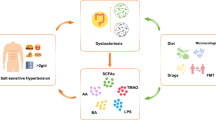
Abstract
Purpose of Review
The role and underlying mechanisms mediated by dietary salt in modulating the gut microbiota and contributing to heart failure (HF) are not clear. This review summarizes the mechanisms of dietary salt and the gut-heart axis in HF.
Recent Findings
The gut microbiota has been implicated in several cardiovascular diseases (CVDs) including HF. Dietary factors including high consumption of salt play a role in influencing the gut microbiota, resulting in dysbiosis. An imbalance of microbial species due to a reduction in microbial diversity with accompanying immune cell activation has been implicated in the pathogenesis of HF via several mechanisms.
Summary
The gut microbiota and gut-associated metabolites contribute to HF by reducing gut microbiota biodiversity and activating several signaling pathways. High dietary salt modulates the gut microbiota composition and exacerbate or induce HF by increasing the expression of the epithelial sodium/hydrogen exchanger isoform 3 in the gut, cardiac expression of beta myosin heavy chain, activation of the myocyte enhancer factor/nuclear factor of activated T cell, and salt-inducible kinase 1. These mechanisms explain the resulting structural and functional derangements in patients with HF.
Graphical Abstract





Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Drazner MH, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. J Am Coll Cardiol. 2013;62:e147–239.
Roger VL, Weston SA, Redfield MM, Hellermann-Homan JP, Killian J, Yawn BP, et al. Trends in heart failure incidence and survival in a community-based population. JAMA. 2004;292:344–50.
Levy D, Kenchaiah S, Larson MG, Benjamin EJ, Kupka MJ, Ho KKL, et al. Long-term trends in the incidence of and survival with heart failure. N Engl J Med. 2002;347:1397–402.
Patel Y, Joseph J. Sodium intake and heart failure. Int J Mol Sci. 2020;21:9474.
•• Naqvi S, Asar TO, Kumar V, Al-Abbasi FA, Alhayyani S, Kamal MA, et al. A cross-talk between gut microbiome, salt and hypertension. Biomed Pharmacother. 2021;134:111156. This paper highlights the role of gut-associated metabolites in modulating blood pressure. They also give insights on the existence of a triangular bridge connecting the gap between dietary salt, hypertension and gut microbiome.
Tang WHW, Li DY, Hazen SL. Dietary metabolism, the gut microbiome, and heart failure. Nat Rev Cardiol. 2019;16:137–54.
Qin J, Li R, Raes J, Arumugam M, Burgdorf KS, Manichanh C, et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature. 2010;464:59–65.
Takagi T, Naito Y, Kashiwagi S, Uchiyama K, Mizushima K, Kamada K, et al. Changes in the Gut Microbiota are Associated with Hypertension, Hyperlipidemia, and Type 2 Diabetes Mellitus in Japanese Subjects. Nutrients. 2020;12:2996.
• Shi G, Lin Y, Wu Y, Zhou J, Cao L, Chen J, et al. Bacteroides fragilis supplementation deteriorated metabolic dysfunction, inflammation, and aorta atherosclerosis by inducing gut microbiota dysbiosis in animal model. Nutrients. 2022;14:2199. Experimental study using an animal model that demonstrates that gut microbiota dysbiosis increases Inflammation and aorta atherosclerosis.
Khan I, Bai Y, Zha L, Ullah N, Ullah H, Shah SRH, et al. Mechanism of the gut microbiota colonization resistance and enteric pathogen infection. Front Cell Infect Microbiol. [Internet]. 2021 [cited 2023 Mar 1];11. Available from: https://www.frontiersin.org/articles/10.3389/fcimb.2021.716299.
Sandek A, Bauditz J, Swidsinski A, Buhner S, Weber-Eibel J, von Haehling S, et al. Altered intestinal function in patients with chronic heart failure. J Am Coll Cardiol. 2007;50:1561–9.
Maynard N, Bihari D, Beale R, Smithies M, Baldock G, Mason R, et al. Assessment of splanchnic oxygenation by gastric tonometry in patients with acute circulatory failure. JAMA. 1993;270:1203–10.
Frangogiannis NG. The inflammatory response in myocardial injury, repair, and remodelling. Nat Rev Cardiol. 2014;11:255–65.
