Abstract
Microplastics (MPs) have become a growing concern in the context of environmental pollution, with an increasing focus on their presence in indoor environments, including university facilities. This study investigates the presence and characteristics of MPs in different university indoor environments. Initial examination of indoor ambient MPs involved physical characterization through optical microscopy, focusing on classifying MPs by shape and color. Various types of MPs, including fibers, fragments, pellets, foams, films, and lines, were identified, with the most common colors being black, red, blue, and brown. Fragments were the predominant type of MPs found, although accurately quantifying their numbers proved challenging due to the dense sample content. These MPs displayed rough and irregular margins suggestive of abrasion. Subsequent chemical and elemental characterization was conducted using micro-Raman and SEM-EDX, revealing the presence of 25 different types of MPs, including PA 66, PTFE, PP, HDPE, and PE. The study indicates that university inhabitants are exposed to airborne MPs (≥ 2.5–336.89 μm) at inhalation rates of 13.88–18.51 MPs/m3 and 180–240 MPs daily. These MPs exhibited significant variations in size, and their distribution varied among the different indoor environments studied. SEM-EDX analysis revealed common elements in the identified MPs, with C, O, F, Na, Cl, Al, Si, and others consistently detected. This research is the first to comprehensively analyze MPs in nine different indoor university environments using active sampling. Identifying and reducing MP contamination in these facilities might stimulate more awareness, promote extensive scientific investigation, and facilitate the development of informed policies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The widespread occurrence of microplastics (MPs) in ecosystems worldwide has become a prominent environmental concern in recent decades. The Presence of MPs in diverse environments, ranging from oceanic (Liu et al. 2019b; Zhang et al. 2023; Thacharodi et al. 2024a) to remote mountainous regions (Allen et al. 2019; Dong et al. 2021), has prompted concerns regarding their potential effects on ecosystems, wildlife, and human health (Eraslan et al. 2021; Bhat et al. 2022b, 2023c). The investigation into the prevalence of MPs has prompted increased research efforts, including in indoor environments associated with educational and research institutions (Amato-Lourenço et al. 2022; Kashfi et al. 2022; Bhat 2023a). The importance of understanding MP contamination within indoor educational environments can be attributed to the potential health risks they pose to large, dense populations of students, faculty, and workers, coupled with the unique indoor activities and materials used in such environments. MPs are commonly defined as plastic particles that are smaller than 5 mm in size. These MPs are derived from a wide range of sources. Primary MPs are those intentionally manufactured on a small scale for utilization in various products, including cosmetics, personal care items, and industrial abrasives (Thompson et al. 2004; Bhat et al. 2021, 2023a). Alternatively, secondary MPs may arise due to the breakdown of larger plastic objects through physical, biological, or chemical mechanisms. The extensive utilization and subsequent disposal of plastic goods, combined with their enduring characteristics, guarantee the eventual infiltration of these particles into diverse ecological compartments encompassing water, soil, and air (Bhat 2023b; Bhat et al. 2023c; Eraslan et al. 2023).
Historically, indoor air quality has emphasized various factors such as volatile organic compounds, particulate matter, and bioaerosols (Can et al. 2015; Bhat et al. 2022a; Bhat 2024a). Nevertheless, recent research indicates that MPs have the potential to become airborne due to their small size and ability to float, thereby adding to the indoor aerosol burden (Habibi et al. 2022; Bhat 2024b; Thacharodi et al. 2024b). Due to their large populations, regular renovations, significant foot traffic, and specific material utilization in laboratories and workshops, universities can serve as focal points for the emission and accumulation of MPs. The comprehension of the occurrence of MPs in indoor university environments is not solely an academic endeavor; rather, it represents a vital undertaking in evaluating potential health hazards. When MPs are inhaled or ingested, they have the potential to introduce harmful additives, plasticizers, and pathogens into the human body (Wright and Kelly 2017; Bhat et al. 2023c). While the health consequences of MPs are currently under investigation, preliminary research suggests potential inflammatory reactions, disruption of the endocrine system, and potentially harmful effects on genetic material (Prata 2018; Prata et al. 2020).
The potential risks of spending substantial time within university premises are paramount for students and faculty members. Furthermore, universities, being at the forefront of research and intellectual discourse, possess significant power in sha** societal perspectives and influencing policy-making processes. Identifying and mitigating MP pollution within these establishments can catalyze increased consciousness, expanded scientific inquiry, and well-informed policy formulation. Moreover, this can potentially stimulate universities to embrace sustainable practices, thereby mitigating their plastic impact and serving as a model for other educational establishments and the general populace. Before delving into the core of the issue, clarifying the mechanisms by which MPs may infiltrate these enclosed environments is imperative. Potential sources of MP generation encompass a broad spectrum, including the gradual deterioration of synthetic carpets due to regular use, the shedding of fibers from garments, the disintegration of equipment and materials composed of plastic-based substances, and the introduction of pollutants from outdoor air through ventilation systems. In addition, it should be noted that specific university settings, such as research laboratories, may unintentionally introduce plastic pellets or powders into the surrounding environment.
The MPs have become emerging pollutants of increasing global concern due to their ubiquitous detection in several environmental compartments, including air, water, soil, sediment, and biota (Abbasi et al. 2021; Liong et al. 2021; Choi et al. 2022). The dust, air, and deposition in indoor environments had been examined for the presence of MPs. In-door duts had been explored more than ambient and deposition MPs in-house dust. In indoor air, Gaston et al. (2020) found polyvinyl chloride (PVC), polyethylene (PE), polystyrene (PS), polycarbonate (PC), polyamide (PA), and acrylonitrile butadiene styrene (ABS) (fibers 3.3 ± 2.9 and 12.6 ± 8.0 fragments m− 3) in university and hospital air samples. Uddin et al. (2022) found polyester (PL) and PA concentrations of 3.24 to 27.13 MP m3 in government buildings, residential dwellings, hospitals, and mosque air samples in Kuwait. In an indoor deposition, Yao et al. (2021) found PS, polyethylene terephthalate (PET), PE, PVC, and polypropylene (PP) with concentrations of (6.20 ± 0.57)×103 - (1.96 ± 1.09)×104 fibers m− 2day− 1 university (office, hallway, classroom) and house. Fang et al. (2022) found PET, PE, and PA (7.6 ± 3.9)×105 MPs m− 2 day− 1 in the dining room in an apartment, dining hall in campus, restaurant, office, and classroom deposition samples in China. This analysis aims to present a thorough and all-encompassing examination of MP pollution in indoor university environments. The study aimed to analyze, compare, and assess the prevalence and abundance of MPs based on their color, shape, size, and chemical composition in ambient samples collected from various indoor environments. Through a comprehensive analysis of the sources, routes, and potential health ramifications, the aim is to cultivate a comprehension that extends beyond the confines of academia, thereby fostering societal consciousness and facilitating practical transformation.
