Abstract
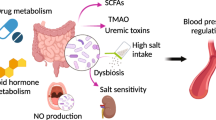
Salt-sensitivity hypertension (SSH) is an independent predictor of cardiovascular event-related death. Despite the extensiveness of research on hypertension, which covers areas such as the sympathetic nervous system, the renin-angiotensin system, the vascular system, and the immune system, its pathogenesis remains elusive, with sub-optimal blood pressure control in patients. The gut microbiota is an important component of nutritional support and constitutes a barrier in the host. Long-term high salt intake can lead to gut microbiota dysbiosis and cause significant changes in the expression of gut microbiota-related metabolites. Of these metabolites, short chain fatty acids (SCFAs), trimethylamine oxide, amino acids, bile acids, and lipopolysaccharide are essential mediators of microbe-host crosstalk. These metabolites may contribute to the incidence and development of SSH via inflammatory, immune, vascular, and nervous pathways, among others. In addition, recent studies, including those on the histone deacetylase inhibitory mechanism of SCFAs and the blood pressure-decreasing effects of H2S via vascular activation, suggest that several proteins and factors in the classical pathway elicit their effects through multiple non-classical pathways. This review summarizes changes in the gut microbiota and its related metabolites in high-salt environments, as well as corresponding treatment methods for SSH, such as diet management, probiotic and prebiotic use, antibiotic use, and fecal transplantation, to provide new insights and perspectives for understanding SSH pathogenesis and the development of strategies for its treatment.




Similar content being viewed by others
References
Carey RM, Moran AE, Whelton PK. Treatment of hypertension: a review. JAMA. 2022;328:1849.
**e Y, Qi H, Peng W, Li B, Wen F, Zhang F, et al. SNPs in lncRNA KCNQ1OT1 modulate its expression and confer susceptibility to salt sensitivity of blood pressure in a Chinese Han population. Nutrients. 2022;14:3990.
Mente A, O’Donnell M, Rangarajan S, McQueen M, Dagenais G, Wielgosz A, et al. Urinary sodium excretion, blood pressure, cardiovascular disease, and mortality: a community-level prospective epidemiological cohort study. Lancet. 2018;392:496–506.
Lu X, Crowley SD. Inflammation in salt-sensitive hypertension and renal damage. Curr Hypertens Rep. 2018;20:103.
Wu Q, Yang H, Zheng Q, Chen Q, Li X, Guo J. κ-Opioid receptors improve vascular endothelial dysfunction in salt-sensitive hypertension via PI3K/Akt/eNOS signaling pathway. Oxid Med Cell Longev. 2023;2023:1–13.
DeLalio LJ, Sved AF, Stocker SD. Sympathetic nervous system contributions to hypertension: updates and therapeutic relevance. Can J Cardiol. 2020;36:712–20.
Ayuzawa N, Fujita T. The mineralocorticoid receptor in salt-sensitive hypertension and renal injury. J Am Soc Nephrol. 2021;31:279–89.
Manosroi W, Williams GH. Genetics of human primary hypertension: focus on hormonal mechanisms. Endocr Rev. 2018;40:825–56.
Fujita T. Mechanism of salt-sensitive hypertension: focus on adrenal and sympathetic nervous systems. J Am Soc Nephrol. 2014;25:1148–55.
Yang X-F, Wang H, Huang Y, Huang J-H, Ren H-L, Xu Q, et al. Myeloid angiotensin II type 1 receptor mediates macrophage polarization and promotes vascular injury in DOCA/salt hypertensive mice. Front Pharm. 2022;13:879693.
David LA, Maurice CF, Carmody RN, Gootenberg DB, Button JE, Wolfe BE, et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature. 2014;505:559–63.
Partridge L, Deelen J, Slagboom PE. Facing up to the global challenges of ageing. Nature. 2018;561:45–56.
** M, Qian Z, Yin J, Xu W, Zhou X. The role of intestinal microbiota in cardiovascular disease. J Cell Mol Med. 2019;23:2343–50.
Liu X-Y, Li J, Zhang Y, Fan L, **a Y, Wu Y, et al. Kidney microbiota dysbiosis contributes to the development of hypertension. Gut Microbes. 2022;14:2143220.