Al-Sadi R, Ye D, Boivin M, Guo S, Hashimi M, Ereifej L, et al. Interleukin-6 modulation of intestinal epithelial tight junction permeability is mediated by JNK pathway activation of claudin-2 gene. PLoS ONE. 2014;9:e85345.
Al-Sadi R, Guo S, Ye D, Ma TY. TNF-α modulation of intestinal epithelial tight junction barrier is regulated by ERK1/2 activation of Elk-1. Am J Pathol. 2013;183:1871–84.
Ma TY, Boivin MA, Ye D, Pedram A, Said HM. Mechanism of TNF-α modulation of Caco-2 intestinal epithelial tight junction barrier: role of myosin light-chain kinase protein expression. Am J Physiol Gastrointest Liver Physiol. 2005;288:G422–30.
Deswal A, Petersen NJ, Feldman AM, Young JB, White BG, Mann DL. Cytokines and cytokine receptors in advanced heart failure. Circulation. 2001;103:2055–9.
Ferrari R, Bachetti T, Confortini R, Opasich C, Febo O, Corti A, et al. Tumor necrosis factor soluble receptors in patients with various degrees of congestive heart failure. Circulation. 1995;92:1479–86.
Ma TY, Tran D, Hoa N, Nguyen D, Merryfield M, Tarnawski A. Mechanism of extracellular calcium regulation of intestinal epithelial tight junction permeability: role of cytoskeletal involvement. Microsc Res Tech. 2000;51:156–68.
Pasini E, Aquilani R, Testa C, Baiardi P, Angioletti S, Boschi F, et al. Pathogenic gut flora in patients with chronic heart failure. JACC Heart Fail. 2016;4:220–7.
Kummen M, Mayerhofer CCK, Vestad B, Broch K, Awoyemi A, Storm-Larsen C, et al. Gut microbiota signature in heart failure defined from profiling of 2 independent cohorts. J Am Coll Cardiol. 2018;71:1184–6.
Luedde M, Winkler T, Heinsen F-A, Rühlemann MC, Spehlmann ME, Bajrovic A, et al. Heart failure is associated with depletion of core intestinal microbiota. ESC Heart Failure. 2017;4:282–90.
Madan S, Mehra MR. Gut dysbiosis and heart failure: navigating the universe within. Eur J Heart Fail. 2020;22:629–37.
Wang Z, Klipfell E, Bennett BJ, Koeth R, Levison BS, DuGar B, et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature. 2011;472:57–63.
Koeth RA, Wang Z, Levison BS, Buffa JA, Org E, Sheehy BT, et al. Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat Med. 2013;19:576–85.
Li Z, Wu Z, Yan J, Liu H, Liu Q, Deng Y, et al. Gut microbe-derived metabolite trimethylamine N-oxide induces cardiac hypertrophy and fibrosis. Lab Investig. 2019;99:346–57.
Makrecka-Kuka M, Volska K, Antone U, Vilskersts R, Grinberga S, Bandere D, et al. Trimethylamine N-oxide impairs pyruvate and fatty acid oxidation in cardiac mitochondria. Toxicol Lett. 2017;267:32–8.
Sun X, Jiao X, Ma Y, Liu Y, Zhang L, He Y, et al. Trimethylamine N-oxide induces inflammation and endothelial dysfunction in human umbilical vein endothelial cells via activating ROS-TXNIP-NLRP3 inflammasome. Biochem Biophys Res Commun. 2016;481:63–70.
Organ CL, Otsuka H, Bhushan S, Wang Z, Bradley J, Trivedi R, et al. Choline diet and its gut microbe–derived metabolite, trimethylamine N-oxide, exacerbate pressure overload–induced heart failure. Circ Heart Fail. 2016;9:e002314.
Blevins HM, Xu Y, Biby S, Zhang S. The NLRP3 inflammasome pathway: a review of mechanisms and inhibitors for the treatment of inflammatory diseases. Front Aging Neurosci. 2022;14:879021.
Chen M, Zhu X, Ran L, Lang H, Yi L, Mi M. Trimethylamine-N-oxide induces vascular inflammation by activating the NLRP3 inflammasome through the SIRT3-SOD2-mtROS signaling pathway. J Am Heart Assoc. 2017;6:e006347.