Materials and methods
Sample collection
University samples were collected from nine indoor environments (air pollution laboratory 1, department corridor, researchers office 1, secretary office, workers office, students laboratory, canteen corridor, air pollution laboratory 2, and researchers office 2) at Eskişehir Technical University Türkiye in December 2021. To better understand the origins of MPs in these indoor environments, the residents filled out a questionnaire that involved the building characteristics, textile contribution, electric and electronic device contribution, activities done at home and in university indoor environments, etc. Detailed information about the characteristics of the indoor university environment is mentioned in the supplementary file under the heading of characteristics of university indoor environments. Active sampling polytetrafluoroethylene (PTFE) filters (25-mm diameter) collected the ambient samples with a 9 L/min Gilian 12 live flow air sampling pump for 8 h. Extensive preliminary testing showed that an eight-hour sample duration guaranteed a sufficient particulate load for MP analysis. The samples were collected at 1.2 m height because this is commonly used to equate to an adult’s breathing height. Filters were weighed before and after the analysis using a micro-analytical balance (AND BM-20) supported by an anti-vibrational table (AD 1671) in a laboratory at an indoor temperature of 17oC and 32% humidity. The weight of these filters in the university indoor environments (Table S1) is shown in a supplementary file. Once the sampling was completed, filters were kept in a petri dish and stored in the fridge until the analysis. Extraction and pretreatment of MPs from samples were not performed to minimize losses in cases where the suspended aerosols concentrated on filters have fewer impurities; it is advisable to exclude density separation and digestion (Gaston et al. 2020; Liu et al., 2019; Wang et al. 2020). The sample may be recognized and processed directly without pretreatment techniques. This method eradicates the possibility of contamination resulting from the preparation procedure (Bhat et al. 2024). It is worth mentioning that the physical properties of MPs may be altered during acidic digestion, potentially affecting their analysis or interpretation. Recently, it was seen that 16.67% of indoor MP studies did not perform any extraction and pretreatment process (Bhat 2023b). To find the chemical composition of MPs, it is essential first to identify whether the samples contain MPs or not and characterize them morphologically. These MPs were identified based on their source, type, shape, and color (Bhat 2023a, 2024b) by using Image J software. The filters were analyzed under an optical microscope, µRaman, and Scanning electron microscopy-energy dispersive X-ray analysis (SEM-EDX).
Optical microscope measurements
For visual characterization of MPs in active samples, the Primotech Zeiss optical microscope with 5x objective (NA = 0.13), 10x objective (NA = 0.23), 20x objective (NA = 0.4), 50x objective (NA = 0.65), and 100x objective (NA = 0.8) was used. The optical microscope was operated by Axiovision SE64 Rel.4.9.1 software embedded with the AxiocamERc5s camera. Under the optical microscope, each filter (entire filter area) was checked from left to right or right to left, then moved down slowly. Each filter should be checked thrice to reduce errors while identifying the MPs and was examined for almost 1–1.5 h. Counting all the MPs from the filters based on the morphological features was impossible. Using an optical microscope for physical identification before the µRaman analysis is a necessary step. It allows us to identify whether our sample contains MPs, making it easy and less time-consuming to do the µRaman analysis. The samples were analyzed for chemical and elemental characterization under µRaman and SEM-EDX.
Micro raman measurements
µRaman measurements of the ambient MPs ≥ 2.5 μm samples in university indoor environments were performed with an alpha 300R confocal Raman microscope (WITec), a grating of G2:600 g/mm BLZ = 500 nm, and a thermoelectrically cooled charge-coupled device (CCD) detector. The 532 nm radiation of a PS laser and a 10× objective (NA = 0.25; WD = 9.3 mm) and 50× objective (NA = 0.8; WD = 0.57 mm) EC Epiplan-Neofluar Disc Zeiss) were used. Thorlabs GmbH laser intensity checker controlled the laser intensity. Raman spectra were recorded in the wavenumber range of 152–4287 cm− 1, with laser power of 9–10 mW and an integration time of 15–20s per scan. For each spectrum, 15–20 scans were accumulated. Increasing the laser power by more than ten mW can harm the sample, as burning marks were seen in some thin MPs. Quantification analysis was performed on 25–30% of the filter area for each sample using the µRaman technique.
The Raman system was operated by control five software (WITec). Under the µRaman, each filter was measured from left to right or right to left, then moved down slowly. Each filter should be read at least two to three times to reduce the error while taking the spectra. For better illustration, smoothing, and baseline correction were done. The spectra obtained in the Raman microscope were compared with reference spectra from the Raman polymer database using open specy (Cowger et al. 2021). The spectra were also cross-checked with the previous literature (Mark 2009; Käppler et al. 2016; Cabernard et al. 2018; Nava et al. 2021). Based on the published research, the highest matching score was selected to identify MPs.
SEM-EDX measurements
Representative MPs from filters were selected and characterized for their morphology and elemental composition using a Backscatter Electron (BSE) - SEM (FEG-SEM; Zeiss Supra 50 VP) coupled with an EDX Microanalyzer (INCA Energy; Oxford Instruments). A Backscatter electron detector (BSD) was used during the analysis. The WD was 10 mm while the 20 kilovolts (kV) was used for electron high tension. The BSE-SEM imaging mode is based on the principle that dark regions represent elements with low atomic numbers, and bright regions represent elements with high atomic numbers. All the samples were sprinkled over double-sided carbon tape and mounted on the SEM, and the surface morphology and micro and nano region elemental composition were determined. The surface morphology and element composition results were printed as black-and-white images and tables. The SEM-EDX measurements were taken after the optical and µRaman microscope analysis. The advantage of doing this is that the carbon covering of filters for SEM-EDX measurements affects the filters and can not be used again if kept for a long time.
Statistical analysis
The current research article utilized Microsoft Excel and Origin as the computational tools for conducting statistical analysis and plotting graphs of the gathered data. The study employed methodological procedures that encompassed the utilization of ANOVA single factor. The research outcomes were subjected to rigorous analytical strategies to ensure optimal accuracy and reliability. An ANOVA analysis was used to determine the variation in MP levels across various indoor environments.
Results and discussion
Airborne MPs (coarse) were collected from nine different Eskişehir Technical University indoor environments (air pollution laboratory 1, department corridor, researchers office 1, secretary office, workers office, students laboratory, canteen corridor, air pollution laboratory 2, and researchers office 2). In the supplementary file, more information is provided regarding the characteristics of these indoor university sample points under the headings of characteristics of university indoor environments.