Qin J, Li R, Raes J, Arumugam M, Burgdorf KS, Manichanh C, et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature. 2010;464:59–65.
Chakraborty S, Mandal J, Cheng X, Galla S, Hindupur A, Saha P, et al. Diurnal timing dependent alterations in gut microbial composition are synchronously linked to salt-sensitive hypertension and renal damage. Hypertension. 2020;76:59–72.
Bier A, Braun T, Khasbab R, Di Segni A, Grossman E, Haberman Y, et al. A high salt diet modulates the gut microbiota and short chain fatty acids production in a salt-sensitive hypertension rat model. Nutrients. 2018;10:1154.
Abais-Battad JM, Saravia FL, Lund H, Dasinger JH, Fehrenbach DJ, Alsheikh AJ, et al. Dietary influences on the Dahl SS rat gut microbiota and its effects on salt-sensitive hypertension and renal damage. Acta Physiol. 2021;232:e13662.
Palmu J, Lahti L, Niiranen T. Targeting gut microbiota to treat hypertension: a systematic review. Int J Environ Res Public Health. 2021;18:1248.
Wilck N, Matus MG, Kearney SM, Olesen SW, Forslund K, Bartolomaeus H, et al. Salt-responsive gut commensal modulates TH17 axis and disease. Nature. 2017;551:585–9.
Chen Y, Zhu Y, Wu C, Lu A, Deng M, Yu H, et al. Gut dysbiosis contributes to high fructose-induced salt-sensitive hypertension in Sprague-Dawley rats. Nutrition. 2020;75–76:110766.
Waghulde H, Cheng X, Galla S, Mell B, Cai J, Pruett-Miller SM, et al. Attenuation of microbiotal dysbiosis and hypertension in a CRISPR/Cas9 gene ablation rat model of GPER1. Hypertension. 2018;72:1125–32.
Li L, Zhong S-J, Hu S-Y, Cheng B, Qiu H, Hu Z-X. Changes of gut microbiome composition and metabolites associated with hypertensive heart failure rats. BMC Microbiol. 2021;21:141.
Mei X, Mell B, Manandhar I, Aryal S, Tummala R, Kyoung J, et al. Repurposing a drug targeting inflammatory bowel disease for lowering hypertension. JAHA. 2022;11:e027893.
Avery EG, Bartolomaeus H, Maifeld A, Marko L, Wiig H, Wilck N, et al. The gut microbiome in hypertension: recent advances and future perspectives. Circ Res. 2021;128:934–50.
Liu P, Wang Y, Yang G, Zhang Q, Meng L, **n Y, et al. The role of short-chain fatty acids in intestinal barrier function, inflammation, oxidative stress, and colonic carcinogenesis. Pharmacol Res. 2021;165:105420.
Sasaki K, Sasaki D, Hannya A, Tsubota J, Kondo A. In vitro human colonic microbiota utilises D-β-hydroxybutyrate to increase butyrogenesis. Sci Rep. 2020;10:8516.
Alexander C, Swanson KS, Fahey GC, Garleb KA. Perspective: physiologic importance of short-chain fatty acids from nondigestible carbohydrate fermentation. Adv Nutr. 2019;10:576–89.
Chen L, He FJ, Dong Y, Huang Y, Wang C, Harshfield GA, et al. Modest sodium reduction increases circulating short-chain fatty acids in untreated hypertensives: a randomized, double-blind, placebo-controlled trial. Hypertension. 2020;76:73–79.
Muralitharan RR, Jama HA, **e L, Peh A, Snelson M, Marques FZ. Microbial peer pressure: the role of the gut microbiota in hypertension and its complications. Hypertension. 2020;76:1674–87.
Wu W, Sun M, Chen F, Cao AT, Liu H, Zhao Y, et al. Microbiota metabolite short-chain fatty acid acetate promotes intestinal IgA response to microbiota which is mediated by GPR43. Mucosal Immunol. 2017;10:946–56.
Furusawa Y, Obata Y, Fukuda S, Endo TA, Nakato G, Takahashi D, et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature. 2013;504:446–50.
