials generated or analyzed during this study are included in this published article.
References
WHO. (2014). Control of residual malaria parasite transmission. Technical Note WHO/HTM/GMP/MPAC/2014.5, pp 1–5.
Baranitharan, M., Tamizhazhagan, V., & Kovendan, K. (2019). Medicinal plants as potent power for malaria control: Review. Entomology and Applied Science Letters, 5(1), 28–44.
WHO. (2017). Global Malaria Programme (GMP) - World Malaria Report, pp. 1–160.
Kovendan, K., Chandramohan, B., Govindarajan, M., Jebanesan, A., Kamalakannan, S., Vincent, S., & Benelli, G. (2018). Orchids as sources of novel nanoinsecticides? Efficacy of Bacillus sphaericus and Zeuxine gracilis-fabricated silver nanoparticles against dengue, malaria and filariasis mosquito vectors. Journal of Cluster Science, 29, 345–357.
Sanil, D., Shetty, V., & Shetty, N. J. (2014). Differential expression of glutathione s-transferase enzyme in different life stages of various insecticide-resistant strains of Anopheles stephensi: A malaria vector. Journal of Vector Borne Diseases, 51, 97–105.
Pimnon, S., & Bhumiratana, A. (2018). Adaptation of Anopheles vectors to anthropogenic malaria-associated rubber plantations and indoor residual spraying: Establishing population dynamics and insecticide susceptibility”. Canadian Journal of Infectious Diseases and Medical Microbiology, 2018, 17.
Sanil, D., & Shetty, N. J. (2012). The effect of sub-lethal exposure to temephos and propoxur on reproductive fitness and its influence on circadian rhythms of pupation and adult emergence in Anopheles stephensi Liston-a malaria vector. Parasitology Research, 111, 423–432.
Baranitharan, M., Krishnappa, K., Elumalai, K., Pandiyan, J., Gokulakrishnan, J., Kovendan, K., & Tamizhazhagan, V. (2020). Citrus limetta (Risso) - borne compound as novel mosquitocides: Effectiveness against medical pest and acute toxicity on non-target fauna. South African Journal of Botany, 128, 218–224.
Karunamoorthi, K., & Sabesan, S. (2013). Insecticide resistance in insect vectors of disease with special reference to mosquitoes: A potential threat to global public health. Health Scope, 2, 4–18.
Singh, R. K., Dhiman, R. C., Mittal, P. K., & Das, M. K. (2010). Susceptibility of malaria vectors to insecticides in Gumla district, Jharkhand state, India. Journal of Vector Borne Diseases, 47, 116–118.
Shi, L., Hu, H., Ma, K., Zhou, D., Yu, J., Zhong, D., Fang, F., Chang, X., Hu, S., Zou, F., & Wang, W. (2015). Development of resistance to pyrethroid in Culex pipiens pallens population under different insecticide selection pressures. PLOS Neglected Tropical Diseases, 9, e0003928.
Dash, A. P., Raghavendra, K., & Pillai, M. K. K. (2007). Resurrection of DDT: A critical appraisal. Indian Journal of Medical Research, 126, 1–4.
Singh, K.V., & Bansal, S.K. (2007). Map** of insecticide resistance in vectors of malaria in Rajasthan. Annual report 2007, Available online: http://www.dmrcjodhpur.org/AR0708/p1-6.pdf
Mohammed, B. R., Abdulsalam, Y. M., & Deeni, Y. Y. (2015). Insecticide resistance to Anopheles spp. mosquitoes (Diptera: Culicidae) in Nigeria: A review. International Journal of Mosquito Research, 2, 56–63.
Campanhola, C., McCutchen, B. F., Baehrecke, E. H., & Plapp, F. W. (1991). Biological constraints associated with resistance to pyrethroids in the tobacco bud worm (Lepidoptera: Noctuidae). Journal of Economic Entomology, 84, 1404–1411.
Ferrari, J. A., & Georghiou, G. R. (1981). Effects of insecticidal selection and treatment on reproductive potential of resistant, susceptible, and heterozygous strains of the southern house mosquito. Journal of Economic Entomology, 74, 323–327.