Seldin MM, Meng Y, Qi H, Zhu W, Wang Z, Hazen SL, et al. Trimethylamine N-oxide promotes vascular inflammation through signaling of mitogen-activated protein kinase and nuclear factor-κB. J Am Heart Assoc. 2016;5:e002767.
Tang WHW, Wang Z, Fan Y, Levison B, Hazen JE, Donahue LM, et al. Prognostic value of elevated levels of intestinal microbe-generated metabolite trimethylamine-N-oxide in patients with heart failure. J Am Coll Cardiol. 2014;64:1908–14.
Silva YP, Bernardi A, Frozza RL. The role of short-chain fatty acids from gut microbiota in gut-brain communication. Front Endocrinol [Internet]. 2020 [cited 2023 Mar 1];11. Available from: https://www.frontiersin.org/articles/10.3389/fendo.2020.00025.
Usami M, Kishimoto K, Ohata A, Miyoshi M, Aoyama M, Fueda Y, et al. Butyrate and trichostatin A attenuate nuclear factor κB activation and tumor necrosis factor α secretion and increase prostaglandin E2 secretion in human peripheral blood mononuclear cells. Nutr Res. 2008;28:321–8.
Huang J, Wang L, Dahiya S, Beier UH, Han R, Samanta A, et al. Histone/protein deacetylase 11 targeting promotes Foxp3+ Treg function. Sci Rep. 2017;7:8626.
Brown AJ, Goldsworthy SM, Barnes AA, Eilert MM, Tcheang L, Daniels D, et al. The orphan G protein-coupled receptors GPR41 and GPR43 are activated by propionate and other short chain carboxylic acids *. J Biol Chem. 2003;278:11312–9.
Koh A, Vadder FD, Kovatcheva-Datchary P, Bäckhed F. From dietary fiber to host physiology: short-chain fatty acids as key bacterial metabolites. Cell. 2016;165:1332–45.
Marques FZ, Mackay CR, Kaye DM. Beyond gut feelings: how the gut microbiota regulates blood pressure. Nat Rev Cardiol. 2018;15:20–32.
Kelly CJ, Zheng L, Campbell EL, Saeedi B, Scholz CC, Bayless AJ, et al. Crosstalk between microbiota-derived short-chain fatty acids and intestinal epithelial HIF augments tissue barrier function. Cell Host Microbe. 2015;17:662–71.
Palm CL, Nijholt KT, Bakker BM, Westenbrink BD. Short-chain fatty acids in the metabolism of heart failure – rethinking the fat stigma. Front Cardiovasc Med [Internet]. 2022 [cited 2023 Mar 1];9. Available from: https://www.frontiersin.org/articles/10.3389/fcvm.2022.915102.
Pakhomov N, Baugh JA. The role of diet-derived short-chain fatty acids in regulating cardiac pressure overload. Am J Physiol Heart Circ Physiol. 2021;320:H475–86.
Winston JA, Theriot CM. Diversification of host bile acids by members of the gut microbiota. Gut Microbes. 2020;11:158–71.
Begley M, Gahan CGM, Hill C. The interaction between bacteria and bile. FEMS Microbiol Rev. 2005;29:625–51.
Kliewer SA, Mangelsdorf DJ. Bile acids as hormones: the FXR-FGF15/19 pathway. Dig Dis. 2015;33:327–31.
Inagaki T, Moschetta A, Lee Y-K, Peng L, Zhao G, Downes M, et al. Regulation of antibacterial defense in the small intestine by the nuclear bile acid receptor. Proc Natl Acad Sci. 2006;103:3920–5.
Lee FY, Lee H, Hubbert ML, Edwards PA, Zhang Y. FXR, a multipurpose nuclear receptor. Trends Biochem Sci. 2006;31:572–80.
Vasavan T, Ferraro E, Ibrahim E, Dixon P, Gorelik J, Williamson C. Heart and bile acids – Clinical consequences of altered bile acid metabolism. Biochim Biophys Acta (BBA) - Mol Basis Dis. 2018;1864:1345–55.
Bishop-Bailey D, Walsh DT, Warner TD. Expression and activation of the farnesoid X receptor in the vasculature. Proc Natl Acad Sci. 2004;101:3668–73.
Gao Y, Zhao Y, Yuan A, Xu L, Huang X, Su Y, et al. Effects of farnesoid-X-receptor SUMOylation mutation on myocardial ischemia/reperfusion injury in mice. Exp Cell Res. 2018;371:301–10.