Sun M, Wu W, Chen L, Yang W, Huang X, Ma C, et al. Microbiota-derived short-chain fatty acids promote Th1 cell IL-10 production to maintain intestinal homeostasis. Nat Commun. 2018;9:3555.
Qiu M, Shu H, Li L, Shen Y, Tian Y, Ji Y, et al. Interleukin 10 attenuates angiotensin II-induced aortic remodelling by inhibiting oxidative stress-induced activation of the vascular p38 and NF-κB pathways. Oxid Med Cell Longev. 2022;2022:1–15.
Onyszkiewicz M, Gawrys-Kopczynska M, Konopelski P, Aleksandrowicz M, Sawicka A, Koźniewska E, et al. Butyric acid, a gut bacteria metabolite, lowers arterial blood pressure via colon-vagus nerve signaling and GPR41/43 receptors. Pflug Arch. 2019;471:1441–53.
Singh N, Gurav A, Sivaprakasam S, Brady E, Padia R, Shi H, et al. Activation of Gpr109a, receptor for niacin and the commensal metabolite butyrate, suppresses colonic inflammation and carcinogenesis. Immunity. 2014;40:128–39.
Lednovich K, Priyadarshini M, Kotlo K, Xu K, Priyamvada S, Dudeja P, et al. OR31-3 role of a novel short chain fatty acid receptor OLFR78 in mediating gluco-metabolic hormone secretion. J Endocr Soc. 2019;3:OR31–3.
Poll BG, Cheema MU, Pluznick JL. Gut microbial metabolites and blood pressure regulation: focus on SCFAs and TMAO. Physiology. 2020;35:275–84.
Griesler B, Schuelke C, Uhlig C, Gadasheva Y, Grossmann C. Importance of micromilieu for pathophysiologic mineralocorticoid receptor activity—when the mineralocorticoid receptor resides in the wrong neighborhood. IJMS. 2022;23:12592.
Wang J, Zhu N, Su X, Gao Y, Yang R. Gut-microbiota-derived metabolites maintain gut and systemic immune homeostasis. Cells. 2023;12:793.
Kim S, Goel R, Kumar A, Qi Y, Lobaton G, Hosaka K, et al. Imbalance of gut microbiome and intestinal epithelial barrier dysfunction in patients with high blood pressure. Clin Sci. 2018;132:701–18.
Liu K, Li F, Sun Q, Lin N, Han H, You K, et al. p53 β-hydroxybutyrylation attenuates p53 activity. Cell Death Dis. 2019;10:243.
Han Y, Bedarida T, Ding Y, Somba BK, Lu Q, Wang Q, et al. β-hydroxybutyrate prevents vascular senescence through hnRNP A1-mediated upregulation of oct4. Mol Cell. 2018;71:1064–.e5.
Reigstad CS, Salmonson CE, Iii JFR, Szurszewski JH, Linden DR, Sonnenburg JL, et al. Gut microbes promote colonic serotonin production through an effect of short‐chain fatty acids on enterochromaffin cells. FASEB J. 2015;29:1395–403.
Zubcevic J, Richards EM, Yang T, Kim S, Sumners C, Pepine CJ, et al. Impaired autonomic nervous system-microbiome circuit in hypertension: a premise for hypertension therapy. Circ Res. 2019;125:104–16.
Day-Walsh P, Shehata E, Saha S, Savva GM, Nemeckova B, Speranza J, et al. The use of an in-vitro batch fermentation (human colon) model for investigating mechanisms of TMA production from choline, l-carnitine and related precursors by the human gut microbiota. Eur J Nutr. 2021;60:3987–99.
Kiouptsi K, Ruf W, Reinhardt C. Microbiota-derived trimethylamine: the missing jigsaw piece in thrombosis? Circ Res. 2018;123:1112–4.
Rath S, Rud T, Pieper DH, Vital M. Potential TMA-producing bacteria are ubiquitously found in mammalia. Front Microbiol. 2020;10:2966.
Janeiro M, Ramírez M, Milagro F, Martínez J, Solas M. Implication of trimethylamine N-Oxide (TMAO) in disease: potential biomarker or new therapeutic target. Nutrients. 2018;10:1398.