Visual identification of ambient microplastics
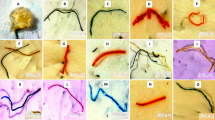
The university indoor ambient MP (coarse) samples were initially examined under an optical microscope for physical characterization. These ambient MPs were classified by shape and color (Fig. 1). Fibers, fragments, pellets, foams, films, and lines were the different MPs detected. The most common colors for these MPs were black, red, blue, transparent, magenta, and brown. Fragments were the most common type of MPs found indoors. However, it was difficult to determine the actual number of these identified MPs based on their morphological features. The sample content was very dense, and the chances of miscounting or mixing MPs with non-MP particles are always high. Hence, the main aim of using an optical microscope was to analyze whether these samples contained MPs or not. The general view of filters under the optical microscope is shown in the supplementary file (Fig. S1-S3). The samples were further analyzed for chemical and elemental characterization under micro-Raman and SEM-EDX. The fact that they were found indicates that the university’s indoor environments are sinks due to the airborne deposition of the MPs from outside. The identified MPs were characterized based on their size air pollution laboratory 1 (≥ 2.5–538.18 μm), department corridor (≥ 2.5–115.9 μm), researchers office 1 (≥ 2.5–678.59 μm), secretary office (≥ 2.5–89.53 μm), workers office (≥ 2.5–321.98 μm), students laboratory (≥ 2.5–282.41 μm), canteen corridor (≥ 2.5–285.96 μm), air pollution laboratory 2 (≥ 2.5–93.21 μm), and researchers office 2 (≥ 2.5–626.22 μm). The average size range of identified MPs in these indoor environments was ≥ 2.5–336.89 μm. The morphological changes in the MPs were evident upon examination using an optical microscope. The modifications involved the existence of cracks, fractured boundaries, ridges, grooves, and coarse and irregular surfaces. The presence of these prominent indicators signifies the deterioration of MPs, which will eventually result in the creation of smaller plastic particles, including nanoplastics, as time progresses. As a result, there will be a rise in the concentration of nanoplastics within indoor university environments.
Examples of some ambient microplastics found in the university indoor environments and classified by shape and color (A, B, C, I, J, L, N, O, P, R): Black, red, and blue fragments, (D, G, K, Q, S, W): Blue and black fibers, (E, H, V, X): Transparent, blue, and magenta, films, (F) Red pellet, (M, U) Brown and red foam, (T): Transparent line
Few previous works have reported the presence of MPs in indoor air but are limited to a few indoor environments. Indeed, the current work was the first to analyze nine indoor environments in the university for the presence of MPs. The results followed the literature findings of Dris et al. (2017), who identified the fiber type of MPs in apartments and offices with a size range of 50–4850 μm the first time. Gaston et al. (2020) found fiber and fragments in a university and a hospital with a size range of < 100 – > 3100 μm. Liao et al. (2021) characterized the fiber and fragments in an apartment, office, classroom, hospital, and transit station waiting hall with a 5–4665 μm size range. The lower limit of detection of MP size in all three studies was mainly higher than the lower size (2.5 μm) found in this study; however, the maximum average size (337 μm) found in this study was lower than all these studies. None of these studies have characterized the MPs based on color and have just characterized the MPs as fiber and fragment. This represents the difficulty in the characterizations of MPs. Significant differences are clear in the size ranges of MPs; this might be due to the usage of different types of instruments. Recently, **e et al. (2022) identified the MPs based on their color and have characterized one more MP type (bead) apart from fiber and fragment living room and office room. The lower size (2.40 μm) found by **e et al. (2022) was close to the lower size (2.5 μm) found in this study; however, their maximum size of MPs (2181.48 μm) was higher than the maximum average size (336.89 μm) found in this study.
Polymeric identification of ambient microplastics
The µRaman analysis of ambient university indoor samples showed the presence of different MPs. 25–30% of filters were scanned for the µRaman analysis, which showed that 20, 15, 19, 20, 20, 20, 19, 19, and 20 MPs were identified in nine university indoor samples. Although only 25–30% of the filter area was scanned under the µRaman, we consider that the filters’ MPs were equally distributed. A total of 25 different polymer types of MPs were identified. Their spectrums were plotted with the match degrees (Fig. 2) and (Fig. 3). These identified MPs include polyamide 66 (PA 66), PTFE, polybutadiene acrylonitrile (PBAN), ethylene vinyl acetate (EVA), 1 2 polybutadiene (1,2-PB), poly(ethylene oxide) (PEO), polyethylene terephthalate (PET), polyamide 12 (PA 12), polymethyl methacrylate (PMMA), polyacetal (POM), polyamide resin (PA resin), polyvinyl acetate (PVAc), polypropylene isotactic (PP-it), low-density polyethylene (LDPE), polymethylpentene (PMP), Poly(1 butene) isotactic (PB-1), polysulfone (PSU), polyamide 6 (PA 6), PE, high-density polyethylene (HDPE), PP, PVC, PE, 1-octene copolymer, ethylene propylene (EP), and polyisoprene (PI). The Anova single factor of the data for the identified MPs among the university indoor environments showed a significant difference (P = 0.02). These identified MPs have comprehensive source applications and are used daily. It was found that most of the sources were present in these indoor university environments, like textiles, food packaging, electronics, paint, shoe soles, adhesives, coatings, inks, ceramics, furnishings, plastic bags, printers, desktops etc. It is important to highlight the matching score of identified MPs (Fig. 2) and (Fig. 3). Few studies have addressed the matching score, and the findings have ranged from 27 to 97% (Cai et al. 2017; Tunahan Kaya et al. 2018; Liu et al. 2019a; Song et al. 2021). Matching scores can be significantly boosted by using more complete commercial libraries if the algorithm leverages multicomponent correlations in the matching process. Perhaps part of the difficulty is that commercial and custom libraries only include spectra from polymers that the environment has not degraded.
The distribution of ambient MPs varied among the indoor university environments (Fig. 4). The MPs found in most indoor university environments were PP, HDPE, PA 6, PMMA, PE, PEO, PTFE, PET, and PE, 1-octene copolymer. MPs identified under µRaman were further characterized based on their type and color (Fig. 5). The fiber was the dominant type of MP seen under µRaman. However, significant differences were seen in the colors. In ambient indoor university environments 1, 2, 3, 4, 5, 6, 7, 8, and 9, the concentrations of MPs were 18.51, 13.88, 17.59, 18.51, 18.51, 18.51, 17.59, 17.59, and 18.51 MPs/m3. These concentration results represent the whole filter area and the total amount of air collected. Based on the µRaman analysis, residents in these university indoor environments are exposed to airborne MPs (≥ 2.5–336.89 μm), with inhalation estimates ranging from 13.88 to 18.51 MPs/m3 and 180–240 MPs daily. In total, 171 particles were identified as MPs, consisting of 25 different types of MPs. According to the author, this is the first indoor study where 25 different MPs were found in indoor university environments using active sampling; only a standard or a limited number of MPs were seen in other indoor studies using active sampling. The results agreed with other findings like Gaston et al. (2020) found PVC, PE, PS, PC, PA, acrylonitrile butadiene styrene, resin, PET, and acrylic in university and hospital in California, United States, using µFTIR and µRaman. Liao et al. (2021) found PL, PA, PP, PE, PS, and PVC in apartment, office, classroom, hospital, and transit station waiting hall by using µFTIR. Recently, **e et al. (2022). identified PE, PL, resin, PVC, cotton, PP, polyurethane, and rubber using Raman in the living room and office room. However, differences were seen in the composition and concentration of MPs within these studies compared with the MPs found in this study; this might be due to the difference in the sampling locations, appropriate sources, sampling volume, and the type of instrument used. Only these three studies (Gaston et al. 2020; Liao et al. 2021; **e et al. 2022) have used active sampling to collect the MPs from university indoor environments. All these three studies have used different instruments (µRaman, µFTIR, and Raman) to analyze MPs.