**a Y, Zhang F, Zhao S, Li Y, Chen X, Gao E, et al. Adiponectin determines farnesoid X receptor agonism-mediated cardioprotection against post-infarction remodelling and dysfunction. Cardiovasc Res. 2018;114:1335–49.
Mayerhofer CCK, Ueland T, Broch K, Vincent RP, Cross GF, Dahl CP, et al. Increased secondary/primary bile acid ratio in chronic heart failure. J Card Fail. 2017;23:666–71.
Mozaffarian D, Fahimi S, Singh GM, Micha R, Khatibzadeh S, Engell RE, et al. Global sodium consumption and death from cardiovascular causes. N Engl J Med. 2014;371:624–34.
Wang C, Huang Z, Yu K, Ding R, Ye K, Dai C, et al. High-salt diet has a certain impact on protein digestion and gut microbiota: a sequencing and proteome combined study. Front Microbiol [Internet]. 2017 [cited 2023 Feb 28];8. Available from: https://www.frontiersin.org/articles/10.3389/fmicb.2017.01838.
Miranda PM, De Palma G, Serkis V, Lu J, Louis-Auguste MP, McCarville JL, et al. High salt diet exacerbates colitis in mice by decreasing Lactobacillus levels and butyrate production. Microbiome. 2018;6:57.
Bäckhed F, Ding H, Wang T, Hooper LV, Koh GY, Nagy A, et al. The gut microbiota as an environmental factor that regulates fat storage. Proc Natl Acad Sci. 2004;101:15718–23.
Jose PA, Raj D. Gut microbiota in hypertension. Curr Opin Nephrol Hypertens. 2015;24:403.
Grillo A, Salvi L, Coruzzi P, Salvi P, Parati G. Sodium intake and hypertension. Nutrients. 2019;11:1970.
Shimosawa T. Salt, the renin–angiotensin–aldosterone system and resistant hypertension. Hypertens Res. 2013;36:657–60.
•• Yan X, ** J, Su X, Yin X, Gao J, Wang X, et al. Intestinal flora modulates blood pressure by regulating the synthesis of intestinal-derived corticosterone in high salt-induced hypertension. Circ Res. 2020;126:839–53. This experimental animal study demonstrates a novel mechanism different from inflammation/immunity by which intestinal flora in high salt diet regulated BP.
• Ferguson JF, Aden LA, Barbaro NR, Beusecum JPV, **ao L, Simmons AJ, et al. High dietary salt–induced DC activation underlies microbial dysbiosis-associated hypertension. JCI Insight [Internet]. American Society for Clinical Investigation; 2019 [cited 2023 Mar 15];4. Available from: https://insight.jci.org/articles/view/126241. This study uses both human and murine models to demonstrate immune mechanisms underlying dysbiosis-associated hypertension induced by high salt diets.
Wilck N, Matus MG, Kearney SM, Olesen SW, Forslund K, Bartolomaeus H, et al. Salt-responsive gut commensal modulates TH17 axis and disease. Nature. 2017;551:585–9.
Singh V, Raheja G, Borthakur A, Kumar A, Gill RK, Alakkam A, et al. Lactobacillus acidophilus upregulates intestinal NHE3 expression and function. Am J Physiol Gastrointest Liver Physiol. 2012;303:G1393–401.
Engevik MA, Engevik KA, Yacyshyn MB, Wang J, Hassett DJ, Darien B, et al. Human Clostridium difficile infection: inhibition of NHE3 and microbiota profile. Am J Physiol Gastrointest Liver Physiol. 2015;308:G497–509.
Larmonier CB, Laubitz D, Hill FM, Shehab KW, Lipinski L, Midura-Kiela MT, et al. Reduced colonic microbial diversity is associated with colitis in NHE3-deficient mice. Am J Physiol Gastrointest Liver Physiol. 2013;305:G667–77.
Hecht G, Hodges K, Gill RK, Kear F, Tyagi S, Malakooti J, et al. Differential regulation of Na+/H+ exchange isoform activities by enteropathogenic E. coli in human intestinal epithelial cells. Am J Physiol Gastrointest Liver Physiol. 2004;287:G370–8.
