Abstract
The primary aim of this study was to formulate a cake with high antioxidant activity through the incorporation of centrifugally spun gelatin-based fiber which was enriched with caffeic acid. Characterization analyses were conducted to evaluate fibers with different concentrations of caffeic acid (2% and 4%), while simultaneously the effects of thermal and citric acid crosslinking on the physical and functional properties of the encapsulated fibers were investigated. The study revealed varying encapsulation efficiencies of caffeic acid in gelatin fibers (56.34–94.55%), markedly affected by substantial reduction of thermal crosslinking, contrasting with citric acid’s minimal impact. Additionally, citric acid increased total phenolic content (TPC), but thermal treatment notably decreased antioxidant activity (AOA) due to its impact on radical scavenging and phenolic group dissociation. The addition of citric acid significantly reduced water vapor permeability by 22% suggesting an induced crosslinking in both thermal-treated caffeic acid and citric acid samples. The study highlighted reduced AOA and phenolic content in thermally treated fibers (thermally treated gelatin with 2% caffeic acid vs thermally treated gelatin with 4% caffeic acid), suggesting lower water solubility and improved thermal stability with approximately 24% remaining weight after thermogravimetric analysis. Despite this, cakes with thermally treated fibers had higher AOA due to improved heat resistance of fibers. Generally, adding fibers to cakes decreased hardness and pH while increasing TPC, AOA, and volume index, showing a novel approach using centrifugally spun fibers to enrich foods with antioxidants.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Functional foods, which are also known as nutraceuticals, offer additional health benefits beyond basic nutrition (Banerjee, 2019). They can be described as natural or processed foods with enhanced nutrients or bioactive compounds which have positive impacts on health. In other words, functional foods may promote the optimum health conditions and decrease the risk of human diseases. In recent years, one of the most researched functional food ingredients was antioxidants (Granato et al., 2020). Antioxidant properties of polyphenols are very high, which can protect the human body from diseases such as inflammation, diabetes, and cancer (Cizmarova et al., 2020). Moreover, polyphenols have the ability to prevent people from viruses such as SARS-CoV-2, which caused a recent pandemic and affected too many people worldwide (Adem et al., 2021).
Caffeic acid, a member of the phenolic acid family of polyphenols, is classified as hydroxycinnamic acid and is found mostly in fruits, vegetables, coffee, and propolis (Mirzaei et al., 2021). In addition to its antioxidant activity, caffeic acid has also anti-inflammatory, antiproliferative, and neuroprotective properties (Kadar et al., 2021). Some studies have revealed that consuming caffeic acid can decrease not only the risk of several cancer types but also metastases in tumor cells (Alam et al., 2022). Thus, the addition of caffeic acid into food can make it functional. Russo et al. (2017) conclude that prostate cancer risk can be decreased by involving caffeic acid in the daily diet. According to research, caffeic acid can be absorbed by the intestines and interact with the targeted tissue. Therefore, oral consumption of caffeic acid may be an attractive way to get it into the human body (Kadar et al., 2021). However, the usage of caffeic acid is limited due to some drawbacks such as forming an undesirable taste in foods and being susceptible to environmental conditions. In order to ease the usage and preserve the antioxidant activity of caffeic acid, different encapsulation techniques such as electrospinning and electrospraying have been shown to be used (Khoshnoudi-Nia et al., 2020). Although there are few studies on the encapsulation of caffeic acid by electrospinning, there is no study on the usage of centrifugal spinning on encapsulation of any phenolic acid. The disadvantages of electrospinning such as high voltage usage, low production rate, and limited appropriate solvent types can be overcome by centrifugal spinning, which is a novel technology (Zhiming & Jun., 2017).
Centrifugal spinning is a safer, cheaper, and faster way for encapsulation, and it also enables the use of various polymer solutions (Fig. 1). Although it offers a wide range of applications in terms of polymer solution, there are some factors that can affect the morphological structure of fibers, such as viscosity, surface tension, and concentration of the solution. There are two main parts in the centrifugal spinning system: the rotating spinning head and the nanofiber collecting part. The polymer solution goes to the rotating spinning head, which has nozzles on it, and then, it produces fibers by ejecting the polymer solution from nozzles. The rotational speed of the head is adjustable and affects not only the centrifugal force but also the diameter of fibers. Thinner fibers can be formed with high centrifugal and air frictional force, by increasing the rotating speed. However, in case of the excess rotating speed over its critical value, thick fibers may be obtained since the decreased time is not sufficient for the solution to form fiber. When the centrifugal force exceeds the surface tension of the solution, a fiber is formed by the evaporation of the solvent. On the other hand, the collecting part is a cylinder that is located under the rotating spinning head, and it collects produced fibers on it. It rotates during the process and a vacuum placed inside the cylinder helps to collect fiber without spreading it around. This novel method has been used in different studies such as tissue engineering, wound dressing, and drug delivery (Chen et al., 2019). Cremar et al. (2018) showed that chitosan-based fiber scaffolds produced by centrifugal spinning provided a potential wound dressing with its antimicrobial activity. Similarly, according to another study done by Gungor et al. (2021), AgNO3-added gelatin-based centrifugal spun biomats provided a wound dressing with its antibacterial activity.
Gelatin, which is a protein-based polymer derived from collagen, is preferred in encapsulation studies, since it can easily form films via casting and pouring methods and fibers, as mentioned in this study, via spinning processes (Baranauskaite et al., 2019). Besides its low cost, commercial availability, and non-toxic and biodegradable properties (Okutan et al., 2014; Xu et al., 2021), it provides consistency and stability to food products. However, due to the high water solubility of gelatin, its usage in food packaging and food products may be difficult. In order to solve this problem, crosslinking methods can be applied (Tavassoli-Kafrani et al., 2018). In this way, chemical bonds are formed between polymer chains, and solubility is decreased. Crosslinking can be done in different ways such as chemical and physical treatment (Siimon et al., 2014). Citric acid, as being non-toxic, cheap, and water soluble, is one of the chemical crosslinking materials to be used in food products. For proteins, crosslinking with citric acid is based on the formation of amide bonds by nucleophilic substitution, as can be seen in Fig. 2 (Jone Uranga et al., 2020). In the literature, citric acid–crosslinked fibers were produced in different studies such as gelatin-based electrospun nanofibers containing curcumin (Hasan et al., 2023), chitosan and chickpea flour-based films with curcumin (Yildiz et al., 2022c), faba bean flour and chitosan-based films with curcumin (Yildiz et al., 2022b), and fish gelatin films (Uranga et al., 2016). The common result of these studies was the improvement of film properties by adding citric acid as a crosslinking agent. On the other hand, thermal treatment (Fig. 3) is preferable for physical crosslinking, because of its easy and chemical-free applicability (Gungor et al., 2021). The thermal crosslink method was applied in the study of develo** active food packaging nanofibers by centrifugal spinning with the addition of lavender essential oil. It was shown that thermal treatment decreased the water solubility of fibers and enabled a controlled release of lavender essential oil (Doğan et al., 2022a).
Up to now, there are some studies about centrifugal spinning in various areas by using gelatin. For instance, gelatin-based antibacterial nanofibers (Loordhuswamy et al., 2014) and fibrous scaffolds to regenerate tissue (Akhtar et al., 2022) were developed to be used in biomedical applications. In addition, gelatin-based N95 mask was produced by centrifugal spinning, which has become widespread due to the COVID-19 pandemic (Arican et al., 2022). Also, there are studies about develo** gelatin-based active food packaging by centrifugal spinning with the addition of lemon peel oil and lavender essential oil (Doğan et al., 2022a, b). However, no food application has been shown in these studies. Furthermore, while the encapsulation of caffeic acid via electrospinning has been studied, as elucidated by Zeren et al. (2022), there remains an unexplored aspect in the encapsulation of caffeic acid or any other phenolic acid through centrifugal spinning, an area yet to be investigated. In addition, to the best of our knowledge, this will be the first study in which centrifugally spun antioxidant fibers were incorporated in a food product, namely cake.
The aim of this study was to encapsulate caffeic acid in gelatin-based fibers by centrifugal spinning method and subsequently to characterize them, with a particular emphasis on understanding the impact of crosslinking techniques on these fibers. This characterization involved a wide range of tests including morphology, color measurement, thermogravimetric analysis (TGA), water vapor permeability (WVP), water solubility, TPC, AOA, encapsulation efficiency (EE), and Fourier transform infrared spectroscopy (FTIR) analysis. The other objective of this study was to use these antioxidant fibers to produce antioxidant-enriched food products. It is worth noting that the application of centrifugal spinning to integrate encapsulated caffeic acid fibers into gelatin for functional food production is an unexplored area in current research, emphasizing the novelty of this study. By that, an opportunity was presented to evaluate the impact of heat treatment during the baking process on both encapsulated and non-encapsulated caffeic acid in real food systems. To evaluate the efficiency of these crosslinking methods and the influence of added fibers on the cake, various quality parameters were measured in cakes.
Materials and Methods
Materials
Bovine gelatin with 200 Bloom was provided from Eti, Food Industry Co. Inc. (Eskisehir, Turkey). Caffeic acid, citric acid, acetic acid, and 2,2-diphenyl-1-picrylhydrazy were obtained from Sigma-Aldrich ChemieGmbh (Germany). Ethanol, Tween 80, Folin-Ciocalteu’s phenol reagent, sodium carbonate, and gallic acid were purchased by Merck (EMSURE; ACS, Reag. Ph Eur., Darmstadt, Germany).
Methods
Solution Preparation
Gelatin powder was dissolved in acetic acid by adjusting the concentration of solution to 25% (w/v). Magnetic stirrer (MaxTir 500, Daihan Scientific, Seoul, Kore) was used overnight at 700 rpm and at 40 °C in order to get a homogenous solution. Caffeic acid was dissolved in ethanol:water (80:20 v/v) mixture with different concentrations: 0%, 2%, and 4% (w/w) based on gelatin. Gelatin and caffeic acid solutions were mixed, and Tween 80 (4% (w/w)) were added. Then, citric acid (0.5% (w/v)) was added to form a crosslink and mixed at 700 rpm, at 25 °C for 15 min. Sample abbreviations with their content are given in Table 1.