Typical representation of ambient microplastics (coarse) seen under micro Raman and are categorized by type and color (PA66, 1,2 PB, PET, and PA 6); Transparent film, (PTFE, PEO, LDPE, and PP); Orange, red, fragment, and tyrian purple, (PBAN, PA 12, PMMA, PA resin, PVAc, PP-it, PB-1, PSU, PE, HDPE, PVC, PE, 1-octene copolymer, EP, and PI); Blue, black, transparent, pink, tyrian purple, and red fiber, (EVA and PMP), Brown foam and (POM); transparent pellet
Passive sampling has also been done in indoor environments like universities apart from active sampling. The results showed that the follow-up to these indoor passive sampling studies, like Yao et al. (2021), found that MPs like PS, PET, PE, PVC, and PP were identified in indoor environments like offices, hallways, classrooms, and single-family houses. Nematollahi et al. (2022) found PET, PP, and PS MPs in schools. The indoor exposure of MPs in different environments like kindergartens, primary schools, middle schools, high schools, and the university was conducted by Ouyang et al. (2021); five different MPs were found: PET, polyacrylonitrile, PVC, PP, and PA. Even MPs like polyacrylonitrile, PMMA, PE copolymer, acrylic, PP, PA, and PET have been seen in households (Jenner et al. 2021). Like active sampling studies, slight differences were seen in the MPs in these passive sampling studies compared with those in this study. Apart from the chemical characterization of MPs, it is noteworthy to identify the structural elements, additives added to them, or the additional contaminants they may carry because these pollutants make them extra toxic.
Identification of structural elements, additives, or adsorbed contaminants
The surface properties of MPs evaluated by SEM and the indicative elemental concentrations revealed by EDX in air pollution laboratory one are illustrated in Fig. 6. The common elements in MPs (A-line and B-fragment) of air pollution laboratory one were are carbon (C), oxygen (O), fluorine (F), aluminum (Al), silicon (Si), and gold (Au). C, O, F, and Si were dominant elements with high concentrations. However, the elements with lower concentrations were potassium (K), calcium (Ca), zinc (Zn), and Au.
Figure S4 depicts the results of the SEM-EDX analysis conducted on MPs (A-fragment, B-film) present in the Department of Environmental Engineering corridor. The common elements in these identified Env Eng department corridor MPs are C, O, (F), Al, Si, K, Ca, Zn, and Au. C, O, F, and Si were dominant elements with high concentrations. However, the elements with lower concentrations were K and Zn. The SEM-EDX analysis of MPs (A-fragment, B-fiber) in researchers office one is shown in Fig. S5. The common elements in these identified MPs of researchers office one were C, O, F, Al, Si, and Au. C, O, F, and Si were dominant elements with high concentrations. However, the elements with lower concentrations were sodium (Na), magnesium (Mg), Ca, and iron (Fe) in the fragment type of MP, while Au had a lower concentration in the fiber type of MP. Fig. S6 displays the results of the SEM-EDX elemental analysis conducted on MPs (A, B-fragment) found in the secretary’s office. The common elements in these identified MPs are C, O, F, Si, Ca, and Au. Dominant elements with high concentrations were C, O, F, and Ca. However, the elements with lower concentrations were Si, Au, Mg, Al, Si, and sulfur (S). The elemental analysis conducted by SEM-EDX of MPs (A-fiber and B-fragment) in the worker’s office is shown in Fig. S7. The common elements present in these identified MPs are C, O, F, Na, Mg, Si, S, chlorine (Cl), and Au. C, O, and F had the highest concentration in both MPs. However, the elements with lower concentrations were Na, Mg, Si, S, Cl, Ca, and Au. Figure S8 depicts the SEM-EDX analysis performed on MPs (A, B-fiber) present in the student’s laboratory. The common elements present in these identified MPs are C, O, F, Na, Mg, Al, Si, S, Cl, K, Ca, and Au. C, O, F, and Si had the highest concentration in both MPs. However, in MP (A-fiber), the elements with lower concentration were Mg, S, and K; in MP (B-fiber), the elements with lower concentration were Na, Mg, S, Cl, and Ca. The SEM-EDX analysis of MPs (A-fragment, B-pellet) in the canteen corridor is shown in Fig. S9. The common elements present in these identified MPs of the canteen corridor are C, O, F, Ca, and Au. C, O, F, and Ca had the highest concentration in both MPs. However, in MP (A-fragment), the Au had a lower concentration, while in the MP (B-pellet), the elements with lower concentrations were Mg, Si, Cl, niobium (Nb), and Au. The elemental analysis performed by SEM-EDX of MPs (A-line, B-fragment) in air pollution laboratory two is shown in Fig. S10. The common elements present in these identified MPs of air pollution laboratory two are C, O, F, and Au. C, O, F, and Si had the highest concentration, while Al, K, and Ca were in MP (A-line). However, in MP (B-fragment), C, O, F, Ni, and Cu had the highest concentrations, while Fe had the lowest concentrations. The SEM-EDX analysis of MPs (A-pellet, B-fragment) found in the researcher’s office two is shown in Fig. S11. The common elements in these identified MPs of researcher’s office two are C, O, F, and Au. C, O, F, Fe, and Au had the highest concentration, while chromium (Cr), manganese (Mn), and nickel (Ni) had the lowest concentration in MP (A-pellet). However, in MP (B-fragment), C, O, F, and Au had the highest concentrations, while Na, Cl, and Ca had the lowest concentrations.
C, O, F, Na, Cl, Al, Si, K, Ca, Zn, barium (Ba), Au, Mg, Fe, Cl, S, Nb, Cu, Ni, and Mn were the common elements seen in the identified MPs in university indoor environments. The presence of elements and their composition of the higher or minor percentage within MPs among the samples was not constant; variations were seen among these elements and percentages. This variation can be due to different MPs identified and their compositions or the presence of contaminants on their surface. SEM-EDX analysis also revealed that fragments were the dominant type of MPs. The margins of fiber, fragment, pellet, film, and line-type MPs are rougher and more irregular, consistent with the consequences of abrasion and disintegration. None of the indoor ambient MP studies have used SEM-EDX for the elemental analysis of MPs. However, our findings agreed with the SEM-EDX analysis of indoor dust samples in the classroom (Abbasi et al. 2022) and hospital, mosque, kindergarten, university, and house (Kashfi et al. 2022) and school (Nematollahi et al. 2022). The results followed the findings of Abbasi et al. (2022); they found C, nitrogen (N), O, Na, Al, Si, Cl, titanium (Ti), Mn, Cu, Zn, Antimony (Sb), and polybutene (Pb), while the C, N, O, Na, were the dominant elements. Furthermore, the results also agreed with the results of Kashfi et al. (2022), who found C and O as dominant elements in all MPs, while N, phosphorus, iodine, Cl, Al, Ca, Mg, Na, and Si were the other elements. Besides these two indoor dust studies, the results also agreed with the indoor dust samples of schools (Nematollahi et al. 2022). Nematollahi et al. (2022) found that MPs were composed of a high percentage of C and O with SEM-EDS, while the MPs had a minor percentage of other elements, including N, Na, Mg, Al, Si, Cl, Ti, Mn, copper (Cu), Zn, tin, Sb, mercury (Hg), and lead (Pb). C, N, and O are the predominant elements, reflecting the polymeric matrix’s composition.