Ge X, Zheng L, Zhuang R, Yu P, Xu Z, Liu G, et al. The gut microbial metabolite trimethylamine N-oxide and hypertension risk: a systematic review and dose-response meta-analysis. Adv Nutr. 2020;11:66–76.
Nie J, **e L, Zhao B, Li Y, Qiu B, Zhu F, et al. Serum trimethylamine N-Oxide concentration is positively associated with first stroke in hypertensive patients. Stroke. 2018;49:2021–8.
Hosseinkhani F, Heinken A, Thiele I, Lindenburg PW, Harms AC, Hankemeier T. The contribution of gut bacterial metabolites in the human immune signaling pathway of non-communicable diseases. Gut Microbes. 2021;13:e1882927.
Verhaar BJH, Prodan A, Nieuwdorp M, Muller M. Gut microbiota in hypertension and atherosclerosis: a review. Nutrients. 2020;12:2982.
Yan M, Guo X, Ji G, Huang R, Huang D, Li Z, et al. Mechanism based role of the intestinal microbiota in gestational diabetes mellitus: a systematic review and meta-analysis. Front Immunol. 2023;13:1097853.
Jiang S, Shui Y, Cui Y, Tang C, Wang X, Qiu X, et al. Gut microbiota dependent trimethylamine N-oxide aggravates angiotensin II–induced hypertension. Redox Biol. 2021;46:102115.
Lin R, Liu W, Piao M, Zhu H. A review of the relationship between the gut microbiota and amino acid metabolism. Amino Acids. 2017;49:2083–90.
Rinschen MM, Palygin O, Guijas C, Palermo A, Palacio-Escat N, Domingo-Almenara X, et al. Metabolic rewiring of the hypertensive kidney. Sci Signal. 2019;12:eaax9760.
Rinschen MM, Palygin O, El-Meanawy A, Domingo-Almenara X, Palermo A, Dissanayake LV, et al. Accelerated lysine metabolism conveys kidney protection in salt-sensitive hypertension. Nat Commun. 2022;13:4099.
Katsyuba E, Mottis A, Zietak M, De Franco F, van der Velpen V, Gariani K, et al. De novo NAD+ synthesis enhances mitochondrial function and improves health. Nature. 2018;563:354–9.
Ralto KM, Rhee EP, Parikh SM. NAD+ homeostasis in renal health and disease. Nat Rev Nephrol. 2020;16:99–111.
Senchukova MA. Microbiota of the gastrointestinal tract: friend or foe? World J Gastroenterol. 2023;29:19–42.
Rashid J, Kumar SS, Job KM, Liu X, Fike CD, Sherwin CMT. Therapeutic potential of citrulline as an arginine supplement: a clinical pharmacology review. Pediatr Drugs. 2020;22:279–93.
Michonneau D, Latis E, Curis E, Dubouchet L, Ramamoorthy S, Ingram B, et al. Metabolomics analysis of human acute graft-versus-host disease reveals changes in host and microbiota-derived metabolites. Nat Commun. 2019;10:5695.
Adak A, Khan MR. An insight into gut microbiota and its functionalities. Cell Mol Life Sci. 2019;76:473–93.
Dilek N, Papapetropoulos A, Toliver-Kinsky T, Szabo C. Hydrogen sulfide: an endogenous regulator of the immune system. Pharmacol Res. 2020;161:105119.
Munteanu C. Hydrogen sulfide and oxygen homeostasis in atherosclerosis: a systematic review from molecular biology to therapeutic perspectives. IJMS. 2023;24:8376.
Zhang Y, Yue T, Gu W, Liu A, Cheng M, Zheng H, et al. pH-responsive hierarchical H2S-releasing nano-disinfectant with deep-penetrating and anti-inflammatory properties for synergistically enhanced eradication of bacterial biofilms and wound infection. J Nanobiotechnol. 2022;20:55.
Spiller F, Orrico MIL, Nascimento DC, Czaikoski PG, Souto FO, Alves-Filho JC, et al. Hydrogen sulfide improves neutrophil migration and survival in sepsis via K + ATP channel activation. Am J Respir Crit Care Med. 2010;182:360–8.
Farahat S, Kherkheulidze S, Nopp S, Kainz A, Borriello M, Perna AF, et al. Effect of hydrogen sulfide on essential functions of polymorphonuclear leukocytes. Toxins. 2023;15:198.
