Abstract
Purpose of Review
Clinically significant portal hypertension (CSPH) is a serious clinical condition causing decompensation and potentially fatal complications especially in the presence of advanced liver disease. This article aims to critically review the current literature on non-invasive assessment of CSPH.
Recent Findings
The Baveno VII consensus encouraged non-invasive assessment of CSPH to identify patients at risk and avoid unnecessary screening endoscopies. Novel machine learning and omics-based laboratory scores have been introduced, which can be combined with liver stiffness measurement (LSM). Spleen stiffness measurement (SSM) is an increasingly used novel elastography modality. Elastography and cross-sectional imaging methods have reached similar predictive power, while the accuracy of non-invasive tests can only be improved when used sequentially.
Summary
In this review, we provide a detailed discussion of advantages and limitations of non-invasive assessment of CSPH, highlighting their diagnostic accuracy, reproducibility, and feasibility in clinical practice.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chronic advanced liver disease, irrespective of etiology, is characterized by hepatic fibrogenesis leading to an increase of hepatic vascular resistance and increased pressure in the portal vein and its branches. Less frequently, increased portal pressure can also be observed during hepatic venous outflow obstructions or vascular disorders.
Portal hypertension (PH) is defined as an increase of portal venous pressure > 5 mmHg. The gold standard to measure portal venous pressure is the evaluation of hepatic venous pressure gradient (HVPG). Portal venous pressure can be assessed by HVPG, which is measured invasively by a balloon catheter inserted through the right jugular vein and assessed by the difference between free hepatic venous pressure and wedged hepatic venous pressure.
A HVPG of 10 mmHg or higher is considered to be clinically significant portal hypertension (CSPH) and is associated with an increased risk of complications like gastrointestinal varices, ascitic decompensation, gastrointestinal hemorrhage from portal hypertensive collaterals and hepatic encephalopathy. Early diagnosis of CSPH is mandatory to optimize patient care and prevent hepatic decompensation [1].
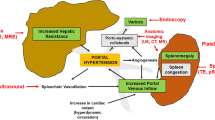
Non-invasive strategies to determine PH are crucial to stratify patient care and to plan their clinical management. Since healthcare resources are limited, HVPG measurement, as a complex and invasive procedure, is only available in specialized centers and contains a periprocedural risk of bleeding and organ injury. Non-invasive tests (NIT) for CSPH are needed to guide patients’ management from a clinicians point-of-view, being useful in ruling out CSPH and therewith avoiding unnecessary examinations. On the other hand, they can rule in CSPH and can identify patients requiring further examinations or referral to a hepatologist. This review aims to summarize the advances achieved in the past 5 years to assess CSPH non-invasively with specific regard to the recently published consensus criteria of the Baveno VII Faculty [2]. A diagram of tests in use to diagnose CSPH is shown in Fig. 1.
Diagram of non-invasive assessment of clinically significant portal hypertension (CSPH). The liver and the spleen are both connected by the portal vein tract. Ultrasound-based techniques, such as LSM or SSM, are the best validated techniques for the diagnosis of CSPH. Several blood-based algorithms (e.g., FIB-4/FIB-4 +) using platelets, albumin, and ALT and AST levels can be used as screening tools for CSPH assessment. Experimental methods, such as MRE and algorithms including circulating metabolites, are emerging and are likely to enter clinical practice in the future. Image was created with BioRender.com
Blood-Based Tests
Serum-Derived Tests
Non-invasive assessment of CSPH through laboratory tests is a convenient tool since these tests do not require technical expertise and/or access to particular devices, resulting in numerous attempts to develop calculation algorithms in order to predict CSPH. A brief overview of tests and suggested cut-offs for ruling in or out of CSPH is displayed in Table 1.
The most established parameter is the platelet count as a single surrogate for the presence of CSPH. Platelets decrease during PH progression probably due to PH-derived hypersplenism but also other unknown mechanisms [9]. Platelets alone have a moderate predictive capacity with an AUROC of 0.72, as confirmed recently [3]. Since laboratory values vary depending on etiology of the underlying liver disease and may be influenced by comorbidities, serum tests alone were considered to only have modest predictive values. However, recent studies could significantly improve diagnostic accuracy using machine learning models and their combination with other diagnostic modalities, especially liver stiffness mostly measured by transient elastography (TE). Common scores used for prediction of fibrosis or mortality, such as alanine aspartate-to-platelet ratio, model for end-stage liver disease (MELD) and albumin-bilirubin score, show only modest diagnostic value for prediction of esophageal varices with an AUROC between 0.6 and 0.7 [10].
The Fibrosis-4 index (FIB-4) is a serum-based non-invasive score for liver fibrosis prediction (including age, alanine aminotransferase (ALT) level, aspartate aminotransferase (AST) level, and platelet count) which was extensively investigated in the context of CSPH. Initially introduced for liver fibrosis prediction in HIV/HCV-coinfected patients, its predictive value was later confirmed for liver fibrosis of other etiologies and can be used with adjusted threshold for CSPH prediction, as demonstrated by a recent retrospective study [3].
Recently, a further modification of FIB-4 was published, using FIB-4 together with serum albumin (FIB4 +) in order to guide clinicians without access to TE with an AUC of 0.8 for prediction of CSPH in patients with cirrhosis due to non-alcoholic steatohepatitis (NASH) [4].
Recent approaches including machine-based learning models using standard laboratory tests reached similar predictive values as liver stiffness measurements (see below) [11].
Blood-Based Biomarkers
Apart from standard laboratory analyses, further blood-derived biomarkers have been identified during the development of PH or in populations receiving treatment for that. Serum levels of bone sialoprotein were inversely correlated to HVPG and could therefore be used in the diagnosis of CSPH [12]. Chemokines are chemotactic cytokines which conduct leukocyte migration and are involved in various homeostatic or inflammatory processes. The CXCR3 ligand CXCL9 is elevated in the portal vein blood in cirrhotic patients with CSPH; CXCL11 correlates with the severity of PH and could be used as a biomarker of CSPH [13, 14]. Higher circulating CXCL10 levels were associated with ascites and IL-6, IL-8, and sIL-33R were correlated with HVPG and therefore possibly reflect systemic inflammation in this collective [15, 16]. These chemokines decrease after TIPS implantation and their decrease may be a beneficial prognostic marker after treatment of CSPH.