Rheological Properties of Solution
Controlled strain rheometer (Kinexus dynamic rheometer, Malvern Instruments Ltd, Worcestershire, UK) was used at 25 °C in order to measure the rheological properties of solutions. It has a 4° cone angle and plate with a 40-mm diameter. Solution samples were put into to device, and shear stress values were recorded from 0.1 to 100 s−1 shear rate (Aslaner et al., 2021).
Centrifugal Spinning of Solution
Nanocentrino L1.0™ (Areka Group LLC, Istanbul, Turkey) was used in order to develop fibers. Needles with a diameter of 23 G were used in the rotating head of the device. Operating conditions, which were the rotational speed of the spindle and volumetric flow rate, were adjusted to 1800 rpm and 10 mL/h, respectively. For each turn, solution was fed to the syringe (20 mL). To gather the fibers, a nonwoven polyester spunbond was placed on the collector. The centrifugal spinning system is shown in Fig. 1.
Thermal Crosslinking Treatment
Thermal treatment was done with some modifications according to the study of Doğan et al. (2022a). Gelatin fibers, which were without citric acid, were placed in an oven at 160 °C for 6 h for the formation of crosslink. Thermal-treated fibers were named as GEL-T, GEL-2CA-T, and GEL-4CA-T for containing 0%, 2%, and 4% caffeic acid (w/w), respectively.
Characterization of Fibers
Morphological Analysis of Fibers
Field emission scanning electron microscopy (FESEM) (JEOL, Japan) was used at a magnification of × 2000. Average fiber diameters and their distributions were determined by using ImageJ (MD, USA) with 100 random measurements from different images (Aslaner et al., 2021).
Total Phenolic Content (TPC)
TPC of gelatin fibers was determined by a modified version of the Folin-Ciocalteu method given by Luca et al. (2013). Gelatin fibers were weighted as 0.01 g and mixed with 15 mL ethanol:water (80:20 v/v) solution. Then, the mixture was treated with high speed homogenizer (IKA T25 Digital Ultra-Turrax; IKA®-Werke GmbH&Co. KG, Staufen, Germany) at 3000 rpm for 1 min. 2.5 mL of Folin-Ciocalteu (0.2 N) solution was mixed with 0.5 mL of sample, after filtering the sample solution with a chromatography syringe filter (ISOLAB—PTFE—45/25). The mixture was kept in the dark for 5 min, and then, 2 mL of sodium carbonate solution (7.5% (w/v)) was added. Before measuring the absorbance values at 760 nm with UV/VIS spectrophotometer (UV 2450, Shimadzu, Columbia, USA), mixed samples were kept in the dark for 1 h.
In order to make the calibration curve, different ratios (10, 20, 40, 60, 80, 100 mg/L) of gallic acid dissolved in ethanol:water (80:20 v/v). Then, the same procedure was performed. Results were calculated with Eq. (1) and given as mg gallic acid equivalent (GAE).
where C, V, D, and \({W}_{\text{s}}\) are the concentration of the measured absorbance value from the calibration curve (mg/L), volume of the solution used for dissolving the fiber (L), dilution ratio, and weight of the film (g), respectively.
Encapsulation Efficiency (EE)
EE values were determined by modifying the method of Yao et al. (2016). Theoretical TPC of gelatin fibers was measured by dissolving caffeic acid in 5 mL ethanol:water (80:20 v/v) solution and conducting the same procedure of the TPC analysis given in the “Total Phenolic Content (TPC)” section. For experimental phenolic content, results were taken from the “Total Phenolic Content (TPC)” section. Final percentage values of EE were determined by using Eq. (2).
DPPH Radial Scavenging Activity
DPPH radical scavenging activity was determined as in the method given by Yildiz et al. (2022c) with some changes. 0.03 g fibers were put into 10 mL ethanol:water (80:20 v/v) solution and centrifugated (MIKRO 220R Hettich Zentrifugen, Tuttlingen, Germany) at 1000 rpm for 10 min. Then, 25 ppm 2, 2-diphenyl-1-picrylhydrazyl (DPPH) was mixed with diluted sample solutions by using a vortex (ZX3; VELP Scientifica, Usmate, MB, Italy) for 1 min. Prepared samples were kept in the dark for 30 min, and then, spectrophotometer (UV2450, Shimadzu, Columbia, USA) at 517 nm was used in order to measure the absorbance values. Finally, antioxidant activity was determined by using Eq. (3).
where, \({A}_{\text{control}}\) is the absorbance of the control sample and \({A}_{\text{sample}}\) is the absorbance of the fibers.
Water Vapor Permeability (WVP) of Fibers
WVP of fibers was measured by the modified version of the method given by Emir et al. (2023). Cylindrical-shaped cups with 40-mm internal diameter were filled with 30 mL distilled water in order to obtain 100% relative humidity. Then, trimmed gelatin fibers were placed on the cups after recording the thickness of fibers from six different points by a digital micrometer (LYK 5202, Loyka, Ankara, Turkey). Fibers were fixed on the cups by affixing them from various edges. The initial weight of each cup was measured, and they were put into the desiccator, which had about 10% relative humidity at room temperature. Temperature, relative humidity, and weight of cups were recorded every hour. At the end, WVP was calculated with Eq. (4).
where G, x, t, A, S, \({R}_{1}\), and \({R}_{2}\) represent water vapor flow in grams, fiber thickness (m), time in seconds, area of used fiber (m2), saturated water vapor pressure in Pascal at measured temperature, relative humidity inside the cups, and relative humidity inside the desiccators, respectively.
Color Analysis
The CIE color parameters of fibers were measured by a portable spectrocolorimeter (Serlab SL400, İstanbul, Turkey). Results were given as L, a, and b values. The Euclidean distance (∆E) was calculated by using Eq. (5) with the color data of the control fiber (GEL) serving as the reference point (L* = 122.42, a* = − 0.090, b* = 2.103).
Water Solubility
Water solubility calculation was done by the method given by Gungor et al. (2021) with some modifications. Gelatin fibers were cut in square pieces and weighted. Then, petri dishes were filled with distilled water, and fiber pieces were placed into the water. After 8 h, they were taken out from the water and kept at room temperature to dry. Finally, dried fibers were weighed, and the water solubility ratio was determined according to Eq. (6):
where Mi and Md are the initial weight of the gelatin fiber and dried gelatin fiber after treating with water, respectively.
Thermogravimetric Analysis (TGA)
Thermogravimetric analyzer (Perkin Elmer Pyris 1, Perkin-Elmer Co., Norwalk, USA) was used for thermogravimetric analysis with about 5 mg of fiber samples and gelatin powder. They heated at a rate of 10 °C/min with a 30 mL/min nitrogen flow rate from room temperature to 600 °C. Analyses were done in duplicate (Yildiz et al., 2022c).
Fourier Transform Infrared Spectroscopy (FTIR)
FTIR analysis of gelatin powder (GEL-P), caffeic acid powder (CA-P), and fibers was performed by FTIR device (IR-Affinityl, Shimadzu, Kyoto, Japan). Analysis was done between the wavenumber of 500 and 4000 cm−1 with 16 scans.
Degree of Crosslinking Determination
The degree of crosslinking was determined by the method given Prasertsung et al. (2010), with some modifications. For the experiment, after weighing 5 mg of fiber samples, 1 mL 0.5% (w/v) 2,4,6-trinitrobenzene sulfonic acid (TNBS) and 1 mL 4% (w/v) sodium hydrogen carbonate (pH 8.5) were added. The solution was heated to 40 °C and kept for 2 h. Then, 2 mL 6N HCl was added and heated to 60 °C for 90 min. Finally, the absorbance values of the appropriately diluted solutions were determined at 415 nm by using UV/VIS spectrophotometer (UV 2450, Shimadzu, Columbia, USA). Results were calculated by Eq. (7).
where Acrosslinked and Auncrosslinked were the absorbance of crosslinked and uncrosslinked fiber sample.
Incorporation of Fibers Into Food System
Cake was selected as the model food system for the fibers produced for the encapsulation of caffeic acid and to examine the impact of chemical (citric acid) and physical (heat treatment) crosslinking. The goal was to observe the effect of the heat treatment during baking.
Preparation of Cake
The following cakes were prepared: (a) control cake without fibers, (b) with fibers 25% gelatin (w/v) + 4% caffeic acid (w/w), (c) with thermally crosslinked 25% gelatin (w/v) + 4% caffeic acid (w/w), and (d) with 25% gelatin (w/v) + 4% caffeic acid (w/w) + 0.5% citric acid (w/v) fiber. Cakes were assigned abbreviations as C, CA, CA-T, and CA-C to denote the control, caffeic acid fiber, thermal treatment–crosslinked caffeic acid fiber, and citric acid–crosslinked caffeic acid fiber cakes, respectively. The caffeic acid that was not encapsulated remained undissolved in the medium; thus, direct extract added cake could not be produced. The cake was prepared following a slightly adapted procedure outlined by Hedayati and Mazaheri Tehrani (2018). In the control cake formulation, the ingredients included 100 g of flour, 70 g of sugar, 62.5 g of eggs, 62.5 g of low-fat milk, 30 g of vegetable oil, 3 g of baking powder, 10 mL of distilled (DI) water, and 0.45 g of vanilla extract. An amount of 500 mg of fiber was added per 100 g of batter to cake formulations. Initially, the dry ingredients were combined, followed by the addition of the remaining components. This mixing process was carried out using a mixer (AR1143-K Crust Mix Neo Stand, Istanbul, Turkey) set at speed setting 1 for a duration of 5 min. Subsequently, the resulting batter was poured into silicone baking cups with a diameter of 6 cm. In the control cake, the formulation includes 10 mL of DI water. However, in the cakes where fiber was added, the fibers were first dissolved in 10 mL of DI water before being integrated into the mixture. The cakes were baked in an electrical oven (Teka HE 615 65 LT, Germany) at 170 °C for 30 min. Following the baking process, the cakes were allowed to cool for 30 min in preparation for analysis.