Heikal L, Starr A, Hussein D, Prieto-Lloret J, Aaronson P, Dailey LA, et al. l-phenylalanine restores vascular function in spontaneously hypertensive rats through activation of the GCH1-GFRP complex. JACC: Basic Transl Sci. 2018;3:366–77.
Li M, Wu Y, Ye L. The role of amino acids in endothelial biology and function. Cells. 2022;11:1372.
Yang P, Zhou L, Chen M, Zeng L, Ouyang Y, Zheng X, et al. Supplementation of amino acids and organic acids prevents the increase in blood pressure induced by high salt in Dahl salt-sensitive rats. Food Funct. 2022;13:891–903.
Heinken A, Ravcheev DA, Baldini F, Heirendt L, Fleming RMT, Thiele I. Systematic assessment of secondary bile acid metabolism in gut microbes reveals distinct metabolic capabilities in inflammatory bowel disease. Microbiome. 2019;7:75.
Winston JA, Theriot CM. Diversification of host bile acids by members of the gut microbiota. Gut Microbes. 2020;11:158–71.
Jia E, Liu Z, Pan M, Lu J, Ge Q. Regulation of bile acid metabolism-related signaling pathways by gut microbiota in diseases. J Zhejiang Univ Sci B. 2019;20:781–92.
Wang H, Latorre JD, Bansal M, Abraha M, Al-Rubaye B, Tellez-Isaias G, et al. Microbial metabolite deoxycholic acid controls Clostridium perfringens-induced chicken necrotic enteritis through attenuating inflammatory cyclooxygenase signaling. Sci Rep. 2019;9:14541.
Fiorucci S, Carino A, Baldoni M, Santucci L, Costanzi E, Graziosi L, et al. Bile acid signaling in inflammatory bowel diseases. Dig Dis Sci. 2021;66:674–93.
Ishimwe JA, Dola T, Ertuglu LA, Kirabo A. Bile acids and salt-sensitive hypertension: a role of the gut-liver axis. Am J Physiol-Heart Circ Physiol. 2022;322:H636–H646.
Ye X, Shen S, Xu Z, Zhuang Q, Xu J, Wang J, et al. Sodium butyrate alleviates cholesterol gallstones by regulating bile acid metabolism. Eur J Pharmacol. 2021;908:174341.
Guan B, Tong J, Hao H, Yang Z, Chen K, Xu H, et al. Bile acid coordinates microbiota homeostasis and systemic immunometabolism in cardiometabolic diseases. Acta Pharmaceutica Sin B. 2022;12:2129–49.
Tang WHW, Li DY, Hazen SL. Dietary metabolism, the gut microbiome, and heart failure. Nat Rev Cardiol. 2019;16:137–54.
Kiouptsi K, Reinhardt C. Contribution of the commensal microbiota to atherosclerosis and arterial thrombosis: microbiota and thrombosis. Br J Pharmacol. 2018;175:4439–49.
Kaminsky LW, Al-Sadi R, Ma TY. IL-1β and the intestinal epithelial tight junction barrier. Front Immunol. 2021;12:767456.
Liang C-F, Liu JT, Wang Y, Xu A, Vanhoutte PM. Toll-like receptor 4 mutation protects obese mice against endothelial dysfunction by decreasing NADPH oxidase isoforms 1 and 4. ATVB. 2013;33:777–84.
De Pergola G, D’Alessandro A. Influence of Mediterranean diet on blood pressure. Nutrients. 2018;10:1700.
Chiavaroli L, Viguiliouk E, Nishi S, Blanco Mejia S, Rahelić D, Kahleová H, et al. DASH dietary pattern and cardiometabolic outcomes: an umbrella review of systematic reviews and meta-analyses. Nutrients. 2019;11:338.
Filippou CD, Thomopoulos CG, Kouremeti MM, Sotiropoulou LI, Nihoyannopoulos PI, Tousoulis DM, et al. Mediterranean diet and blood pressure reduction in adults with and without hypertension: a systematic review and meta-analysis of randomized controlled trials. Clin Nutr. 2021;40:3191–200.