Although EDX cannot differentiate between different types of association, relatively high concentrations of certain metals in some samples (F, Si, Mg, Ca, Ni, and Cu) likely reflect the presence of contemporary and historical additives and catalytic residues in polymeric materials. On the other hand, lower and more uniform concentrations of elements that are not frequently added to plastics and/or are more indicative of geogenic material (such as Al, Mg, and Na) were found to have been captured from the environment. In addition, other components may be present in the plastic either as functional additives or reaction residues or as components of extraneous material that is stuck to or adsorbed onto the surface of the plastic. High quantities of C, O, and plastic-specific chemical components demonstrate the correct identification of MPs (e.g., Cl in PVC) (Abbasi 2021). The presence of elements Al, Si, Na, and Mg on MPs are dominant constituents of silicate minerals (e.g., clays) and can likely be caused by silicates adsorbed onto the surface of these polymeric particles. Al, Ca, Si, and Mg, which mainly originated from natural materials such as soil or dust together with Cu and Zn from anthropogenic sources (such as burning fossil fuels and abrasing vehicles) (Arslan 2001; Abbasi et al. 2020), can also adhere to the surface of plastic particles. Na, Mg, K, Al, Si, Ca, Cl, and O adhere to the MP surface (Ganesan et al. 2019). Zn is a well-known urban element and likely originated from anthropogenic activities, including traffic-related and industrial activities (Ahmady-Birgani et al. 2015; Nematollahi et al. 2021). Fe is also widely used as an additive in plastic materials to achieve desired properties, such as colored plastic (Nematollahi et al. 2020). Al, Si, Na, and Mg are likely adsorbed onto the surface of MPs, and silicate minerals such as clays may cause their presence (Nematollahi et al. 2021). To achieve a wide range of colors, textures, and functionality, a wide variety of elements (Si, Zn, Al, and Fe) have been used in paints, which might be pigments, binders, or additives (Kowalczyk et al. 2012; Lopez et al. 2023; Pfaff 2021; Zuin et al. 2014). As these kinds of additives are not chemically bonded to the polymeric matrix, they can be released into the environment due to the weathering process (Hahladakis et al. 2018; Bhat et al. 2023b) and make MPs more toxic. F is used in toothpaste, mouthwash, and the manufacturing of Teflon, which is a nonstick coating for cookware (Vranic et al. 2004; McKeen 2012), while Ba is used in the production of glass and ceramics (Puig et al. 2017); there are chances that F and Ba might have got adsorbed on the surfaces of MPs. Minerals like gypsum contain S naturally (Kong et al. 2020), and these gypsums are used inside indoor university environments. Ni, Cu, Nb, and Mn are used in the production of electronic components (Montero et al. 2012; Bella et al. 2021; Jadhao et al. 2021).
Comparison with literature
MP research has attracted huge attention over the last decade; however, indoor work is limited (Table 1). Only a few research on indoor dust, air, and deposition have been conducted so far, and the types and quantity of indoor environments are likewise restricted. Researchers have adopted different methods for the characterization and optimization of MPs, from techniques to unit measurements (Table 1). This study used an optical microscope, µRaman, and SEM-EDX. The active sampling has been done in a limited number of indoor environments compared to the indoor environments studied in this research, and none of the work has used SEM-EDX to analyze MPs (Table 1). This is the first study where morphological characterization was done by optical microscope, polymeric composition by µRaman, and structural elements or additives, or vectors that are added or adsorbed were done by SEM-EDX instrument simultaneously. Until now, none of the studies used SEM-EDX or these three instruments simultaneously. Other indoor active sampling studies used Raman or FTIR or a combination of a stereomicroscope with Raman or FTIR (Table 1). To characterize the MPs adequately based on their morphology, polymeric composition, structural elements or additives, or added or adsorbed vectors, an optical microscope, FTIR or Raman, and SEM-EDX based on the size of MPs should be used simultaneously. Although all the active indoor sampling studies focused on the micrometer size range, the size range of MPs in this study was relatively lower than the other active sampling studies. None of the studies has done size segregation of indoor ambient MPs (Table 1). Moreover, the MPs’ colors and types identified in this study were higher than in the other active sampling indoor studies, as they mostly identified fiber and fragment types of MPs, and most of the studies did not characterize MPs based on their color. This shows the difficulty in characterizing indoor ambient MPs, as there is a vast gap in the methodology of ambient MPs research. However, differences were seen in the abundance of MPs with the other active sampling indoor microplastic studies (Table 1); this might be due to the difference in the flow rate and duration of sample collection.
People are becoming more worried about MP pollution, and many questions about MPs in indoor environments still need to be answered. One of the biggest problems is that no standards exist to take samples and analyze MPs in indoor environments. This makes it hard to compare the results of different studies. Another gap is that researchers have only looked at a few indoor environments. Some studies have looked at homes, while others have looked at schools, offices, and public buildings. However, there are still a lot of indoor places that have not been looked at in detail. Also, not enough is known about where indoor MPs come from. Some studies have found that indoor sources like plastic products and textiles may be a big part of the problem. However, there is still much to learn about how MPs get into indoor environments and how they move around. Lastly, more studies must be conducted on the possible health effects of MPs in indoor environments. There is some proof that MPs may harm human health. However, more study is needed to determine how severe these effects are and how to stop them.