Moisture Content (%), Baking Loss (%), and pH Analysis
To determine the moisture content percentage, cake samples of the crumb were subjected to a drying process in an electrically heated oven at 105 °C until a consistent weight was achieved, following the guidelines outlined in AOAC (1984). The weight loss observed while baking was determined by the percent change of the weight of the batter before it went into the oven and the weight of the freshly baked cake right after it was taken out of the oven. The pH measurement followed the procedure outlined by Mustafa et al. (2018), where 1 g of cake particles were mixed with 9 mL of distilled water and homogenized for 1 min. The pH was determined using a pH meter (ST10, OHAUS Corp., USA) measured in the supernatant phase. All measurements were conducted in triplicate.
Volume Index
The volume index of the cakes was determined in accordance with the method described by AACC (1983). The cakes were vertically sliced through their center, and the heights at three specific points (A, B, C) along the cross-section were measured using a template. The volume index was then calculated using the following equation:
where B represents the height of the cake at the center point, while A and C correspond to the heights of the cake sample at locations 2 cm away from the center towards the left and right sides of the cake, respectively.
Texture Analysis
The textural characteristics of the cakes were examined using a Texture Analyser (CT3 Brookfield, Middleboro, MA, USA) (Gul et al., 2023). A texture profile analysis (TPA) test was conducted, involving a two-bite compression test at specific settings: a pretest speed of 2 mm/s, a test speed of 1 mm/s, and a strain deformation of 25%. To assess the texture of the crust, cakes were subjected to testing using a cylindrical probe with a diameter of 10 mm. Measurements were conducted under ambient room temperature conditions, and the hardness (N) data was obtained from the TPA diagram representing the average of six distinct measurements.
Color Analysis
The CIE color parameters of the crust, namely L, a, and b, were determined using a handheld color reader (TES 135A Color Reader, TES, Taiwan) by a measurement of six different places (Luca et al., 2014). The Euclidean distance (∆E) was computed using Eq. (8) below, with the color data of the control cake serving as the reference point (L* = 59.901, a* = 2.541, b* = 24.992).
Total Phenolic Content (TPC) Analysis
To extract phenolic compounds and antioxidants, 2 g of the cake sample was mixed with 20 mL of a solution of methanol, HCl, and distilled water (80:10:10, v/v/v), followed by centrifugation at 3000 rpm for 5 min (Yaver, 2022). Subsequently, 20 μL of the supernatant from the extract solution was mixed with 1.58 mL of distilled water and 100 μL of Folin-Ciocalteu reagent, followed by a 6-min incubation at room temperature. To this mixture, 300 μL of sodium carbonate (Na2CO3) stock solution was added, and the samples were kept in the dark at room temperature for 1 h before measuring the absorbance at 760 nm using a spectrophotometer (T80 + , UV/Vis. spectrometer, PG Instrument Ltd.) with two replicates. The TPC was expressed in milligrams of gallic acid equivalents (GAE) per gram of cake.
Antioxidant Activity (AOA)
The AOA of samples was assessed using the DPPH (1, 1-diphenyl-2-picrylhydrazyl) radical inhibition method (González-Aguilar et al., 2007). 0.1 mL of the supernatant was mixed with 3.9 mL of a 0.6 mM DPPH solution in 80% methanol. The mixture was then kept in darkness for 1 h, followed by measuring the absorbance at 515 nm using a spectrophotometer with methanol as control. The percentage of AOA was determined as in Eq. (9):
Here, As represents the absorbance of the sample, and Ac represents the absorbance of the control.
Statistical Analysis
Statistical analysis was performed by using analysis of variance (ANOVA) by MINITAB (Version 16). Tukey’s multiple comparison tests were used to compare the values, in case there was a significant difference (p ≤ 0.05).
Results and Discussion
Rheological Properties of Solutions
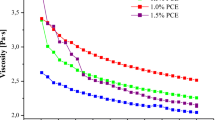
Shear stress versus shear rate curves of solutions is shown in Fig. 4. According to these findings, the rheological behavior of the solutions was characterized as Newtonian, with coefficient of variation (R2) values of 0.999 for the curves. It has been noted that gelatin solutions typically exhibit Newtonian behavior, except when they are at high concentrations and low temperatures. It was previously shown that Newtonian behavior could be observed in gelatin solutions subjected to medium shear rates, typically within the range of 10–350 s−1 (Leuenberger, 1991). Viscosity plays an important role in terms of fiber formation in centrifugal spinning. The production of fibers poses challenges when dealing with both high- and low-viscosity solutions. High-viscosity solutions are impractical for ejection from nozzles, while low-viscosity solutions tend to yield beads instead of fibers (Luz and Santos 2023). Moreover, the spinning jet is generated once the centrifugal force surpasses the resistance posed by the viscosity of the spinning solution (Zhiming et al., 2020). Therefore, the addition of caffeic acid and citric acid was adjusted while taking this aspect into consideration. Table 2 presents the viscosity values of the solutions, which ranged from 0.241 ± 0.04 to 0.326 ± 0.03 Pa.s with no significant differences observed. Similarly, it was previously noted that the addition of 0.5% citric acid had no significant impact on viscosity in the production of gelatin-based electrospun fibers with citric acid (Hasan et al., 2023).
Morphological Analysis of Fibers
Morphological analysis results as FESEM images with their diameter distributions are given in Fig. 5. According to the images, bead free and homogenous fiber structures can be observed in samples with caffeic acid, except for thermal-treated ones. GEL-2CA and GEL-4CA fibers had a more smoother structure in comparison with the GEL. It can be said that the caffeic acid addition did not damage the fiber structure and made it even homogenous and bead free. Similarly, in a study of lemon peel oil–added gelatin-based centrifugally spun fibers, incorporation of lemon peel oil formed a smoother and bead free structure, compared to the gelatin sample. According to Table 3, there was a significant difference between diameters of GEL and GEL-2CA fibers. In another study, it was also shown that the addition of lemon peel oil increased the fiber diameter, but the essential oil concentration did not have any significant effect on the diameter of fibers (Doğan et al., 2022b). Similarly in this study, although the addition of caffeic acid enhanced the fiber diameter, the increase in the caffeic acid concentration from 2 to 4% did not make any significant difference. On the other hand, as can be seen in Table 3, fiber diameters of thermal-treated samples with caffeic acid decreased from 1564.2 ± 352.8 nm and 1415.0 ± 400.0 nm to 1292.7 ± 390.7 nm and 1369.3 ± 435.7 nm for 2% and 4% caffeic acid addition, respectively. Besides their decreased fiber diameter, smoothness and uniformness of the thermal-treated fiber structure were reduced, and broken fibers were observed. This might be due to the fragility effect of heat, which damaged the homogenous fiber structure. Applying heat treatment might change the conformation of the polymer chains or allow esca** water molecules from the structure; therefore, deformation of the smooth structure could be seen (Shi & Yang, 2015). In similar, Gungor et al. (2021) revealed that the cylindrical fiber structure of the centrifugal spun gelatin-based sample was damaged due to the thermal treatment at 170 °C. Citric acid addition increased the fiber thickness from 1254 ± 386 nm (GEL) to 1594.0 ± 382.3 (GEL-0.5C) by having the thickest fiber among all samples. Likewise, in the research about the citric acid–crosslinked electrospun zein fibers (Jiang et al., 2010) and citric acid–crosslinked gelatin-based electrospun gelatin fibers (Hasan et al., 2023), fiber diameter increased by adding citric acid as a crosslinking agent.
Encapsulation Efficiency (EE), Total Phenolic Content (TPC), and Antioxidant Activity (AOA) of Fibers
Encapsulation efficiency is quantified by assessing the concentration of the incorporated active compounds within fibers and the initial concentration of the active compound present in the fiber solution. It denotes the percentage of the active agent effectively entrapped within the fiber structure. Table 4 presents EE, TPC, and AOA of the fibers. The EE of caffeic acid within gelatin fibers exhibited a range, with values spanning from 56.34% (GEL-4CA-T) to 94.55% (GEL-4CA-0.5C), which shows the percentage of how much active agent is trapped in the fiber. Citric acid crosslinking had no substantial impact on EE when compared to caffeic acid–added fibers, whereas thermal crosslinking significantly reduced EE. Furthermore, an increase in the concentration of caffeic acid corresponded to a decrease in EE of thermally treated samples. This might be due to the higher active agent addition. In other words, the ratio of matrix to active agent decreased; therefore, less effective coating was done (Göksen et al., 2020). EE of citric acid–crosslinked and uncrosslinked samples was similar to the results of encapsulation of caffeic acid into nanofibers produced via the electrospinning method with an EE of 94.0% for 10% CA concentration (Zeren et al., 2022). It can be stated that while a 2% CA concentration sufficed to enhance EE in centrifugal spinning, electrospinning required a 10% CA concentration to achieve comparable results. Both of these results were higher than the EE results reported in various other encapsulation methods; for instance, the EE for caffeic acid–loaded solid lipid nanoparticles was 71.21% (Fathi et al., 2013), and the encapsulation of caffeic acid phenethyl ester in skim milk microcapsules achieved an EE of 41.7% through spray-drying (Wang et al., 2020). This might have shown the advantage of using centrifugal spinning in the encapsulation of valuable compounds.