Amin, A. M., & White, G. B. (1984). Relative fitness of organophosphate-resistant and susceptible strains of Culex quinquefasciatus Say (Diptera: Culicidae). Bulletin of Entomological Research, 74, 591–598.
Bonning, B. C., & Hemingway, J. (1991). Identification of reduced fitness associated with an insecticide resistance gene in Culex pipiens by microtitre plate tests. Medical and Veterinary Entomology, 5, 377–380.
Arnaud, L., Brostaux, Y., Assie, L. K., Gaspar, C., & Haubruge, E. (2002). Increased fecundity of malathion-specific resistant beetles in absence of insecticide pressure. Heredity, 89, 425–429.
Okoye, P. N., Brooke, B. D., Hunt, R. H., & Coetzee, M. (2007). Relative developmental and reproductive fitness associated with pyrethroid resistance in the major southern African malaria vector, Anopheles funestus. Bulletin of Entomological Research, 97, 599–605.
Tabbabi, A., & Daaboub, J. (2018). Fitness cost in field Anopheles labranchiae populations associated with resistance to the insecticide deltamethrin. Medical and Veterinary Entomology, 62, 107–111.
Desneux, N., Wajnberg, E., Fauvergue, X., Privet, S., & Kaiser, L. (2004). Sub-lethal effects of a neurotoxic insecticide on the oviposition behavior and the patch-time allocation in two aphid parasitoids, Diaeretiella rapae and Aphidius matricariae. Entomologia Experimentalis et Applicata, 112, 227–235.
Kamalakannan, S., Kovendan, K., Balachandar, V., Gopi Naik, K., & Chauhan, A. (2021). Sources of potential fungi generated biogenic nanoparticles for the control of diseases transmitting mosquitoes: A review. Letters in Applied NanoBioScience, 11, 3523–3536.
Singh, K. R. P., Patterson, R. S., La Brecque, G. C., & Razdan, R. K. (1975). Mass rearing of Culex pipiens fatigans Wied. Journal of Communicable Diseases, 7(1), 31–53.
Christophers, S. R. (1933). The fauna of British India, including Ceylon and Burma. Diptera. IV. Family Culicidae. Tribe Anophelini (p. 371). Taylor & Francis.
Priyalakshmi, B. L., Rajashree, B. H., Ghosh, C., & Shetty, N. J. (1999). Effect of Fenitrothion, Deltamethrin and Cypermethrin on reproductive potential and longevity of life cycle in Anopheles stephensi Liston, a malaria mosquito. Journal of Parasitic Diseases, 23, 125–128.
Zoh, M. G., Tutagata, J., Fodjo, B. K., Mouhamadou, C. S., Sadia, C. G., McBeath, J., Schmitt, F., Horstmann, S., David, J. P., & Reynaud, S. (2022). Exposure of Anopheles gambiae larvae to a sub-lethal dose of an agrochemical mixture induces tolerance to adulticides used in vector control management. Aquatic Toxicology, 248, 106181.
Rodriguez, M. M., Bisset, J. A., & Fernandez, D. (2007). Levels of insecticide resistance and resistance mechanisms in Aedes aegypti from some Latin American countries. Journal of the American Mosquito Control Association, 23, 420–429.
Kumar, S., Gupta, L., Han, Y. S., & Barillas-Mury, C. (2004). Inducible peroxidases mediate nitration of Anopheles midgut cells undergoing apoptosis in response to Plasmodium invasion. Journal of Biological Chemistry, 279, 53475–53482.
Kumar, S., Thomas, A., Sahgal, A., Verma, A., Samuel, T., & Pillai, M. K. K. (2002). Effect of the synergist, piperonyl butoxide, on the development of deltamethrin resistance in yellow fever mosquito, Aedes aegypti L. (Diptera: Culicidae). Archives of Insect Biochemistry and Physiology, 50, 1–8.
Chakravorthy, B. C., & Kalyanasundaram, M. (1992). Selection of permethrin resistance in the malaria vector Anopheles stephensi. Indian Journal of Malariology, 29, 161–165.