Conclusion
The research affirms the widespread occurrence of MPs in university indoor environments. The levels of MP contamination vary greatly throughout indoor areas, suggesting that variables such as building materials and ventilation have a substantial impact. The research highlights the need for more health-related studies due to possible human exposure to MPs in indoor environments. The many types of MPs identified in samples taken from university indoor environments included fibers, fragments, pellets, foams, films, and lines. The predominant colors for the MPs were black, red, blue, transparent, magenta, and brown. Indoor fragments were the dominant form of MPs detected. The mean size of the detected MPs in various indoor settings ranged from ≥ 2.5 to 336.89 μm. Previous studies have provided little information on the occurrence of MPs in indoor air, focusing on just a few indoor environments. Twenty-five distinct varieties of MPs were identified. According to the µRaman study, people living in these indoor environments at the university are exposed to airborne MPs (≥ 2.5–336.89 μm) with estimated inhalation rates ranging from 13.88 to 18.51 MPs/m3 and a daily intake of 180 to 240 MPs. The most often seen MPs in indoor university environments are PP, HDPE, PA 6, PMMA, PE, PEO, PTFE, PET, and PE, 1-octene copolymer. MPs detected by µRaman were further classified according to their type and color. The detected MPs in university indoor environments often consisted of C, O, F, Na, Cl, Al, Si, K, Ca, Zn, Ba, Au, Mg, Fe, Cl, S, F, Nb, Cu, Ni, and Mn. It was discovered that the MP samples’ homogeneity of the presence and proportion of constituent elements was variable, showing noticeable differences between the elements and their percentages. The observed differences may be attributed to the various categories of MPs present, their compositions, or the presence of pollutants that cling to their surfaces. An SEM-EDX investigation revealed that fragments are the predominant form of MP. The edges of MPs with the fiber, fragment, pellet, film, and line types all had ragged and crooked surfaces, indicating abrasion and disintegration. SEM-EDX has not been employed in recent studies investigating the levels of MPs in indoor environments to ascertain the elemental composition of MP samples.
Universities must implement mitigation strategies to address MP contamination, including material choices and indoor air quality improvements. Promoting awareness within university communities about the issue of indoor MPs and encouraging environmentally responsible behaviors is essential. Future research should identify sources, pathways, and long-term impacts of indoor MP contamination and evaluate mitigation measures. The human MP exposure level through inhalation and MP distribution pattern in indoor environments requires more investigations in the future, and their harmful effects on the human body should be investigated. Addressing indoor MPs is part of the global effort to combat MP pollution across different environments. Commitment to sustainable practices, including plastic reduction and recycling, is vital for mitigating MP pollution indoors and in the broader environment.
Data availability
The author confirms that the data supporting the findings of this study are available in the article.
References
Abbasi S (2021) Prevalence and physicochemical characteristics of microplastics in the sediment and water of Hashilan Wetland, a national heritage in NW Iran. Environ Technol Innov 23:101782. https://doi.org/10.1016/j.eti.2021.101782
Abbasi S, Keshavarzi B, Moore F et al (2020) Elemental and magnetic analyses, source identification, and oxidative potential of airborne, passive, and street dust particles in Asaluyeh County, Iran. Sci Total Environ 707:136132. https://doi.org/10.1016/j.scitotenv.2019.136132
Abbasi S, Turner A, Hoseini M, Amiri H (2021) Microplastics in the Lut and Kavir deserts, Iran. Environ Sci Technol 55:5993–6000. https://doi.org/10.1021/acs.est.1c00615
Abbasi S, Turner A, Sharifi R et al (2022) Microplastics in the school classrooms of Shiraz, Iran. Build Environ 207:108562. https://doi.org/10.1016/j.buildenv.2021.108562
Ahmady-Birgani H, Mirnejad H, Feiznia S, McQueen KG (2015) Mineralogy and geochemistry of atmospheric particulates in western Iran. Atmos Environ 119:262–272. https://doi.org/10.1016/j.atmosenv.2015.08.021
Allen S, Allen D, Phoenix VR et al (2019) Atmospheric transport and deposition of microplastics in a remote mountain catchment. Nat Geosci 12:339–344. https://doi.org/10.1038/s41561-019-0335-5
Amato-Lourenço LF, dos Santos Galvão L, Wiebeck H et al (2022) Atmospheric microplastic fallout in outdoor and indoor environments in São Paulo megacity. Sci Total Environ 821. https://doi.org/10.1016/j.scitotenv.2022.153450
Arslan H (2001) Heavy metals in street dust in BURSA, TURKEY. J Trace Microprobe Tech 19:439–445. https://doi.org/10.1081/TMA-100105058
Bella F, De Luca S, Fagiolari L et al (2021) An overview on anodes for magnesium batteries: challenges towards a promising storage solution for renewables. Nanomaterials 11:1–29. https://doi.org/10.3390/nano11030810
Bhat MA (2023a) Identification and characterization of microplastics in indoor environments. Eskişehir Technical University. http://dx.doi.org/10.13140/RG.2.2.22164.88960
Bhat MA (2023b) Indoor microplastics: a comprehensive review and bibliometric analysis. Environ Sci Pollut Res 30:121269–121291. https://doi.org/10.1007/s11356-023-30902-0
Bhat MA (2024a) Indoor Microplastics and Microfibers Sources and Impacts on Human Health. In: Rathinamoorthy R, Balasaraswathi SR (eds) Microfibre Pollution from Textiles Research Advances and Mitigation Strategies, 1st Editio. CRC Press, pp 285–307. http://dx.doi.org/10.1201/9781003331995-16
Bhat MA (2024b) Unveiling the overlooked threat: macroplastic pollution in indoor markets in an urban city. Case Stud Chem Environ Eng 9:100558. https://doi.org/10.1016/j.cscee.2023.100558
Bhat MA, Eraslan FN, Gedik K, Gaga EO (2021) Impact of Textile Product emissions: Toxicological considerations in assessing indoor Air Quality and Human Health. In: Malik JA, Marathe S (eds) Ecological and Health effects of Building materials, 1st edn. Springer Nature Switzerland, pp 505–541. http://dx.doi.org/10.1007/978-3-030-76073-1_27
Bhat MA, Eraslan FN, Awad A et al (2022a) Investigation of indoor and outdoor air quality in a university campus during COVID-19 lock down period. Build Environ J 219:109176. https://doi.org/10.1016/j.buildenv.2022.109176