Notably, the TPC and AOA of samples without caffeic acid, namely GEL, GEL-T, and GEL-0.5C, were found to be undetectable. Similarly, in a study examining the incorporation of various essential oils into gelatin-based film-forming solutions, it was observed that no phenolic content was detectable within the gelatin solution (Alparslan, 2018). According to the results, an increase in the caffeic acid concentration led to an increase in the TPC of all fibers. The TPC for GEL-2CA and GEL-4CA was found to be 19.83 ± 1.36 mg GAE/g and 35.75 ± 0.07 mg GAE/g, respectively, while for GEL-2CA-T and GEL-4CA-T, these values were 16.95 ± 1.29 mg GAE/g and 24.58 ± 1.05 mg GAE/g, respectively. The application of thermal treatment resulted in a significant reduction in the TPC of GEL-4CA; however, no significant alteration was observed between GEL-2CA and GEL-2CA-T. It is known that phenolic compounds are affected by thermal treatment (Maier et al., 2009). The reason for the greater reduction in the TPC by thermal treatment at higher concentrations of caffeic acid might be attributed to the lower EE for these samples. Moreover, it has been demonstrated that the enhanced EE contributed to the enhanced thermal resilience of bioactive compounds in food products (Bodbodak et al., 2022). Similarly, the encapsulated açai extract exhibited significantly improved stability against thermal processes compared to commercial açaí fruit, with only minor phenolic content losses (approximately 5–20%) in contrast to a more than 40% reduction in phenolic content observed in the non-encapsulated açaí fruit, highlighting the protective effect of encapsulation (de Dicastillo et al., 2019).
On the other hand, the incorporation of citric acid resulted in an elevation of the TPC when compared to fibers without citric acid. This increase could potentially be attributed to the influence of citric acid on the degradation of polyphenols into simpler phenolic compounds (Salas-Pérez, 2018). In a similar study investigating the impact of citric acid on the phenolic content of olive oil, it was observed that the addition of citric acid led to an increase in the concentration of phenolic compounds (Aliakbarian et al., 2009).
Consistent with the observed trend in TPC, the AOA results also demonstrated a similar pattern with increasing caffeic acid concentration. The application of thermal treatment led to a reduction in AOA values, decreasing from 69.00 ± 0.00% and 94.62 ± 0.69% to 32.38 ± 1.16% and 59.85 ± 2.19% for fibers containing 2% and 4% caffeic acid concentration, respectively. Thermal crosslinking may have an effect on the reduction of the availability of free amino and hydroxyl groups, resulting in a decline in radical scavenging activity. Heating caffeic acid diminishes its AOA by increasing the dissociation of phenolic groups in the presence of higher temperatures, as evidenced by the inverse correlation between the relative decline in AOA with temperature and the oxidisability of phenolic acids (Réblová, 2012). In this study, citric acid had no effect on AOA of caffeic acid–loaded gelatin fibers. However, a decrease in AOA in faba bean/curcumin/chitosan films resulting from the crosslinking of citric acid was attributed to the interaction of citric acid’s carboxyl group of citric acid with the hydroxyl and amino groups of chitosan and the active sites of curcumin, leading to a reduced number of active sites required for AOA (Yildiz et al., 2022b).
Water Vapor Permeability (WVP) of Fibers
The WVP is an important parameter that quantifies the moisture transfer characteristics of a given fiber to its surrounding environment. It can be affected by various factors, such as the hydrophobic interactions of the fibers, the chemical and structural characteristics of the polymeric backbone, and the presence of additives in the fiber (Yadav et al., 2020). Table 3 displays the WVP values of fibers, which ranged from 3.162 ± 0.54 × 10−12 to 5.561 ± 0.16 × 10−12 g m−1 s−1 Pa−1. According to the results, the presence of caffeic acid reduced the WVP in both thermal-treated and non-thermally treated fibers. However, increasing the concentration of caffeic acid within the same groups did not yield a significant difference. Similar findings were reported in a study investigating the effects of caffeic and ferulic acids on fish gelatin films (Araghi et al., 2015) and gallic acid in gelatin films (Bhatia et al., 2022). The reason for decreasing WVP in the presence of caffeic acid might be the crosslinking ability of phenolic compounds by making irreversible covalent bonds with protein (Erge & Eren, 2021). On the other hand, while the thermal treatment without caffeic acid did not result in a significant difference in WVP (GEL vs GEL-T), the addition of citric acid decreased the WVP by 22% (GEL vs GEL-0.5C). This implies that, within the scope of this analysis, the application of thermal treatment of caffeic acid–added samples and citric acid–added samples has indeed induced crosslinking in the samples, thus significantly reducing the WVP in comparison to the pure gelatin-based samples. As the addition of a crosslinking agent (in this study, citric acid) reduced the mobility of the polymer chains, water diffusion might become more challenging (Azeredo et al., 2015), resulting in an average decrease of 32.9% in WVP. The influence of citric acid in reducing WVP was observed in various studies, including the development of citric acid–crosslinked films with curcumin in a chitosan and chickpea flour-based system 20%, by adding 0.5% citric acid (Yildiz et al., 2022a), and a 27% reduction in WVP in citric acid–crosslinked chitosan/starch composite films (Wu et al., 2021). There was a significant difference between the ΔE* values of GEL-2CA-T and GEL-4CA-T. When the phenolic content was higher, the amount of caffeic acid binding to gelatin was high, so the exposed gelatin remained less, and the color change was less. With a higher concentration of caffeic acid, darker fibers were anticipated. However, due to low EE for higher CA concentrations, this significant decrease in L* value could not be observed.
Water Solubility
In fibers, the presence of hydrophilic components increases water solubility, while the inclusion of hydrophobic components diminishes it. Gelatin exhibits high water solubility due to its water-soluble structure. Furthermore, due to the thin thickness of the fibers, they could be readily dispersed in water. Consequently, it was challenging to assess the water solubility of the GEL, GEL-2CA, and GEL-4CA samples. Similarly, fibers containing citric acid also exhibited prompt solubilization and dispersion in water, rendering it impractical to measure their water solubility. This outcome was unexpected, as citric acid was anticipated to induce crosslinking; the immediate dispersion of the fibers in water posed a surprising observation. This situation could be explained by the presence of residual free citric acid within the fibers, which might impart a plasticizing effect and render them soluble in water (Das et al., 2019). The water solubility values for gelatin films containing nisin and catechin were reported as 82.1 ± 11.7% and 88.7 ± 3.3%, respectively (Kaewprachu et al., 2018).
In this study, as can be seen in Table 3, the water solubility of gelatin films subjected to thermal treatment exhibited a range between 21.40 ± 0.75% and 37.65 ± 1.60%. Consistent with previous findings, the reduction in water solubility of gelatin films following thermal treatment can be attributed to the denaturation of the protein structure due to heat and the subsequent formation of crosslinks (Kim et al., 2002). A decrease in water solubility from 75 to 23% was also obtained in a study with thermal treatment done by fish gelatin films (Kchaou et al., 2019). Significant differences were observed in the water solubility values between GEL-2CA-T and GEL-4CA-T. Despite the general trend of phenolic compounds reducing water solubility through crosslinking (Choi et al., 2018), this study recorded an increase, with values rising from 27.89 ± 1.20 to 37.65 ± 1.60 for GEL-2CA-T and GEL-4CA-T, respectively. As observed in the “Encapsulation Efficiency (EE), Total Phenolic Content (TPC), and Antioxidant Activity (AOA) of Fibers” section, thermal treatment has been shown to degrade phenolic compounds and to decrease EE (Maier et al., 2009). Thus, the lower levels of AOA and TPC in GEL-2CA-T fibers in comparison to GEL-4CA-T films implied a potential reduction in water solubility for GEL-2CA-T with the lowest water solubility values observed for the GEL-T fiber.
Thermogravimetric Analysis (TGA)
Figure 6A–D shows the weight loss versus temperature curve for thermal and citric acid–crosslinked fibers and the rate of material weight changes upon heating for the thermal and citric acid–crosslinked fibers, respectively. According to the curves, two weight loss steps can be observed. The first one, occurred between 25 °C and around 120 °C, was due to the water loss, whereas the second one, from around 230 °C to approximately 500 °C, was corresponded to the thermal decomposition of gelatin due to the breakage of the protein chain and the peptide bonds (Peña et al., 2010). Duan et al. (2023) mentioned in their study about curcumin-added gelatin and chitosan-based electrospun nanofibers that the thermal degradation of gelatin was at around 250 to 600 °C. Also, in a study about develo** microfibrous mats with fish gelatin by blow spinning method, it was stated that weight loss from 30 to 160 °C occurred due to the evaporation of water (Vilches et al., 2019). According to Fig. 6A, moisture loss at the first degradation step took place at higher temperatures for thermal-treated samples, compared with not crosslinked ones. This indicated that hydrogen bonding between fiber component and water molecules was more stable for thermal-treated samples. Apart from not crosslinked and thermal-crosslinked samples, there was a degradation at 190–200 °C for citric acid–crosslinked samples (Fig. 6B), indicating the evaporation of citric acid derivatives (Yildiz et al., 2022b). According to the DTG curves (Fig. 6C and D), while all samples, except GEL, showed a peak at around 400 °C, the peak of the GEL sample is 372 °C. The shift of samples as compared with GEL indicated the strong intermolecular interactions and thermal stability. In addition, according to Fig. 6C, when the temperatures corresponding to 10% weight loss were compared, the thermal-treated samples reached higher temperatures, meaning that their thermal stability was higher. At the end of the thermogravimetric analysis, the remaining weight of all samples was determined as around 24%.