Chareonviriyaphap, T., Rongnoparut, P., & Juntarumporn, P. (2002). Selection for pyrethroid resistance in a colony of Anopheles minimus species A, malaria vector in Thailand. Journal of Vector Ecology, 27, 222–229.
Mittal, P. K., Adak, T., Singh, O. P., Raghavendra, K., & Subbarao, S. K. (2002). Reduced susceptibility to deltamethrin in Anopheles culicifacies sensu lato, in Ramnathapuram district, Tamil Nadu-Selection of a pyrethroid-resistant strain. Cursos e Congresos da Universidade de Santiago de Compostela, 82, 185–188.
Paeporn, P., Komalamisra, N., Deesin, V., Rongsriyam, Y., Eshita, Y., & Thongrungkiat, S. (2003). Temephos resistance in two forms of Aedes aegypti and its significance for the resistance mechanism. Southeast Asian Journal of Tropical Medicine and Public Health, 34, 786–792.
Gayathri, V., & Murthy, P. B. (2006). Reduced susceptibility to deltamethrin and kdr mutation in Anopheles stephensi Liston, a malaria vector in India. Journal of the American Mosquito Control Association, 22, 678–688.
Enayati, A., Hanafi-Bojd, A. A., Sedaghat, M. M., Zaim, M., & Hemingway, J. (2020). Evolution of insecticide resistance and its mechanisms in Anopheles stephensi in the WHO Eastern Mediterranean Region. Malaria Journal, 19, 1–12.
Namias, A., Jobe, N. B., Paaijmans, K. P., & Huijben, S. (2021). The need for practical insecticide-resistance guidelines to effectively inform mosquito-borne disease control programs. eLife, 10, e65655.
Song, A., **feng, L., Wende, Z., Zhen, Z., **xuan, Li., Zhen**, Y., Wing-Leung, W., Kun, Z., Min, C., & Panpan, W. (2023). Novel matrine derivatives as potential larvicidal agents against Aedes albopictus: Synthesis, biological evaluation, and mechanistic analysis. Molecules, 28, 1–18.
Dekker, T., Ignell, R., Ghebru, M., Glinwood, R., & Hopkins, R. (2011). Identification of mosquito repellent odours from Ocimum forskolei. Parasites & Vectors, 4, 1–7.
Cohnstaedt, L. W., & Allan, S. A. (2011). Effects of sub-lethal pyrethroid exposure on the host seeking behavior of female mosquitoes. Journal of Vector Ecology, 36, 395–403.
Lissenden, N., Kont, M. D., Essandoh, J., Ismail, H. M., Churcher, T. S., Lambert, B., Lenhart, A., McCall, P. J., Moyes, C. L., Paine, M. J., & Praulins, G. (2021). Review and meta-analysis of the evidence for choosing between specific pyrethroids for programmatic purposes. Insects, 12, 826.
N’Guessan, R., Darriet, F., Doannio, J. M., Chandre, F., & Carnevale, P. (2001). Olyset net efficacy against pyrethroid resistant Anopheles gambiae and Culex quinquefasciatus after 3 years field use in Cote d’voire. Medical Vet Entomology, 15, 97–104.
Liu, W., Todd, R. G., & Gerberg, E. J. (1986). Effect of three pyrethroids on blood feeding and fecundity of Aedes aegypti. Journal of the American Mosquito Control Association, 2, 310–313.
Lim, M. P. (1995). A study on physiological factors of mosquitoes in relation to the effects of exposure to mosquito coils. M.Sc dissertation, University Sains Malaysia (p. 114).
Yap H. H., Lim, M. P., Chong, N. L., & Lee, C. Y. (1996). Efficacy and sub-lethal effects of mosquito coils on Aedes aegypti and Culex quinquefasciatus (Diptera: Culicidae). In K. B. Wildey (Ed.), Proceeding of the 2nd international conference on insect pests in the urban environment (pp. 177–184). Heriot-Watt University, Edinburgh, Scotland.
Kovendan, K., Murugan, K., Kamalakannan, S., & Vincent, S. (2011). Larvicidal efficacy of Jatropha curcas and bacterial insecticide, Bacillus thuringiensis, against lymphatic filarial vector, Culex quinquefasciatus Say (Diptera: Culicidae). Parasitology Research, 109, 1251–1257.