Bhat MA, Gedik K, Gaga EO (2022b) Environmental Toxicity of Emerging Micro and nanoplastics: a lesson learned from nanomaterials. In: Dar AH, Nayik GA (eds) Nanotechnology interventions in Food Packaging and Shelf Life, 1st edn. Taylor & Francis (CRC, pp 311–337. http://dx.doi.org/10.1201/9781003207641-18
Bhat MA, Eraslan FN, Gaga EO, Gedik K (2023a) Scientometric Analysis of Microplastics across the Globe. In: Vithanage M, Prasad MNV (eds) Microplastics in the Ecosphere: Air, Water, Soil, and Food, 1st edn. John Wiley & Sons Ltd, pp 3–13. http://dx.doi.org/10.1002/9781119879534.ch1
Bhat MA, Gedik K, Gaga EO (2023b) A preliminary study on the natural aging behavior of microplastics in indoor and outdoor environments. Int J Environ Sci Technol 21:1923–1936. https://doi.org/10.1007/s13762-023-05319-4
Bhat MA, Gedik K, Gaga EO (2023c) Atmospheric micro (nano) plastics: future growing concerns for human health. Air Qual Atmos Heal 16:233–262. https://doi.org/10.1007/s11869-022-01272-2
Bhat MA, Gaga EO, Gedik K (2024) How can contamination be prevented during laboratory analysis of atmospheric samples for microplastics ? Environ Monit Assess 196:1–15. https://doi.org/10.1007/s10661-024-12345-3
Cabernard L, Roscher L, Lorenz C et al (2018) Comparison of Raman and Fourier Transform Infrared Spectroscopy for the quantification of Microplastics in the aquatic environment. Environ Sci Technol 52:13279–13288. https://doi.org/10.1021/acs.est.8b03438
Cai L, Wang J, Peng J et al (2017) Characteristic of microplastics in the atmospheric fallout from Dongguan city, China: preliminary research and first evidence. Environ Sci Pollut Res 24:24928–24935. https://doi.org/10.1007/s11356-017-0116-x
Can E, Üzmez ÖÖ, Döǧeroǧlu T, Gaga EO (2015) Indoor air quality assessment in painting and printmaking department of a fine arts faculty building. Atmos Pollut Res 6:1035–1045. https://doi.org/10.1016/j.apr.2015.05.008
Chen EY, Lin KT, Jung CC et al (2022) Characteristics and influencing factors of airborne microplastics in nail salons. Sci Total Environ 806:151472. https://doi.org/10.1016/j.scitotenv.2021.151472
Choi H, Lee I, Kim H et al (2022) Comparison of microplastic characteristics in the indoor and Outdoor Air of Urban areas of South Korea. Water Air Soil Pollut 233:1–10. https://doi.org/10.1007/s11270-022-05650-5
Cowger W, Steinmetz Z, Gray A et al (2021) Microplastic Spectral classification needs an Open Source Community: open specy to the rescue! Anal Chem 93:7543–7548. https://doi.org/10.1021/acs.analchem.1c00123
Dong H, Wang L, Wang X et al (2021) Microplastics in a remote lake basin of the Tibetan Plateau: impacts of atmospheric transport and glacial melting. Environ Sci Technol 55:12951–12960. https://doi.org/10.1021/acs.est.1c03227
Dris R, Gasperi J, Mirande C et al (2017) A first overview of textile fibers, including microplastics, in indoor and outdoor environments. Environ Pollut 221:453–458. https://doi.org/10.1016/j.envpol.2016.12.013
Eraslan FN, Bhat MA, Gaga EO, Gedik K (2021) Comprehensive Analysis of Research Trends in Volatile Organic compounds emitted from building materials: a bibliometric analysis. In: Malik JA, Marathe S (eds) Ecological and Health effects of Building materials, 1st edn. Springer Nature Switzerland, pp 87–109. http://dx.doi.org/10.1007/978-3-030-76073-1_6
Eraslan FN, Bhat MA, Gedik K, Gaga EO (2023) The single-use Plastic Pandemic in the COVID-19 era. In: Vithanage M, Prasad MNV (eds) Microplastics in the Ecosphere: Air, Water, Soil, and Food, 1st edn. John Wiley & Sons Ltd, pp 65–75. http://dx.doi.org/10.1002/9781119879534.ch4
Fang M, Liao Z, Ji X et al (2022) Microplastic ingestion from atmospheric deposition during dining/drinking activities. J Hazard Mater 432:128674. https://doi.org/10.1016/j.jhazmat.2022.128674
Ganesan M, Nallathambi G, Srinivasalu S (2019) Fate and transport of microplastics from water sources. Curr Sci 117:1879–1885. https://doi.org/10.18520/cs/v117/i11/1874-1879
Gaston E, Woo M, Steele C et al (2020) Microplastics Differ between Indoor and Outdoor Air masses: insights from multiple Microscopy methodologies. Appl Spectrosc 74:1079–1098. https://doi.org/10.1177/0003702820920652
Habibi N, Uddin S, Fowler SW, Behbehani M (2022) Microplastics in the atmosphere: a review. J Environ Expo Assess 1:1–20. https://doi.org/10.20517/jeea.2021.07
Hahladakis JN, Velis CA, Weber R et al (2018) An overview of chemical additives present in plastics: Migration, release, fate and environmental impact during their use, disposal and recycling. J Hazard Mater 344:179–199. https://doi.org/10.1016/j.jhazmat.2017.10.014
Jadhao PR, Pandey A, Pant KK, Nigam KDP (2021) Efficient recovery of Cu and Ni from WPCB via alkali leaching approach. J Environ Manage 296:113154. https://doi.org/10.1016/j.jenvman.2021.113154
Jenner LC, Sadofsky LR, Danopoulos E, Rotchell JM (2021) Household indoor microplastics within the Humber region (United Kingdom): quantification and chemical characterisation of particles present. Atmos Environ 259:118512. https://doi.org/10.1016/j.atmosenv.2021.118512
Käppler A, Fischer D, Oberbeckmann S et al (2016) Analysis of environmental microplastics by vibrational microspectroscopy: FTIR, Raman or both? Anal Bioanal Chem 408:8377–8391. https://doi.org/10.1007/s00216-016-9956-3
Kashfi FS, Ramavandi B, Arfaeinia H et al (2022) Occurrence and exposure assessment of microplastics in indoor dusts of buildings with different applications in Bushehr and Shiraz cities, Iran. Sci Total Environ 829:154651. https://doi.org/10.1016/j.scitotenv.2022.154651
Kong M, Liu Q, Fan C et al (2020) Separating sulfur from fuel gas desulfurization gypsum with an oxalic acid solution. ACS Omega 5:16932–16939. https://doi.org/10.1021/acsomega.0c02172
Kowalczyk K, Łuczka K, Grzmil B, Spychaj T (2012) Anticorrosive polyurethane paints with nano- and microsized phosphates. Prog Org Coat 74:151–157. https://doi.org/10.1016/j.porgcoat.2011.12.003
Liao Z, Ji X, Ma Y et al (2021) Airborne microplastics in indoor and outdoor environments of a coastal city in Eastern China. J Hazard Mater 417:126007. https://doi.org/10.1016/j.jhazmat.2021.126007
Liong RMY, Hadibarata T, Yuniarto A et al (2021) Microplastic Occurrence in the Water and Sediment of Miri River Estuary, Borneo Island. Water Air Soil Pollut 232:342. https://doi.org/10.1007/s11270-021-05297-8
Liu K, Wang X, Fang T et al (2019a) Source and potential risk assessment of suspended atmospheric microplastics in Shanghai. Sci Total Environ 675:462–471. https://doi.org/10.1016/j.scitotenv.2019.04.110
Liu K, Wu T, Wang X et al (2019b) Consistent Transport of Terrestrial Microplastics to the Ocean through Atmosphere. Environ Sci Technol 53:10612–10619. https://doi.org/10.1021/acs.est.9b03427