Frame AA, Puleo F, Kim K, Walsh KR, Faudoa E, Hoover RS, et al. Sympathetic regulation of NCC in norepinephrine-evoked salt-sensitive hypertension in Sprague-Dawley rats. Am J Physiol Ren Physiol. 2019;317:F1623–36.
• DeLalio LJ, Hahn S, Katayama PL, Wenner MM, Farquhar WB, Straub AC, et al. Excessive dietary salt promotes aortic stiffness in murine renovascular hypertension. Am J Physiol Heart Circ Physiol. 2020;318:H1346–55. Animal study demonstrating how high salt promotes vascular stiffness in hypertension.
Masenga SK, Pilic L, Malumani M, Hamooya BM. Erythrocyte sodium buffering capacity status correlates with self-reported salt intake in a population from Livingstone, Zambia. PLoS ONE. 2022;17:e0264650.
Kirabo A. A new paradigm of sodium regulation in inflammation and hypertension. Am J Physiol Regul Integr Comp Physiol. 2017;313:R706–10.
Kirabo A, Fontana V, de Faria APC, Loperena R, Galindo CL, Wu J, et al. DC isoketal-modified proteins activate T cells and promote hypertension. J Clin Invest. 2014;124:4642–56.
Barbaro NR, Van Beusecum J, **ao L, do Carmo L, Pitzer A, Loperena R, et al. Sodium activates human monocytes via the NADPH oxidase and isolevuglandin formation. Cardiovasc Res [Internet]. 2020 [cited 2020 Sep 22]; Available from: https://academic.oup.com/cardiovascres/advance-article/doi/10.1093/cvr/cvaa207/5872525.
Kee HJ, Ryu Y, Seok YM, Choi SY, Sun S, Kim GR, et al. Selective inhibition of histone deacetylase 8 improves vascular hypertrophy, relaxation, and inflammation in angiotensin II hypertensive mice. Clin Hypertens. 2019;25:13.
Hu Y, **a W, Li Y, Wang Q, Lin S, Wang B, et al. High-salt intake increases TRPC3 expression and enhances TRPC3-mediated calcium influx and systolic blood pressure in hypertensive patients. Hypertens Res. 2020;43:679–87.
Berger RCM, Benetti A, Girardi ACC, Forechi L, de Oliveira RM, Vassallo PF, et al. Influence of long-term salt diets on cardiac Ca2+ handling and contractility proteins in hypertensive rats. Am J Hypertens. 2018;31:726–34.
Popov S, Venetsanou K, Chedrese PJ, Pinto V, Takemori H, Franco-Cereceda A, et al. Increases in intracellular sodium activate transcription and gene expression via the salt-inducible kinase 1 network in an atrial myocyte cell line. Am J Physiol Heart Circ Physiol. 2012;303:H57-65.
van Oort RJ, van Rooij E, Bourajjaj M, Schimmel J, Jansen MA, van der Nagel R, et al. MEF2 activates a genetic program promoting chamber dilation and contractile dysfunction in calcineurin-induced heart failure. Circulation. 2006;114:298–308.
•• Hsu A, Duan Q, McMahon S, Huang Y, Wood SAB, Gray NS, et al. Salt-inducible kinase 1 maintains HDAC7 stability to promote pathologic cardiac remodeling. J Clin Invest. 130:2966–77. This mechanistic study highlights the role of salt-inducible kinases in pathologic cardiomyocyte remodeling.
Kleinewietfeld M, Manzel A, Titze J, Kvakan H, Yosef N, Linker RA, et al. Sodium chloride drives autoimmune disease by the induction of pathogenic TH17 cells. Nature. 2013;496:518–22.
Rucker AJ, Rudemiller NP, Crowley SD. Salt, hypertension, and immunity. Annu Rev Physiol. 2018;80:283–307.
Wu C, Yosef N, Thalhamer T, Zhu C, **ao S, Kishi Y, et al. Induction of pathogenic TH17 cells by inducible salt-sensing kinase SGK1. Nature. 2013;496:513–7.
Baban B, Liu JY, Mozaffari MS. SGK-1 regulates inflammation and cell death in the ischemic-reperfused heart: pressure-related effects. Am J Hypertens. 2014;27:846–56.
Bozkurt B. Activation of cytokines as a mechanism of disease progression in heart failure. Ann Rheum Dis. 2000;59:i90–3.