Kuppusamy, C., & Murugan, K. (2011). Adult mortality and blood feeding behavioral effects of α-amyrin acetate, a novel bioactive compound on in vivo exposed females of Anopheles stephensi Liston. (Diptera: Culicidae). Parasitology Research, 110, 2117–2124.
Wang, J., Lu, S., Chen, R., & Wang, L. (1998). Relative fitness of three organophosphate-resistant strains of Culex pipiens pallens (Diptera: Culicidae). Journal of Medical Entomology, 35, 716–719.
Lloyd, J. E. (1979). Mating behaviour and natural selection. The Florida Entomologist, 62, 17–34.
Teshome, A., Erko, B., Golassa, L., Yohannes, G., Irish, S. R., Zohdy, S., Yoshimizu, M., & Dugassa, S. (2023). Resistance of Anopheles stephensi to selected insecticides used for indoor residual spraying and long-lasting insecticidal nets in Ethiopia. Malaria Journal, 22, 218.
Sutherland, D. J., Beam, F. D., & Gupta, A. P. (1967). The effects of mosquitoes of sub-lethal exposure to insecticides. I. DDT, dieldrin, malathion and the basal follicles of Aedes aegypti (L.). Mosquito News, 27, 316–323.
Gaaboub, I. A. (1976). Observations on the basal follicle numbers developed per female of two strains of Aedes aegypti after being fed on hosts with different levels of microfilariae of Brugia pahangi. Journal of Invertebrate Pathology, 28, 203–207.
Cutler, G. C. (2013). Insects, insecticides and hormesis: Evidence and considerations for study. Dose Response, 11, 154–177.
Guedes, R. N. C., & Cutler, G. C. (2014). Insecticide-induced hormesis and arthropod pest management. Pest Management Science, 70, 690–697.
Kumar, S., & Pillai, M. K. K. (2010). Reproductive disadvantage in an Indian strain of malarial vector, Anopheles stephensi Liston on selections with deltamethrin/synergized deltamethrin. Acta Entomol Sinica, 53, 1111–1118.
Li, X., Ma, L., Sun, L., & Zhu, C. (2002). Biotic characteristics in the deltamethrin-susceptible and resistant strains of Culex pipiens pallens (Diptera: Culicidae) in China. Applied Entomology and Zoology, 37, 305–308.
De Coursey, J. D., Webster, A. P., & Leopold, R. S. (1953). Studies on the effect of insecticide on the oviposition of Anopheles quadrimaculatus Say. Annals of the Entomological Society of America, 46, 359–365.
Duncan, J. (1963). Post-treatment effects of sub-lethal doses of dieldrin on the mosquito Aedes aegypti L. Annals of Applied Biology, 52, 1–6.
Verma, K. V. S. (1986). Deterrent effect of synthetic pyrethroids on the oviposition of mosquitoes. Cursos e Congresos da Universidade de Santiago de Compostela, 55, 373–375.
Robert, L. L., & Olson, J. K. (1989). Effects of sub-lethal dosages of insecticides on Culex quinquefasciatus. Journal of the American Mosquito Control Association, 5, 239–246.
Reyes-Villanueva, F., Juarez-Eguia, M., & Flores-Leal, A. (1990). Effects of sub-lethal dosages of abate upon adult fecundity and longevity of Aedes aegypti. Journal of the American Mosquito Control Association, 6, 739–741.
Rowland, M. (1991). Behavior and fitness of gamma-hch dieldrin resistant and susceptible female Anopheles gambiae and Anopheles stephensi mosquitos in the absence of insecticide. Medical and Veterinary Entomology, 5, 193–206.
Rao, D. E. G., & Shetty, N. J. (1992). Effect of insecticide resistance on reproductive potential in Anopheles stephensi Liston, a malaria mosquito. International Journal of Occupational and Environmental Health, 1, 48–52.
Mohapatra, R., Ranjit, M. R., & Dash, A. P. (1999). Evaluation of cyfluthrin and fenfluthrin for their insecticidal activity against three vector mosquitoes. Journal of Communicable Diseases, 31, 91–99.