Schwingshackl L, Morze J, Hoffmann G. Mediterranean diet and health status: active ingredients and pharmacological mechanisms. Br J Pharm. 2020;177:1241–57.
Koelman L, Egea Rodrigues C, Aleksandrova K. Effects of dietary patterns on biomarkers of inflammation and immune responses: a systematic review and meta-analysis of randomized controlled trials. Adv Nutr. 2022;13:101–15.
Gibiino G, De Siena M, Sbrancia M, Binda C, Sambri V, Gasbarrini A, et al. Dietary habits and gut microbiota in healthy adults: focusing on the right diet. a systematic review. IJMS. 2021;22:6728.
Jennings A, Berendsen AM, de Groot LCPGM, Feskens EJM, Brzozowska A, Sicinska E, et al. Mediterranean-style diet improves systolic blood pressure and arterial stiffness in older adults: results of a 1-year European multi-center trial. Hypertension 2019;73:578–86.
Carson TL, Buro AW, Miller D, Peña A, Ard JD, Lampe JW, et al. Rationale and study protocol for a randomized controlled feeding study to determine the structural- and functional-level effects of diet-specific interventions on the gut microbiota of non-Hispanic black and white adults. Contemp Clin Trials. 2022;123:106968.
Diao Z, Molludi J, Latef Fateh H, Moradi S. Comparison of the low-calorie DASH diet and a low-calorie diet on serum TMAO concentrations and gut microbiota composition of adults with overweight/obesity: a randomized control trial. Int J Food Sci Nutr. 2023;75:1–14.
Filippou CD, Tsioufis CP, Thomopoulos CG, Mihas CC, Dimitriadis KS, Sotiropoulou LI, et al. Dietary approaches to stop hypertension (DASH) diet and blood pressure reduction in adults with and without hypertension: a systematic review and meta-analysis of randomized controlled trials. Adv Nutr. 2020;11:1150–60.
Bakhoum CY, Anderson CAM, Juraschek SP, Rebholz CM, Appel LJ, Miller ER, et al. The relationship between urine uromodulin and blood pressure changes: The DASH-sodium trial. Am J Hypertens. 2021;34:154–6.
Pavlidou E, Fasoulas A, Mantzorou M, Giaginis C. Clinical evidence on the potential beneficial effects of probiotics and prebiotics in cardiovascular disease. IJMS. 2022;23:15898.
Davani-Davari D, Negahdaripour M, Karimzadeh I, Seifan M, Mohkam M, Masoumi S, et al. Prebiotics: definition, types, sources, mechanisms, and clinical applications. Foods. 2019;8:92.
Lordan C, Thapa D, Ross RP, Cotter PD. Potential for enriching next-generation health-promoting gut bacteria through prebiotics and other dietary components. Gut Microbes. 2020;11:1–20.
Markowiak-Kopeć P, Śliżewska K. The effect of probiotics on the production of short-chain fatty acids by human intestinal microbiome. Nutrients. 2020;12:1107.
Khalesi S, Sun J, Buys N, Jayasinghe R. Effect of probiotics on blood pressure: a systematic review and meta-analysis of randomized, controlled trials. Hypertension. 2014;64:897–903.
Robles‐Vera I, Visitación N, Toral M, Sánchez M, Romero M, Gómez‐Guzmán M, et al. Probiotic Bifidobacterium breve prevents DOCA‐salt hypertension. FASEB J. 2020;34:13626–40.
Aoki R, Kamikado K, Suda W, Takii H, Mikami Y, Suganuma N, et al. A proliferative probiotic Bifidobacterium strain in the gut ameliorates progression of metabolic disorders via microbiota modulation and acetate elevation. Sci Rep. 2017;7:43522.
Zhao T, Zhang L, Zhou N, Sun D, **e J, Xu S. Long‐term use of probiotics for the management of office and ambulatory blood pressure: A systematic review and meta‐analysis of randomized, controlled trials. Food Sci Nutr. 2022;11:101–13.
Leung C-Y, Weitz JS. Not by (good) microbes alone: towards immunocommensal therapies. Trends Microbiol. 2019;27:294–302.
Sanders ME, Merenstein DJ, Reid G, Gibson GR, Rastall RA. Probiotics and prebiotics in intestinal health and disease: from biology to the clinic. Nat Rev Gastroenterol Hepatol. 2019;16:605–16.