Collagen is the main structural protein in the extracellular matrix (ECM) and its hepatic deposition is directly correlated with severity of liver fibrosis. Degradation products of the ECM can be identified in serum. For the diagnosis of CSPH, C4M, C5M, ELM, and PRO-C5 levels are of special interest since these proteins were significantly elevated in patients with CSPH [17, 18]. Circulating levels of elastin fragments increase in the hepatic vein and are associated with ascites as a clinical hallmark of CSPH [19]. PRO-C3 (MMP-degraded n-terminal propeptide of type III collagen) is significantly correlated with HVPG in humans and its increase was demonstrated in animals during ascites development [20, 21]. MMP-2/9-degraded type IV collagen (C4M and C5M) are both increased in serum of patients with PH (HVPG > 5 mmHg) and C4M showed a sex-specific profile and is able to independently predict survival in female patients with decompensated cirrhosis [20, 22].
Lastly, changes in microRNAs (miR) were recently discovered in patients with PH. miR-122 was demonstrated to be inversely correlated with HVPG measurements [23], whereas miR-34a predicted survival in patients receiving TIPS but no correlation with HVPG was observed [24]. Further research is required to better describe the ability of CSPH prediction for miRs.
Omics-Based Tests
While omics have been implemented earlier in decompensated cirrhosis with high predictive value for patient outcome [25], in CSPH, this has only been achieved recently. With the implementation of omics-based tests, experimental models are emerging which might predict liver-related outcomes comparable to invasive HVPG measurement. In a recently published sub-analysis of patients recruited in the PREDESCI study, inclusion of ceramide (d18:1/22:0) and methionine enabled development of models which achieved similar predictive power for hepatic decompensation and liver-related deaths [26]. In the CLIF-C MET score, three serum metabolites, namely, 4-hydroxy-3-methoxyphenylglycol sulfate, hexanoylcarnitine, and galacturonic acid, are predictors of short term mortality and outperformed the MELD and the NaMELD score in short term prediction, rendering these models interesting in a pretransplant setting [27]. Interestingly, these metabolites are linked to hallmarks of the underlying disease. While 4-hydroxy-3-methoxyphenylglycol sulfate is a derivate of norepinephrine and therefore may be a response to systemic inflammation, galacturonic acid and hexanoylcarnitine are both linked to mitochondrial dysfunction. While these experimental models show promising results, they require validation. Interestingly, metabolomic analyses were found to distinguish between patients with idiopathic and cirrhotic PH indicating the need for different models depending on etiology [28].
Lipidomic studies identified biomarkers for treatment response to non-selective betablockers (NSBB) in patients with HVPG-proven CSPH. A model including serum levels of phosphatidylcholine (PC(P-16:0/22:6)) and a free fatty acid (20:2(n-6), eicosadienoic acid) could predict the response to propranolol treatment with an AUROC of 0.801 as defined by HVPG reduction > 10% [29]. Specific models for CSPH prediction have not been established to date.
Area of Uncertainty and Clinical Need
Currently, there are no validated serum-based tests for prediction of CSPH in patients with vascular liver diseases. There is a definite need for future research since the diagnosis of vascular liver diseases and CSPH to date is solely based on exclusion.
Importantly, none of the abovementioned studies included patients with non-cirrhotic PH, e.g., patients with portosinusoidal vascular disease (PSVD).
A multicenter study, published in 2021 including 428 patients with clinical signs of PH, demonstrated the diagnostic accuracy of transient elastography to distinguish PSVD from cirrhosis with PH. Cut-off values ≤ 10 kPa indicated PSVD with a probability of 85%, while values ≥ 20 kPa had a negative predictive value of 97% [30].
Superiority of either liver stiffness measurement (LSM) or spleen stiffness measurement (SSM) in the prediction of CSPH needs to be elucidated in future studies.
Stiffness Measurements
Transient Elastography of the Liver
Liver fibrosis is the main mechanistic driver of portal hypertension. Portal hypertension is further aggravated by splanchnic blood flow and congestion. For a long time, histological analysis of liver biopsy was the most common tool to quantify liver fibrosis in patients with chronic liver disease, while HVPG was the gold standard for the diagnosis of CSPH. However, liver fibrosis and portal hypertension are both reflected in an increase in stiffness of the liver tissue due to congestion and fibrosis itself. In the last decades, non-invasive LSM by TE emerged and almost completely replaced liver biopsy for fibrosis grading. Over this time, studies have demonstrated that TE correlates with HVPG and could therefore be used to first rule out and later diagnose CSPH. To date, TE is widely available and commonly used in the evaluation of liver stiffness.
The European Association for the Study of the Liver (EASL) update of clinical practice guidelines and the American Association for the Study of Liver Disease (AASLD) recommend that a threshold of > 20–25 kPa irrespective of platelet count should be used to rule in CSPH [31, 32]. Within the Baveno VII consensus recommendation, for LSM between 15 and 25, platelet count needs to be taken into account and thresholds for ruling in CSPH varies (see Table 1).
Salavrakos et al. published a single-center study comparing liver biopsy, measurement of HVPG, esophageal endoscopy, and Fibroscan® in 118 patients with alcohol-induced liver disease. A threshold of 30.4 kPa indicated CSPH with a sensitivity of 94%. Moreover, it ruled out the presence of esophageal varices with a negative predictive value of 84% [33].