•• Romano KA, Nemet I, Prasad Saha P, Haghikia A, Li XS, Mohan ML, et al. Gut microbiota-generated phenylacetylglutamine and heart failure. Circ Heart Fail. 2023;16:e009972. This study demonstrates that the gut microbial metabolite Phenylacetylglutamine is clinically and mechanistically linked to HF presence and severity.
• Awoyemi A, Mayerhofer C, Felix AS, Hov JR, Moscavitch SD, Lappegård KT, et al. Rifaximin or Saccharomyces boulardii in heart failure with reduced ejection fraction: Results from the randomized GutHeart trial. EBioMedicine. 2021;70:103511. Clinical trial on effects of modulating the gut microbiota composition in heart failure.
• Moludi J, Saiedi S, Ebrahimi B, Alizadeh M, Khajebishak Y, Ghadimi SS. Probiotics supplementation on cardiac remodeling following myocardial infarction: a single-center double-blind clinical study. J Cardiovasc Transl Res. 2021;14:299–307. Clinical trial on using probiotics to ameliorate cardiac damage after a heart attack.
Dethlefsen L, Huse S, Sogin ML, Relman DA. The pervasive effects of an antibiotic on the human gut microbiota, as revealed by deep 16S rRNA sequencing. PLoS Biol. 2008;6:e280.
Ramirez J, Guarner F, Bustos Fernandez L, Maruy A, Sdepanian VL, Cohen H. Antibiotics as major disruptors of gut microbiota. Front Cell Infect Microbiol. [Internet]. 2020 [cited 2023 Feb 28];10. Available from: https://www.frontiersin.org/articles/10.3389/fcimb.2020.572912.
Han Z-L, Chen M, Fu X-D, Yang M, Hrmova M, Zhao Y-H, et al. Potassium alginate oligosaccharides alter gut microbiota, and have potential to prevent the development of hypertension and heart failure in spontaneously hypertensive rats. Int J Mol Sci. 2021;22:9823.
Gutiérrez-Calabrés E, Ortega-Hernández A, Modrego J, Gómez-Gordo R, Caro-Vadillo A, Rodríguez-Bobada C, et al. Gut microbiota profile identifies transition from compensated cardiac hypertrophy to heart failure in hypertensive rats. Hypertension. 2020;76:1545–54.
Riba A, Deres L, Eros K, Szabo A, Magyar K, Sumegi B, et al. Doxycycline protects against ROS-induced mitochondrial fragmentation and ISO-induced heart failure. PLoS ONE. 2017;12:e0175195.
Palleja A, Mikkelsen KH, Forslund SK, Kashani A, Allin KH, Nielsen T, et al. Recovery of gut microbiota of healthy adults following antibiotic exposure. Nat Microbiol. 2018;3:1255–65.
de Gunzburg J, Ghozlane A, Ducher A, Le Chatelier E, Duval X, Ruppé E, et al. Protection of the human gut microbiome from antibiotics. J Infect Dis. 2018;217:628–36.
Robinson-Cohen C, Newitt R, Shen DD, Rettie AE, Kestenbaum BR, Himmelfarb J, et al. Association of FMO3 variants and trimethylamine N-oxide concentration, disease progression, and mortality in CKD patients. PLoS ONE. 2016;11:e0161074.
Collins MD, Gibson GR. Probiotics, prebiotics, and synbiotics: approaches for modulating the microbial ecology of the gut. Am J Clin Nutr. 1999;69:1052s–7s.
Pais P, Almeida V, Yılmaz M, Teixeira MC. Saccharomyces boulardii: what makes it tick as successful probiotic? J Fungi (Basel). 2020;6:78.
Costanza AC, Moscavitch SD, Neto HCCF, Mesquita ET. Probiotic therapy with Saccharomyces boulardii for heart failure patients: a randomized, double-blind, placebo-controlled pilot trial. Int J Cardiol. 2015;179:348–50.
Bajaj BK, Claes IJJ, Lebeer S. Functional mechanisms of probiotics. J Microbiol Biotechnol Food Sci. 2015;4:321–7.
Wu X, Vallance BA, Boyer L, Bergstrom KSB, Walker J, Madsen K, et al. Saccharomyces boulardii ameliorates Citrobacter rodentium-induced colitis through actions on bacterial virulence factors. Am J Physiol-Gastrointest Liver Physiol. 2008;294:G295–306.