Choi MS, Yu JS, Yoo HH, Kim D-H. The role of gut microbiota in the pharmacokinetics of antihypertensive drugs. Pharmacol Res. 2018;130:164–71.
Jaworska K, Kopacz W, Koper M, Szudzik M, Gawryś-Kopczyńska M, Konop M, et al. Enalapril diminishes the diabetes-induced changes in intestinal morphology, intestinal RAS and blood SCFA concentration in rats. IJMS. 2022;23:6060.
Wu H, Lam TYC, Shum T-F, Tsai T-Y, Chiou J. Hypotensive effect of captopril on deoxycorticosterone acetate-salt-induced hypertensive rat is associated with gut microbiota alteration. Hypertens Res. 2022;45:270–82.
Yonekura S, Terrisse S, Alves Costa Silva C, Lafarge A, Iebba V, Ferrere G, et al. Cancer induces a stress ileopathy depending on β-adrenergic receptors and promoting dysbiosis that contributes to carcinogenesis. Cancer Discov. 2022;12:1128–51.
Galla S, Chakraborty S, Cheng X, Yeo J, Mell B, Zhang H. et al. Disparate effects of antibiotics on hypertension. Physiol Genom. 2018;50:837–45.
Dong Y, Xu T, **ao G, Hu Z, Chen J. Opportunities and challenges for synthetic biology in the therapy of inflammatory bowel disease. Front Bioeng Biotechnol. 2022;10:909591.
Li J, Zhao F, Wang Y, Chen J, Tao J, Tian G, et al. Gut microbiota dysbiosis contributes to the development of hypertension. Microbiome. 2017;5:14.
Suez J, Zmora N, Zilberman-Schapira G, Mor U, Dori-Bachash M, Bashiardes S, et al. Post-antibiotic gut mucosal microbiome reconstitution is impaired by probiotics and improved by autologous FMT. Cell. 2018;174:1406–23.
Costello SP, Hughes PA, Waters O, Bryant RV, Vincent AD, Blatchford P, et al. Effect of fecal microbiota transplantation on 8-week remission in patients with ulcerative colitis: a randomized clinical trial. JAMA. 2019;321:156–64.
Zhang, Mocanu, Cai, Dang, Slater, Deehan, et al. Impact of fecal microbiota transplantation on obesity and metabolic syndrome—a systematic review. Nutrients. 2019;11:2291.
Martinez-Gili L, McDonald JAK, Liu Z, Kao D, Allegretti JR, Monaghan TM, et al. Understanding the mechanisms of efficacy of fecal microbiota transplant in treating recurrent Clostridioides difficile infection and beyond: the contribution of gut microbial-derived metabolites. Gut Microbes. 2020;12:1810531.
Richards EM, Pepine CJ, Raizada MK, Kim S. The gut, its microbiome, and hypertension. Curr Hypertens Rep. 2017;19:36.
Hashimoto T, Perlot T, Rehman A, Trichereau J, Ishiguro H, Paolino M, et al. ACE2 links amino acid malnutrition to microbial ecology and intestinal inflammation. Nature. 2012;487:477–81.
Yang T, Santisteban MM, Rodriguez V, Li E, Ahmari N, Carvajal JM, et al. Gut microbiota dysbiosis is linked to hypertension. Hypertension. 2015;65:1331–40.
Santisteban MM, Qi Y, Zubcevic J, Kim S, Yang T, Shenoy V, et al. Hypertension-linked pathophysiological alterations in the gut. Circ Res. 2017;120:312–23.
Funding
This work was supported by the Natural Science Foundation of Henan Province (222300420089), the National Natural Science Foundation of China (31971065 and 32371170), and the Scientific Research and Innovation Team of the First Affiliated Hospital of Zhengzhou University (QNCXTD2023006).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mu, YF., Gao, ZX., Mao, ZH. et al. Perspectives on the involvement of the gut microbiota in salt-sensitive hypertension. Hypertens Res (2024). https://doi.org/10.1038/s41440-024-01747-y
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41440-024-01747-y
- Springer Nature Singapore Pte Ltd.