Fourier Transform Infrared Spectroscopy (FTIR)
FTIR spectra of samples are shown in Fig. 7A and B. For all samples, except CA-P, there were two main peaks at 1522 cm−1 and 1636 cm−1, which corresponded to amide II and amide I, respectively. There are some factors that affect the infrared absorption at different wavenumber bands. For example, the amide I region is related with C = O stretch and bending of N–H bond. On the other hand, the amide II region indicates the N–H bond deformation (Hassan et al., 2021). Nearly similar FTIR spectra were observed in a study about blood orange peel extract–added edible gelatin films (Jridi et al., 2019). When the amide II peak of samples was compared, GEL had a peak at 1527 cm−1, whereas the others had at 1522 cm−1. The reason of this shift might be the new linkage formation between antioxidants, which was caffeic acid, and polymer chains (Benbettaïeb et al., 2015). In addition, there was a slight peak at 1236 cm−1, which indicates the amide III band. The reason for the smaller peak in the amide III band, in comparison to the other peaks, might be the triple helix state being lost during high temperature gelatin extraction (Hashim et al., 2010). In addition, according to Fig. 7B, the amide III peak shifted from 1236 to 1241 cm−1 in GEL-4CA and GEL-4CA-0.5C might be due to the electrostatic interactions between the active agent and gelatin (Kan et al., 2019). Besides, a peak was observed at 3279 cm−1, which was the characteristic peak of gelatin, indicating the O–H stretching vibrations (Riahi et al., 2021). For the CA-P curve, which was the FTIR spectra of pure caffeic acid, there were intense peaks lower than 1120 cm−1 indicating the C–C-C bending of the aromatic ring. However, peaks at 640 and 812 cm−1 corresponded to the bending of the carbonyl group. Another peak at 1213 and 1270 cm−1 referred to the bending of olefinic and aromatic C-H bonds, whereas peaks at 1447 and 1596 cm−1 were the stretching of olefinic and aromatic C–C bonds. A peak at 1642 cm−1 belonged to stretching vibrations of C = O bonds. Other peaks from 2600 to 4000 cm−1 indicated the OH stretching vibrations (Catauro et al., 2020). Since the intense peak band of CA-P was not observed in gelatin fiber samples, it can be said that caffeic acid encapsulation was done properly.
Although there was no significant difference between the curves of gelatin fibers, the effect of both crosslinking methods, the thermal treatment and citric acid addition, slightly decreased the peak at 3279 cm−1, as compared to GEL. This peak was at 3290 cm−1 for GEL; however, it shifted to 3284 cm−1 and 3279 cm−1 for GEL-2CA, GEL-2CA-T, and GEL-4CA-T and for GEL-4CA, GEL-T, and fibers with citric acid, respectively. The reason for that was because of the OH stretching bands of absorbed water molecules (Rawdkuen et al., 2010). Moreover, in the samples of GEL, GEL-2CA, and GEL-4CA, a peak observed at 3000–3500 cm−1 was significantly more pronounced, while it appeared less prominent in both thermal-crosslinked (Fig. 7A) and citric acid–added samples (Fig. 7B). This observation indicates a reduction in free amino and hydroxyl groups due to crosslinking or chemical reactions. The decline implies that free amino and hydroxyl groups may be forming larger molecular structures or engaging in reactions with other chemical groups. A similar decrease at 3274 cm−1 was observed in a study focusing on the development of centrifugally spun gelatin-based active food packaging fibers incorporating lemon peel oil (Doğan et al., 2022b). Moreover, the addition of caffeic acid to pure gelatin solution exhibited no additional specific peak in the infrared spectra, suggesting the absence of new chemical bonds due to the encapsulation of caffeic acid within the gelatin fiber mats, as observed in similar studies (Cui et al., 2018; Doğan et al., 2022b).
Degree of Crosslinking
Methods for quantitatively assessing chemical crosslinking quantitatively can effectively demonstrate the extent of interaction between the crosslinking agent and the matrix (Wang et al., 2023). Table 3 shows the degree of crosslinking in fibers. The idea behind this approach was to utilize 2,4,6-trinitrobenzene sulfonic acid (TNBS) to interact with unbound amino groups in gelatin, which represented the non-crosslinked entities (Kchaou et al., 2018). A higher degree of crosslinking typically signifies a greater number of covalent bonds formed between gelatin and citric acid. These findings were in accordance with the outcomes of water solubility tests, as GEL-2CA, GEL-4CA, and citric acid–incorporated fibers exhibited less crosslinking, whereas heat-treated fibers displayed a higher percentage of crosslinking. It was observed that as the degree of crosslinking decreased, water solubility also increased (Abedini et al., 2023). Fibers comprising lower amount of caffeic acid in heat-treated samples displayed the most pronounced degree of crosslinking, indicating the fiber’s presence of unbound amino groups.
Overall, fiber properties can be affected from both caffeic acid concentration and crosslinking method. Increasing caffeic acid content maintained the homogenous fiber morphology and enhanced the TPC and AOA of fibers, however, decreased their EE. The addition of caffeic acid led to a decrease in the WVP of fibers, but an increase in the caffeic acid content 2 to 4% did not show any significant difference. From crosslinking methods, thermal treatment was found more effective than citric acid addition in terms of water solubility and degree of crosslinking. However, thermal treatment negatively affected the morphological properties of fibers and decreased EE, TPC, and AOA. Nevertheless, fibers, containing 4% caffeic acid, with and without crosslinking, were selected to be added to cakes with the highest TPC and AOA.
The Effect of Nanofiber Addition on Physicochemical Properties of Cakes
To observe the impact of fibers obtained through centrifugal spinning with and without crosslinking on the physicochemical properties of cakes, four variations were studied: cakes without fibers, cakes with fibers containing 4% added caffeic acid, and cakes with fibers containing 4% caffeic acid subjected to thermal and citric acid crosslinking processes. The moisture content in both the fiber-added and control samples ranged from 40.07 to 42.23%, while the weight loss during baking ranged from 28.55 to 31.23% (Table 6). Importantly, no statistically significant differences were detected among the various samples. Similarly, the moisture content value of the crumb of wheat flour cake was found to be around 40% by Chan et al. (2023). Incorporating 500 mg of caffeic acid fiber per 100 g of batter did not yield any impact on the moisture content and baking loss. However, as expected, the inclusion of caffeic acid decreased the pH value whether crosslinking was applied or not.
The volume index in cake quality assessment is crucial because it reflects the cake’s texture, appearance, and consumer appeal. It measures the amount of air trapped within the cake, and it serves as an indicator of its quality. A higher volume index indicates a fluffier, more visually appealing cake with better moisture retention, contributing to a positive consumer perception and overall product quality. The introduction of thermally crosslinked caffeic acid fibers led to a notable increase in the volume index values of the fiber-added samples, elevating them from 26.28 to 53.31 as seen in Table 6. Crosslinked rice flour was also shown to increase the volume of cake (Lee et al., 2020). Due to thermal crosslinking, the fiber diameters decreased, leading to an increase in the volume of fibers while maintaining a constant weight. This phenomenon is likely to contribute to a significant increase in the volume index as an equal amount of fibers was added to the formula, resulting in a more porous structure. Given that an equal weight of fiber was incorporated into each cake formulation, the thermally crosslinked fibers occupied a larger volume compared to others due to their smaller diameter. This reduction in fiber diameter correspondingly led to an increase in surface area for the same weight of fibers. Similar observations were made with crosslinked chitosan fibers, where decreasing the fiber diameter within these mats led to various beneficial effects, including an increase in the ratio of specific surface area to volume (Schiffman & Schauer, 2007).
Hardness refers to the amount of force needed to alter the shape of the sample, resembling the pressure exerted when chewing food. Cake with a low hardness value is characterized by a soft texture. Table 6 illustrates how cake hardness changes with the addition of various fibers. Whether or not crosslinking was applied, the inclusion of fibers significantly resulted in a reduction in sample hardness from 2.36 to 1.112 N. An expected outcome was a decrease in hardness values of cakes as the volume of cakes increased due to the addition of nanofibers.
The Effect of Nanofiber Addition on Color Properties of Cakes
Color parameter data displayed in Table 7 effectively illustrates the significant color variations (ΔE*) attributed to the integration of thermally crosslinked centrifugal spun fibers in the cakes. Similarly, the L* value of cakes containing thermally crosslinked centrifugally spun fibers was notably lower compared to the other samples. This difference in L* and ΔE* values could be attributed to the darker color of these specific fibers. In another study, the fibers subjected to heating at 170 °C exhibited a yellowish hue, potentially indicating an augmentation in C-N bond formation during the thermal crosslinking process (Gungor et al., 2021). Moreover, the formation of C-N bonds was attributed to the Maillard reaction, a widely accepted method for protein crosslinking, which facilitates the generation of covalent bonds between the aldehyde groups of sugar molecules in cake and the amino groups of gelatin (Saeed et al., 2021). These reactions not only contributed to the color development but also enhanced the overall texture and flavor of cakes. Positive a* values suggest red hues, and negative values imply green hues, while positive b* values indicate the presence of yellow hues, and negative values signify blue hues. These values did not exhibit any significant differences among the samples.
The Effect of Nanofiber Addition on Total Phenolic Content (TPC) and Antioxidant Activities (AOA) of Cakes
TPC values were as follows: 0.8774 ± 0.0972 mg GAE/g for the thermally crosslinked fiber-added cake, 0.7519 ± 0.0219 mg GAE/g for the uncrosslinked fiber-added cake, and 0.7252 ± 0.0292 mg GAE/g for the citric acid–crosslinked fiber-added cakes. The TPC of the control cakes was measured at 0.4768 ± 0.0559 mg GAE/g, primarily due to the presence of phenolic acids naturally occurring in wheat flour. These phenolic acids, such as protocatechuic acid and gallic acid, were found in varying amounts in wheat and contributed to the overall phenolic content (Nikolić et al., 2019). In fact, while a decrease in the naturally occurring phenolic acids in wheat was expected, a significant increase in TPC values was observed due to the encapsulated caffeic acid within the fiber. As discussed in the “Encapsulation Efficiency (EE), Total Phenolic Content (TPC), and Antioxidant Activity (AOA) of Fibers” section, TPC of fibers in mg GAE/g was 35.75 for uncrosslinked, 24.58 for thermal crosslinked, and 41.24 for citric acid crosslinked. Considering that 500 mg of fiber was incorporated into 100 g of batter, the weight loss of the batter was calculated to be approximately 30%, resulting in a 70 g cake. Within the 500 mg of fiber, 20 mg of caffeic acid was encapsulated. This implies an expected increase of ~ 0.3 mg of caffeic acid per 1 g of cake. Despite the low quantity of fiber in the cake formulation, the TPC of the fiber-added cakes remained preserved under high baking temperatures, allowing the assessment of thermal stability in both crosslinked and uncrosslinked samples. The AOA of the cakes ranged from 14.55 ± 0.66 to 58.91 ± 6.58%, with the lowest value observed in the control cake and the highest value in the thermally crosslinked fiber-added cake. When observing the AOA values of the fibers, it was evident that the thermally crosslinked fibers exhibited a lower value. However, as indicated in the TGA results, the fibers subjected to thermal crosslinking displayed a greater heat resistance. Consequently, this attribute led to a higher impact of AOA on the cakes. In detail, upon heating to approximately 170 °C (TGA), the thermally crosslinked fibers exhibited notably lower weight loss and thermal degradation (Fig. 6A).