Castagliuolo I, Riegler MF, Valenick L, LaMont JT, Pothoulakis C. Saccharomyces boulardii protease inhibits the effects of Clostridium difficile toxins A and B in human colonic mucosa. Infect Immun. 1999;67:302–7.
Castagliuolo I, LaMont JT, Nikulasson ST, Pothoulakis C. Saccharomyces boulardii protease inhibits Clostridium difficile toxin A effects in the rat ileum. Infect Immun. 1996;64:5225–32.
Buts J-P, Bernasconi P, Van Craynest M-P, Maldague P, De Meyer R. Response of human and rat small intestinal mucosa to oral administration of Saccharomyces boulardii. Pediatr Res. 1986;20:192–6.
Buts J-P, Bernasconi P, Vaerman J-P, Dive C. Stimulation of secretory IgA and secretory component of immunoglobulins in small intestine of rats treated withSaccharomyces boulardii. Digest Dis Sci. 1990;35:251–6.
Buts J-P, Dekeyser N, Stilmant C, Delem E, Smets F, Sokal E. Saccharomyces boulardii produces in rat small intestine a novel protein phosphatase that inhibits Escherichia coli endotoxin by dephosphorylation. Pediatr Res. 2006;60:24–9.
Brandão RL, Castro IM, Bambirra EA, Amaral SC, Fietto LG, Tropia MJM, et al. Intracellular signal triggered by cholera toxin in Saccharomyces boulardii and Saccharomyces cerevisiae. Appl Environ Microbiol. 1998;64:564–8.
Offei B, Vandecruys P, Graeve SD, Foulquié-Moreno MR, Thevelein JM. Unique genetic basis of the distinct antibiotic potency of high acetic acid production in the probiotic yeast Saccharomyces cerevisiae var. boulardii. Genome Res. 2019;29:1478–94.
Salehi-Abargouei A, Maghsoudi Z, Shirani F, Azadbakht L. Effects of dietary approaches to stop hypertension (DASH)-style diet on fatal or nonfatal cardiovascular diseases—incidence: a systematic review and meta-analysis on observational prospective studies. Nutrition. 2013;29:611–8.
Nguyen HT, Bertoni AG, Nettleton JA, Bluemke DA, Levitan EB, Burke GL. DASH eating pattern is associated with favorable left ventricular function in the multi-ethnic study of atherosclerosis. J Am Coll Nutr. 2012;31:401–7.
Rifai L, Pisano C, Hayden J, Sulo S, Silver MA. Impact of the Dash diet on endothelial function, exercise capacity, and quality of life in patients with heart failure. Bayl Univ Med Cent Proc. 2015;28:151–6.
Khan MS, Khan F, Fonarow GC, Sreenivasan J, Greene SJ, Khan SU, et al. Dietary interventions and nutritional supplements for heart failure: a systematic appraisal and evidence map. Eur J Heart Fail. 2021;23:1468–76.
Barrea L, Muscogiuri G, Frias-Toral E, Laudisio D, Pugliese G, Castellucci B, et al. Nutrition and immune system: from the Mediterranean diet to dietary supplementary through the microbiota. Crit Rev Food Sci Nutr. 2021;61:3066–90.
Fu J, Liu Y, Zhang L, Zhou L, Li D, Quan H, et al. Nonpharmacologic interventions for reducing blood pressure in adults with prehypertension to established hypertension. J Am Heart Assoc. 2020;9:e016804.
•• Juraschek SP, Kovell LC, Appel LJ, Miller ER, Sacks FM, Chang AR, et al. Effects of diet and sodium reduction on cardiac injury, strain, and inflammation: The DASH-Sodium Trial. J Am Coll Cardiol. 2021;77:2625–34. Clinical trial highlighting effects of reducing salt on cardiac function.
Hummel SL, Seymour EM, Brook RD, Kolias TJ, Sheth SS, Rosenblum HR, et al. Low-sodium dietary approaches to stop hypertension diet reduces blood pressure, arterial stiffness, and oxidative stress in hypertensive heart failure with preserved ejection fraction. Hypertension. 2012;60:1200–6.
Estruch R, Ros E, Salas-Salvadó J, Covas M-I, Corella D, Arós F, et al. Primary prevention of cardiovascular disease with a Mediterranean diet supplemented with extra-virgin olive oil or nuts. N Engl J Med. 2018;378:e34.
