Abstract
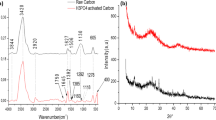
The present study investigated both the feasibility of activated carbon (AC) production from olive tree branches and its application as an adsorbent for aqueous phase phenol removal. The response surface methodology (RSM) based modeling approach was also employed for both activated carbon production and phenol adsorption process optimization. Several olive branches activated carbon (OBAC) samples were synthesized under a varying set of chemical activation conditions. The OBAC synthesized at carbonation temperature of 700 °C, impregnation ratio (I-R) of 2.0 mL/g and acid strength of 60% yielded maximum BET specific surface area (SSA) of 1420 m2/g along with an average pore diameter of 43.46 Å. The study also revealed that the produced activated carbon has a high thermal stability and possesses several active surface functional groups. Furthermore, the application of olive branches activated carbon (with the highest SSA) showed good phenol removal efficiency from synthetic wastewater under a varying set of operating conditions for pH, initial phenol concentration and adsorbent dosage. The kinetic study showed that the adsorption process follows pseudo-First-order kinetics (R2 = 0.9872) with a rate constant of 0.127 min−1. The developed RSM models for activated carbon production and phenol adsorption had R2 values of 0.9829 and 0.9113, respectively, with good modeling and optimization outcomes. In summary, the present results show that the olive branches-based activated carbon possesses a high specific surface area and can be successfully used as an adsorbent for toxic aqueous phase pollutants such as phenol under a varying set of process conditions.







Similar content being viewed by others
References
Abdelwahab O, Amin NK (2013) Adsorption of phenol from aqueous solutions by Luffa cylindrica fibers: kinetics, isotherm and thermodynamic studies. Egypt J Aquat Res 39:215–223. https://doi.org/10.1016/j.ejar.2013.12.011
Acosta KZ et al (2019) Immobilization of p. stutzeri on activated carbons for degradation of hydrocarbons from oil-in-saltwater emulsions. Nanomaterials 9:1–15. https://doi.org/10.3390/nano9040500
Afsharnia M et al (2016) Phenol removal from aqueous environment by adsorption onto pomegranate peel carbon. Electron Phys 8:3248–3256. https://doi.org/10.19082/3248
Ashouri R et al (2021) Dynamic and static removal of benzene from air based on task-specific ionic liquid coated on MWCNTs by sorbent tube-headspace solid-phase extraction procedure. Int J Environ Sci Technol 18:2377–2390. https://doi.org/10.1007/s13762-020-02995-4
Baytar O et al (2020) High-performance gas-phase adsorption of benzene and toluene on activated carbon: response surface optimization, reusability, equilibrium, kinetic, and competitive adsorption studies. Environ Sci Pollut Res 27:26191–26210. https://doi.org/10.1007/s11356-020-08848-4
Bohli T et al (2013) Adsorption on activated carbon from olive stones: kinetics and equilibrium of phenol removal from aqueous solution. J Chem Eng Process Technol. https://doi.org/10.4172/2157-7048.1000165
Cuhadaroglu D, Uygun OA (2008) Production and characterization of activated carbon from a bituminous coal by chemical activation. Afr J Biotech 7:3706–3713. https://doi.org/10.4314/ajb.v7i20.59416
Dastgheib SA, Karanfil T, Cheng W (2004) Tailoring activated carbons for enhanced removal of natural organic matter from natural waters. Carbon 42:547–557. https://doi.org/10.1016/j.carbon.2003.12.062
Duarte CM, Jaremko L, Jaremko M (2020) Hypothesis: potentially systemic impacts of elevated CO2 on the human proteome and health. Front Public Health. https://doi.org/10.3389/fpubh.2020.543322
Goher ME et al (2015) Removal of aluminum, iron and manganese ions from industrial wastes using granular activated carbon and Amberlite IR-120H. Egypt J Aquat Res 41:155–164. https://doi.org/10.1016/j.ejar.2015.04.002
Hamadneh I, Abu-Zurayk RA, Al-Dujaili AH (2020) Removal of phenolic compounds from aqueous solution using MgCl2-impregnated activated carbons derived from olive husk: the effect of chemical structures. Water Sci Technol 81:2351–2367. https://doi.org/10.2166/wst.2020.297
Heydari M et al (2018) Data for efficiency comparison of raw pumice and manganese-modified pumice for removal phenol from aqueous environments—application of response surface methodology. Data Brief 20:1942–1954. https://doi.org/10.1016/j.dib.2018.09.027
Jibril B et al (2008) Effects of H3PO4 and KOH in carbonization of lignocellulosic material. J Anal Appl Pyrol 83:151–156. https://doi.org/10.1016/j.jaap.2008.07.003
Khare P, Kumar A (2012) Removal of phenol from aqueous solution using carbonized Terminalia chebula-activated carbon: process parametric optimization using conventional method and Taguchi’s experimental design, adsorption kinetic, equilibrium and thermodynamic study. Appl Water Sci 2:317–326. https://doi.org/10.1007/s13201-012-0047-0
Kumar A, Jena HM (2016) Preparation and characterization of high surface area activated carbon from Fox nut (Euryale ferox) shell by chemical activation with H3PO4. Results in Physics 6:651–658. https://doi.org/10.1016/j.rinp.2016.09.012
Kutluay S, Temel F (2021) Silica gel based new adsorbent having enhanced VOC dynamic adsorption/desorption performance. Colloids Surf, A 609:125848. https://doi.org/10.1016/j.colsurfa.2020.125848
Lachowicz JI et al (2020) Improving metal adsorption on triethylenetetramine (TETA) functionalized SBA-15 mesoporous silica using potentiometry, EPR and ssNMR. Adv. Mater. Interfaces. doi: https://doi.org/10.1002/admi.202000544.