Zin, T., & Shetty, N. J. (2008). Sub-lethal effect of bifenthrin and neem on fecundity, hatchability and sex ratio of Anopheles stephenesi Liston, a malaria mosquito. Pestology, 32, 39–44.
Minn, Z., & Shetty, N. (2008). Toxicological effect of malathion and alphamethrin on reproductive potential in Aedes aegypti, a yellow fever mosquito. Pestology, 32, 39–43.
Packer, M. J., & Corbet, P. S. (1989). Size variation and reproductive success of female Aedes punctor (Diptera: Culicidae). Ecological Entomology, 14, 297–309.
Chadee, D. D., & Beier, J. C. (1996). Natural variation in blood-feeding kinetics of four mosquito vectors. Journal of Vector Ecology, 21, 150–155.
Xue, R. D., Ali, A., & Barnard, D. R. (2005). Effects of forced egg-retention in Aedes albopictus on adult survival and reproduction following application of DEETas an oviposition deterrent. Journal of Vector Ecology, 30, 45–48.
Ohashi, K., Nakada, K., Ishiwatari, T., Miyaguchi, J. I., Shono, Y., Lucas, J. R., & Mito, N. (2012). Efficacy of pyriproxyfen-treated nets in sterilizing and shortening the longevity of Anopheles gambiae (Diptera: Culicidae). Journal of Medical Entomology, 49, 1052–1058.
Maddrell, S. H. P. (1972). Reynolds, S.E. Release of hormones in insects after poisoning with insecticides. Nature, 236, 404–406.
Lee, C. Y. (2000). Sub-lethal effects of insecticides on longevity, fecundity and behaviour of insect pests: A review. Journal of Biosciences, 11, 107–112.
Spencer, M., Blaustein, L., & Cohen, J. E. (2002). Oviposition habitat selection by mosquitoes (Culiseta longiareolata) and consequences for population size. Ecology, 83, 669–679.
Reisen, W. K., Milby, M. M., & Bock, M. E. (1984). The effects of immature stress on selected events in the life history of Culex tarsalis. Mosquito News, 44, 385–395.
Clements, A. N. (1992). The biology of mosquitoes: Development, nutrition and reproduction (p. 536). Chapman and Hall.
Rodcharoen, J., & Mulla, S. M. (1997). Biological fitness of Culex quinquefasciatus (Diptera: Culicidae) susceptible and resistant to Bacillus sphaericus. Journal of Medical Entomology, 34, 5–10.
Olayemi, I. K., Maduegbuna, E. N., Ukubuiwe, A. C., & Chukwuemeka, V. I. (2012). Laboratory studies on developmental responses of the filarial vector mosquito, Culex pipiens (Diptera: Culicidae), to urea fertilizer. Journal of Medical Sciences, 12, 175–181.
Olayemi, I. K., Akpan, B., Ejima, I. A. A., Ukubuiwe, A. C., & Olorunfemi, O. J. (2014). Influence of rice-farming herbicide (2, 4-dichlorophenoxyl acetic acid) on the development of Culex pipiens (Diptera: Culicidae), a major swamp-breeding mosquito vector of filariasis. Advance in Agriculture and Biology, 1, 131–134.
Gunasekaran, K., Vijayakumar, T., & Kalyanasundaram, M. (2009). Larvicidal and emergence inhibitory activities of Neem Azal T/S 1.2 percent EC against vectors of malaria, filariasis & dengue. Indian Journal of Medical Research, 130, 138–145.
Mordue, L. A. J., Morgan, E. D., & Nisbet, A. J. (2005). In L. I. Gilbert, K. Iatrou, & S. S. Gill (Eds.), Comprehensive molecular insect science (Vol. 6, pp. 117–135), Elsevier.
Invest, J. F., & Lucas, J. R. (2008). Pyriproxyfen as a mosquito larvicide. In W. H. Robinson & D. Bajomi (Eds.), Proceedings of the 6th International Conference on Urban Pests (ICUP), Budapest, Hungary (pp. 239–245).
Mahyoub, J. A. (2013). Evaluation of the IGRs Alsystin and Pyriproxyfen as well as the plant extract jojoba oil against the mosquito Aedes aegypti. Journal of Pure and Applied Microbiology, 7, 3225–3229.