Conclusions
In this study, various concentrations of caffeic acid were encapsulated through centrifugal spinning, employing two different crosslinking methodologies. By considering the higher TPC and AOA of these fibers, an antioxidant-enriched cake was developed as a functional food. While citric acid inclusion had no impact on fiber water solubility, thermal treatment notably increased water insolubility and the degree of fiber crosslinking. Thermal treatment induced alterations in fiber color and significantly reduced not only the TPC and AOA but also the EE. EE in thermally treated fibers was lower than in both uncrosslinked and citric acid–crosslinked fibers. Increasing the caffeic acid concentration showed higher TPC and AOA in fibers; however, it decreased the EE. Still, by having the highest TPC and AOA, fibers with 4% caffeic acid were added into cakes to enhance the TPC and AOA of cakes. Incorporation of fibers to cakes did not make any difference in moisture content and baking loss, while it decreased the pH and hardness. The addition of thermal-crosslinked fibers to the cake showed the highest volume index value among the other cake samples. As a conclusion, incorporation of thermally crosslinked and 4% caffeic acid–added gelatin nanofiber in cake had the potential of giving functional properties to cakes with its high TPC and AOA.
Data Availability
Data is available upon request.
References
AACC. (1983). American Association of Cereals Chemists. Approved methods of the AACC. (11th ed.) The Association. St. Paul, Mc.
Abedini, A. A., Pircheraghi, G., & Kaviani, A. (2023). The role of calcium crosslinking and glycerol plasticizing on the physical and mechanical properties of superabsorbent: Alginate/quince seed gum films. Journal of Polymer Research, 30(1), 1–14. https://doi.org/10.1007/s10965-022-03397-5
Adem, Ş, Eyupoglu, V., Sarfraz, I., Rasul, A., Zahoor, A. F., Ali, M., et al. (2021). Caffeic acid derivatives (CAFDs) as inhibitors of SARS-CoV-2: CAFDs-based functional foods as a potential alternative approach to combat COVID-19. Phytomedicine, 85, 1–17. https://doi.org/10.1016/j.phymed.2020.153310
Akhtar, M., Ahmed, S., Hussain, R., Wadood, A., Roy, I., Rehman, A. U., & M., & Boccaccini, A. R. (2022). Centrifugal spinning of polyvinyl alcohol/sodium alginate-di -aldehyde-gelatin based antibacterial nanofibers intended for skin tissue engineering. Materials Letters, 323, 1–5. https://doi.org/10.1016/j.matlet.2022.132530
Alam, M., Ahmed, S., Elasbali, A. M., Adnan, M., Alam, S., Hassan, M. I., & Pasupuleti, V. R. (2022). March 10). Therapeutic implications of caffeic acid in cancer and neurological diseases. Frontiers in Oncology, 12, 1–18. https://doi.org/10.3389/fonc.2022.860508
Aliakbarian, B., Dehghani, F., & Perego, P. (2009). The effect of citric acid on the phenolic contents of olive oil. Food Chemistry, 116(3), 617–623. https://doi.org/10.1016/j.foodchem.2009.02.077
Alparslan, Y. (2018). Antimicrobial and antioxidant capacity of biodegradable gelatin film forming solutions incorporated with different essential oils. Journal of Food Measurement and Characterization, 12(1), 317–322. https://doi.org/10.1007/s11694-017-9643-x
AOAC. (1984). AOAC Official methods of analysis Association of Official Analytical Chemists (14th ed.). Washington, DC: Association of Official Analytical Chemists.
Araghi, M., Moslehi, Z., Nafchi, A. M., Mostahsan, A., Salamat, N., & Garmakhany, A. D. (2015). Cold water fish gelatin modification by a natural phenolic cross-linker (ferulic acid and caffeic acid). Food Science and Nutrition, 3(5), 370–375. https://doi.org/10.1002/fsn3.230
Arican, F., Uzuner-Demir, A., Polat, O., Sancakli, A., & Ismar, E. (2022). Fabrication of gelatin nanofiber webs via centrifugal spinning for N95 respiratory filters. Bulletin of Materials Science, 45, 1–8. https://doi.org/10.1007/s12034-022-02668-7S
Aslaner, G., Sumnu, G., & Sahin, S. (2021). Encapsulation of grape seed extract in rye flour and whey protein-based electrospun nanofibers. Food and Bioprocess Technology, 14(6), 1118–1131. https://doi.org/10.1007/s11947-021-02627-w/Published
Azeredo, H. M. C., Kontou-Vrettou, C., Moates, G. K., Wellner, N., Cross, K., Pereira, P. H. F., & Waldron, K. W. (2015). Wheat straw hemicellulose films as affected by citric acid. Food Hydrocolloids, 50, 1–6. https://doi.org/10.1016/j.foodhyd.2015.04.005
Banerjee, P. (2019). Functional food: a brief overview. International Journal of Bioresource Science, 6(2), 57–60. https://doi.org/10.30954/2347-9655.02.2019.2
Baranauskaite, J., Kopustinskiene, D. M., & Bernatoniene, J. (2019). Impact of gelatin supplemented with gum Arabic, Tween 20, and β-cyclodextrin on the microencapsulation of Turkish oregano extract. Molecules, 24(1), 1–16. https://doi.org/10.3390/molecules24010176
Benbettaïeb, N., Karbowiak, T., Brachais, C. H., & Debeaufort, F. (2015). Coupling tyrosol, quercetin or ferulic acid and electron beam irradiation to cross-link chitosan-gelatin films: A structure-function approach. European Polymer Journal, 67, 113–127. https://doi.org/10.1016/j.eurpolymj.2015.03.060
Bhatia, S., Al-Harrasi, A., Al-Azri, M. S., Ullah, S., Makeen, H. A., Meraya, A. M., et al. (2022). Gallic acid crosslinked gelatin and casein based composite films for food packaging applications. Polymers, 14(19), 1–15. https://doi.org/10.3390/polym14194065
Bodbodak, S., Nejatian, M., Ghandehari Yazdi, A. P., Kamali Rousta, L., Rafiee, Z., Jalali-Jivan, M., et al. (2022). Improving the thermal stability of natural bioactive ingredients via encapsulation technology. Critical Reviews in Food Science and Nutrition, 64(10), 1–23. https://doi.org/10.1080/10408398.2022.2127145
Catauro, M., Barrino, F., Dal Poggetto, G., Crescente, G., Piccolella, S., & Pacifico, S. (2020). New SiO2/Caffeic acid hybrid materials: Synthesis, spectroscopic characterization, and bioactivity. Materials, 13(2), 1–12. https://doi.org/10.3390/ma13020394
Chan, D. S., Wang, S. T., Chen, M. Y., & Sung, W. C. (2023). The effect of okra (Abelmoschus esculentus l.) powder addition on qualities of gluten-free chiffon cake. Food Science and Technology International, 108201322311621, 1–10. https://doi.org/10.1177/10820132231162164
Chen, C., Dirican, M., & Zhang, X. (2019). Centrifugal spinning-high rate production of nanofibers. Electrospinning: Nanofabrication and Applications, 321–338. https://doi.org/10.1016/B978-0-323-51270-1.00010-8
Choi, I., Lee, S. E., Chang, Y., Lacroix, M., & Han, J. (2018). Effect of oxidized phenolic compounds on cross-linking and properties of biodegradable active packaging film composed of turmeric and gelatin. LWT, 93, 427–433. https://doi.org/10.1016/j.lwt.2018.03.065
Cizmarova, B., Hubkova, B., Bolerazska, B., Marekova, M., & Birkova, A. (2020). Caffeic acid: a brief overview of its presence, metabolism, and bioactivity. Bioactive Compounds in Health and Disease, 3(4), 74–81. https://doi.org/10.31989/bchd.v3i4.692
Cremar, L., Gutierrez, J., Martinez, J., Materon, L., Gilkerson, R., Xu, F., & Lozano, K. (2018). Development of antimicrobial chitosan based nanofiber dressings for wound healing applications. Nanomedicine Journal, 5(1), 6–14. https://doi.org/10.22038/nmj.2018.05.002
Cui, H., Bai, M., Li, C., Liu, R., & Lin, L. (2018). Fabrication of chitosan nanofibers containing tea tree oil liposomes against Salmonella spp. in chicken. Food Science and Technology, 96, 671–678. https://doi.org/10.1016/j.lwt.2018.06.026
Das, A., Uppaluri, R., & Das, C. (2019). Feasibility of poly-vinyl alcohol/starch/glycerol/citric acid composite films for wound dressing applications. International Journal of Biological Macromolecules, 131, 998–1007. https://doi.org/10.1016/j.ijbiomac.2019.03.160
de Dicastillo, C. L., Piña, C., Garrido, L., Arancibia, C., & Galotto, M. J. (2019). Enhancing thermal stability and bioaccesibility of açaí fruit polyphenols through electrohydrodynamic encapsulation into zein electrosprayed particles. Antioxidants, 8(10), 1–16. https://doi.org/10.3390/antiox8100464
Doğan, C., Doğan, N., Gungor, M., Eticha, A. K., & Akgul, Y. (2022a). Novel active food packaging based on centrifugally spun nanofibers containing lavender essential oil: Rapid fabrication, characterization, and application to preserve of minced lamb meat. Food Packaging and Shelf Life, 34, 100942. https://doi.org/10.1016/j.fpsl.2022.100942
Doğan, N., Doğan, C., Eticha, A. K., Gungor, M., & Akgul, Y. (2022b). Centrifugally spun micro-nanofibers based on lemon peel oil/gelatin as novel edible active food packaging: Fabrication, characterization, and application to prevent foodborne pathogens E. coli and S. aureus in cheese. Food Control, 139, 109081. https://doi.org/10.1016/j.foodcont.2022.109081
Duan, M., Sun, J., Huang, Y., Jiang, H., Hu, Y., Pang, J., & Wu, C. (2023). Electrospun gelatin/chitosan nanofibers containing curcumin for multifunctional food packaging. Food Science and Human Wellness, 12(2), 614–621. https://doi.org/10.1016/j.fshw.2022.07.064
Emir, A. A., Yildiz, E., Aydogdu, Y., & Sumnu, G. (2023). Active films based on faba bean (Vicia faba L.) flour incorporated with sumac (Rhus coriaria): Assessment of antioxidant and antimicrobial performances of packaging for shelf life of chicken breast. Food and Bioprocess Technology, 16(2), 327–341. https://doi.org/10.1007/s11947-022-02940-y