Lorenc-Grabowska E (2016) Effect of micropore size distribution on phenol adsorption on steam activated carbons. Adsorption 22:599–607. https://doi.org/10.1007/s10450-015-9737-x
Lu Q, Sorial GA (2004) The role of adsorbent pore size distribution in multicomponent adsorption on activated carbon. Carbon 42:3133–3142. https://doi.org/10.1016/j.carbon.2004.07.025
Makoś P, Słupek E, Małachowska A (2020) Silica gel impregnated by deep eutectic solvents for adsorptive removal of BTEX from Gas Streams. Materials 13:1894. https://doi.org/10.3390/MA13081894
Mameri N et al (2000) Preparation of activated carbon from olive mill solid residue. J Chem Technol Biotechnol 75:625–631. https://doi.org/10.1002/1097-4660(200007)75:7%3c625::AID-JCTB257%3e3.0.CO;2-9
Molina-Sabio M, Gonçalves M, Rodríguez-Reinoso F (2011) Oxidation of activated carbon with aqueous solution of sodium dichloroisocyanurate: effect on ammonia adsorption. Microporous Mesoporous Mater 142:577–584. https://doi.org/10.1016/j.micromeso.2010.12.045
Moradi M et al (2016) Response surface methodology (RSM) and its application for optimization of ammonium ions removal from aqueous solutions by pumice as a natural and low cost adsorbent. Arch Environ Protect 42:33–43. https://doi.org/10.1515/aep-2016-0018
Moradi M et al (2018) Kinetic and modeling data on phenol removal by Iron-modified Scoria Powder (FSP) from aqueous solutions. Data Brief 20:957–968. https://doi.org/10.1016/j.dib.2018.08.068
Nouri M (2021) Potentials and challenges of date pits as alternative environmental clean-up ingredients. Biomass Convers Biorefinery. https://doi.org/10.1007/s13399-020-01215-w
Ould-Idriss A et al (2011) Preparation of activated carbons from olive-tree wood revisited. I. Chemical activation with H3PO4. Fuel Process Technol 92:261–265. https://doi.org/10.1016/j.fuproc.2010.05.011
Rahman MM et al (2014) Removal of heavy metal ions with acid activated carbons derived from oil palm and coconut shells. Materials 7:3634–3650. https://doi.org/10.3390/ma7053634
Raissi S, Farsani RE (2009) Statistical process optimization through multi-response surface methodology. World Acad Sci Eng Technol 39:280–284
Shirzad-Siboni M et al (2013) Removal of phenol from aqueous solutions by activated red mud: equilibrium and kinetics studies. Environ Eng Res 18:247–252. https://doi.org/10.4491/eer.2013.18.4.247
Tan KL, Hameed BH (2017) Insight into the adsorption kinetics models for the removal of contaminants from aqueous solutions. J Taiwan Inst Chem Eng 74:25–48. https://doi.org/10.1016/j.jtice.2017.01.024
Tsang DCW et al (2007) Activated carbon produced from waste wood pallets: adsorption of three classes of dyes. Water Air Soil Pollut 184:141–155. https://doi.org/10.1007/s11270-007-9404-2
Vohra M, Al-Suwaiyan M, Hussaini M (2020) Gas phase toluene adsorption using date palm-tree branches based activated carbon. Int J Environ Res Public Health 17:9287. https://doi.org/10.3390/ijerph17249287
Vohra MS (2015) Adsorption-based removal of gas-phase benzene using granular activated carbon (GAC) produced from date palm pits. Arab J Sci Eng 40:3007–3017. https://doi.org/10.1007/s13369-015-1683-0
Vohra MS, Labaran BA (2020) Photocatalytic treatment of mixed selenocyanate and phenol streams: process modeling, optimization, and kinetics. Environ Prog Sustain Energy 39:1–11. https://doi.org/10.1002/ep.13401
**e B et al (2020) Adsorption of phenol on commercial activated carbons: Modelling and interpretation. Int J Environ Res Public Health 17:789. https://doi.org/10.3390/ijerph17030789
Yadav N et al (2020) Adsorption and equilibrium studies of phenol and para-nitrophenol by magnetic activated carbon synthesised from cauliflower waste. Environ Eng Res 25:742–752. https://doi.org/10.4491/eer.2019.238
Yuney K et al (2020) CuO coated olive cake nanocomposites for rapid phenol removal and effective discoloration of high strength olive mill wastewater. Chemosphere 253:126703. https://doi.org/10.1016/j.chemosphere.2020.126703
Zheng T et al (2016) Microwave regeneration of spent activated carbon for the treatment of ester-containing wastewater. RSC Adv 6:60815–60825. https://doi.org/10.1039/c6ra05211h
Acknowledgements
The authors are thankful to the Deanship of Research at King Fahd University of Petroleum & Minerals (KFUPM) for providing the support for this work through project SB191028, and also to the Civil and Environmental Engineering Department at KFUPM for providing the laboratory facilities.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Vohra, M., Hussaini, M. & Mohammad, T. Olive branches activated carbon: synthesis, phenol adsorption and modeling. Chem. Pap. 77, 485–498 (2023). https://doi.org/10.1007/s11696-022-02457-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11696-022-02457-w