Sihunincha, M., Zamora-Perea, E., Orellana-Rios, W., Stancil, J. D., Lopez-Sifuentes, V., & Vidal-Ore, C. (2005). Potential use of pyriproxyfen for control of Aedes aegypti (Diptera: Culicidae) in Iquitos, Peru. Journal of Medical Entomology, 42, 620–630.
Yapabandara, A. M., & Curtis, C. F. (2004). Control of vectors and incidence of malaria in an irrigated settlement scheme in Sri Lanka by using the insect growth regulator Pyriproxyfen. Journal of the American Mosquito Control Association, 20, 395–400.
Ansari, M. A., Razdan, R. K., & Sreehari, U. (2005). Laboratory and field evaluation of Hilmilin against mosquitoes. Journal of the American Mosquito Control Association, 21, 432–436.
Mulla, M. S. (1995). The future of insect growth regulators in vector control. Journal of the American Mosquito Control Association, 1, 269–273.
Gillies, M. T., & De Meillon, B. (1998). The Anophelinae of Africa South of the Sahara. South African Institute for Medical Research, 54, 1343.
Christiansen-Jucht, C. D., Parham, P. E., Saddler, A., Koella, J. C., & Basanez, M. G. (2015). Larval and adult environmental temperatures influence the adult reproductive traits of Anopheles gambiae ss. Parasites & Vectors, 8, 456.
Waldock, J., Chandra, N. L., Lelieveld, J., Proestos, Y., Michael, E., Christophides, G., & Parham, P. E. (2013). The role of environmental variables on Aedes albopictus biology and chikungunya epidemiology. Pathogens and Global Health, 107, 224–241.
Martins, A. J., Ribeiro, C. D. M., Bellinato, D. F., Peixoto, A. A., & Valle, D. (2012). Effect of insecticide resistance on development, longevity and reproduction of field or laboratory selected Aedes aegypti populations. PLoS ONE, 7, e31889.
Protopopoff, N., Bortel, W. V., Speybroeck, N., Geertruyden, J. P. V., Baza, D., D’Alessandro, U., & Coosemans, M. (2009). Ranking malaria risk factors to guide malaria control efforts in African highlands. PLoS ONE, 4, e8022.
Kovendan, K., Fabiola, M., Jebanesan, A., & Rajaganesh, R. (2024). Green synthesis of Malvastrum coromandelianum fabricated AgNPs: Anti-dengue and mosquitocidal studies. Inorganic Chemistry Communications, 161, 112067.
Acknowledgements
The authors would like to thank the authorities of Annamalai University. The authors are grateful to Dr. A. Subramaniyan, Professor and Head, Department of Zoology for the laboratory facilities provided.
Funding
The authors are thankful to University Grants Commission (UGC), Government of India, New Delhi under the scheme of Dr. D.S. Kothari Post Doctoral Fellowship (DSKPDF), (BL/19–20/0208) for providing financial support for the present work.
Author information
Authors and Affiliations
Contributions
Dr. Palani Aarumugam is the prime investigator who worked in the project. Writing—methodology and formal analysis. He helped in collection and rearing of mosquitoes and assisted in statistical analyses. Dr. Kalimuthu Kovendan has supervised conceptualization, designing, writing—original draft preparation, review, editing, scientific investigation and project administration. Dr. Siva Kamalakannan have evaluated scientific review and consultation. Dr. Arulsamy Jebanesan have evaluated for standard formal analysis and scientific consultation. List of the authors have fully read and agreed to prepare the manuscript.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
Informed consent was obtained from all individual participants included in this study.
Consent for publication
The participants have given their consent to submit this manuscript in this esteemed journal.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Aarumugam, P., Kovendan, K., Kamalakannan, S. et al. Chemical Exposure of Synthetic Pyrethroid on Deltamethrin Under the Selection Pressure over the Generations: A Reproductive Potential Study of Anopheles stephensi. Appl Biochem Biotechnol (2024). https://doi.org/10.1007/s12010-024-04911-9
Accepted:
Published:
DOI: https://doi.org/10.1007/s12010-024-04911-9