A multicenter study published in 2021 evaluated the validation of LSM in diagnosing CSPH in the most common etiologies of patients with compensated advanced chronic liver disease. Especially in patients with viral hepatitis or alcoholic liver disease, the positive predictive value was ≥ 90% in predicting CSPH by LSM with ≥ 25 kPa as cut-off. However, evidence was given that in patients with obese non-alcoholic steatohepatitis, LSM is not an effective tool to assess PH, with the correlation being only moderate in this patient group (positive predictive value of only 62.8%) [34].
Another study published in 2022 including 418 hepatitis C patients with PH prior to antiviral treatment, who achieved sustained virological response, analyzed non-invasive LSM in monitoring CSPH after hepatitis C treatment. LSM ≤ 12 kPa was able to rule in CSPH after antiviral therapy with a sensitivity of 93.6%, while LSM ≥ 25 kPa accurately ruled out CSPH (specificity 99.2%) [35]. Moreover, LSM is adequate in predicting further decompensation after treatment of chronic hepatitis B. Patients with HBV-derived CSPH and suppressed HBV replication are at low risk for further decompensation if LSM is < 25 kPa [36].
Furthermore, LSM by TE can display the course of CSPH after therapeutic interventions. As demonstrated by several studies, LSM usually decreases by 20% after TIPS implantation as a surrogate parameter of CSPH [37, 38]. Interestingly, in a small proportion of patients, LSM by TE increases after TIPS implantation, possibly due to an increased inflammatory response. An increase of LSM ≥ 10% after TIPS insertion is associated with an increased mortality [38]. Changes in LSM by TE after initiating treatment with NSBB are not significant. If changes are observed, they cannot assess the dynamic changes in PH [39, 40].
In summary, LSM by TE is a very accurate and promising tool to predict CSPH. TE is especially valuable for ruling in or ruling out of CSPH leading to a significant proportion of unclassified patients. However, it must be noted that the cut-off values differ greatly depending on etiology of liver disease, patient characteristics, and study design.
Shear Wave Elastography of the Liver
Similarly to TE, shear wave elastography (SWE) is used to determine liver fibrosis. Comparability of studies is hindered by different manufacturers and different elastography methods (two-dimensional (2D-SWE) vs. point-SWE) depending on the respective device. SWE is widely used since it can be frequently and easily performed with regular ultrasound machines [41].
The main advantage of SWE compared to TE is that this modality can be independently performed irrespective of the presence of ascites. Several older studies found that SWE was superior in diagnosing CSPH in ascitic patients [42, 43]. Non-inferiority to vibration controlled TE was demonstrated in a study including 127 patients [5]. Cut-offs for the diagnosis of CSPH depend on the ultrasound device used and may vary due to the etiology of the underlying disease.
Hristov et al. investigated whether 2D-SWE was capable of identifying presence and severity of esophageal varices. In this study with 86 patients, only end-stage varices could be predicted with 2D-SWE, implying that 2D-SWE can identify patients with very severe PH [44].
In total, a recent meta-analysis including nine studies on 2D-SWE performed up to August 2021 for CSPH prediction revealed a summary sensitivity of 83%, a summary specificity of 78% and a summary AUROC of 0.88. The authors rated 2D-SWE as a good tool for CSPH prediction [45]. However, since this meta-analysis included studies performed on different elastography devices and used different references (advanced cirrhosis, CSPH, or presence of varices which is not interchangeable), AUROC might be overestimated.
Spleen Stiffness Measurement
Firstly introduced in 2011, SSM by transient elastography has rapidly evolved in recent years. It was commercially introduced 2020 and is currently being studied extensively. During PH, splenic vein pressure increases and is conveyed to the spleen pulp. Splenic blood congestion leads to an increased spleen size and stiffness, suggesting spleen stiffness as a good surrogate parameter for CSPH. While in healthy adults, mean SSM is estimated to be 18.35 kPa, it is significantly increased in patients with CSPH [6].
A spleen stiffness < 35.8 kPa was demonstrated to exclude the presence of high-risk varices [7]. In a study with 260 patients, Stefanescu et al. showed that SSM > 41.3 kPa was associated with an increased risk of variceal bleeding [46]. Dajti et al. showed that the number of patients unclassified according to LSM, the so-called grey zone (see above), could be lowered by the addition of SSM with a cut-off of 40 kPa. Of note, all hepatic decompensations occurred in the “rule-in CSPH” group determined by SSM in this population [47]. Thus, it was proposed to supplement the Baveno VI recommendation, whereby screening endoscopy can be avoided in patients with LSM > 20 kPa and/or platelet count < 150/nl, and this was adapted in the new consensus statement [2]. Interestingly, a recent study evaluating LSM and SSM for the diagnosis of CSPH determined by HVPG showed better predictive values for LSM than for SSM [48]. Presumably, the combination of both LSM and SSM will reach the highest predictive accuracy since increased spleen stiffness should be adjusted to the increased LSM, thereby excluding patients with increased SSM values based on concomitant diseases. Hematological disorders, such as acute myeloid leukemia and bone marrow fibrosis, were identified as factors increasing spleen stiffness [49, 50].
Furthermore, SSM was proposed to monitor improvement of PH after interventions. In a pilot study with 20 patients by Marasco et al., changes in SSM were observed after initiation of NSBB treatment. SSM decreased and the authors concluded that SSM can be used to assess hemodynamic response [51]. In a study with 24 patient receiving TIPS, a statistically significant decrease of SSM by TE can be observed one and 28 days after implantation [52]. Irrespective of changes in LSM by TE or SWE after TIPS implantation, all patients showed a decrease of SSM by SWE seven days after TIPS implantation as demonstrated by a study with 67 patients [38].
To date, SSM is recommended for ruling in or ruling out CSPH in chronic advanced liver disease due to viral hepatitis according to the Baveno VII consensus criteria [2]. We believe that application of SSM by TE will further increase with publication of positive studies in the future.
Due to lack of data, further validation and technical studies, particular in determining acceptable IQR variation and required measurement attempts, are urgently needed. Based on the available knowledge, SSM represents a promising tool in the diagnosis and monitoring of CSPH.