Erge, A., & Eren, Ö. (2021). Chicken gelatin modification by caffeic acid: A response surface methodology investigation. Food Chemistry, 351, 1–6. https://doi.org/10.1016/j.foodchem.2021.129269
Fathi, M., Mirlohi, M., Varshosaz, J., & Madani, G. (2013). Novel caffeic acid nanocarrier: Production, characterization, and release modeling. Journal of Nanomaterials, 2013, 1–9. https://doi.org/10.1155/2013/434632
Göksen, G., Fabra, M. J., Ekiz, H. I., & López-Rubio, A. (2020). Phytochemical-loaded electrospun nanofibers as novel active edible films: Characterization and antibacterial efficiency in cheese slices. Food Control, 112, 1–11. https://doi.org/10.1016/j.foodcont.2020.107133
González-Aguilar, G. A., Villegas-Ochoa, M. A., Martínez-Téllez, M. A., Gardea, A. A., & Ayala-Zavala, J. F. (2007). Improving antioxidant capacity of fresh-cut mangoes treated with UV-C. Journal of Food Science, 72(3), 197–202. https://doi.org/10.1111/j.1750-3841.2007.00295.x
Granato, D., Barba, F. J., Bursac Kovačevic, D., Lorenzo, J. M., Cruz, A. G., & Putnik, P. (2020). Functional foods: Product development, technological trends, efficacy testing, and safety. Annual Review of Food Science and Technology, 11, 93–118. https://doi.org/10.1146/annurev-food-032519
Gul, M. R., Ince, A. E., Ozel, B., Uslu, A. K., Çetin, M., Mentes, D., et al. (2023). Effect of microwave-vacuum drying on the physicochemical properties of a functional tomato snack bar. Journal of the Science of Food and Agriculture, 104(1), 83–92. https://doi.org/10.1002/jsfa.12894
Gungor, M., Sagirli, M. N., Calisir, M. D., Selcuk, S., & Kilic, A. (2021). Develo** centrifugal spun thermally cross-linked gelatin based fibrous biomats for antibacterial wound dressing applications. Polymer Engineering and Science, 61(9), 2311–2322. https://doi.org/10.1002/pen.25759
Hasan, R., Sumnu, G., Sahin, S., Oz, E., & Oz, F. (2023). The effects of citric acid crosslinking on fabrication and characterization of gelatin/curcumin-based electrospun antioxidant nanofibers. Antioxidants, 12(7), 1–17. https://doi.org/10.3390/antiox12071387
Hashim, D. M., Man, Y. B. C., Norakasha, R., Shuhaimi, M., Salmah, Y., & Syahariza, Z. A. (2010). Potential use of Fourier transform infrared spectroscopy for differentiation of bovine and porcine gelatins. Food Chemistry, 118(3), 856–860. https://doi.org/10.1016/j.foodchem.2009.05.049
Hassan, N., Ahmad, T., Zain, N. M., & Awang, S. R. (2021). Identification of bovine, porcine and fish gelatin signatures using chemometrics fuzzy graph method. Scientific Reports, 11(1), 1–10. https://doi.org/10.1038/s41598-021-89358-2
Hedayati, S., & Mazaheri Tehrani, M. (2018). Effect of total replacement of egg by soymilk and lecithin on physical properties of batter and cake. Food Science and Nutrition, 6(4), 1154–1161. https://doi.org/10.1002/fsn3.656
Jiang, Q., Reddy, N., & Yang, Y. (2010). Cytocompatible cross-linking of electrospun zein fibers for the development of water-stable tissue engineering scaffolds. Acta Biomaterialia, 6(10), 4042–4051. https://doi.org/10.1016/j.actbio.2010.04.024
Jridi, M., Boughriba, S., Abdelhedi, O., Nciri, H., Nasri, R., Kchaou, H., et al. (2019). Investigation of physicochemical and antioxidant properties of gelatin edible film mixed with blood orange (Citrus sinensis) peel extract. Food Packaging and Shelf Life, 21, 1–9. https://doi.org/10.1016/j.fpsl.2019.100342
Kadar, N. N., Ahmad, F., Teoh, S. L., & Yahaya, M. F. (2021). Caffeic acid on metabolic syndrome: A review. Molecules, 26(18), 1–14. https://doi.org/10.3390/molecules26185490
Kaewprachu, P., Ben Amara, C., Oulahal, N., Gharsallaoui, A., Joly, C., Tongdeesoontorn, W., et al. (2018). Gelatin films with nisin and catechin for minced pork preservation. Food Packaging and Shelf Life, 18, 173–183. https://doi.org/10.1016/j.fpsl.2018.10.011
Kan, J., Liu, J., Yong, H., Liu, Y., Qin, Y., & Liu, J. (2019). Development of active packaging based on chitosan-gelatin blend films functionalized with Chinese hawthorn (Crataegus pinnatifida) fruit extract. International Journal of Biological Macromolecules, 140, 384–392. https://doi.org/10.1016/j.ijbiomac.2019.08.155
Kchaou, H., Benbettaïeb, N., Jridi, M., Abdelhedi, O., Karbowiak, T., Brachais, C. H., et al. (2018). Enhancement of structural, functional and antioxidant properties of fish gelatin films using Maillard reactions. Food Hydrocolloids, 83, 326–339. https://doi.org/10.1016/j.foodhyd.2018.05.011
Kchaou, H., Benbettaieb, N., Jridi, M., Nasri, M., & Debeaufort, F. (2019). Influence of Maillard reaction and temperature on functional, structure and bioactive properties of fish gelatin films. Food Hydrocolloids, 97, 1–14. https://doi.org/10.1016/j.foodhyd.2019.105196
Khoshnoudi-Nia, S., Sharif, N., & Jafari, S. M. (2020). Loading of phenolic compounds into electrospun nanofibers and electrosprayed nanoparticles. Trends in Food Science and Technology, 95, 59–74. https://doi.org/10.1016/j.tifs.2019.11.013
Kim, K. M., Weller, C. L., Hanna, M. A., & Gennadios, A. (2002). Heat curing of soy protein films at selected temperatures and pressures. LWT, 35(2), 140–145. https://doi.org/10.1006/fstl.2001.0825
Lee, C. E., No, J., Lee, K., & Shin, M. (2020). Improvement of resistant starch content and baking quality of cross-linked soft rice flour. Food Science and Biotechnology, 29(12), 1695–1703. https://doi.org/10.1007/s10068-020-00826-3
Leuenberger, B. H. (1991). Investigation of viscosity and gelation properties of different mammalian and fish gelatins. Topics in Catalysis, 5(4), 353–361. https://doi.org/10.1016/S0268-005X(09)80047-7
Loordhuswamy, A. M., Krishnaswamy, V. R., Korrapati, P. S., Thinakaran, S., & Rengaswami, G. D. V. (2014). Fabrication of highly aligned fibrous scaffolds for tissue regeneration by centrifugal spinning technology. Materials Science and Engineering C, 42, 799–807. https://doi.org/10.1016/j.msec.2014.06.011
Luca, A., Cilek, B., Hasirci, V., Sahin, S., & Sumnu, G. (2013). Effect of degritting of phenolic extract from sour cherry pomace on encapsulation efficiency-production of nano-suspension. Food and Bioprocess Technology, 6(9), 2494–2502. https://doi.org/10.1007/s11947-012-0880-z
Luca, A., Cilek, B., Hasirci, V., Sahin, S., & Sumnu, G. (2014). Storage and baking stability of encapsulated sour cherry phenolic compounds prepared from micro- and nano-suspensions. Food and Bioprocess Technology, 7(1), 204–211. https://doi.org/10.1007/s11947-013-1048-1
Luz, H. Z., Santos, L., & a. L. D. (2023). Centrifugal spinning for biomedical use: A review. Critical Reviews in Solid State and Materials Sciences, 48(4), 519–534. https://doi.org/10.1080/10408436.2022.2080640
Maier, T., Fromm, M., Schieber, A., Kammerer, D. R., & Carle, R. (2009). Process and storage stability of anthocyanins and non-anthocyanin phenolics in pectin and gelatin gels enriched with grape pomace extracts. European Food Research and Technology, 229(6), 949–960. https://doi.org/10.1007/s00217-009-1134-9
Melike, G., Calisir, M. D., Akgul, Y., Selcuk, S., Ali, D., & Kilic, A. (2021). Submicron aerosol filtration performance of centrifugally spun nanofibrous polyvinylpyrrolidone media. Journal of Industrial Textiles, 50(10), 1545–1558. https://doi.org/10.1177/1528083719865041
Mirzaei, S., Gholami, M. H., Zabolian, A., Saleki, H., Farahani, M. V., Hamzehlou, S., et al. (2021). Caffeic acid and its derivatives as potential modulators of oncogenic molecular pathways: New hope in the fight against cancer. Pharmacological Research, 171, 1–18. https://doi.org/10.1016/j.phrs.2021.105759
Mustafa, R., He, Y., Shim, Y. Y., & Reaney, M. J. T. (2018). Aquafaba, wastewater from chickpea canning, functions as an egg replacer in sponge cake. International Journal of Food Science and Technology, 53(10), 2247–2255. https://doi.org/10.1111/ijfs.13813
Nikolić, N., Mitrović, J., Karabegović, I., Savić, S., Petrović, S., Lazić, M., & Stojanović, G. (2019). A comparison between wheat and different kinds of corn flour based on minerals, free phenolic acid composition and antioxidant activity. Quality Assurance and Safety of Crops and Foods, 11(4), 341–349. https://doi.org/10.3920/QAS2018.1411
Okutan, N., Terzi, P., & Altay, F. (2014). Affecting parameters on electrospinning process and characterization of electrospun gelatin nanofibers. Food Hydrocolloids, 39, 19–26. https://doi.org/10.1016/j.foodhyd.2013.12.022
Peña, C., de la Caba, K., Eceiza, A., Ruseckaite, R., & Mondragon, I. (2010). Enhancing water repellence and mechanical properties of gelatin films by tannin addition. Bioresource Technology, 101(17), 6836–6842. https://doi.org/10.1016/j.biortech.2010.03.112
Prasertsung, I., Mongkolnavin, R., Kanokpanont, S., & Damrongsakkul, S. (2010). The effects of pulsed inductively coupled plasma (PICP) on physical properties and biocompatibility of crosslinked gelatin films. International Journal of Biological Macromolecules, 46(1), 72–78. https://doi.org/10.1016/j.ijbiomac.2009.11.001
Rawdkuen, S., Sai-Ut, S., & Benjakul, S. (2010). Properties of gelatin films from giant catfish skin and bovine bone: A comparative study. European Food Research and Technology, 231(6), 907–916. https://doi.org/10.1007/s00217-010-1340-5
Réblová, Z. (2012). Effect of temperature on the antioxidant activity of phenolic acids. Czech J. Food Sci, 30, 171–177.