Holscher HD. Diet affects the gastrointestinal microbiota and health. J Acad Nutr Diet. 2020;120:495–9.
Trefflich I, Jabakhanji A, Menzel J, Blaut M, Michalsen A, Lampen A, et al. Is a vegan or a vegetarian diet associated with the microbiota composition in the gut? Results of a new cross-sectional study and systematic review. Crit Rev Food Sci Nutr. 2020;60:2990–3004.
Rautmann AW, de La Serre CB. Microbiota’s role in diet-driven alterations in food intake: satiety, energy balance, and reward. Nutrients. 2021;13:3067.
Taladrid D, de Celis M, Belda I, Bartolomé B, Moreno-Arribas MV. Hypertension- and glycaemia-lowering effects of a grape-pomace-derived seasoning in high-cardiovascular risk and healthy subjects. Interplay with the gut microbiome. Food Funct. 2022;13:2068–82.
He J, Ogden LG, Bazzano LA, Vupputuri S, Loria C, Whelton PK. Dietary sodium intake and incidence of congestive heart failure in overweight US men and women: first National Health and Nutrition Examination Survey epidemiologic follow-up study. Arch Intern Med. 2002;162:1619–24.
Strazzullo P, D’Elia L, Kandala N-B, Cappuccio FP. Salt intake, stroke, and cardiovascular disease: meta-analysis of prospective studies. BMJ. 2009;339:b4567.
Cook NR, Cutler JA, Obarzanek E, Buring JE, Rexrode KM, Kumanyika SK, et al. Long term effects of dietary sodium reduction on cardiovascular disease outcomes: observational follow-up of the trials of hypertension prevention (TOHP). BMJ. 2007;334:885.
He FJ, Li J, MacGregor GA. Effect of longer‐term modest salt reduction on blood pressure. Cochrane Database Syst Rev [Internet]. John Wiley & Sons, Ltd; 2013 [cited 2023 Feb 28]; Available from: https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD004937.pub2/full.
Jula AM, Karanko HM. Effects on left ventricular hypertrophy of long-term nonpharmacological treatment with sodium restriction in mild-to-moderate essential hypertension. Circulation. 1994;89:1023–31.
Levitan EB, Wolk A, Mittleman MA. Consistency with the DASH diet and incidence of heart failure. Arch Intern Med. 2009;169:851–7.
Parrinello G, Pasquale PD, Licata G, Torres D, Giammanco M, Fasullo S, et al. Long-term effects of dietary sodium intake on cytokines and neurohormonal activation in patients with recently compensated congestive heart failure. J Card Fail. 2009;15:864–73.
Gupta D, Georgiopoulou VV, Kalogeropoulos AP, Dunbar SB, Reilly CM, Sands JM, et al. Dietary sodium intake in heart failure. Circulation. 2012;126:479–85.
Masaki H, Sako H, Kadambi VJ, Sato Y, Kranias EG, Yatani A. Overexpression of phospholamban alters inactivation kinetics of L-type Ca2+channel currents in mouse atrial myocytes. J Mol Cell Cardiol. 1998;30:317–25.
Kadambi VJ, Ponniah S, Harrer JM, Hoit BD, Dorn GW, Walsh RA, et al. Cardiac-specific overexpression of phospholamban alters calcium kinetics and resultant cardiomyocyte mechanics in transgenic mice. J Clin Invest. 1996;97:533–9.
Funding
This work was supported by the Fogarty International Center of the National Institutes of Health grants R03HL155041, R01HL147818 and R01HL144941 (AK) and 2D43TW009744, D43 TW009744 and D43 TW009337 (SKM). The content is solely the responsibility of the authors and does not represent the official views of the National Institutes of Health.
Author information
Authors and Affiliations
Contributions
SKM conceptualized the study and wrote the draft manuscript. SKM and AK wrote and edited different sections of the manuscript. SKM created all the figures. AK conceptualized the framework and finalized the manuscript and obtained funding for the manuscript. All authors contributed to article reviews, edited, and approved the final version of this manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no conflicts of interest relevant to this manuscript.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Masenga, S.K., Kirabo, A. Salt and Gut Microbiota in Heart Failure. Curr Hypertens Rep 25, 173–184 (2023). https://doi.org/10.1007/s11906-023-01245-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11906-023-01245-5