Other Imaging-Based Tests
Computational tomography (CT) and magnetic resonance elastography (MRE) were evaluated for CSPH prediction but are rarely used in clinical practice for CSPH assessment. Especially MRE is a promising tool, since it has already been established in the diagnosis for liver fibrosis via computing of the extracellular volume fraction (ECV) by T1-weighted sequences [41].
Studies on the capability of CT for predicting CSPH are scarce. However, contrast-enhanced CT- or MR-based measurement of whole-vessel volume in the portal tract together with their length resulted in a recently published model with a good diagnostic value for HVPG [53]. As with the advances in TE, MRE of the spleen was evaluated. In a prospective study with 36 patients, 3D-MRE of spleen stiffness correlated best with HVPG and had the best predictive value followed by 2D-MRE of spleen stiffness, 3D-MRE of liver stiffness and, finally, 2D-SWE, with AUCs of 0.911, 0.845, 0.804, and 0.583 respectively [54]. Interestingly, 3D-MRE of liver and spleen stiffness can predict PH but do not reflect HVPG response after NSBB treatment according to a study with 52 patients [55]. In chronic hepatitis B and C patients, viscoelastic parameters in MRE were demonstrated not only to be correlated with HVPG, but might have a role in the detection of early necroinflammation in these patients [56]. A recent meta-analysis evaluated the combination of MRE and FIB-4 (MEFIB) which demonstrated the association with hepatic decompensation and an excellent negative predictive value for hepatic decompensations [57]. Overall, MRE in combination with blood-based tests shows very promising results in non-invasive assessment of CSPH, but require further validation and standardized protocols in order to obtain comparable results.
Briefly, all available imaging modalities perform well in predicting CSPH according to the published data. This was confirmed by a Bayesian network meta-analytic approach which included 45 studies where imaging modalities (CT, MRI, MRE, TE, SWE, and acoustic radiation force impulse imaging, inter alia) were compared to HVPG. This analysis revealed that the AUC of all imaging methods exceeded 0.8, indicating very good performance [58].
Algorithms
The best predictive results in non-invasive assessment of CSPH are achieved when combining different modalities simultaneously or sequentially. We will therefore present the current best available algorithms in the assessment of CSPH.
For ruling in or ruling out of CSPH, the best validated method is Fibroscan®, which is also recommended by the Baveno VII consensus as guidance for the necessity of esophageal endoscopy for varices screening [2]. The consensus is that LSM < 10 kPa can be used to rule out chronic advanced liver disease and LSM < 15 kPa and platelet count > 150/nl can be used to rule out CSPH.
Recently, Jachs et al. provided a novel score to reduce the number of patients in the “gray zone”, i.e., between 15 kPa ≤ LSM + platelets ≥ 150/nl and LSM < 25 kPa, where CSPH could be neither ruled in nor ruled out. It consists of the addition of Willebrand factor antigen to platelet ratio (VITRO) to LSM, which could safely increase the ratio of CSPH ruled out patients, since no patient allocated additionally experienced hepatic decompensation in the follow-up period [59]. When LSM is used together with FIB-4 and sex, sensitivity can be improved by up to 94%, while specificity remains low at 67% [3].
The ANTICIPATE model including TE and platelet count and the ANTICIPATE-NASH model (ANTICIPATE + body mass index) were recently validated in a cohort of patients with NASH cirrhosis and were first introduced in 2016 [4, 60, 61].
In order to stratify the monitoring of patients with CSPH, a large multi-center study including 2148 patients revealed that the combination of MELD score and liver SWE was able to predict patient mortality and risk of decompensation. According to the thresholds, this algorithm was named M10S20, i.e., MELD score ≥ 10 and liver SWE ≥ 20 kPa, indicating a high risk of 2-year mortality (38.8%) and an overall risk of development or worsening of decompensation or death of 61.8% within 2 years [8].
Studies on linear wave SWE have been performed more rarely with similar results to 2D-SWE. It was proposed that sequential linear wave SWE of liver and spleen may increase diagnostic accuracy [62].
Conclusions
While HVPG measurement remains the gold-standard for the diagnosis of CSPH, it could eventually be replaced with NITs. LSM by TE along with platelet count, which is already used in clinical practice, is currently considered to be the best method to evaluate CSPH in most patients. In the era of continuously striving for less invasive and economic fast-track medicine, TE seems to be a promising cost-efficient tool in identifying and monitoring CSPH and thereby adjusting the individual patient follow-up.
Moreover, the prognostic value of NIT in predicting liver-related mortality has been increasingly examined. As mentioned above, the M10S20 algorithm validly differentiates patients with advance chronic liver disease in a high- and low-risk group, with a significantly worse outcome in the high-risk group [59]. The use of TE, in combination with platelet count, is also recommended by the EASL to rule out high-risk varices. Especially high liver stiffness by TE is shown to increase the risk of HCC and liver-related death [63, 64].
Several limitations in NIT must be acknowledged. Tests can be false positive, e.g., in the case of acute hepatitis, extrahepatic cholestasis, or food intake and there is only limited data about retest reliability. As for TE, its results are less accurate when ascites is present and it does not mirror changes in HVPG due to medical therapy. Therefore, a significant number of unclassified patients remains in whom invasive measurement is still indispensable. Soon, it appears likely that the value of HVPG measurements will be further reduced by implementation of combinations of established and introduction of novel tests in CSPH assessment.
SSM is an emerging and promising tool which requires more extensive validation with regard to the underlying etiology of liver disease, sex, concomitant diseases, and periprocedural circumstances (e.g., necessity of fasting period). Also, blood-based biomarkers and omics techniques are emerging and may supplement established algorithms in future.
Possibly, invasive HVPG measurement will decrease in value for CSPH identification. However, it will remain an important tool in unclear conditions and can be expanded to include direct portal pressure measurement via punctuation of the portal vein during a single examination process.