Riahi, Z., Priyadarshi, R., Rhim, J. W., & Bagheri, R. (2021). Gelatin-based functional films integrated with grapefruit seed extract and TiO2 for active food packaging applications. Food Hydrocolloids, 112, 1–10. https://doi.org/10.1016/j.foodhyd.2020.106314
Russo, G. I., Campisi, D., Mauro, M. D., Regis, F., Reale, G., Marranzano, M., et al. (2017). Dietary consumption of phenolic acids and prostate cancer: A case-control study in Sicily. Southern Italy. Molecules, 22(12), 1–9. https://doi.org/10.3390/molecules22122159
Saeed, M., Beigi-Boroujeni, S., Rajabi, S., Rafati Ashteiani, G., Dolatfarahi, M., & Özcan, M. (2021). A simple, green chemistry technology for fabrication of tissue-engineered scaffolds based on mussel-inspired 3D centrifugal spun. Materials Science and Engineering C, 121, 1–11. https://doi.org/10.1016/j.msec.2020.111849
Salas-Pérez, L. (2018). The effect of citric acid on the phenolic compounds, flavonoids and antioxidant capacity of wheat sprouts. Revista de la Facultad de Ciencias Agrarias UNCuyo, 50(2), 119–127. https://www.researchgate.net/publication/328968766
Schiffman, J. D., & Schauer, C. L. (2007). One-step electrospinning of cross-linked chitosan fibers. Biomacromolecules, 8(9), 2665–2667. https://doi.org/10.1021/bm7006983
Shi, J., & Yang, E. (2015). Green electrospinning and crosslinking of polyvinyl alcohol/ citric acid. Journal of Nano Research, 32, 32–42. https://doi.org/10.4028/www.scientific.net/JNanoR.32.32
Siimon, K., Reemann, P., Põder, A., Pook, M., Kangur, T., Kingo, K., et al. (2014). Effect of glucose content on thermally cross-linked fibrous gelatin scaffolds for tissue engineering. Materials Science and Engineering C, 42, 538–545. https://doi.org/10.1016/j.msec.2014.05.075
Tavassoli-Kafrani, E., Goli, S. A. H., & Fathi, M. (2018). Encapsulation of orange essential oil using cross-linked electrospun gelatin nanofibers. Food and Bioprocess Technology, 11(2), 427–434. https://doi.org/10.1007/s11947-017-2026-9
Uranga, J., Leceta, I., Etxabide, A., Guerrero, P., & De La Caba, K. (2016). Cross-linking of fish gelatins to develop sustainable films with enhanced properties. European Polymer Journal, 78, 82–90. https://doi.org/10.1016/j.eurpolymj.2016.03.017
Uranga, J., Nguyen, B. T., Si, T. T., Guerrero, P., & De la Caba, K. (2020). The effect of cross-linking with citric acid on the properties of agar/fish gelatin films. Polymers, 12(2), 1–12. https://doi.org/10.3390/polym12020291
Vilches, J. L., De Souza Filho, M. D. S. M., De Freitas Rosa, M., Sanches, A. O., & Malmonge, J. A. (2019). Fabrication of fish gelatin microfibrous mats by solution blow spinning. Materials Research, 22, 1–5. https://doi.org/10.1590/1980-5373-MR-2019-0158
Wang, A., Leible, M., Lin, J., Weiss, J., & Zhong, Q. (2020). Caffeic acid phenethyl ester loaded in skim milk microcapsules: Physicochemical properties and enhanced in vitro bioaccessibility and bioactivity against colon cancer cells. Journal of Agricultural and Food Chemistry, 68(50), 14978–14987. https://doi.org/10.1021/acs.jafc.0c05143
Wang, Y., Chen, S., Yao, Y., Wu, N., Xu, M., Yin, Z., Zhao, Y., & Tu, Y. (2023). Effects of citric acid crosslinking on the structure and properties of ovotransferrin and chitosan composite films. International Journal of Biological Macromolecules, 229, 268–281. https://doi.org/10.1016/j.ijbiomac.2022.12.187
Wu, H., Lei, Y., Lu, J., Zhu, R., **ao, D., Jiao, C., **a, R., Zhang, Z., Shen, G., Liu, Y., Li, S., & Li, M. (2019). Effect of citric acid induced crosslinking on the structure and properties of potato starch/chitosan composite films. Food Hydrocolloids, 97, 1–10. https://doi.org/10.1016/j.foodhyd.2019.105208
Xu, J., Li, X., Xu, Y., Wang, A., Xu, Z., Wu, X., Li, D., Mu, C., & Ge, L. (2021). Dihydromyricetin-loaded pickering emulsions stabilized by dialdehyde cellulose nanocrystals for preparation of antioxidant gelatin–based edible films. Food and Bioprocess Technology, 14(9), 1648–1661. https://doi.org/10.1007/s11947-021-02664-5
Yadav, S., Mehrotra, G. K., Bhartiya, P., Singh, A., & Dutta, P. K. (2020). Preparation, physicochemical and biological evaluation of quercetin based chitosan-gelatin film for food packaging. Carbohydrate Polymers, 227, 1–9. https://doi.org/10.1016/j.carbpol.2019.115348
Yao, Z. C., Chang, M. W., Ahmad, Z., & Li, J. S. (2016). Encapsulation of rose hip seed oil into fibrous zein films for ambient and on demand food preservation via coaxial electrospinning. Journal of Food Engineering, 191, 115–123. https://doi.org/10.1016/j.jfoodeng.2016.07.012
Yaver, E. (2022). Novel crackers incorporated with carob and green lentil flours: Physicochemical, textural, and sensory attributes. Journal of Food Processing and Preservation, 46(10), 1–8. https://doi.org/10.1111/jfpp.16911
Yildiz, E., Emir, A. A., Sumnu, G., & Kahyaoglu, L. N. (2022a). Citric acid cross-linked curcumin/chitosan/chickpea flour film: An active packaging for chicken breast storage. Food Bioscience, 50, 1–9. https://doi.org/10.1016/j.fbio.2022.102121
Yildiz, E., Ilhan, E., Kahyaoglu, L. N., Sumnu, G., & Oztop, M. H. (2022b). The effects of crosslinking agents on faba bean flour–chitosan-curcumin films and their characterization. Legume Science, 4(1), 1–13. https://doi.org/10.1002/leg3.121
Yildiz, E., Sumnu, G., & Kahyaoglu, L. N. (2022c). Assessment of curcumin incorporated chickpea flour/PEO (polyethylene oxide) based electrospun nanofiber as an antioxidant and antimicrobial food package. Food and Bioproducts Processing, 135, 205–216. https://doi.org/10.1016/j.fbp.2022.08.002
Zeren, S., Sahin, S., & Sumnu, G. (2022). Encapsulation of caffeic acid in carob bean flour and whey protein-based nanofibers via electrospinning. Foods, 11(13), 1–18. https://doi.org/10.3390/foods11131860
Zhiming, Z., Boya, C., Zilong, L., Jiawei, W., & Yaoshuai, D. (2020). Spinning solution flow model in the nozzle and experimental study of nanofibers fabrication via high speed centrifugal spinning. Polymer, 205, 1–10. https://doi.org/10.1016/j.polymer.2020.122794
Zhiming, Z., & Jun, S. (2017). Research on the development of the centrifugal spinning. MATEC Web of Conferences, 95, 07003. https://doi.org/10.1051/matecconf/20179507003
Funding
Open access funding provided by the Scientific and Technological Research Council of Türkiye (TÜBİTAK).
Author information
Authors and Affiliations
Contributions
Gunes Su Guler took a part in conceptualization, methodology, investigation, and writing. Gulum Sumnu took a part in conceptualization, supervision, reviewing, and editing. Nalan Yazicioglu took a part in conceptualization, methodology, reviewing, and editing.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Guler, G.S., Sumnu, G. & Yazicioglu, N. Caffeic Acid Encapsulated Centrifugally Spun Antioxidant Fibers: Characterization and Incorporation in Cakes. Food Bioprocess Technol (2024). https://doi.org/10.1007/s11947-024-03455-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11947-024-03455-4