Lopez GP, Gallegos MV, Peluso MA et al (2023) ZnO recovered from spent alkaline batteries as antimicrobial additive for waterborne paints. Emergent Mater 6:147–158. https://doi.org/10.1007/s42247-022-00443-2
Mark JE (2009) Polymer Data Book, 2nd edn. Oxford University Press
McKeen LW (2012) Fluoropolymers. In: Permeability Properties of Plastics and Elastomers. pp 195–231
Montero R, Guevara A, De La Torre E (2012) Recovery of gold, silver, copper and niobium from printed circuit boards using leaching column technique. J Earth Sci Eng 2:590–595
Nava V, Frezzotti ML, Leoni B (2021) Raman Spectroscopy for the analysis of microplastics in aquatic systems. Appl Spectrosc 75:1341–1357. https://doi.org/10.1177/00037028211043119
Nematollahi MJ, Moore F, Keshavarzi B et al (2020) Microplastic particles in sediments and waters, south of Caspian Sea: frequency, distribution, characteristics, and chemical composition. Ecotoxicol Environ Saf 206:111137. https://doi.org/10.1016/j.ecoenv.2020.111137
Nematollahi MJ, Dehdaran S, Moore F, Keshavarzi B (2021) Potentially toxic elements and polycyclic aromatic hydrocarbons in street dust of Yazd, a central capital city in Iran: contamination level, source identification, and ecological–health risk assessment. Springer Netherlands
Nematollahi MJ, Zarei F, Keshavarzi B et al (2022) Microplastic occurrence in settled indoor dust in schools. Sci Total Environ 807:150984. https://doi.org/10.1016/j.scitotenv.2021.150984
Ouyang Z, Mao R, Hu E et al (2021) The indoor exposure of microplastics in different environments. Gondwana Res 108:193–199. https://doi.org/10.1016/j.gr.2021.10.023
Pfaff G (2021) Iron oxide pigments. Phys Sci Rev 6:535–548. https://doi.org/10.1515/psr-2020-0179
Prata JC (2018) Airborne microplastics: consequences to human health? Environ Pollut 234:115–126. https://doi.org/10.1016/j.envpol.2017.11.043
Prata JC, Castro JL, da Costa JP et al (2020a) The importance of contamination control in airborne fibers and microplastic sampling: experiences from indoor and outdoor air sampling in Aveiro, Portugal. Mar Pollut Bull 159:111522. https://doi.org/10.1016/j.marpolbul.2020.111522
Prata JC, da Costa JP, Lopes I et al (2020) Environmental exposure to microplastics: an overview on possible human health effects. Sci Total Environ 702:134455. https://doi.org/10.1016/j.scitotenv.2019.134455
Puig J, Prange A, Arati B et al (2017) Optimization of the synthesis route of a barium boron aluminosilicate sealing glass for SOFC applications. Ceram Int 43:9753–9758. https://doi.org/10.1016/j.ceramint.2017.04.151
Song Z, Liu K, Wang X et al (2021) To what extent are we really free from airborne microplastics? Sci Total Environ 754:142118. https://doi.org/10.1016/j.scitotenv.2020.142118
Thacharodi A, Hassan S, Meenatchi R et al (2024a) Mitigating microplastic pollution: a critical review on the effects, remediation, and utilization strategies of microplastics. J Environ Manage 351:119988. https://doi.org/10.1016/j.jenvman.2023.119988
Thacharodi A, Meenatchi R, Hassan S et al (2024b) Microplastics in the environment: a critical overview on its fate, toxicity, implications, management, and bioremediation strategies. J Environ Manage 349:119433. https://doi.org/10.1016/j.jenvman.2023.119433
Thompson RC, Olsen Y, Mitchell RP et al (2004) Lost at sea: where is all the plastic? Sci (80-) 304:838–838. https://doi.org/10.1111/sum.12058
Tunahan Kaya A, Yurtsever M, Çiftçi Bayraktar S (2018) Ubiquitous exposure to microfiber pollution in the air. Eur Phys J Plus. https://doi.org/10.1140/epjp/i2018-12372-7. 133:
Uddin S, Fowler SW, Habibi N et al (2022) A preliminary assessment of size-fractionated microplastics in indoor aerosol — Kuwait ’ s baseline. Toxics 10:2–17
Vianello A, Jensen RL, Liu L, Vollertsen J (2019) Simulating human exposure to indoor airborne microplastics using a Breathing Thermal Manikin. Sci Rep 9:1–11. https://doi.org/10.1038/s41598-019-45054-w
Vranic E, Lacevic A, Mehmedagic A, Uzunovic A (2004) Mouthwash and toothpaste formulation. Bosn J Basic Med Sci 4:51–58
Wang X, Li C, Liu K et al (2020) Atmospheric microplastic over the South China Sea and East Indian Ocean: abundance, distribution and source. J Hazard Mater 389:121846. https://doi.org/10.1016/j.jhazmat.2019.121846
Wright SL, Kelly FJ (2017) Plastic and human health: a Micro Issue? Environ Sci Technol 51:6634–6647. https://doi.org/10.1021/acs.est.7b00423
**e Y, Li Y, Feng Y et al (2022) Inhalable microplastics prevails in air: exploring the size detection limit. Environ Int 162:107151. https://doi.org/10.1016/j.envint.2022.107151
Yao Y, Glamoclija M, Murphy A, Gao Y (2021) Characterization of microplastics in indoor and ambient air in northern New Jersey. Environ Res 112142. https://doi.org/10.1016/j.envres.2021.112142
Zhang Y, Wu P, Xu R et al (2023) Plastic waste discharge to the global ocean constrained by seawater observations. Nat Commun 14:1372. https://doi.org/10.1038/s41467-023-37108-5
Zuin S, Gaiani M, Ferrari A, Golanski L (2014) Leaching of nanoparticles from experimental water-borne paints under laboratory test conditions. J Nanoparticle Res 16:1–17. https://doi.org/10.1007/s11051-013-2185-1
Acknowledgements
This work is a part of my Ph.D. dissertation. I am very thankful to my supervisor, Eftade Emine Gaga (Ph.D.), and co-supervisor, Kadir Gedik (Ph.D.), for their support during my Ph.D. dissertation. The author further expresses his gratitude to Gül Ipek Selimoğlu (Ph.D.) Feridun AY (Ph.D.), Nihan Kosku Perkgöz (Ph.D.), and Servet Turan (Ph.D.) for providing access to an optic microscope, µRaman microscope and SEM-EDX, and Akif Ari (Ph.D.) for providing filters and a pump for the sampling.
Funding
The present research received support from the Research Fund of Eskişehir Technical University under project 21DRP106. I am grateful to the Presidency for Turks Abroad and Related Communities for providing me with the Ph.D. grant.
Open access funding provided by the Scientific and Technological Research Council of Türkiye (TÜBİTAK).
Author information
Authors and Affiliations
Contributions
Mansoor Ahmad Bhat: Conceptualization; Data curation; Formal analysis; Funding acquisition; Investigation; Methodology; Project administration; Resources; Software; Supervision; Validation; Visualization; Roles/Writing - original draft; and Writing - review & editing.
Corresponding author
Ethics declarations
Conflict of interest
The author has no conflicts of interest to declare.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bhat, M.A. Airborne microplastic contamination across diverse university indoor environments: A comprehensive ambient analysis. Air Qual Atmos Health (2024). https://doi.org/10.1007/s11869-024-01548-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11869-024-01548-9