References
Groszmann RJ, Garcia-Tsao G, Bosch J, Grace ND, Burroughs AK, Planas R, Escorsell A, Garcia-Pagan JC, Patch D, Matloff DS, et al. Beta-blockers to prevent gastroesophageal varices in patients with cirrhosis. N Engl J Med. 2005;353:2254–61. https://doi.org/10.1056/NEJMoa044456.
de Franchis R, Bosch J, Garcia-Tsao G, Reiberger T, Ripoll C, Abraldes JG, Albillos A, Baiges A, Bajaj J, Bañares R, et al. Baveno VII – renewing consensus in portal hypertension. J Hepatol. 2022;76:959–74. https://doi.org/10.1016/j.jhep.2021.12.022.
Banini BA, Patel S, Yu JW, Kang L, Bailey C, Strife BJ, Siddiqui MS, Patel V, Matherly SC, Lee H, et al. Derivation and validation of a model to predict clinically significant portal hypertension using transient elastography and FIB-4. J Clin Gastroenterol. 2023;57:189–97. https://doi.org/10.1097/MCG.0000000000001664.
Rabiee A, Deng Y, Ciarleglio M, Chan JL, Pons M, Genesca J, Garcia-Tsao G. Noninvasive predictors of clinically significant portal hypertension in NASH cirrhosis: validation of ANTICIPATE Models and development of a lab-based model. Hepatol Commun. 2022;6:3324–34. https://doi.org/10.1002/hep4.2091.
Stefanescu H, Rusu C, Lupsor-Platon M, Nicoara Farcau O, Fischer P, Grigoras C, Horhat A, Stancu O, Ardelean A, Tantau M, et al. Liver stiffness assessed by ultrasound shear wave elastography from general electric accurately predicts clinically significant portal hypertension in patients with advanced chronic liver disease. Ultraschall Med. 2020;41:526–33. https://doi.org/10.1055/a-0965-0745.
Kani̇ HT, Kekli̇kkiran Ç, Ergenç İ, Yilmaz Y (2022) Evaluation of spleen stiffness in healthy population: a vibration-controlled transient elastography study. J Health Sci Med 5:689–692https://doi.org/10.32322/jhsm.1074776
Karagiannakis DS, Voulgaris T, Koureta E, Chloupi E, Papatheodoridis GV, Vlachogiannakos J. Role of spleen stiffness measurement by 2D-shear wave elastography in ruling out the presence of high-risk varices in cirrhotic patients. Dig Dis Sci. 2019;64:2653–60. https://doi.org/10.1007/s10620-019-05616-4.
Trebicka J, Gu W, de Ledinghen V, Aubé C, Krag A, Praktiknjo M, Castera L, Dumortier J, Bauer DJM, Friedrich-Rust M, et al. Two-dimensional shear wave elastography predicts survival in advanced chronic liver disease. Gut. 2022;71:402–14. https://doi.org/10.1136/gutjnl-2020-323419.
Iwakiri Y, Trebicka J. Portal hypertension in cirrhosis: pathophysiological mechanisms and therapy. JHEP Rep. 2021;3:100316. https://doi.org/10.1016/j.jhepr.2021.100316.
Glisic T, StojkovicLalosevic M, Milovanovic T, Rankovic I, Stojanovic M, Toplicanin A, Aleksic M, Milivojevic V, MartinovNestorov J, Lolic I, et al. Diagnostic value of non-invasive scoring systems in the prediction of esophageal varices in patients with liver cirrhosis-single center experience. Medicina (Kaunas). 2022;58:158. https://doi.org/10.3390/medicina58020158.
Reiniš J, Petrenko O, Simbrunner B, Hofer BS, Schepis F, Scoppettuolo M, Saltini D, Indulti F, Guasconi T, Albillos A, et al. Assessment of portal hypertension severity using machine learning models in patients with compensated cirrhosis. J Hepatol. 2023;78:390–400. https://doi.org/10.1016/j.jhep.2022.09.012.
Benz F, Bogen A, Praktiknjo M, Jansen C, Meyer C, Wree A, Demir M, Loosen S, Vucur M, Schierwagen R, et al. Serum levels of bone sialoprotein correlate with portal pressure in patients with liver cirrhosis. PLoS One. 2020;15:e0231701. https://doi.org/10.1371/journal.pone.0231701.
Berres M-L, Asmacher S, Lehmann J, Jansen C, Görtzen J, Klein S, Meyer C, Strunk HM, Fimmers R, Tacke F, et al. CXCL9 is a prognostic marker in patients with liver cirrhosis receiving transjugular intrahepatic portosystemic shunt. J Hepatol. 2015;62:332–9. https://doi.org/10.1016/j.jhep.2014.09.032.
Berres M-L, Lehmann J, Jansen C, Görtzen J, Meyer C, Thomas D, Zimmermann HW, Kroy D, Schumacher F, Strassburg CP, et al. Chemokine (C-X-C Motif) ligand 11 levels predict survival in cirrhotic patients with transjugular intrahepatic portosystemic shunt. Liver Int. 2016;36:386–94. https://doi.org/10.1111/liv.12922.
Lehmann JM, Claus K, Jansen C, Pohlmann A, Schierwagen R, Meyer C, Thomas D, Manekeller S, Claria J, Strassburg CP, et al. Circulating CXCL10 in cirrhotic portal hypertension might reflect systemic inflammation and predict ACLF and mortality. Liver Int. 2018;38:875–84. https://doi.org/10.1111/liv.13610.
Praktiknjo M, Monteiro S, Grandt J, Kimer N, Madsen JL, Werge MP, William P, Brol MJ, Turco L, Schierwagen R, et al. Cardiodynamic state is associated with systemic inflammation and fatal acute-on-chronic liver failure. Liver Int. 2020;40:1457–66. https://doi.org/10.1111/liv.14433.
Leeming DJ, Karsdal MA, Byrjalsen I, Bendtsen F, Trebicka J, Nielsen MJ, Christiansen C, Møller S, Krag A. Novel serological neo-epitope markers of extracellular matrix proteins for the detection of portal hypertension. Aliment Pharmacol Ther. 2013;38:1086–96. https://doi.org/10.1111/apt.12484.
Leeming DJ, Veidal SS, Karsdal MA, Nielsen MJ, Trebicka J, Busk T, Bendtsen F, Krag A, Møller S. Pro-C5, a marker of true type V collagen formation and fibrillation, correlates with portal hypertension in patients with alcoholic cirrhosis. Scand J Gastroenterol. 2015;50:584–92. https://doi.org/10.3109/00365521.2014.996590.
Nielsen MJ, Lehmann J, Leeming DJ, Schierwagen R, Klein S, Jansen C, Strassburg CP, Bendtsen F, Møller S, Sauerbruch T, et al. Circulating elastin fragments are not affected by hepatic, renal and hemodynamic changes, but reflect survival in cirrhosis with TIPS. Dig Dis Sci. 2015;60:3456–64. https://doi.org/10.1007/s10620-015-3783-9.
Jansen C, Leeming DJ, Mandorfer M, Byrjalsen I, Schierwagen R, Schwabl P, Karsdal MA, Anadol E, Strassburg CP, Rockstroh J, et al. PRO-C3-levels in patients with HIV/HCV-co-infection reflect fibrosis stage and degree of portal hypertension. PLoS One. 2014;9:e108544. https://doi.org/10.1371/journal.pone.0108544.
Praktiknjo M, Lehmann J, Nielsen MJ, Schierwagen R, Uschner FE, Meyer C, Thomas D, Strassburg CP, Bendtsen F, Møller S, et al. Acute decompensation boosts hepatic collagen type III deposition and deteriorates experimental and human cirrhosis. Hepatol Commun. 2018;2:211–22. https://doi.org/10.1002/hep4.1135.
Lehmann J, Praktiknjo M, Nielsen MJ, Schierwagen R, Meyer C, Thomas D, Violi F, Strassburg CP, Bendtsen F, Møller S, et al. Collagen type IV remodelling gender-specifically predicts mortality in decompensated cirrhosis. Liver Int. 2019;39:885–93. https://doi.org/10.1111/liv.14070.
Jansen C, Reiberger T, Huang J, Eischeid H, Schierwagen R, Mandorfer M, Anadol E, Schwabl P, Schwarze-Zander C, Warnecke-Eberz U, et al. Circulating MiRNA-122 levels are associated with hepatic necroinflammation and portal hypertension in HIV/HCV coinfection. PLoS One. 2015;10:e0116768. https://doi.org/10.1371/journal.pone.0116768.
Jansen C, Eischeid H, Goertzen J, Schierwagen R, Anadol E, Strassburg CP, Sauerbruch T, Odenthal M, Trebicka J. The role of MiRNA-34a as a prognostic biomarker for cirrhotic patients with portal hypertension receiving TIPS. PLoS One. 2014;9:e103779. https://doi.org/10.1371/journal.pone.0103779.
Moreau R, Clària J, Aguilar F, Fenaille F, Lozano JJ, Junot C, Colsch B, Caraceni P, Trebicka J, Pavesi M, et al. Blood metabolomics uncovers inflammation-associated mitochondrial dysfunction as a potential mechanism underlying ACLF. J Hepatol. 2020;72:688–701. https://doi.org/10.1016/j.jhep.2019.11.009.
Nicoară-Farcău O, Lozano JJ, Alonso C, Sidorova J, Villanueva C, Albillos A, Genescà J, Llop E, Calleja JL, Aracil C, et al. Metabolomics as a tool to predict the risk of decompensation or liver related death in patients with compensated cirrhosis. Hepatology. 2023. https://doi.org/10.1097/HEP.0000000000000316.
Weiss E, de la Peña-Ramirez C, Aguilar F, Lozano J-J, Sánchez-Garrido C, Sierra P, Martin PI-B, Diaz JM, Fenaille F, Castelli FA et al (2023) Sympathetic nervous activation, mitochondrial dysfunction and outcome in acutely decompensated cirrhosis: the metabolomic prognostic models (CLIF-C MET). Gut gutjnl-2022–328708. https://doi.org/10.1136/gutjnl-2022-328708
Seijo S, Lozano JJ, Alonso C, Reverter E, Miquel R, Abraldes JG, Martinez-Chantar ML, Garcia-Criado A, Berzigotti A, Castro A, et al. Metabolomics discloses potential biomarkers for the noninvasive diagnosis of idiopathic portal hypertension. Am J Gastroenterol. 2013;108:926–32. https://doi.org/10.1038/ajg.2013.11.
Reverter E, Lozano JJ, Alonso C, Berzigotti A, Seijo S, Turon F, Baiges A, Martínez-Chantar ML, Mato JM, Martínez-Arranz I, et al. Metabolomics discloses potential biomarkers to predict the acute HVPG response to propranolol in patients with cirrhosis. Liver Int. 2019;39:705–13. https://doi.org/10.1111/liv.14042.
Elkrief L, Lazareth M, Chevret S, Paradis V, Magaz M, Blaise L, Rubbia-Brandt L, Moga L, Durand F, Payancé A, et al. Liver stiffness by transient elastography to detect porto-sinusoidal vascular liver disease with portal hypertension. Hepatology. 2021;74:364–78. https://doi.org/10.1002/hep.31688.
Garcia-Tsao G, Abraldes JG, Berzigotti A, Bosch J. Portal hypertensive bleeding in cirrhosis: risk stratification, diagnosis, and management: 2016 Practice Guidance by the American Association for the study of liver diseases. Hepatology. 2017;65:310–35. https://doi.org/10.1002/hep.28906.
Berzigotti A, Tsochatzis E, Boursier J, Castera L, Cazzagon N, Friedrich-Rust M, Petta S, Thiele M. EASL Clinical practice guidelines on non-invasive tests for evaluation of liver disease severity and prognosis – 2021 update. J Hepatol. 2021;75:659–89. https://doi.org/10.1016/j.jhep.2021.05.025.
Salavrakos M, Piessevaux H, Komuta M, Lanthier N, Stärkel P. Fibroscan reliably rules out advanced liver fibrosis and significant portal hypertension in alcoholic patients. J Clin Gastroenterol. 2019;53:772–8. https://doi.org/10.1097/MCG.0000000000001119.
Pons M, Augustin S, Scheiner B, Guillaume M, Rosselli M, Rodrigues SG, Stefanescu H, Ma MM, Mandorfer M, Mergeay-Fabre M, et al. Noninvasive diagnosis of portal hypertension in patients with compensated advanced chronic liver disease. Off J Am College Gastroenterol | ACG. 2021;116:723. https://doi.org/10.14309/ajg.0000000000000994.
Semmler G, Lens S, Meyer EL, Baiges A, Alvardo-Tapias E, Llop E, Tellez L, Schwabl P, Mauro E, Escudé L, et al. Non-invasive tests for clinically significant portal hypertension after HCV cure. J Hepatol. 2022;77:1573–85. https://doi.org/10.1016/j.jhep.2022.08.025.
Jachs M, Hartl L, Bauer D, Simbrunner B, Stättermayer AF, Strassl R, Trauner M, Mandorfer M, Reiberger T. Long-term outcome of HBV-infected patients with clinically significant portal hypertension achieving viral suppression. J Pers Med. 2022;12:239. https://doi.org/10.3390/jpm12020239.
Piecha F, Paech D, Sollors J, Seitz H-K, Rössle M, Rausch V, Mueller S. Rapid change of liver stiffness after variceal ligation and TIPS implantation. Am J Physiol Gastrointest Liver Physiol. 2018;314:G179–87. https://doi.org/10.1152/ajpgi.00239.2017.
Jansen C, Möller P, Meyer C, Kolbe CC, Bogs C, Pohlmann A, Schierwagen R, Praktiknjo M, Abdullah Z, Lehmann J, et al. Increase in liver stiffness after transjugular intrahepatic portosystemic shunt is associated with inflammation and predicts mortality. Hepatology. 2018;67:1472–84. https://doi.org/10.1002/hep.29612.
Reiberger T, Ferlitsch A, Payer BA, Pinter M, Homoncik M, Peck-Radosavljevic M. Vienna Hepatic hemodynamic lab non-selective β-blockers improve the correlation of liver stiffness and portal pressure in advanced cirrhosis. J Gastroenterol. 2012;47:561–8. https://doi.org/10.1007/s00535-011-0517-4.
Zaghloul SG, Wahab EA, Seleem WM, Hanafy AS, Gomaa AF, Lakouz K, Amin AI. Impact of non-selective beta blockers on portal hypertension and hepatic elasticity in hepatitis C virus-related liver cirrhosis. Drug Discov Ther. 2019;13:108–13. https://doi.org/10.5582/ddt.2019.01009.
Brol MJ, Drebber U, Luetkens JA, Odenthal M, Trebicka J (2022) “The pathogenesis of hepatic fibrosis: basic facts and clinical challenges”—assessment of liver fibrosis: a narrative review. Dig Med Res 5. https://doi.org/10.21037/dmr-22-9
Elkrief L, Rautou P-E, Ronot M, Lambert S, Dioguardi Burgio M, Francoz C, Plessier A, Durand F, Valla D, Lebrec D, et al. Prospective comparison of spleen and liver stiffness by using shear-wave and transient elastography for detection of portal hypertension in cirrhosis. Radiology. 2015;275:589–98. https://doi.org/10.1148/radiol.14141210.
Leung VY, Shen J, Wong VW, Abrigo J, Wong GL, Chim AM, Chu SH, Chan AW, Choi PC, Ahuja AT, et al. Quantitative elastography of liver fibrosis and spleen stiffness in chronic hepatitis B carriers: comparison of shear-wave elastography and transient elastography with liver biopsy correlation. Radiology. 2013;269:910–8. https://doi.org/10.1148/radiol.13130128.
Hristov B, Andonov V, Doykov D, Doykova K, Valova S, Nacheva-Georgieva E, Uchikov P, Kostov G, Doykov M, Tilkian E. Evaluation of liver stiffness measurement by means of 2D-SWE for the diagnosis of esophageal varices. Diagnostics (Basel). 2023;13:356. https://doi.org/10.3390/diagnostics13030356.
Dong B, Chen Y, Chen Y, Wang H, Lyu G. Diagnostic accuracy of liver stiffness on two-dimensional shear wave elastography for detecting clinically significant portal hypertension: a meta-analysis. Expert Rev Med Devices. 2023;20:141–9. https://doi.org/10.1080/17434440.2022.2077642.
Stefanescu H, Marasco G, Calès P, Fraquelli M, Rosselli M, Ganne-Carriè N, de Ledinghen V, Ravaioli F, Colecchia A, Rusu C, et al. A novel spleen-dedicated stiffness measurement by FibroScan® improves the screening of high-risk oesophageal varices. Liver Int. 2020;40:175–85. https://doi.org/10.1111/liv.14228.
Dajti E, Ravaioli F, Marasco G, Alemanni LV, Colecchia L, Ferrarese A, Cusumano C, Gemini S, Vestito A, Renzulli M, et al. A combined Baveno VII and spleen stiffness algorithm to improve the noninvasive diagnosis of clinically significant portal hypertension in patients with compensated advanced chronic liver disease. Am J Gastroenterol. 2022;117:1825–33. https://doi.org/10.14309/ajg.0000000000001887.
Grgurevic I, Madir A, Trkulja V, Bozin T, Aralica G, Podrug K, Mikolaševic I, Tsochatzis E, O’Beirne J, Pinzani M. Assessment of clinically significant portal hypertension by two-dimensional shear wave elastography. Eur J Clin Invest. 2022;52:e13750. https://doi.org/10.1111/eci.13750.
Moia R, Cittone MG, Boggione P, Manfredi GF, Favini C, Awikeh B, Pedrinelli AR, Mahmoud AM, Nicolosi M, Bellan M, et al. Stiffer spleen predicts higher bone marrow fibrosis and higher JAK2 allele burden in patients with myeloproliferative neoplasms. Front Oncol. 2021;11:777730. https://doi.org/10.3389/fonc.2021.777730.
Zhao C-S, Wang N-F, You Y-M, Wang Y-J, Liu F, Cai F-F. The value of CD44+ mononuclear cells and spleen stiffness measurement in minimal residual disease in acute myeloid leukemia and its prognosis. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2021;29:715–9. https://doi.org/10.19746/j.cnki.issn.1009-2137.2021.03.010.
Marasco G, Dajti E, Ravaioli F, Alemanni LV, Capuano F, G**i K, Colecchia L, Puppini G, Cusumano C, Renzulli M, et al. Spleen stiffness measurement for assessing the response to β-blockers therapy for high-risk esophageal varices patients. Hepatol Int. 2020;14:850–7. https://doi.org/10.1007/s12072-020-10062-w.
Buechter M, Manka P, Theysohn JM, Reinboldt M, Canbay A, Kahraman A. Spleen stiffness is positively correlated with HVPG and decreases significantly after TIPS implantation. Dig Liver Dis. 2018;50:54–60. https://doi.org/10.1016/j.dld.2017.09.138.
Wang C, Huang Y, Liu C, Liu F, Hu X, Kuang X, An W, Liu C, Liu Y, Liu S et al (2023) Diagnosis of clinically significant portal hypertension using CT- and MRI-based vascular model. Radiology 221648. https://doi.org/10.1148/radiol.221648
Kennedy P, Stocker D, Carbonell G, Said D, Bane O, Hectors S, Abboud G, Cuevas J, Bolster BD, Friedman SL, et al. MR elastography outperforms shear wave elastography for the diagnosis of clinically significant portal hypertension. Eur Radiol. 2022;32:8339–49. https://doi.org/10.1007/s00330-022-08935-9.
Danielsen KV, Hove JD, Nabilou P, Yin M, Chen J, Zhao M, Kallemose T, Teisner AS, Siebner HR, Ehman RL, et al. Using MR elastography to assess portal hypertension and response to beta-blockers in patients with cirrhosis. Liver Int. 2021;41:2149–58. https://doi.org/10.1111/liv.14981.
Shi Y, Qi Y-F, Lan G-Y, Wu Q, Ma B, Zhang X-Y, Ji R-Y, Ma Y-J, Hong Y. Three-dimensional MR elastography depicts liver inflammation, fibrosis, and portal hypertension in chronic hepatitis B or C. Radiology. 2021;301:154–62. https://doi.org/10.1148/radiol.2021202804.
Ajmera V, Kim BK, Yang K, Majzoub AM, Nayfeh T, Tamaki N, Izumi N, Nakajima A, Idilman R, Gumussoy M, et al. Liver stiffness on magnetic resonance elastography and the MEFIB index and liver-related outcomes in nonalcoholic fatty liver disease: a systematic review and meta-analysis of individual participants. Gastroenterology. 2022;163:1079-1089.e5. https://doi.org/10.1053/j.gastro.2022.06.073.
Hai Y, Chong W, Eisenbrey JR, Forsberg F. Network meta-analysis: noninvasive imaging modalities for identifying clinically significant portal hypertension. Dig Dis Sci. 2022;67:3313–26. https://doi.org/10.1007/s10620-021-07168-y.
Jachs M, Hartl L, Simbrunner B, Bauer D, Paternostro R, Scheiner B, Balcar L, Semmler G, Stättermayer AF, Pinter M, et al. The sequential application of Baveno VII criteria and VITRO score improves diagnosis of clinically significant portal hypertension. Clin Gastroenterol Hepatol. 2022;S1542–3565(22):00957. https://doi.org/10.1016/j.cgh.2022.09.032.
Abraldes JG, Bureau C, Stefanescu H, Augustin S, Ney M, Blasco H, Procopet B, Bosch J, Genesca J, Berzigotti A, et al. Noninvasive tools and risk of clinically significant portal hypertension and varices in compensated cirrhosis: the “Anticipate” study. Hepatology. 2016;64:2173–84. https://doi.org/10.1002/hep.28824.
Pons M, Augustin S, Scheiner B, Guillaume M, Rosselli M, Rodrigues SG, Stefanescu H, Ma MM, Mandorfer M, Mergeay-Fabre M, et al. Noninvasive diagnosis of portal hypertension in patients with compensated advanced chronic liver disease. Am J Gastroenterol. 2021;116:723–32. https://doi.org/10.14309/ajg.0000000000000994.
Jansen C, Bogs C, Verlinden W, Thiele M, Möller P, Görtzen J, Lehmann J, Vanwolleghem T, Vonghia L, Praktiknjo M, et al. Shear-wave elastography of the liver and spleen identifies clinically significant portal hypertension: a prospective multicentre study. Liver Int. 2017;37:396–405. https://doi.org/10.1111/liv.13243.
Jung KS, Kim SU, Ahn SH, Park YN, Kim DY, Park JY, Chon CY, Choi EH, Han K-H. Risk assessment of hepatitis B virus-related hepatocellular carcinoma development using liver stiffness measurement (FibroScan). Hepatology. 2011;53:885–94. https://doi.org/10.1002/hep.24121.
Wang J, Li J, Zhou Q, Zhang D, Bi Q, Wu Y, Huang W. Liver stiffness measurement predicted liver-related events and all-cause mortality: a systematic review and nonlinear dose–response meta-analysis. Hepatol Commun. 2018;2:467–76. https://doi.org/10.1002/hep4.1154.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Brol, M.J., Gödiker, J., Uschner, F.E. et al. Non-invasive Assessment of Clinically Significant Portal Hypertension. Curr Hepatology Rep 22, 206–215 (2023). https://doi.org/10.1007/s11901-023-00609-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11901-023-00609-4